Antiparasitic Bromotyrosine Derivatives from the Marine Sponge Verongula rigida
Abstract
:1. Introduction
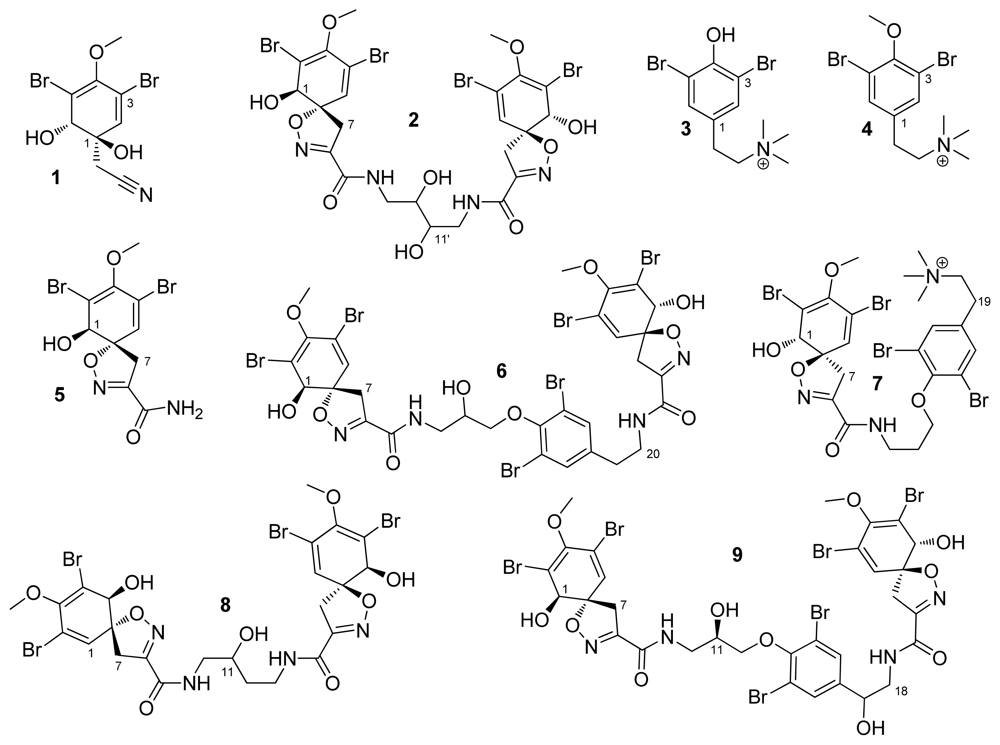
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Sponge Material
3.3. Extraction and Isolation
3.4. Bioassays
3.4.1. In Vitro Leishmanicidal Activity on Axenic and Intracellular Amastigotes
3.4.1.1. Activity against Axenic Amastigotes
3.4.1.2. Activity against Intracellular Amastigotes
3.4.2. Antimalarial Activity against Plasmodium Falciparum
3.4.3. Trypanocidal Activity
3.4.4. In Vitro Cytotoxic Activity in Mammalian Cells
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- World Health Organization, World Malaria Report 2010; WHO Press: Geneve, Switzerland, 2010; p. 238.
- World Health Organization, Control of the Leishmaniasis: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases; WHO Press: Geneve, Switzerland, 2010; Volume 949, p. 202.
- World Health Organization, Innovation for Health: Research that Makes a Difference: TDR Annual Report 2009; WHO Press: Geneve, Switzerland, 2010; p. 76.
- Orhan, I; Şener, B; Kaiser, M; Brun, R; Tasdemir, D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs 2010, 8, 47–58. [Google Scholar]
- Galeano, E; Martínez, A. Antimicrobial activity of marine sponges from Urabá Gulf, Colombian Caribbean region. J. Mycol. Med 2007, 17, 21–24. [Google Scholar]
- Galeano, E; Rojas, JJ; Martínez, A. Pharmacological developments obtained from marine natural products and current pipeline perspective. Nat. Prod. Commun 2011, 6, 287–300. [Google Scholar]
- Zabala, DA; Echavarria, B; Martínez, A. Inhibitory activity of some marine sponge extracts from Urabá Gulf on dihydrofolate reductase enzyme. Vitae 2008, 15, 285–289. [Google Scholar]
- Kochanowska, AJ; Rao, KV; Childress, S; El-Alfy, A; Matsumoto, RR; Kelly, M; Stewart, GS; Sufka, KJ; Hamann, MT. Secondary metabolites from three Florida sponges with antidepressant activity. J. Nat. Prod 2008, 71, 186–189. [Google Scholar]
- Ciminiello, P; Dell’Aversano, C; Fattorusso, E; Magno, S; Pansini, M. Chemistry of Verongida sponges. 9.1 Secondary metabolite composition of the Caribbean sponge Aplysina cauliformis. J. Nat. Prod 1999, 62, 590–593. [Google Scholar]
- Ciminiello, P; Dell’Aversano, C; Fattorusso, E; Magno, S; Pansini, M. Chemistry of Verongida sponges. 10.1 Secondary metabolite composition of the Caribbean sponge Verongula gigantea. J. Nat. Prod 2000, 63, 263–266. [Google Scholar]
- Xu, M; Andrews, KT; Birrell, GW; Tran, TL; Camp, D; Davis, RA; Quinn, RJ. Psammaplysin H, a new antimalarial bromotyrosine alkaloid from a marine sponge of the genus Pseudoceratina. Bioorg. Med. Chem. Lett 2011, 21, 846–848. [Google Scholar]
- Shaker, KH; Zinecker, H; Ghani, MA; Imhoff, JF; Schneider, B. Bioactive metabolites from the sponge Suberea sp. Chem. Biodivers 2010, 7, 2880–2887. [Google Scholar]
- Buchanan, MS; Carroll, AR; Wessling, D; Jobling, M; Avery, VM; Davis, RA; Feng, Y; Hooper, JNA; Quinn, RJ. Clavatadines C–E, guanidine alkaloids from the Australian sponge Suberea clavata. J. Nat. Prod 2009, 72, 973–975. [Google Scholar]
- Sepčić, K; Kauferstein, S; Mebs, D; Turk, T. Biological activities of aqueous and organic extracts from tropical marine sponges. Mar. Drugs 2010, 8, 1550–1566. [Google Scholar]
- Gorshkov, BA; Gorshkova, IA; Makarieva, TN; Stonik, VA. Inhibiting effect of cytotoxic bromine-containing compounds from sponges (Aplysinidae) on Na+-K+-ATPase activity. Toxicon 1982, 20, 1092–1094. [Google Scholar]
- Fendert, T; Wray, V; van Soest, RWM; Proksch, P. Bromoisoxazoline alkaloids from the Caribbean sponge Aplysina insularis. Z. Naturforsch. C 1999, 54, 246–252. [Google Scholar]
- Gunasekera, M; Gunasekera, SP. Dihydroxyaerothionin and Aerophobin 1. Two brominated tyrosine metabolites from the deep water marine sponge Verongula rigida. J. Nat. Prod 1989, 52, 753–756. [Google Scholar]
- Fulmor, W; van Lear, GE; Morton, GO; Mills, RD. Isolation and absolute configuration of the aeroplysinin I enantiomorphic pair from Ianthella ardis. Tetrahedron Lett 1970, 11, 4551–4552. [Google Scholar]
- Córdoba, R; Tormo, NS; Medarde, AF; Plumet, J. Antiangiogenic versus cytotoxic activity in analogues of aeroplysinin-1. Bioorg. Med. Chem 2007, 15, 5300–5315. [Google Scholar]
- Rodríguez-Nieto, S; González-Iriarte, M; Carmona, R; Muñoz-Chápuli, R; Medina, MA; Quesada, AR. Antiangiogenic activity of aeroplysinin-1, a brominated compound isolated from a marine sponge. FASEB J 2001, 16, 261–263. [Google Scholar]
- Kaul, PN; Sindermann, CJ. Drugs and Food from the Sea: Myth or Reality? University of Oklahoma Press: Norman, OK, USA, 1978; p. 448. [Google Scholar]
- Ciminiello, P; Fattorusso, E; Magno, S; Pansini, M. Chemistry of Verongida sponges, III. Constituents of a Caribbean Verongula sp. J. Nat. Prod 1994, 57, 1564–1569. [Google Scholar]
- Kobayashi, J; Honma, K; Sasaki, T; Tsuda, M. Purealidins J–R, new bromotyrosine alkaloids from the Okinawan marine sponge Psammaplysilla purea. Chem. Pharm. Bull 1995, 43, 403–407. [Google Scholar]
- Mancini, I; Guella, G; Laboute, P; Debitus, C; Pietra, F. Hemifistularin 3: A degraded peptide or biogenetic precursor? Isolation from a sponge of the order Verongida from the coral sea or generation from base treatment of 11-oxofistularin 3. J Chem Soc Perkin Trans 1 1993, 3121–3125. [Google Scholar]
- Kobayashi, J; Tsuda, M; Agemi, K; Shigemori, H; Ishibashi, M; Sasaki, T; Mikami, Y. Purealidins B and C, new bromotyrosine alkaloids from the Okinawan marine sponge Psammaplysilla purea. Tetrahedron 1991, 47, 6617–6622. [Google Scholar]
- Benharref, A; Païs, M. Bromotyrosine alkaloids from the aponge Pseudoceratina verrucosa. J. Nat. Prod 1996, 59, 177–180. [Google Scholar]
- Kernan, MR; Cambie, RC; Bergquist, PR. Chemistry of Sponges, VII. 11,19-Dideoxyfistularin 3 and 11-Hydroxyaerothionin, bromotyrosine derivatives from Pseudoceratina durissima. J. Nat. Prod 1990, 53, 615–622. [Google Scholar]
- El Sayed, KA; Bartyzel, P; Shen, X; Perry, TL; Zjawiony, JK; Hamann, MT. Marine natural products as nntituberculosis agents. Tetrahedron 2000, 56, 949–953. [Google Scholar]
- Acosta, AL; Rodríguez, AD. 11-Oxoaerothionin: A cytotoxic antitumor bromotyrosine-derived alkaloid from the Caribbean marine sponge Aplysina lacunosa. J. Nat. Prod 1992, 55, 1007–1012. [Google Scholar]
- Kalaitzis, JA; Leone, PA; Hooper, JNA; Quinn, RJ. Ianthesine E, a new bromotyrosine-derived metabolite from the Great Barrier Reef sponge Pseudoceratina sp. Nat. Prod. Res 2008, 22, 1257–1263. [Google Scholar]
- Gopichand, Y; Schmitz, FJ. Marine natural products: Fistularin-1, -2 and -3 from the sponge Aplysina fistularis forma fulva. Tetrahedron Lett 1979, 20, 3921–3924. [Google Scholar]
- de Oliveira, MF; de Oliveira, JHHL; Galetti, FCS; de Souza, AO; Silva, CL; Hajdu, E; Peixinho, S; Berlinck, RGS. Antimycobacterial brominated metabolites from two species of marine sponges. Planta Med 2006, 72, 437–441. [Google Scholar]
- Compagnone, RS; Avila, R; Suárez, AI; Abrams, OV; Rangel, HR; Arvelo, F; Piña, IC; Merentes, E. 11-Deoxyfistularin-3, a new cytotoxic metabolite from the Caribbean sponge Aplysina fistularis insularis. J. Nat. Prod 1999, 62, 1443–1444. [Google Scholar]
- Gunasekera, SP; Cross, SS. Fistularin 3 and 11-Ketofistularin 3. Feline Leukemia Virus active bromotyrosine metabolites from the marine sponge Aplysina archeri. J. Nat. Prod 1992, 55, 509–512. [Google Scholar]
- Blumer, C; Haas, D. Mechanism, regulation, and ecological role of bacterial cyanide biosynthesis. Arch. Microbiol 2000, 173, 170–177. [Google Scholar]
- Fattorusso, E; Minale, L; Sodano, G. Aeroplysinin-1, an antibacterial bromo-compound from the sponge Verongia aerophoba. J Chem Soc Perkin Trans 1 1972, 16–18. [Google Scholar]
- Granato, AC; de Oliveira, JHHL; Seleghim, MHR; Berlinck, RGS; Macedo, ML; Ferreira, AG; da Rocha, RM; Hajdu, E; Peixinho, S; Pessoa, CO; Moraes, MO; Cavalcanti, BC. Natural products from the ascidian Botrylloides giganteum, from the sponges Verongula gigantea, Ircinia felix, Cliona delitrix and from the nudibranch Tambja eliora, from the Brazilian coastline. Quim. Nova 2005, 28, 192–198. [Google Scholar]
- Rogers, EW; de Oliveira, MF; Berlinck, RGS; König, GM; Molinski, TF. Stereochemical heterogeneity in verongid sponge metabolites. Absolute stereochemistry of (+)-Fistularin-3 and (+)-11-epi-Fistularin-3 by microscale LCMS-Marfey’s analysis. J. Nat. Prod 2005, 68, 891–896. [Google Scholar]
- Taylor, VM; Muñoz, DL; Cedeño, DL; Vélez, ID; Jones, MA; Robledo, SM. Leishmania tarentolae: Utility as an in vitro model for screening of antileishmanial agents. Exp. Parasitol 2010, 126, 471–475. [Google Scholar]
- Varela M, RE; Lorena Muñoz, D; Robledo, SM; Kolli, BK; Dutta, S; Chang, KP; Muskus, C. Leishmania (Viannia) panamensis: An in vitro assay using the expression of GFP for screening of antileishmanial drug. Exp. Parasitol 2009, 122, 134–139. [Google Scholar]
- Agudelo, C; Corena-McLeod, M; Robledo, S. Carbonic anhydrase in Plasmodium falciparum: A useful target for antimalarial drug designing and malaria blocking transmission compounds. Vitae 2010, 17, 91–100. [Google Scholar]
- Buckner, F; Verlinde, C; La Flamme, A; van Voorhis, W. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother 1996, 40, 2592–2597. [Google Scholar]
| Compound | % of inhibition of the growth a | ||||
|---|---|---|---|---|---|
| U-937 cells (20 μM) | L. panamensis | P. falciparum Total forms (5 μM) | T. cruzi Intracellular amastigotes (10 μM) | ||
| Axenic amastigotes (20 μM) | Intracellular amastigotes (10 μM) | ||||
| 1 | 94.8 ± 3.6 | 0 | NE | 35.3 ± 3.5 | 29.1 ± 0.4 |
| 2 | 8.2 ± 1.7 | 0.3 ± 0.06 | 2.1 ± 0.4 | 7.9 ± 1.2 | 0 |
| 3 | 0 | 0 | NE | 0 | 0 |
| 4 | 5.3 ± 1.1 | 0 | NE | 0 | 0 |
| 5 | 45.3 ± 13.5 | 0 | NE | 7.1 ± 1.2 | 1.6 ± 0.3 |
| 6 | 0 | 0 | NE | 0 | 0.2 ± 0.03 |
| 7 | 0 | 1.6 ± 0.4 | 0 | 23.2 ± 1.0 | 0 |
| 8 | 0 | 0.0 | 12.6 ± 0.9 | 8.0 ± 0.5 | 0 |
| 9 | 58.2 ± 12.0 | 7.7 ± 1.6 | NE | 10.8 ± 1.5 | 6.3 ± 1.3 |
| Amphotericin B b | 53.2 | 60.4 ± 5.7 | 44.9 ± 7.1 | NA | NA |
| Chloroquine c | NA | NA | NA | 66.8 ± 1.3 | NA |
| Benznidazole d | NA | NA | NA | NA | 44.5 ± 2.7 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Galeano, E.; Thomas, O.P.; Robledo, S.; Munoz, D.; Martinez, A. Antiparasitic Bromotyrosine Derivatives from the Marine Sponge Verongula rigida. Mar. Drugs 2011, 9, 1902-1913. https://doi.org/10.3390/md9101902
Galeano E, Thomas OP, Robledo S, Munoz D, Martinez A. Antiparasitic Bromotyrosine Derivatives from the Marine Sponge Verongula rigida. Marine Drugs. 2011; 9(10):1902-1913. https://doi.org/10.3390/md9101902
Chicago/Turabian StyleGaleano, Elkin, Olivier P. Thomas, Sara Robledo, Diana Munoz, and Alejandro Martinez. 2011. "Antiparasitic Bromotyrosine Derivatives from the Marine Sponge Verongula rigida" Marine Drugs 9, no. 10: 1902-1913. https://doi.org/10.3390/md9101902
APA StyleGaleano, E., Thomas, O. P., Robledo, S., Munoz, D., & Martinez, A. (2011). Antiparasitic Bromotyrosine Derivatives from the Marine Sponge Verongula rigida. Marine Drugs, 9(10), 1902-1913. https://doi.org/10.3390/md9101902






