Marine Polysaccharides in Pharmaceutical Applications: An Overview
Abstract
:1. Introduction
2. Production, Applications and Modification Strategies of Marine Polysaccharides
2.1. Biotechnology of Marine Extremophylic Bacteria
2.2. Hydrogels and Superporous Hydrogels
2.3. Bioadhesivs and Mucoadhesives from Marine Sources
- maintain intimate contact with the site of application for 1 to 24 hours;
- be sufficiently adhesive and cohesive;
- guarantee controlled delivery of the active ingredients in wet and moist environments;
- be non-toxic, non irritating;
- be easily removable.
2.4. General Strategies of Modification of Marine Polysaccharides
2.4.1. Blending
2.4.2. Chemical Modifications
2.4.2.1. Hydrophobic Modification
2.4.2.2. Depolymerization
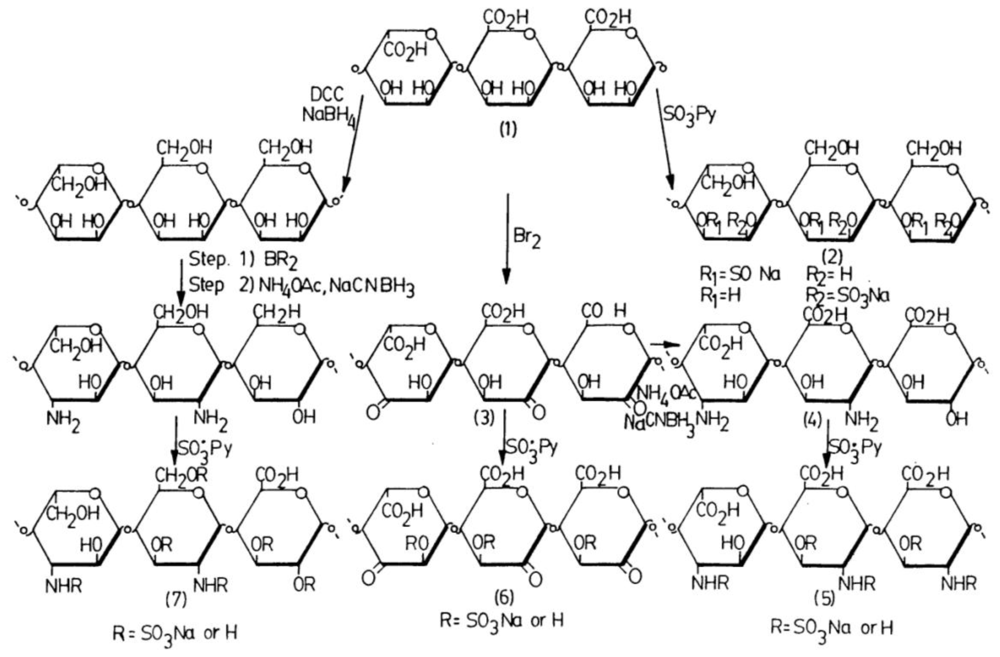
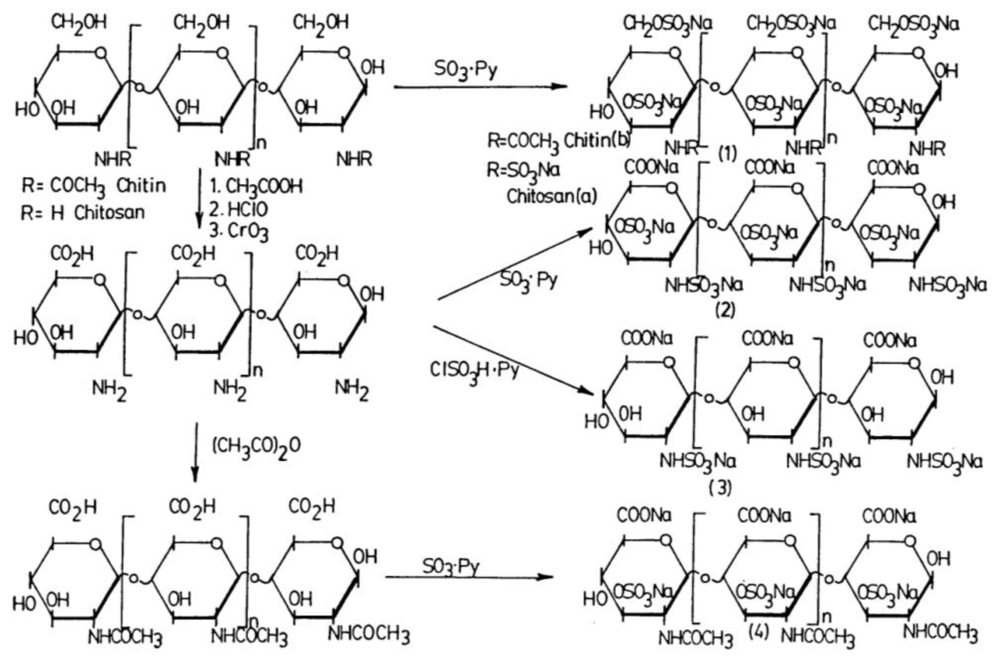
2.4.2.3. Sulfation
3. Examples of Applications of More Abundant Marine Polysaccharides in Pharmaceuticals
3.1. Alginate
Alginate for Wound Healing
3.2. Chitosan
3.3. Agar/Agarose and Carrageenans
3.4. Exopolysaccharides (EPS)
3.4.1. Biological Activity of EPSs
3.4.2. Exopolysaccharides from Cyanobacteria
4. Conclusions
- Samples Availability: Available from the authors.
References
- Nigrelli, RF; Stempien, MF; Ruggirei, GD; Liguori, VR; Cecil, JT. Substances of potential biomedical importance from marine organisms. Fed. Proc 1967, 26, 1197–1205. [Google Scholar]
- Immirzi, B; Laurienzo, P; Liquori, AM; Malinconico, M; Martuscelli, E; Orsello, G; Volpe, MG. Inorganic components of the macroalga ulva. Agro Food Ind. Hi Tec 1995, 6, 42–44. [Google Scholar]
- Carlsson, AS; van Beilen, J; Möller, R; Clayton, D. Bowles, D, Ed.; Micro- and macro-algae: Utility for industrial applications. In Outputs from the EPOBIO Project; CPL Science: Newbury, UK, 2007. [Google Scholar]
- De Pauw, N; Persoone, G. Borowitzka, MA, Borowitzka, LJ, Eds.; Microalgae for aquaculture. In Microalgal Biotechnology; Cambridge University: Cambridge, UK, 1988; pp. 197–221. [Google Scholar]
- Ramus, JS. The production of extracellular polysaccharides by the unicellular red alga Porphyridium eurugineum. J. Phycol 1972, 8, 97–111. [Google Scholar]
- Lewis, RA. Extracellular polysaccharides of green algae. Can. J. Microbiol 1956, 2, 665–672. [Google Scholar]
- Moore, BG; Tischer, RG. Extracellular polysaccharides of algae: Effects on life-support system. Science 1964, 145, 586–587. [Google Scholar]
- Ramus, JS. Algae biopolymer production. US Patent 4,236,349, 2 December 1980. [Google Scholar]
- Colwell, RR. Fulfilling the promise of biothechnology. Biotechnol. Adv 2002, 20, 215–228. [Google Scholar]
- Staley, JT; Castenholz, RW; Colwell, RR; Holt, JG; Kane, MD; Pace, NR; Salyers, AA; Tiedje, JM. The microbial world: Foundation of the biosphere. In Report from the American Academy of Microbiology; American Society for Microbiology: Washington, DC, USA, 1997. [Google Scholar]
- Burgess, JG; Jordan, EM; Bregu, M; Mearns-Spragg, A; Boyd, KG. Microbial antagonism: A neglected avenue of natural products research. J. Biotechnol 1999, 70, 27–32. [Google Scholar]
- Jensen, PR; Fenical, W. Strategies for the discovery of secondary metabolites from marine bacteria: Ecological perspectives. Annu. Rev. Microbiol 1994, 48, 559–584. [Google Scholar]
- Omidian, H; Rocca, JG; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar]
- Guilherme, MR; Reis, AV; Paulino, AT; Fajardo, AR; Muniz, EC; Tambourgi, EB. Superabsorbent hydrogel based on modified polysaccharide for removal of Pb2+ and Cu2+ from water with excellent performance. J. Appl. Polym. Sci 2007, 105, 2903–2909. [Google Scholar]
- Omidian, H; Rocca, JG; Park, K. Elastic, superporous hydrogel hybrids of polyacrylamide and sodium alginate. Macromol. Biosci 2006, 6, 703–710. [Google Scholar]
- Pourjavadi, A; Soleyman, R; Bardajee, GR; Ghavami, S. Novel superabsorbent hydrogel based on natural hybrid backbone: Optimized synthesis and its swelling behavior. Bull. Korean Chem. Soc 2009, 30, 2680–2686. [Google Scholar]
- Pourjavadi, A; Farhadpour, B; Seidi, F. Synthesis and investigation of swelling behavior of new agar based superabsorbent hydrogel as a candidate for agrochemical delivery. J. Polym. Res 2009, 16, 655–665. [Google Scholar]
- Pourjavadi, A; Barzegar, Sh; Mahdavinia, GR. MBA-crosslinked Na-Alg/CMC as a smart full-polysaccharide superabsorbent hydrogel. Carbohydr. Polym 2006, 66, 386–395. [Google Scholar]
- Hoffmann, B; Volkmer, E; Kokott, A; Augat, P; Ohnmacht, M; Sedlmayr, N; Schieker, M; Claes, L; Mutschle, W; Ziegler, G. Characterisation of a new bioadhesives system based on polysaccharides with the potential to be used as bone glue. J. Mater. Sci. Mater. Med 2009, 20, 2001–2009. [Google Scholar]
- Sever, MJ; Weisser, JT; Monahan, J; Srinivasan, S; Wilker, JJ. Metal-mediated cross-linking in the generation of a marine-mussel adhesive. Angew. Chem. Int. Ed. Engl 2004, 43, 448–450. [Google Scholar]
- Yu, M; Deming, TJ. Synthetic polypeptide mimics of marine adhesives. Macromolecules 1998, 31, 4739–4745. [Google Scholar]
- Deming, TJ. Mussel byssus and biomolecular materials. Curr. Opin. Chem. Biol 1999, 3, 100–105. [Google Scholar]
- Montanaro, L; Arciola, CR; Cenni, E; Ciapetti, G; Ravioli, F; Filippini, F; Barsanti, LA. Cytotoxicity, blood compatibility and antimicrobial activity of two cyanoacrylate glues for surgical use. Biomaterials 2001, 22, 59–66. [Google Scholar]
- Holte, O; Ons⊘yen, E; Myrvold, R; Karlsen, J. Sustained release of water-soluble drug from directly compressed alginate tablets. Eur. J. Pharm. Sci 2003, 20, 403–407. [Google Scholar]
- Tonnesen, HH; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm 2002, 28, 621–630. [Google Scholar]
- Rogovina, SZ; Vikhoreva, GA. Polysaccharide-based polymer blends: Methods of their production. Glycoconj. J 2006, 23, 611–618. [Google Scholar]
- Lee, YL; Shin, DS; Kwon, OW; Park, WH; Choi, HG; Lee, YR; Han, SS; Noh, SK; Lyoo, WS. Preparation of atactic poly(vinyl alcohol)/sodium alginate blend nanowebs by electrospinning. J. Appl. Polym. Sci 2007, 106, 1337–1342. [Google Scholar]
- Cho, SH; Oh, SH; Lee, JH. Fabrication and characterization of porous alginate/polyvinyl alcohol hybrid scaffolds for 3D cell culture. J. Biomater. Sci. Polym. Ed 2005, 16, 933–947. [Google Scholar]
- Avella, M; Di Pace, E; Immirzi, B; Impallomeni, G; Malinconico, M; Santagata, G. Addition of glycerol plasticizer to seaweeds derived alginates: influence of microstructure on chemical-physical properties. Carbohydr. Polym 2007, 69, 503–511. [Google Scholar]
- Daia, YN; Lia, P; Zhangc, JP; Wangc, AQ; Wei, Q. A novel pH sensitive N-succinyl chitosan/alginate hydrogel bead for nifedipine delivery. Biopharm. Drug Dispos 2008, 29, 173–184. [Google Scholar]
- Nobile, MR; Pirozzi, V; Somma, E; Gomez d’Ayala, G; Laurienzo, P. Development and rheological investigation of novel alginate/N-succinylchitosan hydrogels. J. Polym. Sci. B 2008, 46, 1167–1182. [Google Scholar]
- Gomez d’Ayala, G; De Rosa, A; Laurienzo, P; Malinconico, M. Development of a new calcium sulfate-based composite using alginate and chemically modified chitosan for bone regeneration. J. Biomed. Mater. Res. A 2007, 81, 811–820. [Google Scholar]
- Laurienzo, P; Di Stasio, M; Malinconico, M; Volpe, MG. Dehydration of apples by innovative bio-films drying. J. Food Eng 2010, 97, 491–496. [Google Scholar]
- Akiyoshi, K; Sunamoto, J. Supramolecular assembly of hydrophobized polysaccharides. Supramol. Sci 1996, 3, 157–163. [Google Scholar]
- Sato, T. Targetability of cell-specific liposomes coated with polysaccharide-cholesterol derivatives. Nippon Rinsho 1989, 47, 1402–1407. [Google Scholar]
- Matsukawa, S; Yamamoto, M; Ichinose, K; Ohata, N; Ishii, N; Kohji, T; Akiyoshi, K; Sunamoto, J; Kanematsu, T. Selective uptake by cancer cells of liposomes coated with polysaccharides bearing 1-aminolactose. Anticancer Res 2000, 20, 2339–2344. [Google Scholar]
- Shaikh, VAE; Maldar, NN; Lonikar, SV; Rajan, CR; Ponrathnam, S. Thermotropic behavior of cholesterol-linked polysaccharides. J. Appl. Polym. Sci 1998, 70, 195–201. [Google Scholar]
- Wang, Y; Hollingsworth, RI; Kasper, DL. Oxidative depolymerization of polysaccharides in aqueous solutions. Carbohydr. Res 1999, 319, 141–147. [Google Scholar]
- Uchida, K; Kawakishi, S. Oxidative depolymerization of polysaccharides induced by the ascorbic acid-copper ion systems. Agric. Biol. Chem 1986, 50, 2579–2583. [Google Scholar]
- Balakrishnan, B; Lesieur, S; Labarre, D; Jayakrishnan, A. Periodate oxidation of sodium alginate in water and in ethanol-water mixtures: a comparative study. Carbohydr. Res 2005, 340, 1425–1429. [Google Scholar]
- Dutton, GGS; Savage, AV; Mignon, M. Use of a bacteriophage to depolymerize a polysaccharide to an oligosaccharide; comparison of the 1H and 13C nuclear magnetic resonance spectra of the polymer and its hexasaccharide repeating unit. Can. J. Chem 1980, 58, 2588–2591. [Google Scholar]
- Karstens, T; Kettenbach, G; Seger, T; Stein, A; Steinmeyer, H; Mauer, G. Method for carrying out the targeted depolymerization of polysaccharides. US Patent WO/2001/007485, 1 February 2001. [Google Scholar]
- Chrinstensen, BE; Smidsr⊘d, O; Elgsaeter, A; Stokke, BT. Depolymerization of double-stranded xanthan by acid hydrolysis: characterization of partially degraded double strands and single-stranded oligomers released from the ordered structures. Macromolecules 1993, 26, 6111–6120. [Google Scholar]
- El-Sawy, NM; El-Rheim, HAA; Elbarbary, AM; Hegazy, ESA. Radiation induced degradation of chitosan for possible use as a growth promoter for agricultural purposes. Carbohydr. Polym 2010, 79, 555–562. [Google Scholar]
- Pawlowski, A; Svenson, SB. Electron beam fragmentation of bacterial polysaccharides as a method of producing oligosaccharides for the preparation of conjugate vaccines. FEMS Microbiol. Lett 1999, 174, 255–263. [Google Scholar]
- Trinchero, J; Ponce, NM; Cordoba, OL; Flores, ML; Pampuro, S; Stortz, CA; Salomon, H; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother. Res 2009, 23, 707–712. [Google Scholar]
- Schwartz-Albiez, R; Adams, Y; von der Lieth, CW; Mischnick, P; Andrews, KT; Kirschfink, M. Regioselectively modified sulfated cellulose as prospective drug for treatment of malaria tropica. Glycoconj. J 2007, 24, 57–65. [Google Scholar]
- Kaneko, Y; Havlik, I. Medicinal compositions, dose and method for treating malaria. European Patent 132,945,2A1, 2003. [Google Scholar]
- Kydonieus, A; Elson, C; Thanou, M. Drug delivery using sulfated chitinous polymers. US Patent 20,040,038,870, 26 February 2004. [Google Scholar]
- Lange, LG, III; Spilburg, CA. Use of sulfated polysaccharides to decrease cholesterol and fatty acid absorption. US Patent 5,063,210, 5 November 1991. [Google Scholar]
- Lewis, JG; Stanley, NF; Guist, GG. Lembi, CA, Waaland, JR, Eds.; Commercial production and applications of algal hydrocolloids. In Algae and Human Affairs; Cambridge University Press: Cambridge, UK, 1988; pp. 205–236. [Google Scholar]
- Russo, R; Malinconico, M; Santagata, G. Effect on cross-linking with calcium ions on the physical properties of alginate films. Biomacromolecules 2007, 8, 3193–3197. [Google Scholar]
- Merrill, EW; Salzman, EW. Polyethylene oxide as a biomaterial. Am. Soc. Artif. Intern. Organs J 1983, 6, 60–64. [Google Scholar]
- Han, DK; Park, KD; Ahn, KD; Jeong, SY; Kim, YH. Preparation and surface characterization of PEO-grafted and heparin-immobolized polyurethanes. J. Biomed. Mater. Res 1989, 23, 87–104. [Google Scholar]
- Tu, R; Lu, CL; Thyagarajan, K; Wang, E; Nguyen, H; Shen, S; Hata, C; Quijano, RC. Kinetic study of collagen fixation with polyepoxy fixatives. J. Biomed. Mater. Res 1993, 27, 3–9. [Google Scholar]
- Chen, JP; Chu, IM; Shiao, MY; Hsu, BRS; Fu, SH. Microencapsulation of islets in PEG-amine modified alginate-poly(l-lysine)-alginate microcapsules for constructing bioartificial pancreas. J. Ferment. Bioeng 1998, 86, 185–190. [Google Scholar]
- Chandy, T; Mooradian, DL; Rao, GHR. Chitosan/polyethylene glycol-alginate microcapsules for oral delivery of hirudin. J. Appl. Polym. Sci 1998, 70, 2143–2153. [Google Scholar]
- Qurrat-ul-Ain; Sharma, S; Khuller, GK; Garg, SK. Alginate-based oral drug delivery system for tuberculosis: Pharmacokinetics and therapeutic effects. J. Antimicrob. Chemother 2003, 51, 931–938. [Google Scholar]
- Jayant, RD; McShane, MJ; Srivastava, R. Polyelectrolyte-coated alginate microspheres as drug delivery carriers for dexamethasone release. Drug Deliv 2009, 16, 331–340. [Google Scholar]
- Kevadiya, BD; Joshi, GV; Patel, HA; Ingole, PG; Mody, HM; Bajaj, HC. Montmorillonite-alginate nanocomposites as a drug delivery system: Intercalation and in vitro release of vitamin B1 and vitamin B6. J. Biomater. Appl 2010, 25, 161–177. [Google Scholar]
- Miyazaki, S; Nakayama, A; Oda, M; Takada, M; Attwood, D. Chitosan and sodium alginate based bioadhesive tablets for intraoral drug delivery. Biol. Pharm. Bull 1994, 17, 745–747. [Google Scholar]
- Tapia, C; Escobar, Z; Costa, E; Sapag-Hagar, J; Valenzuela, F; Basualto, C; Gai, MN; Yazdani-Pedram, M. Comparative studies on polyelectrolyte complexes and mixtures of chitosan-alginate and chitosan-carrageenan as prolonged diltiazem clorohydrate release systems. Eur. J. Pharm. Biopharm 2004, 57, 65–75. [Google Scholar]
- Gavini, E; Sanna, V; Juliano, C; Bonferoni, MC; Giunchedi, P. Mucoadhesive vaginal tablets as veterinary delivery system for the controlled release of an antimicrobial drug, acriflavine. AAPS PharmSciTech 2002, 3, E20. [Google Scholar]
- El-Kamel, A; Sokar, M; Naggar, V; Al Gamal, S. Chitosan and sodium alginate-based bioadhesive vaginal tablets. AAPS PharmSci 2002, 4, E44. [Google Scholar]
- Strand, BL; Gaserod, O; Kulseng, B; Espevik, T; Skjak-Braek, G. Alginate-polylisine-alginate microcapsules: Effect of size reduction on capsule properties. J. Microencapsul 2002, 19, 612–630. [Google Scholar]
- Mumper, RJ; Hoffman, AS; Puolakkainen, PA; Bouchard, LS; Gombotz, WR. Calcium-alginate beads for the oral delivery of transforming growth factor-β1 (TGF-β1): stabilization of TGF-β1 by the addition of polyacrylic acid within acid-treated beads. J. Control. Release 1994, 30, 241–251. [Google Scholar]
- Shojaei, AH; Paulson, J; Honary, S. Evaluation of poly(acrylic acid-co-ethylhexyl acrylate) films for mucoadhesive transbuccal drug delivery: factors affecting the force of mucoadhesion. J. Control. Release 2000, 67, 223–232. [Google Scholar]
- Laurienzo, P; Malinconico, M; Mattia, G; Russo, R; La Rotonda, MI; Quaglia, F; Capitani, D; Mannina, L. Novel alginate-acrylic polymers as a platform for drug delivery. J. Biomed. Mater. Res. A 2006, 78, 523–531. [Google Scholar]
- Kang, HA; Shin, MS; Yang, JW. Preparation and characterization of hydrophobically modified alginate. Polym. Bull 2002, 47, 429–435. [Google Scholar]
- Rowley, JA; Madlambayan, G; Mooney, DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 1999, 20, 45–53. [Google Scholar]
- Seifert, DB; Phillips, JA. Porous alginate-poly(ethylene glycol) entrapment system for the cultivation of mammalian cells. Biotechnol. Prog 1997, 13, 569–576. [Google Scholar]
- Laurienzo, P; Malinconico, M; Motta, A; Vicinanza, A. Synthesis of a novel alginate-poly(ethylene glycol) graft copolymer for cell immobilization. Carbohydr. Polym 2005, 62, 274–282. [Google Scholar]
- Gilchrist, T; Martin, AM. Wound treatment with Sorban-an alginate fibre dressing. Biomaterials 1983, 4, 317–320. [Google Scholar]
- Motta, GJ. Calcium alginate topical wound dressings: a new dimension in the cost-effective treatment for exudating dermal wounds and pressure sores. Ostomy Wound Manage 1989, 25, 52–56. [Google Scholar]
- Doyle, JW; Roth, TP; Smith, RM; Li, Y-Q; Dunn, RM. Effects of calcium alginate on cellular wound healing processes modelled in vitro. J. Biomed. Mater. Res 1996, 32, 561–568. [Google Scholar]
- Berry, DP; Bale, S; Harding, KG. Dressings for treating cavity wounds. J. Wound Care 1996, 5, 10–17. [Google Scholar]
- Segan, HT; Hunt, BJ; Gilding, K. The effects of alginate and non-alginate wound dressings on blood coagulation and platelet activation. J. Biomater. Appl 1998, 12, 249–257. [Google Scholar]
- Odell, EW; Oades, P; Lombardi, T. Symptomatic foreign body reaction to haemostatic alginate. Br. J. Oral Maxillofac. Surg 1994, 32, 178–179. [Google Scholar]
- Burrows, F; Louime, C; Abazinge, M; Onokpise, O. Extraction and evaluation of chitin from crub exoskeleton as a seed fungicide and plant growth enhancer. Amer.-Eurasian J. Agric. Environ. Sci 2007, 2, 103–111. [Google Scholar]
- Muzzarelli, RAA; Muzzarelli, C. Chitosan chemistry: Relevance to the biomedical sciences. Adv. Polym. Sci 2005, 186, 151–209. [Google Scholar]
- Kumar, MNV; Muzzarelli, RAA; Muzzarelli, C; Sashiwa, H; Domb, AJ. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev 2004, 104, 6017–6084. [Google Scholar]
- Geng, X; Kwon, OH; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar]
- Chow, KS; Khor, E. Novel fabrication of open-pore chitin matrices. Biomacromolecules 2000, 1, 61–67. [Google Scholar]
- Suh, JKF; Matthew, HWT. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar]
- Di Martino, A; Sittinger, M; Risbud, MV. Chitosan: A versatile biopolymer for orthopaedic tissue engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar]
- Chenite, A; Chaput, C; Wang, D; Combes, C; Buschmann, MD; Hoemann, CD; Leroux, JC; Atkinson, BL; Binette, F; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar]
- Wang, X; Yan, Y; Zhang, R. A comparison of chitosan and collagen sponges as hemostatic dressings. J. Bioact. Compat. Polym 2006, 21, 39–54. [Google Scholar]
- Tuzlakoglu, K; Alves, CM; Mano, JF; Reis, RL. Production and characterization of chitosan fibers and 3-D fiber mesh scaffolds for tissue engineering applications. Macromol. Biosci 2004, 4, 811–819. [Google Scholar]
- Zhang, Y; Zhang, M. Cell growth and function on calcium phosphate reinforced chitosan scaffolds. J. Mater. Sci. Mater. Med 2004, 15, 255–260. [Google Scholar]
- Xia, W; Liu, W; Cui, L; Liu, Y; Zhong, W; Liu, D; Wu, J; Chua, K; Cao, Y. Tissue engineering of cartilage with the use of chitosan–gelatin complex scaffolds. J. Biomed. Mater. Res 2004, 71B, 373–380. [Google Scholar]
- Risbud, M; Ringe, J; Bhonde, R; Sittinger, M. In vitro expression of cartilage-specific markers by chondrocytes on a biocompatible hydrogel: implications for engineering cartilage tissue. Cell Transplant 2001, 10, 755–763. [Google Scholar]
- Hu, Q; Li, B; Wang, M; Shen, J. Preparation and characterization of biodegradable chitosan/hydroxyapatite nanocomposite rods via in situ hybridization: a potential material as internal fixation of bone fracture. Biomaterials 2004, 25, 779–785. [Google Scholar]
- Iwasaki, N; Yamane, S-T; Majima, T; Kasahara, Y; Minami, A; Harada, K; Nonaka, S; Maekawa, N; Tamura, H; Tokura, S; Shiono, M; Monde, K; Nishimura, S-I. Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules 2004, 5, 828–833. [Google Scholar]
- Gåser⊘d, O; Smidsr⊘d, O; Skjåsk-Bræk, G. Microcapsules of alginate-chitosan-I. A quantitative study of the interaction between alginate and chitosan. Biomaterials 1998, 19, 1815–1825. [Google Scholar]
- Peniche, C; Arguëlles-Monal, W; Davidenko, N; Sastre, R; Gallardo, A; San Román, J. Self-curing membranes of chitosan/PAA IPNs obtained by radical polymerization: preparation, characterization and interpolymer complexation. Biomaterials 1999, 20, 1869–1878. [Google Scholar]
- Leroux, L; Hatim, Z; Freche, M; Lacout, JL. Effects of various adjuvants (lactic acid, glycerol, and chitosan) on the injectability of a calcium phosphate cement. Bone 1999, 25, 31S–34S. [Google Scholar]
- Zong, Z; Kimura, Y; Takahashi, M; Yamane, H. Characterization of chemical and solid state structures of acylated chitosans. Polymer 2000, 41, 899–906. [Google Scholar]
- Hirano, S; Ohe, Y; Ono, H. Selective N-acylation of chitosan. Carbohydr. Res 1976, 47, 315–320. [Google Scholar]
- Moore, GK; Roberts, GAF. Reactions of chitosan: 2. Preparation and reactivity of N-acyl derivatives of chitosan. Int. J. Biol. Macromol 1981, 3, 292–296. [Google Scholar]
- Nishimura, SI; Kohgo, O; Kurita, K. Chemospecific manipulations of a rigid polysaccharide: Syntheses of novel chitosan derivatives with excellent solubility in common organic solvents by regioselective chemical modifications. Macromolecules 1991, 24, 4745–4748. [Google Scholar]
- Yalpani, M; Hall, LD. Some chemical and analytical aspects of polysaccharide modifications. III. Formation of branched-chain, soluble chitosan derivatives. Macromolecules 1984, 17, 272–281. [Google Scholar]
- Chenite, A; Chaput, C; Wang, D; Combes, C; Buschmann, MD; Hoemann, CD; Leroux, JC; Atkinson, BL; Binette, F; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar]
- Mi, FL; Sung, HW; Shyu, SS; Su, CC; Peng, CK. Synthesis and characterization of biodegradable TPP/genipin co-crosslinked chitosan gel beads. Polymer 2003, 44, 6521–6530. [Google Scholar]
- Akao, T; Kobashi, K; Aburada, M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull 1994, 17, 1573–1576. [Google Scholar]
- Mi, FL; Shyu, SS; Lee, ST; Wong, TB. Kinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method. J. Polym. Sci. B Polym. Phys 1999, 37, 1551–1564. [Google Scholar]
- Shu, XZ; Zhu, KJ. A novel approach to prepare tripolyphosphate/chitosan complex beads for controlled drug delivery. Int. J. Pharm 2000, 201, 51–58. [Google Scholar]
- Shu, XZ; Zhu, KJ. Chitosan/gelatin microspheres prepared by modified emulsification and ionotropic gelation. J. Microencapsul 2001, 18, 237–245. [Google Scholar]
- Shu, XZ; Zhu, KJ; Song, W. Novel pH-sensitive citrate cross-linked chitosan film for drug controlled release. Int. J. Pharm 2001, 212, 19–28. [Google Scholar]
- Koa, JA; Park, HJ; Hwang, SJ; Park, JB; Lee, JS. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm 2002, 249, 165–174. [Google Scholar]
- Aral, C; Akbuđa, J. Alternative approach to the preparation of chitosan beads. Int. J. Pharm 1998, 168, 9–15. [Google Scholar]
- Hejazi, R; Amiji, M. Chitosan-based gastrointestinal delivery systems. J. Control. Release 2003, 89, 151–165. [Google Scholar]
- Illum, L; Jabbal-Gill, I; Hinchcliffe, M; Fisher, AN; Davis, SS. Chitosan as a novel nasal delivery system for vaccines. Adv. Drug Deliv. Rev 2001, 51, 81–96. [Google Scholar]
- Yao, KD; Xu, MX; Yin, YJ; Zhao, JY; Chen, XL. pH-sensitive chitosan/gelatin hybrid polymer network microspheres for delivery of cimetidine. Polym. Int 1996, 39, 333–337. [Google Scholar]
- Chen, SC; Wu, YC; Mi, FL; Lin, YH; Yu, LC; Sung, HW. A novel pH-sensitive hydrogel composed of N,O-carboxymethyl chitosan and alginate cross-linked by genipin for protein drug delivery. J. Control. Release 2004, 96, 285–300. [Google Scholar]
- Liu, L; Li, Y; Liu, H; Fang, Y. Synthesis and characterization of chitosan-graftpolycaprolactone copolymers. Eur. Polym. J 2004, 40, 2739–2744. [Google Scholar]
- Yu, H; Wang, W; Chen, X; Deng, C; Jing, X. Synthesis and characterization of the biodegradable polycaprolactone-graft-chitosan amphiphilic copolymers. Biopolymers 2006, 83, 233–242. [Google Scholar]
- Guan, X; Quan, D; Shuai, X; Liao, K; Mai, K. Chitosan-graft-poly(ε-caprolactone)s: An optimized chemical approach leading to a controllable structure and enhanced properties. J. Polym. Sci. A Polym. Chem 2007, 45, 2556–2568. [Google Scholar]
- Bhattarai, N; Ramay, HR; Gunn, J; Matsen, FA; Zhang, M. PEG-graft-chitosan as an injectable thermosensitive hydrogel for sustained protein release. J. Control. Release 2005, 103, 609–624. [Google Scholar]
- Lu, Y; Liu, L; Guo, S. Novel amphiphilic ternary polysaccharide derivates chitosan-g-PCL-g-mPEG: Syntesis, characterization and aggregation in aqueous solutions. Biopolymers 2007, 86, 403–408. [Google Scholar]
- Duan, K; Zhang, X; Tang, X; Yu, J; Liu, S; Wang, D; Li, Y; Huang, J. Fabrication of cationic nanomicelle from chitosan-graft-polycaprolactone as the carrier of 7-ethyl-10-hydroxy-camptothecin. Colloids Surf. B 2010, 76, 475–482. [Google Scholar]
- Wong, TW. Chitosan and Its Use in Design of Insulin Delivery System. Recent Pat. Drug Deliv. Formul 2009, 3, 8–25. [Google Scholar]
- Ma, Z; Lim, LY. Uptake of chitosan and associated insulin in Caco-2 cell monolayers: A comparison between chitosan molecules and chitosan nanoparticles. Pharm. Res 2003, 20, 1812–1819. [Google Scholar]
- Morille, M; Passirani, C; Vonarbourg, A; Clavreul, A; Benoit, JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials 2008, 29, 3477–3496. [Google Scholar] [Green Version]
- Brown, MD; Schätzlein, AG; Uchegbu, IF. Gene delivery with synthetic (non viral) carriers. Int. J. Pharm 2001, 229, 1–21. [Google Scholar]
- Erbacher, P; Zou, S; Bettinger, T; Steffan, AM; Remy, JS. Chitosan-based vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability. Pharm. Res 1998, 15, 1332–1339. [Google Scholar]
- Koping-Hoggard, M; Tubulekas, I; Guan, H; Edwards, K; Nilsson, M; Varum, KM; Artursson, P. Chitosan as a nonviral gene delivery system: structure-property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther 2001, 8, 1108–1121. [Google Scholar]
- Ravi Kumar, MNV; Bakowsky, U; Lehr, CM. Preparation and characterization of cationic PLGA nanospheres as DNA carriers. Biomaterials 2004, 25, 1771–1777. [Google Scholar]
- Katas, H; Alpar, HO. Development and characterisation of chitosan nanoparticles for siRNA delivery. J. Control. Release 2006, 115, 216–225. [Google Scholar]
- Sarasam, AR; Brown, P; Khajotia, SS; Dmytryk, JJ; Madihally, SV. Antibacterial activity of chitosan-based matrices on oral pathogens. J. Mater. Sci. Mater. Med 2008, 19, 1083–1090. [Google Scholar]
- Aimin, C; Chunlin, H; Juliang, B; Tinyin, Z; Zhichao, D. Antibiotic loaded chitosan bar. An in vitro, in vivo study of a possible treatment for osteomyelitis. Clin. Orthop 1999, 366, 239–247. [Google Scholar]
- Hu, SG; Jou, CH; Yang, MC. Protein adsorption, fibroblast activity and antibacterial properties of poly(3-hydroxybutyric acid-co-3-hydroxyvaleric acid) grafted with chitosan and chitooligosaccharide after immobilized with hyaluronic acid. Biomaterials 2003, 24, 2685–2693. [Google Scholar]
- El, Salmawi KM. Gamma-radiation-induced crosslinked PVA/chitosan blends for wound dressing. J. Macromol. Sci. A Pure Appl. Chem 2007, 44, 541–545. [Google Scholar]
- Gupta, KC; Ravi Kumar, MNV. Drug release behaviour of beads and microgranules of chitosan. Biomaterials 2000, 21, 1115–1119. [Google Scholar]
- Boddohi, S; Almodóvar, J; Zhang, H; Johnson, PA; Kipper, MJ. Layer-by-layer assembly of polysaccharide-based nanostructured surfaces containing polyelectrolyte complex nanoparticles. Colloids Surf. B 2010, 77, 60–68. [Google Scholar]
- Araki, C. Some recent studies on the polysaccharides of agarophytes. Proc. Int. Seaweed Symp 1966, 5, 3–19. [Google Scholar]
- Stephen, AM; Phillips, GO; Williams, PA. Food Polysaccharides and Their Applications; Marcel Dekker: New York, NY, USA, 1995; pp. 187–203. [Google Scholar]
- Glicksman, M. Blanshard, JMV, Mitchell, JR, Eds.; Gelling hydrocolloids in product applications. In Polysaccharides in Foods; Butterworths: London, UK, 1979. [Google Scholar]
- Armisen, R; Galatas, F. Philips, GO, Williams, PA, Eds.; Agar. In Handbook of Hydrocolloids; CRC: New York, NY, USA, 2000. [Google Scholar]
- Norziah, MH; Foo, SL; Karim, A. Abd. Rheological studies on mixtures of agar (Gracilaria changii) and k-carrageenan. Food Hydrocol 2006, 20, 204–217. [Google Scholar]
- Morris, VJ. Mitchell, JA, Ledwards, DA, Eds.; Gelation of polysaccharide. In Functional Properties of Food Macromolecules; Elsevier: London, UK, 1986; pp. 121–170. [Google Scholar]
- Schafer, SF; Steven, FS. A reexamination of the double-helix model for agarose gel using optical rotation. Biopolymers 1995, 36, 103–108. [Google Scholar]
- Medina-Esquivel, R; Freile-Pelegrin, Y; Quintana-Owen, P; Yáñez-Limón, JM; Alvarado-Gil, JJ. Measurement of the Sol-Gel Transition Temperature in Agar. Int. J. Thermophys 2008, 29, 2036–2045. [Google Scholar]
- Arnott, S; Fulner, A; Scott, WE; Dea, ICM; Morehouse, R; Rees, DA. The agarose double helix and its function in agarose gel structure. J. Mol. Biol 1974, 90, 269–284. [Google Scholar]
- Bao, L; Yang, W; Mao, X; Mou, S; Tang, S. Agar/collagen membrane as skin dressing for wounds. Biomed. Mater 2008, 3, 044108. [Google Scholar] [CrossRef]
- Dumitriu, S. Polysaccharides: Structural Diversity and Functional Versatility; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Adachi, M; Watanabe, S. Evaluation of combined deactivators-supplemented agar medium (CDSAM) for recovery of dermatophytes from patients with tinea pedis. Med. Mycol 2007, 45, 347–349. [Google Scholar]
- Knox, R; Woodroffe, R. Semi-solid agar media for rapid drug sensitivity tests on cultures of Mycobacterium tuberculosis. J. Gen. Microbiol 1957, 16, 647–659. [Google Scholar]
- Yew, WW; Tonb, SCW; Lui, KS; Leung, SKF; Chau, CH; Wang, EP. Comparison of MB/BacT system and agar proportion method in drug susceptibility testing of Mycobacterium tuberculosis. Diagn. Microbiol. Infect. Dis 2001, 39, 229–232. [Google Scholar]
- Nakano, M; Nakamur, Y; Takikawa, K; Kouketsu, M; Arita, T. Sustained release of sulfamethizole from agar beads. J. Pharm. Pharmacol 1979, 31, 869–872. [Google Scholar]
- Kojima, T; Hashida, M; Muranishi, S; Sezaki, H. Antitumor activity of timed-release derivative of mitomycin C, agarose bead conjugate. Chem. Pharm. Bull 1978, 26, 1818–1824. [Google Scholar]
- El-Raheem El-Helw, A; El-Said, Y. Preparation and characterization of agar beads containing phenobarbitone sodium. J. Microencapsul 1988, 5, 159–163. [Google Scholar]
- Prasad, K; Mehta, G; Meena, R; Siddhanta, AK. Hydrogel-forming Agar-graft-PVP and k-Carrageenan-graft-PVP blends: Rapid synthesis and characterization. J. Appl. Polym. Sci 2006, 102, 3654–3663. [Google Scholar]
- Lugao, AB; Machado, LDB; Miranda, LF; Alveraz, MR; Roziak, JM. Study of wound dressing structure and hydration/dehydration properties. Radiat. Phys. Chem 1998, 52, 319–322. [Google Scholar]
- Nichols, CA; Guezennec, J; Bowman, JP. Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice and deep-sea hydrothermal vents: A review. Mar. Biotechnol 2005, 7, 253–271. [Google Scholar]
- Weiner, R; Langille, S; Quintero, E. Structure, function an immunochemistry of bacterial exopolysaccharides. J. Ind. Microbiol 1995, 15, 339–346. [Google Scholar]
- Sun, C; Wang, JW; Fang, L; Gao, XD; Tan, RX. Free radical scavenging and antioxidant activities of EPS2, an exopolysaccharide produced by a marine filamentous fungus Keissleriella sp. YS 4108. Life Sci 2004, 75, 1063–1073. [Google Scholar]
- Sun, C; Shan, CY; Gao, XD; Tan, RX. Protection of PC12 cells from hydrogen peroxide-induced injury by EPS2, an exopolysaccharide from a marine filamentous fungus Keissleriella sp. YS 4108. J. Biotechnol 2005, 115, 137–144. [Google Scholar]
- Liu, F; Ng, TB. Antioxidative and free radical scavenging activities of selected medicinal herbs. Life Sci 2000, 66, 725–735. [Google Scholar]
- Schinella, GR; Tournier, HA; Prieto, JM; Mordujovich de Buschiazzo, P; Ríos, JL. Antioxidant activity of anti-inflammatory plant extracts. Life Sci 2002, 70, 1023–1033. [Google Scholar]
- Grice, HC. Safety evaluation of butylated hydroxyanisole from the perspective of effects on forestomach and oesophageal squamous epithelium. Food Chem. Toxicol 1988, 26, 717–723. [Google Scholar]
- Qi, HM; Zhang, QB; Zhao, TT; Chen, R; Zhang, H; Niu, X; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int. J. Biol. Macromol 2005, 37, 195–199. [Google Scholar]
- Sun, H-H; Mao, W-J; Chen, Y; Guo, S-D; Li, H-Y; Qi, X-H; Chen, Y-L; Xu, J. Isolation, chemical characteristics and antioxidant properties of the polysaccharides from marine fungus Penicillium sp. F23-2. Carbohydr. Polym 2009, 78, 117–124. [Google Scholar]
- Ofek, I; Beachey, EH; Sharon, N. Surface sugars of animal cells as determinants of recognition in bacterial adherence. Trends Biochem. Sci 1978, 3, 159–160. [Google Scholar]
- Bergey, E; Stinson, M. Heparin-inhibitable basement membrane-binding protein of Streptococcus pyogenes. Infect. Immun 1988, 56, 1715–1721. [Google Scholar]
- Bellamy, F; Horton, D; Millet, J; Picart, F; Samreth, S; Chana, JB. Glycosylated derivatives of benzophenone, benzhydrol, and benzhydril as potential venous antithrombotic agents. J. Med. Chem 1993, 36, 898–903. [Google Scholar]
- Cassaro, CMF; Dietrich, CP. Distribution of sulfated mucopolysaccharides in invertebrates. J. Biol. Chem 1977, 252, 2254–2261. [Google Scholar]
- Höök, M; Kjellen, L; Johansson, S; Robinson, J. Cell surface glycosaminoglycans. Ann. Rev. Biochem 1984, 53, 847–869. [Google Scholar]
- Wight, TN; Kinsella, MG; QwarnstroÈm, E. The role of proteglycans in cell adhesion, migration and proliferation. Curr. Opin. Cell Biol 1992, 4, 793–801. [Google Scholar]
- Guzman-Murillo, MA; Ascencio, F. Anti-adhesive activity of sulfated exopolysaccharides of microalgae on attachment of red sore disease-associated bacteria and Helicobacter pylori to tissue culture cells. Lett. Appl. Microbiol 2000, 30, 473–478. [Google Scholar]
- Ascencio, F; Fransson, LA; Wadström, T. Affinity of the gastric pathogen Helicobacter pylori for the N-sulfated glycosaminoglycan heparan sulfate. J. Med. Microbiol 1993, 38, 240–244. [Google Scholar]
- SjÖustrÖum, JE; Larsson, H. Factors affecting growth and antibiotic susceptibility of Helicobacter pylori: Effect of pH and urea on the survival of a wild-type strain and a urease-deficient mutant. J. Med. Microbiol 1996, 44, 425–433. [Google Scholar]
- SjÖustrÖum, JE; Fryklund, J; KoÈhler, T; Larsson, H. In vitro antibacterial activity of omeprazole and its selectivity for Helicobacter spp. are dependent on incubation conditions. Antimicrob. Agents Chemother 1996, 40, 621–626. [Google Scholar]
- Chihara, G; Hamuro, J; Maeda, Y; Araki, Y; Fukuoka, F. Fractionation and purification of the polysaccharides with marked antitumor activity, especially lentinan, from Lentinus edodes (Berk) Sing (an edible mushroom). Cancer Res 1970, 30, 2776–2781. [Google Scholar]
- Yoshizawa, Y; Enomoto, A; Todoh, H; Ametani, A; Kaminogawa, S. Activation of murine macrophages by polysaccharide fractions from marine algae (Porphyra yezoensis). Biosci. Biotechnol. Biochem 1993, 57, 1862–1866. [Google Scholar]
- Matsuda, M; Yamori, T; Naitoh, M; Okutani, K. Structural revision of sulfated polysaccharide B-1 isolated from a marine pseudomonas species and its cytotoxic activity against human cancer cell lines. Mar. Biotechnol 2003, 5, 13–19. [Google Scholar]
- Colliec-Jouault, S; Zanchetta, P; Helley, D; Ratiskol, J; Sinquin, C; Fischer, AM; Guezennec, J. Les polysaccharides microbiens d’origine marine et leur potentiel en thérapeutique humaine. Pathologie Biologie 2004, 52, 127–130. [Google Scholar]
- Guezennec, J; Pignet, P; Lijour, Y; Gentric, E; Ratiskol, J; Colliec-Jouault, S. Sulfation and depolymerization of a bacterial exopolysaccharide of hydrothermal origin. Carbohydr. Polym 1998, 37, 19–24. [Google Scholar]
- De Philippis, R; Sili, C; Paperi, R; Vincenzini, M. Exopolysaccharide-producing cyanobacteria and their possibile exploitation: A review. J. Appl. Phycol 2001, 13, 293–299. [Google Scholar]
- Nicolaus, B; Panico, A; Lama, L; Romano, I; Manca, MC; De Giulio, A; Gambacorta, A. Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous cyanobacteria. Phytochemistry 1999, 52, 639–647. [Google Scholar]
- Sutherland, IW. Biotechnology of Microbial Exopolysaccharides; Cambridge University Press: Cambridge, UK, 1990; p. 163. [Google Scholar]
- Atkins, EDT. Biomolecular structures of naturally occurring carbohydrate polymers. Int. J. Biol. Macromol 1986, 8, 323–329. [Google Scholar]
- Sutherland, IW. Novel and established applications of microbial polysaccharides. Tibtech 1998, 16, 41–46. [Google Scholar]
- De Vuyst, L; Degeest, B. Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol. Rev 1999, 23, 153–177. [Google Scholar]
- Flaibani, A; Olsen, Y; Painter, TJ. Polysaccharides in desert reclamation: Composition of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydr. Res 1989, 190, 235–248. [Google Scholar]
- Shepherd, R; Rockey, J; Sutherland, IW; Roller, S. Novel bioemulsifiers from microorganisms for use in foods. J. Biotechnol 1995, 40, 207–217. [Google Scholar]
- Sutherland, IW. Structure-function relationships in microbial exopolysaccharides. Biotech. Adv 1994, 12, 393–448. [Google Scholar]
- Hayashi, K; Hayashi, T; Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retrovir 1996, 12, 1463–1471. [Google Scholar]
- Hayashi, T; Hayashi, K. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. (Lloydia) 1996, 59, 83–87. [Google Scholar]
| Product | Production (t y−1) | Algae Harvested (t y−1) | Comments |
|---|---|---|---|
| Carrageenan | 33,000 | 168,400 | Mainly Eucheuma and Kappaphycus |
| Alginate | 30,000 | 126,500 | Laminaria, Macrocystis, Lessonia, Ascophyllum and others |
| Agar | 7,630 | 55,650 | Mainly Gelidium and Gracilaria |
| Role of Exopolymer | Example |
|---|---|
| Assists in attachment to surfaces | Exopolymers of marine Vibrio MH3 were involved in reversible attachment. Cross-linking of adjacent polysaccharide chains aided in permanent adhesion. |
| Facilitates biochemical interactions between cells | Exopolymer mediated bacterial attachment to the polar end of blue-green N2-fixing alga. EPS aided attachment to symbiotic host such as vent tube worm to absorb metals and detoxify microenvironment. Exopolymer buffered against sudden osmotic changes. |
| Provides protective barrier around the cell | Bacteria in aggregates were less preferred by grazers than freely suspended bacteria. EPS-producing deep-sea hydrothermal vent bacteria showed resistance to heavy metals. Metal binding involves cell wall components as well as polysaccharides. Exopolymer in sea-ice brine channels provided cryoprotection by interacting with water at low temperature to depress freezing point. Nutrient uptake by bacteria in aggregates was higher than for free-living cells in low nutrient systems. |
| Absorbs dissolved organic material | Porous and hydrated matrix acts like a sponge and sequesters and concentrates dissolved organics. |
| Type | Component | Example | Mode of Linkage |
|---|---|---|---|
| Sugar | Pentoses | d-Arabinose | |
| d-Ribose | |||
| d-Xylose | |||
| Hexoses | d-Glucose | ||
| d-Mannose | |||
| d-Galactose | |||
| d-Allose | |||
| l-Ramnose | |||
| l-Fucose | |||
| Amino sugars | d-Glucosamine | ||
| d-Galactosamine | |||
| Uronic acids | d-Glucuronic acid | ||
| d-Galacturonic acid | |||
| Non sugar | Acetic acid | O-acyl, N-acyl | |
| Succinic acid | O-acyl | ||
| Pyruvic acid | Acetal | ||
| Phosphoric acid | Ester, Diester | ||
| Sulfuric acid | Ester |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Laurienzo, P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Mar. Drugs 2010, 8, 2435-2465. https://doi.org/10.3390/md8092435
Laurienzo P. Marine Polysaccharides in Pharmaceutical Applications: An Overview. Marine Drugs. 2010; 8(9):2435-2465. https://doi.org/10.3390/md8092435
Chicago/Turabian StyleLaurienzo, Paola. 2010. "Marine Polysaccharides in Pharmaceutical Applications: An Overview" Marine Drugs 8, no. 9: 2435-2465. https://doi.org/10.3390/md8092435
APA StyleLaurienzo, P. (2010). Marine Polysaccharides in Pharmaceutical Applications: An Overview. Marine Drugs, 8(9), 2435-2465. https://doi.org/10.3390/md8092435





