Antimutagenicity and Antiproliferative Studies of Lipidic Extracts from White Shrimp (Litopenaeus vannamei)
Abstract
:1. Introduction
2. Results and Discussion
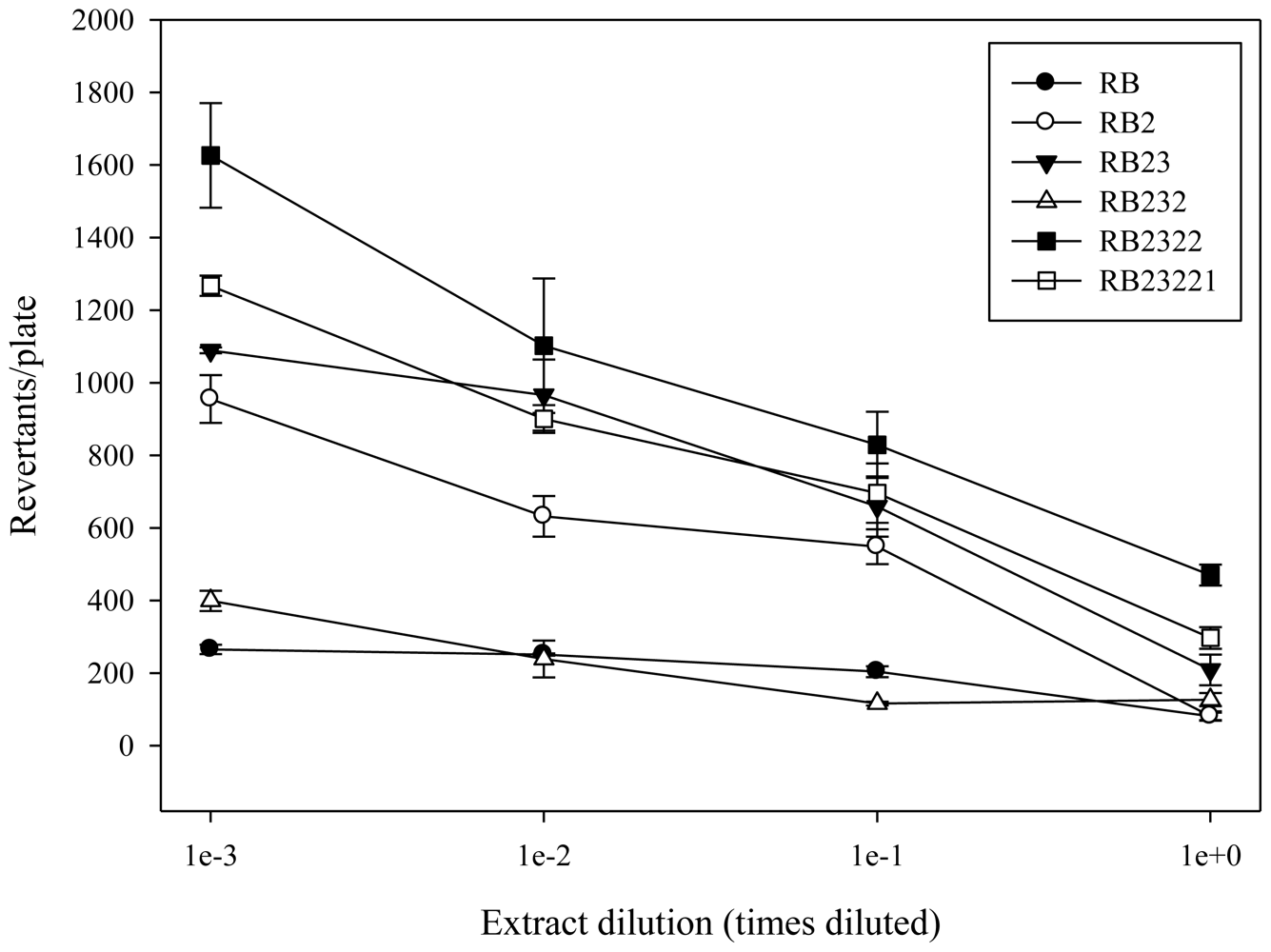
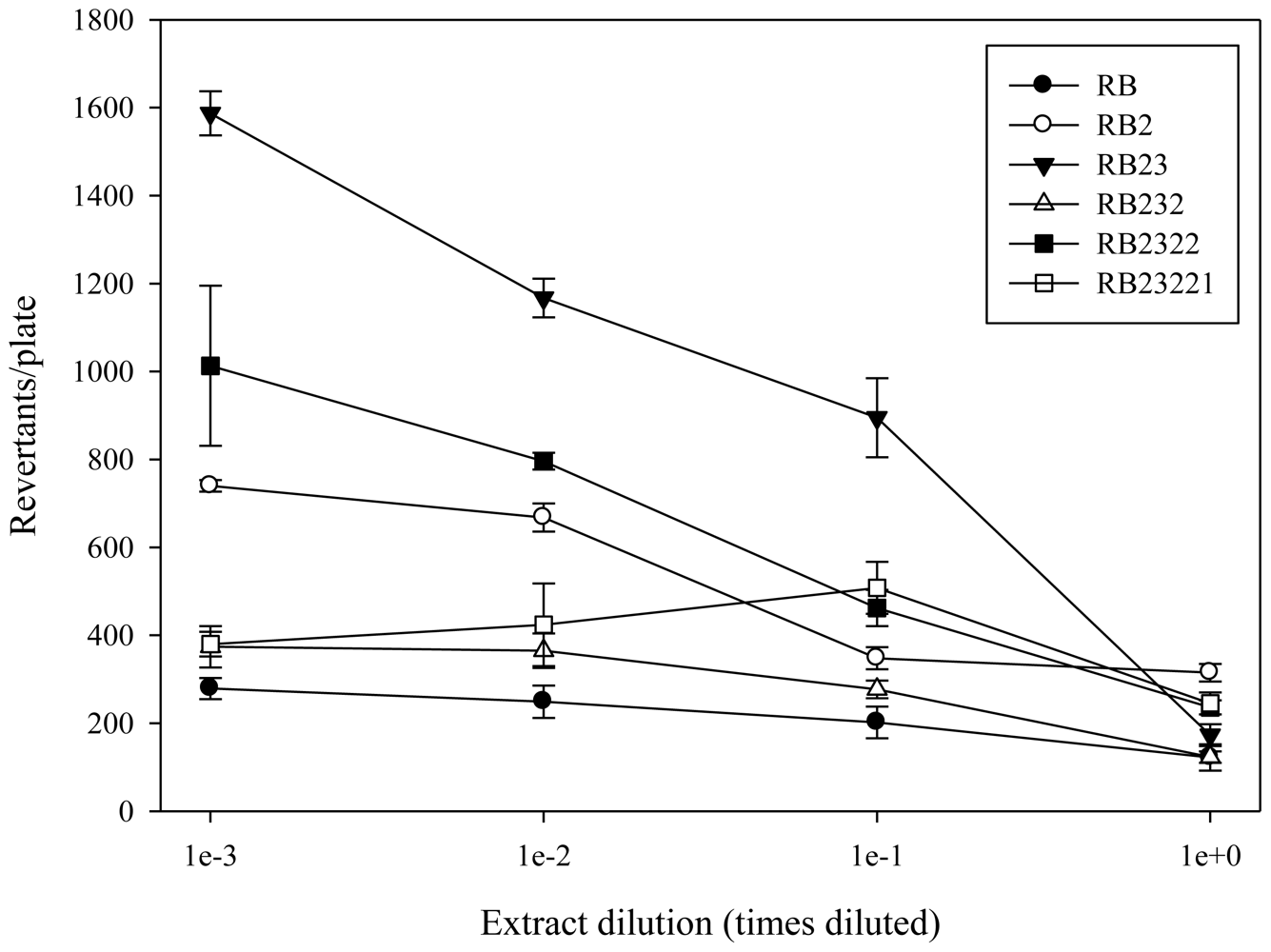
2.1. Antimutagenicity
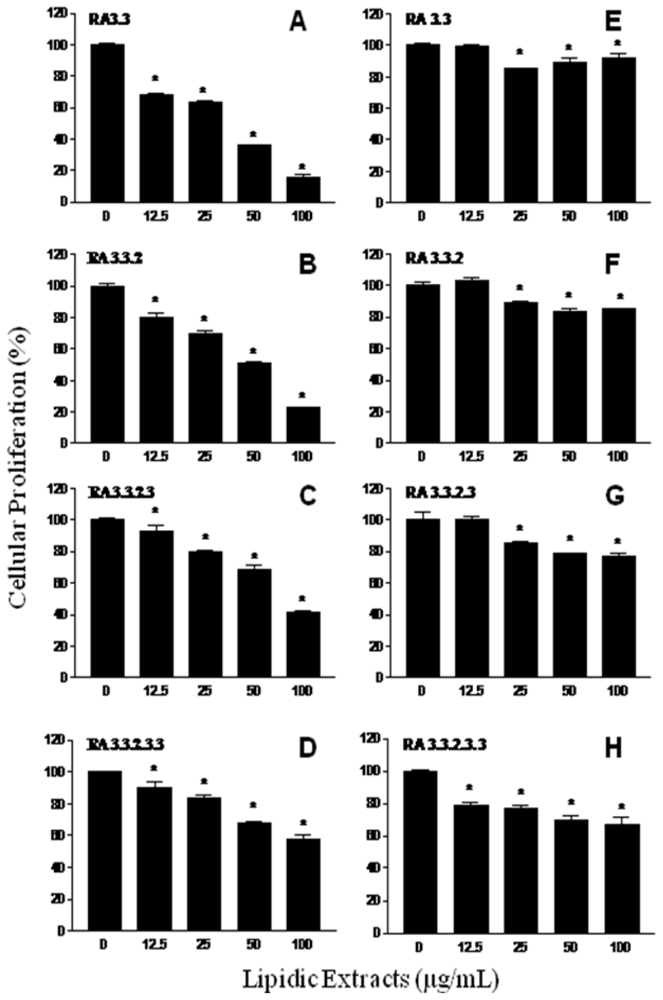
2.2. Antiproliferative activity
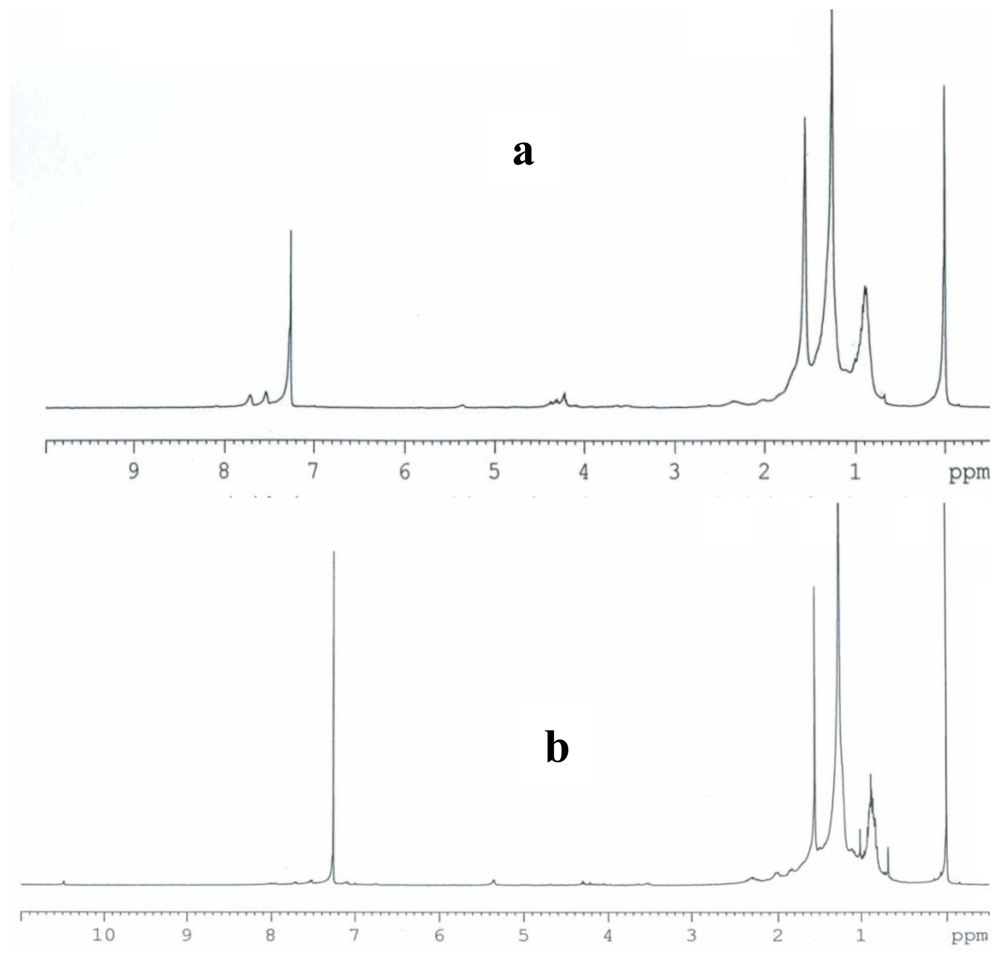
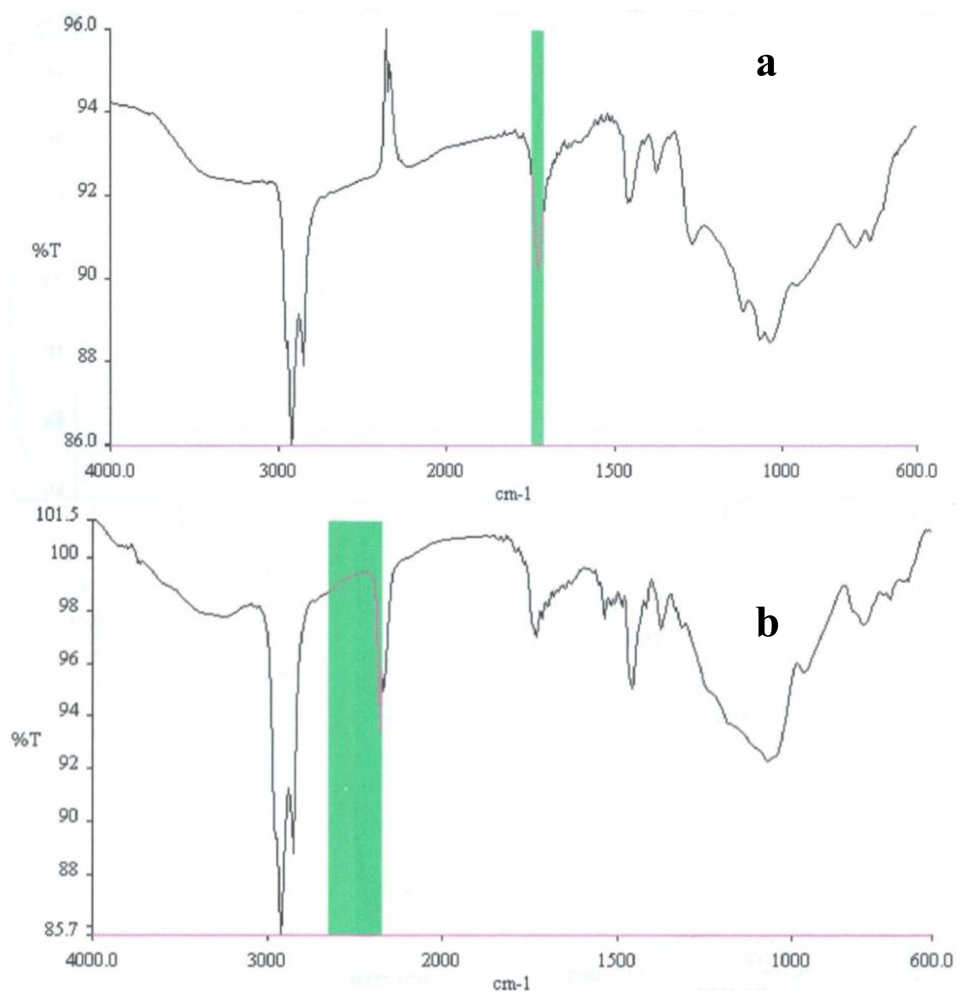
2.3. Partial chemical/structural characterization studies
3. Experimental Section
3.1. Testing species
Shrimp extract
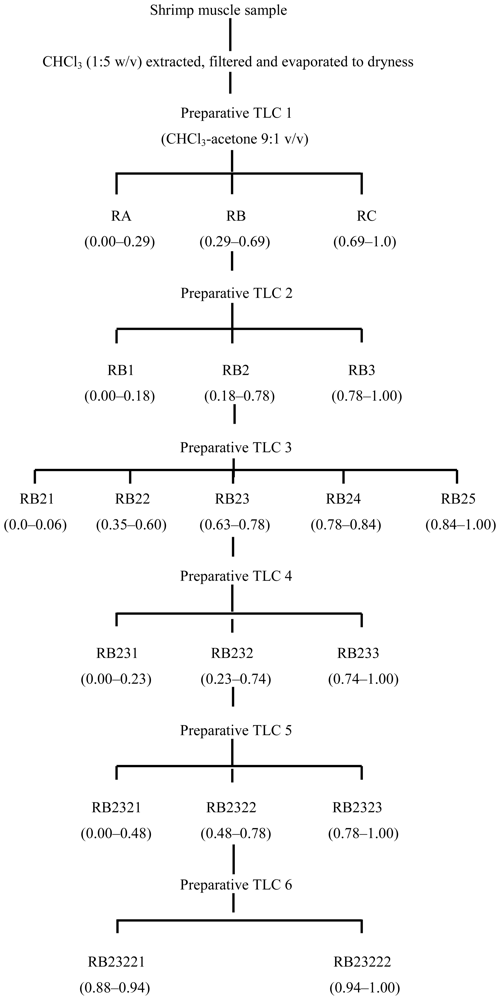
3.2. Fractionation of shrimp extract
3.3. Bacterial cultures
3.4. Antimutagenicity test
3.5. Cell lines
3.6. Antiproliferation assay
3.7. Partial chemical/structural characterization studies
4. Conclusions
Acknowledgements
References
- Watanabe, K; Ohta, T; Shirasu, Y. Antimutagenic effects of benzaldehyde and its derivatives on mutagenesis induced by 4-Nitroquinoline-1-oxide in Escherichia coli. Agric Biol Chem 1988, 52, 1041–1045. [Google Scholar]
- Hernandez, J; Goycoolea, FM; Quintero, J; Acosta, A; Castaneda, M; Dominguez, Z; Robles, R; Vazquez-Moreno, L; Velazquez, EF; Astiazaran, H; Lugo, E; Velazquez, C. Sonoran propolis: chemical composition and antiproliferative activity on cancer cell lines. Planta Med 2007, 73, 1469–1474. [Google Scholar]
- Renner, HW; Delincee, H. Different antimutagenic actions of linoleic and linolenic acid derivatives on bisulfan-induced genotoxicity in Chinese hamsters. Nutr Res 1988, 8, 635–642. [Google Scholar]
- Fritsche, KL; Johnston, PV. Effect of dietary alpha-linolenic acid on growth, metastasis, fatty acid profile and prostaglandin production of two murine mammary adenocarcinomas. J Nutr 1990, 120, 2015–2019. [Google Scholar]
- Rose, DP; Connolly, JM. Omega-3 fatty acids as cancer chemopreventing agents. Pharm Therap 1999a, 83, 217–244. [Google Scholar]
- Burgos-Hernández, A; López-García, R; Njapau, H; Park, DL. Antimutagenic compounds from corn. Food Add. Contam. 2001a, 18, 797–809. [Google Scholar]
- Burgos-Hernández, A; López-García, R; Njapau, H; Park, DL. Partial chemical/structural elucidation of anti-mutagenic compounds from corn. Toxicology 2001b, 166, 161–170. [Google Scholar]
- Burgos-Hernandez, A; Pena-Sarmiento, M; Moreno-Ochoa, M. Mutagenicity and Antimutagenicity Studies of Lipidc Extracts from Yellowtail Fish (Seriola lalandi), Lisa Fish (Mugil cephalus), and Cazón Fish (Mustelus lunulatus). Food Chem Toxicol 2002, 40, 1469–1474. [Google Scholar]
- Ito, Y; Shimizu, H; Yoshimura, T; Ross, RK; Kabuto, M; Takatsuka, N; Tokui, N; Suzuki, K; Shinohara, R. Serum concentrations of carotenoids, alpha-tocopherol, fatty acids, and lipid peroxides among Japanesse in Japan, and Japanesse and Caucasians in the US. Int J Vit Nutr Res 1999, 69, 385–395. [Google Scholar]
- Simopoulos, AP. Evolutionary aspects of amoega-3 fatty acids in the food supply. Prost Leuk Essent Fat Acids 1999, 60, 421–429. [Google Scholar]
- Burns, CP; Halabi, S; Clamon, GH; Hars, V; Wagner, BA; Hohl, RJ; Lester, E; Kirshner, JJ; Vinciguerra, V; Paskett, E. Phase I clinical study of fish oil fatty acid capsules for patients with cancer cachexia: cancer and leukemia group B study 9473. Clin Cancer Res 1999, 5, 3942–3947. [Google Scholar]
- Rose, DP; Connolly, JM. Antiangiogenicity of docosahexaenoic acid and its role in the suppression of breast cancer cell growth in nude mice. Int. J. Oncol. 1999b, 15, 1011–1015. [Google Scholar]
- Norrish, AE; Skeaff, CM; Arribas, GL; Sharpe, SJ; Jackson, RT. Prostate cancer risk and consumption of fish oils: a dietary biomarker-based case-control study. Brit J Cancer 1999, 81, 1238–1242. [Google Scholar]
- Yang, YJ; Lee, SH; Hong, SJ; Chung, BC. Comparison of fatty acid profiles in the serum of patients with prostate cancer and benign prostatic hyperplasia. Clin Biochem 1999, 32, 405–409. [Google Scholar]
- Maron, DM; Ames, BN. Revised methods for the Salmonella mutagenicity test. Mut Res 1983, 113, 173–215. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival-application to proliferation and cyto-toxicity assays. J Immun Meth 1983, 65, 55–63. [Google Scholar]
- Sacchi, R; Medina, I; Aubourg, SP; Addeo, F; Paolillo, L. Proton nuclear magnetic resonance rapid and structure-specific determination of ω-3 polyunsaturated fatty acids in fish lipids. J Am Oil Chem Soc 1993, 70, 225–228. [Google Scholar]


© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wilson-Sanchez, G.; Moreno-Félix, C.; Velazquez, C.; Plascencia-Jatomea, M.; Acosta, A.; Machi-Lara, L.; Aldana-Madrid, M.-L.; Ezquerra-Brauer, J.-M.; Robles-Zepeda, R.; Burgos-Hernandez, A. Antimutagenicity and Antiproliferative Studies of Lipidic Extracts from White Shrimp (Litopenaeus vannamei). Mar. Drugs 2010, 8, 2795-2809. https://doi.org/10.3390/md8112795
Wilson-Sanchez G, Moreno-Félix C, Velazquez C, Plascencia-Jatomea M, Acosta A, Machi-Lara L, Aldana-Madrid M-L, Ezquerra-Brauer J-M, Robles-Zepeda R, Burgos-Hernandez A. Antimutagenicity and Antiproliferative Studies of Lipidic Extracts from White Shrimp (Litopenaeus vannamei). Marine Drugs. 2010; 8(11):2795-2809. https://doi.org/10.3390/md8112795
Chicago/Turabian StyleWilson-Sanchez, Griselda, Carolina Moreno-Félix, Carlos Velazquez, Maribel Plascencia-Jatomea, Anita Acosta, Lorena Machi-Lara, María-Lourdes Aldana-Madrid, Josafat-Marina Ezquerra-Brauer, Ramón Robles-Zepeda, and Armando Burgos-Hernandez. 2010. "Antimutagenicity and Antiproliferative Studies of Lipidic Extracts from White Shrimp (Litopenaeus vannamei)" Marine Drugs 8, no. 11: 2795-2809. https://doi.org/10.3390/md8112795
APA StyleWilson-Sanchez, G., Moreno-Félix, C., Velazquez, C., Plascencia-Jatomea, M., Acosta, A., Machi-Lara, L., Aldana-Madrid, M.-L., Ezquerra-Brauer, J.-M., Robles-Zepeda, R., & Burgos-Hernandez, A. (2010). Antimutagenicity and Antiproliferative Studies of Lipidic Extracts from White Shrimp (Litopenaeus vannamei). Marine Drugs, 8(11), 2795-2809. https://doi.org/10.3390/md8112795



