Brominated Selinane Sesquiterpenes from the Marine Brown Alga Dictyopteris divaricata
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Algal Material
3.3. Extraction and Isolation
Acknowledgements
- Sample Availability: Samples of compounds 1–7 are available from the authors.
References and Notes
- Song, F; Fan, X; Xu, X; Zhao, J; Yang, Y; Shi, J. Cadinane sesquiterpenes from the brown alga Dictyopteris divaricata. J Nat Prod 2004, 67, 1644–1649. [Google Scholar]
- Song, F; Xu, X; Li, S; Wang, S; Zhao, J; Cao, P; Yang, Y; Fan, X; Shi, J; He, L; Lü, Y. Norsesquiterpenes from the brown alga Dictyopteris divaricata. J Nat Prod 2005, 68, 1309–1313. [Google Scholar]
- Song, F; Xu, X; Li, S; Wang, S; Zhao, J; Yang, Y; Fan, X; Shi, J; He, L. Minor sesquiterpenes with new carbon skeleton from the brown alga Dictyopteris divaricata. J Nat Prod 2006, 69, 1261–1266. [Google Scholar]
- Wen, W; Li, F; Ji, NY; Li, XM; Cui, CM; Li, XD; Zhang, LN; Xue, QZ; Wang, BG. A new cadinane sesquiterpene from the marine brown alga Dictyopteris divaricata. Molecules 2009, 14, 2273–2277. [Google Scholar]
- Takahashi, K; Takani, M. Studies on constituents of medical plants. XVII. Constituents of Schizandra nigra Max. and their carbon-13 nuclear magnetic resonance spectra. Chem Pharm Bull 1976, 24, 2000–2006. [Google Scholar]
- Williams, HJ; Sattler, I; Moyna, G; Scott, AI; Bell, AA; Vinson, SB. Diversity in cyclic sesquiterpene production by Gossypium hirsutum. Phytochemistry 1995, 40, 1633–1636. [Google Scholar]
- Hu, L; Chen, Z. Sesquiterpenoid alcohols from Chrysanthemum morifolium. Phytochemistry 1997, 44, 1287–1290. [Google Scholar]
- Xu, F; Morikawa, T; Matsuda, H; Ninomiya, K; Yoshikawa, M. Structures of new sesquiterpenes and hepatoprotective constituents from the Egyptian herbal medicine Cyperus longus. J Nat Prod 2004, 67, 569–576. [Google Scholar]
- Howard, BM; Fenical, W. Structure, chemistry, and absolute configuration of 1(S)-bromo-4(R)-hydroxy-(–)-selin-7-ene from a marine red alga Laurencia sp. J Org Chem 1977, 42, 2518–2520. [Google Scholar]
- Ji, NY; Li, XM; Wang, BG. Unpublished data.
- Paul, VJ; Cronan, JM, Jr; Cardellina, JH, II. Isolation of new brominated sesquiterpene feeding deterrents from tropical green alga Neomeris annulata (Dasycladaceae: Chlorophyta). J Chem Ecol 1993, 19, 1847–1860. [Google Scholar]
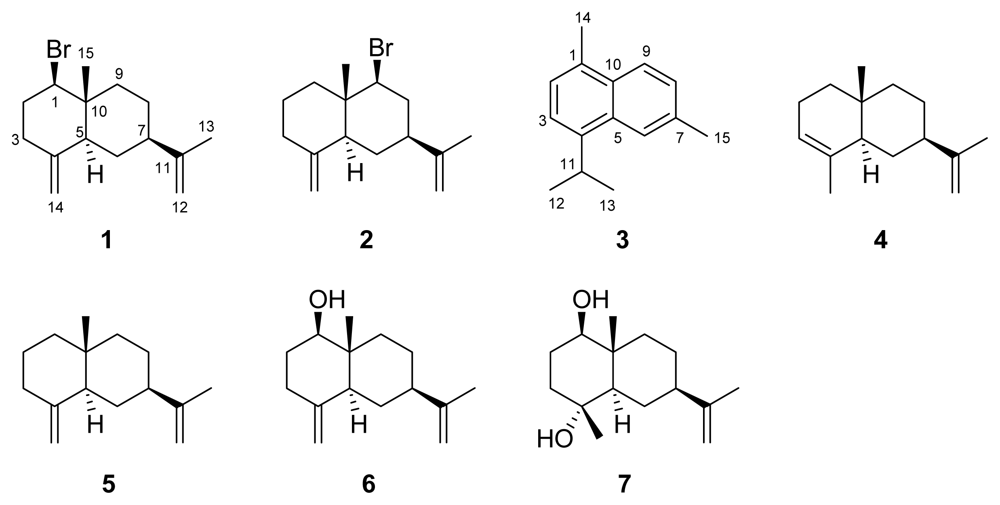
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | |
| 1a | 67.9 d | 4.09 (dd, 11.8, 4.4) | 42.8 t | 1.14 (m) | 131.8 s | |
| 1b | 1.91 (m) | |||||
| 2a | 35.0 t | 2.15 (m) | 27.1 t | 1.43 (m) | 125.6 d | 7.22 (d, 7.2) |
| 2b | 2.21 (m) | 1.61 (m) | ||||
| 3a | 37.2 t | 2.15 (m) | 39.1 t | 2.17 (m) | 121.4 d | 7.28 (d, 7.2) |
| 3b | 2.32 (m) | 2.17 (m) | ||||
| 4 | 147.5 s | 147.8 s | 142.1 s | |||
| 5 | 49.7 d | 1.91 (m) | 49.1 d | 1.99 (m) | 131.6 s | |
| 6a | 30.1 t | 1.41 (m) | 30.7 t | 1.35 (m) | 122.9 d | 7.92 (d, 1.7) |
| 6b | 1.63 (m) | 1.62 (m) | ||||
| 7 | 45.4 d | 1.95 (m) | 45.7 d | 1.95 (m) | 134.7 s | |
| 8a | 26.8 t | 1.40 (m) | 37.7 t | 2.39 (m) | 127.2 d | 7.35 (dd, 8.5, 1.7) |
| 8b | 1.66 (m) | 2.39 (m) | ||||
| 9a | 39.8 t | 1.18 (m) | 52.2 d | 4.30 (dd, 12.0, 4.0) | 124.8 d | 7.92 (d, 8.5) |
| 9b | 2.04 (m) | |||||
| 10 | 41.2 s | 41.0 s | 131.2 s | |||
| 11 | 150.0 s | 149.9 s | 28.3 d | 3.72 (h, 7.0) | ||
| 12a | 108.6 t | 4.73 (br s) | 108.6 t | 4.73 (br s) | 23.6 q | 1.27 (d, 7.0) |
| 12b | 4.73 (br s) | 4.73 (br s) | ||||
| 13 | 21.0 q | 1.75 (s) | 21.0 q | 1.75 (s) | 23.6 q | 1.27 (d, 7.0) |
| 14a | 107.7 t | 4.55 (br s) | 107.6 t | 4.56 (br s) | 19.4 q | 2.65 (s) |
| 14b | 4.78 (br s) | 4.73 (br s) | ||||
| 15 | 12.0 q | 0.85 (s) | 14.1 q | 0.86 (s) | 22.0 q | 2.56 (s) |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ji, N.-Y.; Wen, W.; Li, X.-M.; Xue, Q.-Z.; Xiao, H.-L.; Wang, B.-G. Brominated Selinane Sesquiterpenes from the Marine Brown Alga Dictyopteris divaricata. Mar. Drugs 2009, 7, 355-360. https://doi.org/10.3390/md7030355
Ji N-Y, Wen W, Li X-M, Xue Q-Z, Xiao H-L, Wang B-G. Brominated Selinane Sesquiterpenes from the Marine Brown Alga Dictyopteris divaricata. Marine Drugs. 2009; 7(3):355-360. https://doi.org/10.3390/md7030355
Chicago/Turabian StyleJi, Nai-Yun, Wei Wen, Xiao-Ming Li, Qin-Zhao Xue, Hua-Ling Xiao, and Bin-Gui Wang. 2009. "Brominated Selinane Sesquiterpenes from the Marine Brown Alga Dictyopteris divaricata" Marine Drugs 7, no. 3: 355-360. https://doi.org/10.3390/md7030355
APA StyleJi, N.-Y., Wen, W., Li, X.-M., Xue, Q.-Z., Xiao, H.-L., & Wang, B.-G. (2009). Brominated Selinane Sesquiterpenes from the Marine Brown Alga Dictyopteris divaricata. Marine Drugs, 7(3), 355-360. https://doi.org/10.3390/md7030355







