Fibrinolytic Compounds Isolated from a Brown Alga, Sargassum fulvellum
Abstract
:1. Introduction
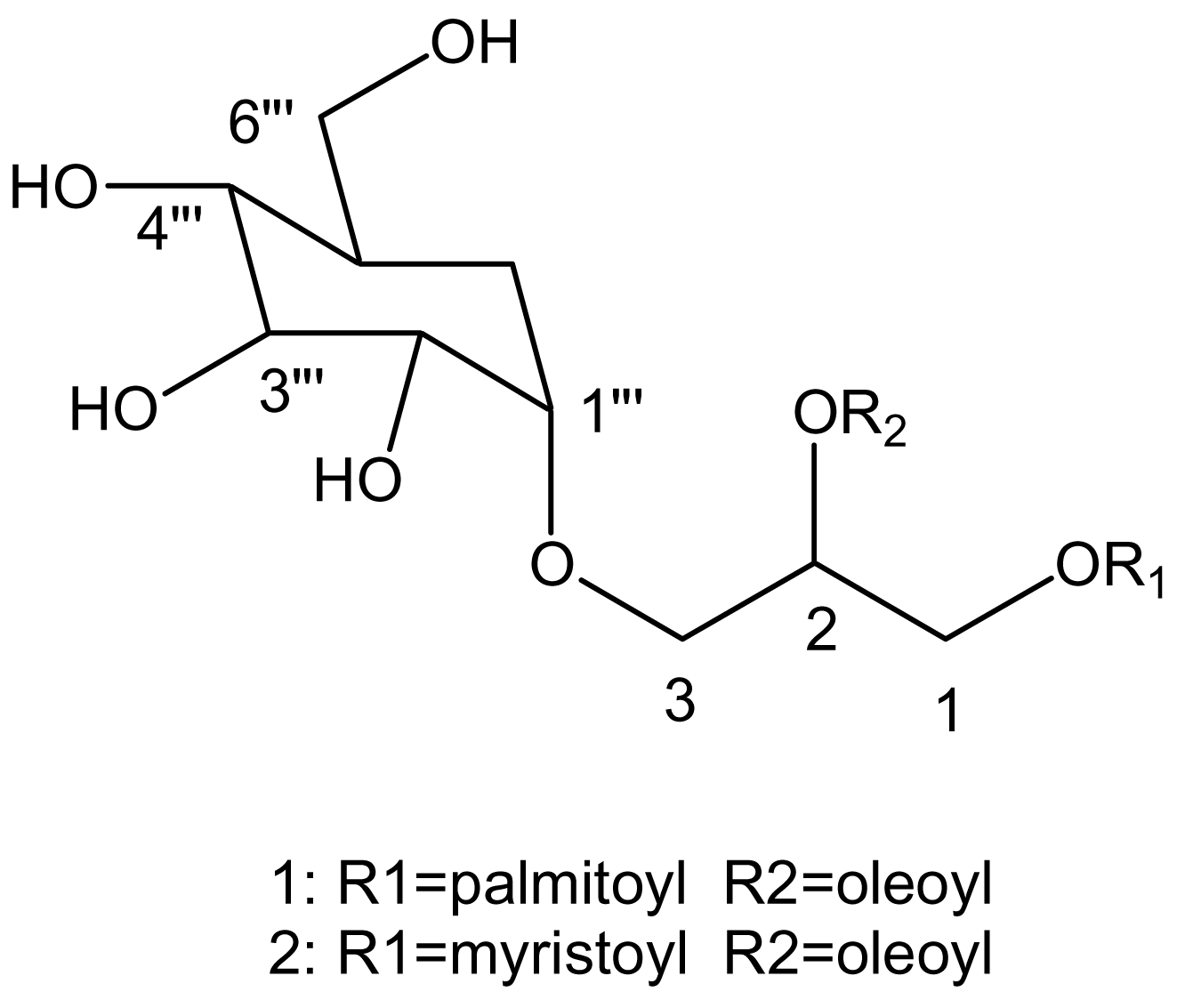
2. Results and discussion
3. Conclusions
4. Experimental section
4.1 General experimental procedures
4.2 Isolation of biophysiological substance
4.3 Methanolysis of the compound
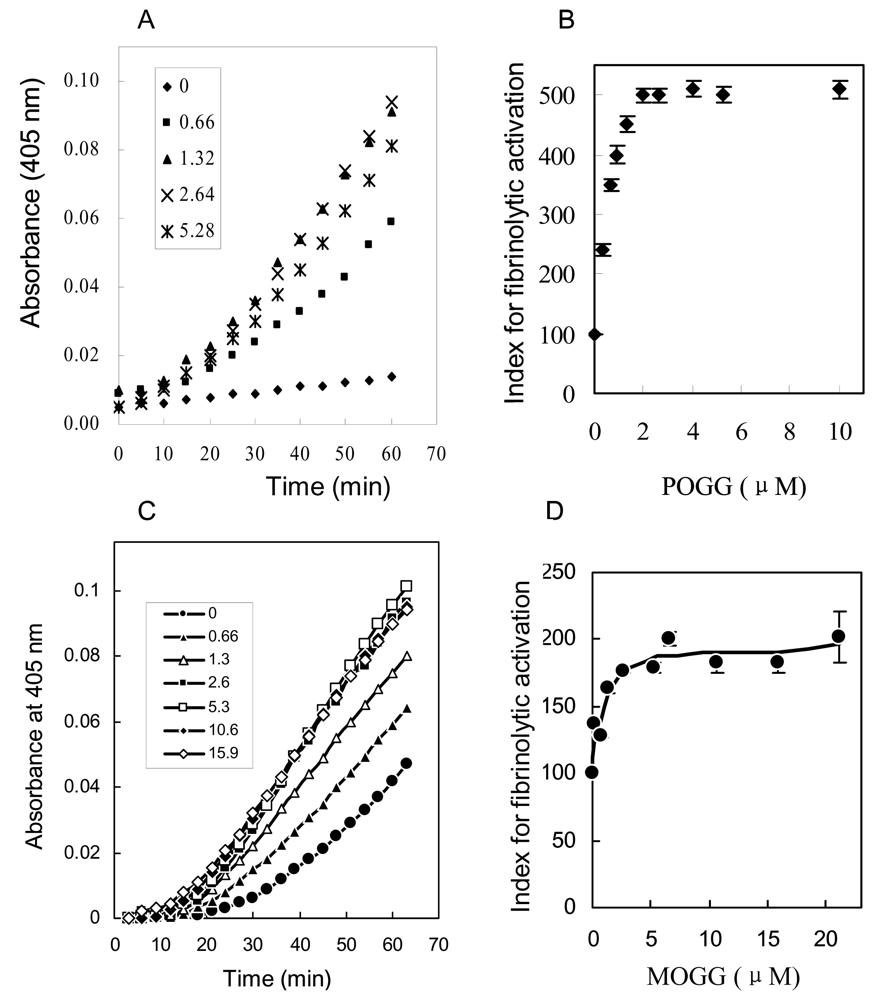
4.4 Analysis of fibrinolytic activity
Acknowledgement
- Samples Availability: Available from the authors.
References
- Potin, P; Bouarab, K; Salaun, JP; Pohnert, G; Kloareg, B. Biotic interactions of marine algae. Curr Opin Plant Biol 2002, 5, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Donia, M; Hamann, MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis 2003, 3, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 2002, 19, 1–49. [Google Scholar] [PubMed]
- Carte, BK. Biomedical potential of marine natural products. Bioscience 1996, 46, 271–286. [Google Scholar] [CrossRef]
- Garg, HS; Sharma, T; Bhakuni, DS; Pramanik, BN; Bose, AK. An antiviral sphingosine derivative from the green alga Ulva fasciata. Tetrahedron lett 1992, 33, 1641–1644. [Google Scholar] [CrossRef]
- Gerwick, WH; Fenical, W. Ichthyotoxic and cytotoxic metabolites of the tropical brown alga Stypopodium zonale. J Org Chem 1981, 46, 21–27. [Google Scholar]
- Ireland, C; Copp, B; Foster, M; McDonald, L; Radisky, D; Swersey, J. Attaway, D, Zeborsky, O, Eds.; Marine Biology; Plenum Press: New York, 1993; Volume I, pp. 1–43. [Google Scholar]
- Tang, HF; Yi, YH; Yao, XS; Xu, QZ; Zhang, SY; Lin, HW. Bioactive steroids from the brown alga Sargassum carpophyllum. J Asian Nat Prod Res 2002, 4, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, WH; Bernart, MW. Attaway, D, Zaborsky, O, Eds.; Marine Biotechnology; Plenum Press: New York, 1993; Volume I, pp. 101–152. [Google Scholar]
- Yamamoto, I; Nagumo, T; Fujihara, M; Takahashi, M; Ando, Y. Antitumor effect of seaweeds. II. Fractionation and partial characterization of the polysaccharide with antitumor activity from Sargassum fulvellum. Jpn J Exp Med 1977, 47, 133–140. [Google Scholar] [PubMed]
- Kim, SH; Choi, DS; Athukorala, Y; ·Jeon, YJ; Senevirathne, M; Cho, KR. Antioxidant activity of sulfated polysaccharides isolated from Sargassum fulvellum. J Food Sci Nutr 2007, 12, 65–73. [Google Scholar] [CrossRef]
- Kang, JY; Khan, MN; Park, NH; Cho, JY; Lee, MC; Fuji, H; Hong, YK. Antipyretic, analgesic, and anti-inflammatory activities of the seaweed Sargassum fulvellum and Sargassum thunbergii in mice. J Ethnopharmacol 2008, 116, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Batrakov, SG; Nikitin, DI; Sheichenko, VI; Ruzhitsky, AO. Unusual lipid composition of the gram-negative, freshwater, stalked bacterium Caulobacter bacteroides NP-105. Biochim Biophys Acta 1997, 1347, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Wu, WH; Narasaki, R; Maeda, F; Hasumi, K. Glucosyldiacylglycerol enhances peciprocal activation of prourokinase and plasminogen. Biosci Biotenol Biochem 2004, 7, 1549–1556. [Google Scholar]
- Li, CJ; Li, YX; Guan, HS. Progress in study on bioactivities of glycoglycerolipids. Chinese J Marin Drug 2003, 22, 47–52. [Google Scholar]
- Matsumoto, K; Okada, M; Horikoshi, Y; Matsuzaki, H; Kishi, T; Itaya, M; Shibuya, I. Cloning, sequencing, and disruption of the bacillus subtilis psd gene coding for phosphatidylserine decarboxylase. J Bacteriol 1998, 180, 100–106. [Google Scholar] [PubMed]
- Rauvala, H; Finne, J; Krusius, T; Karkkainen, J; Jarnefelt, J. Methylation techniques in the structural analysis of glycoproteins and glycolipids. Adv Carbohydr Chem Biochem 1981, 38, 389–416. [Google Scholar] [PubMed]
- Bock, K; Meldal, M; Bundle, DR; Iversen, T; Pinto, BM; Garegg, PJ; Kvanstrom, I; Norberg, T; Lindberg, AA; Svenson, SB. The conformation of Salmonella O-antigenic oligosaccharides of serogroups A, B, and D1 inferred from 1H- and 13C-nuclear magnetic resonance spectroscopy. Carbohydr Res 1984, 130, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Bourgault, R; Oakley, AJ; Bewley, JD; Wilce, MCJ. Three-dimensional structure of (1, 4)-β-D-mannan mannanohydrolase from tomato fruit. Protein Sci 2005, 14, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Manns, D. Linalool and cineole type glucosides from Cunila spicata. Phytochembtry 1995, 39, 1115–1118. [Google Scholar] [CrossRef]
- Seifert, K; Preiss, A; Johne, S; Schmidt, J; Lien, NT; Lavaud, C; Massiot, G. Triterpene saponins from Verbascum songaricum. Phytochemistry 1991, 30, 3395–3400. [Google Scholar] [CrossRef] [PubMed]
- Gaver, RC; Sweeley, CC. Methods for methanolysis of sphingolipids and direct determination of long-chain bases by gas chromatography. J Am Oil Chem Soc 1965, 42, 294–298. [Google Scholar] [CrossRef] [PubMed]
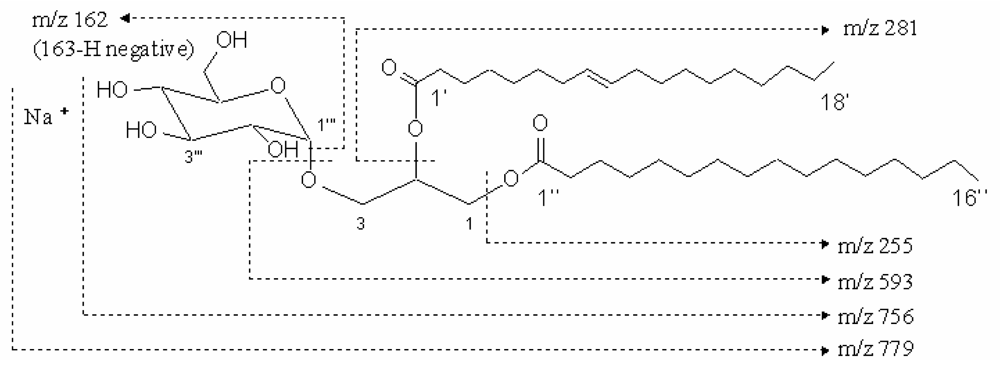
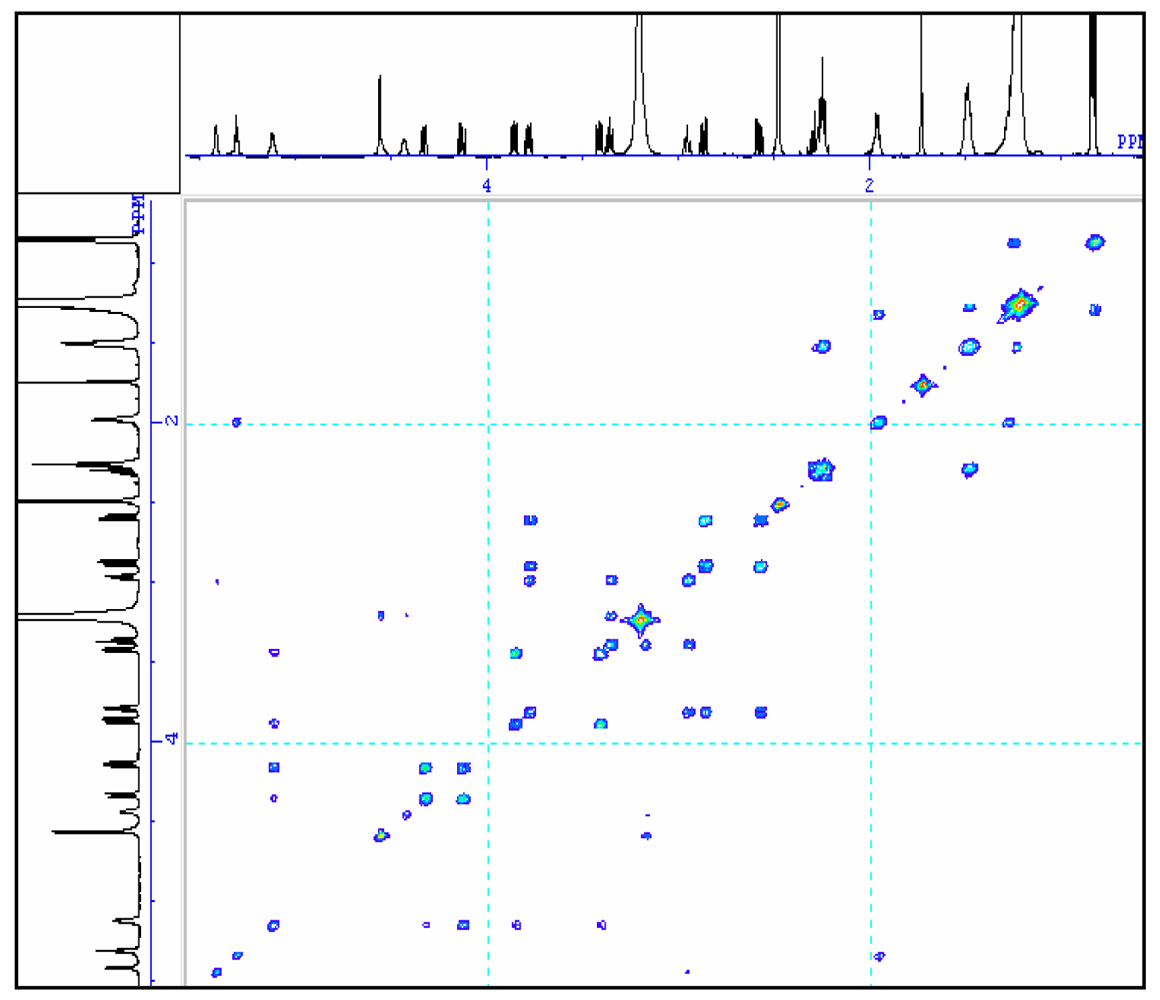
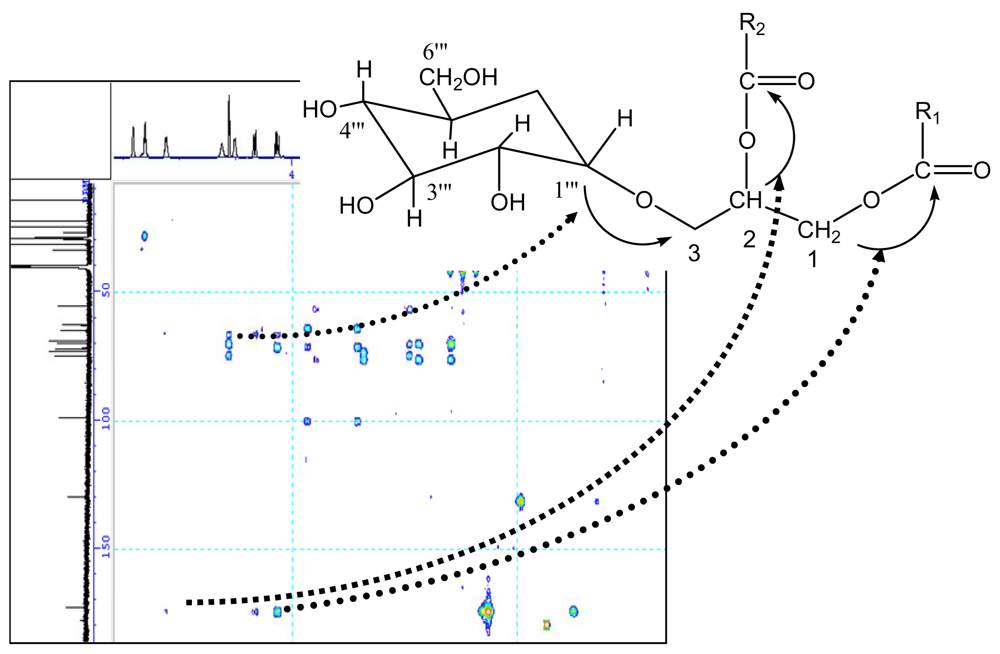
| No. | δC | δH |
|---|---|---|
| 1 | 62.5 | 4.34 (1H, dd, J = 2.6, 12.1), 4.21 (1H, dd, J = 2.6, 12.1) |
| 2 | 69.7 | 5.12 (1H, m) |
| 3 | 64.7 | 3.87 (1H, dd, J = 5.8, 10.6), 3.41 (1H, dd, J1=5.5, 10.6) |
| 1‴ | 98.3 | 4.56 (1H, d, J = 3.7) |
| 2‴ | 71.6 | 3.18 (1H, dd, J = 4.4, 9.2) |
| 3‴ | 72.8 | 3.36 (1H, t, J = 9.2) |
| 4‴ | 74.4 | 2.94 (1H, t, J = 9.2) |
| 5‴ | 68.4 | 3.78 (1H, ddd, J = 5.1, 5.5, 9.5) |
| 6‴ | 54.9 | 2.58 (1H, dd, J = 4.8, 13.9), 2.57 (1H, dd, J = 6.2, 13.9) |
| 1′, 1″ | 172.4, 172.2 | |
| 2′, 2″ | 33.5, 33.3 | 2.26 (4H, m) |
| 3′, 3″ | 24.3 | 1.49 (4H, m) |
| 4′ – 7′, 12′ – 15′, 4″ – 13″ | 28.3 – 28.9 | 1.2 – 1.3 (m) |
| 8′, 11′ | 26.5, 26.4 | 1.97 (4H, m) |
| 9′, 10′ | 129.5, 129.4 | 5.04 (2H, t, J = 4.8) |
| 16′, 14″ | 31.2 | 1.2 – 1.3 (m) |
| 17′, 15″ | 21.9 | 1.2 – 1.3 (m) |
| 18′, 16″ | 13.7 | 0.84 (6H, t, J = 6.8) |
| No. | δC | δH |
|---|---|---|
| 1 | 62.6 | 4.09 (1H, dd, J = 7.4, 11.4), 4.30 (1H, d, J = 11.8) |
| 2 | 69.7 | 5.10 (1H, d, J = 5.0) |
| 3 | 64.6 | 3.36 (1H, dd, J = 5.9, 10.3), 3.85(1H, dd, J = 5.8, 10.5) |
| 1‴ | 98.3 | 4.54 (1H, d, J = 3.5) |
| 2‴ | 71.6 | 3.15 (1H, m) |
| 3‴ | 72.9 | 3.32 (1H, t, J = 8.9) |
| 4‴ | 74.2 | 2.88 (1H, m) |
| 5‴ | 68.5 | 3.74 (1H, m) |
| 6‴ | 54.5 | 2.52 (1H, dd, J = 6.8, 12.5), 2.88 (1H, m) |
| 1′, 1″ | 172.3, 172.4 | |
| 2′, 2″ | 33.4, 33.6 | 2.22 (2H, t, J = 7.1), 2.26(2H, m) |
| 3′, 3″ | 24.4 | 1.46 (4H, dd, J = 6.7, 13.4) |
| 4″ – 7″ | 28.4–29.1 | 1.2 – 1.3 (m) |
| 8″, 11″ | 26.5, 26.6 | 1.2 – 1.3 (m), 1.94 (2H, m) |
| 9″, 10″ | 129.4, 129.5 | 5.27 (1H, t, J = 4.5), 5.35 (1H, d, J = 2.5) |
| 12″ – 15″ | 28.4–29.1 | 1.2 – 1.3 (m) |
| 4′ – 11′ | 28.4–29.1 | 1.2 – 1.3 (m) |
| 16″, 12′ | 31.3 | 1.2 – 1.3 (m) |
| 17″, 13′ | 22.1 | 1.2 – 1.3 (m) |
| 18″, 14′ | 13.9 | 0.81 (6H, t, J = 6.7) |
Abbreviations
| COSY | Correlation spectroscopy |
| HMQC | 1H detected multiple quantum coherence |
| HMBC | 1H detected multiple bound connectivity |
| DEPT | Distorsionless enhancenment by polarization transfer |
| pro-u-PA | single chain urokinase-type plasminogen activator |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, W.; Hasumi, K.; Peng, H.; Hu, X.; Wang, X.; Bao, B. Fibrinolytic Compounds Isolated from a Brown Alga, Sargassum fulvellum. Mar. Drugs 2009, 7, 85-94. https://doi.org/10.3390/md7020085
Wu W, Hasumi K, Peng H, Hu X, Wang X, Bao B. Fibrinolytic Compounds Isolated from a Brown Alga, Sargassum fulvellum. Marine Drugs. 2009; 7(2):85-94. https://doi.org/10.3390/md7020085
Chicago/Turabian StyleWu, Wenhui, Keiji Hasumi, Hui Peng, Xianwen Hu, Xichang Wang, and Bin Bao. 2009. "Fibrinolytic Compounds Isolated from a Brown Alga, Sargassum fulvellum" Marine Drugs 7, no. 2: 85-94. https://doi.org/10.3390/md7020085
APA StyleWu, W., Hasumi, K., Peng, H., Hu, X., Wang, X., & Bao, B. (2009). Fibrinolytic Compounds Isolated from a Brown Alga, Sargassum fulvellum. Marine Drugs, 7(2), 85-94. https://doi.org/10.3390/md7020085




