Aplysinopsins - Marine Indole Alkaloids: Chemistry, Bioactivity and Ecological Significance
Abstract
:1. Introduction
2. Stereochemistry
3. Biological Activity
3.1. Neurotransmission
3.2. Antineoplastic Activity
3.3. Antiplasmodial Activity
3.4. Antimicrobial Activity
4. Ecological Significance
5. Summary
Acknowledgements
References
- Kazlauskas, R; Murphy, PT; Quinn, RJ; Wells, RJ. Aplysinopsin, a new tryptophan derivative from a sponge. Tetrahedron Lett 1977, 1, 61–64. [Google Scholar]
- Hollenbeak, KH; Schmitz, FJ. Aplysinopsin: Antineoplastic tryptophan derivative from marine sponge Verongia spengelii. Lloydia 1977, 40, 479–481. [Google Scholar] [PubMed]
- Djura, P; Faulkner, DJ. Metabolites of the marine sponge Derictus sp. J Org Chem 1980, 45, 737–738. [Google Scholar] [CrossRef]
- Djura, P; Stierle, DB; Sullivan, B; Faulkner, DJ. Some metabolites of the marine sponges Smenospongia aurea and Smenospongia (Polyfibrospongia) echina. J. Org. Chem 1980, 45, 1435–1441. [Google Scholar] [CrossRef]
- Tymiak, AA; Rinehart, KL. JR Constituents of morphologically similar sponges. Tetrahedron 1985, 41, 1039–1047. [Google Scholar] [CrossRef]
- Hu, JF; Schetz, JA; Kelly, M; Peng, J-N; Ang, KKH; Flotow, H; Yan Leong, C; Ng, SB; Buss, AD; Wilkins, SP; Hamann, MT. New antiinfective and human 5-HT2 receptor binding natural and semisynthetic compounds from the Jamaican sponge Smenospongia aurea. J Nat Prod 2002, 65, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Kochanowska, AJ; Rao, KV; Childress, S; El-Alfy, A; Matsumoto, RR; Kelly, M; Stewart, GS; Sufka, KJ; Hamann, MT. Secondary metabolites from three Florida sponges with antidepressant activity. J Nat Prod 2008, 71, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, PR; Wells, RJ. Schauer, PJ, Ed.; Marine Natural Products: Chemical and Biological Perspectives; Academic Press: New York, 1983; Volume V, p. 1. [Google Scholar]
- Baker, JT; Wells, RJ. Beal, JL, Reinhard, E, Eds.; Biological active substances from Australian marine organisms. In Natural Products as Medicinal Agents; Hipocrates Verlag: Stuttgart, 1981; p. 281. [Google Scholar]
- Kondo, K; Nishi, J; Ishibashi, M; Kobayashi, J. Two new tryptophan-derived alkaloids from the Okinawan marine sponge Aplysina sp. J Nat Prod 1994, 57, 1008–1011. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S; Ye, Y; Higuchi, K; Takashima, A; Tanaka, Y; Kitagawa, I; Kobayashi, M. Novel neuronal nitric oxide synthase (nNOS) selective inhibitor, aplysinopsin-type indole alkaloid, from marine sponge Hyrtios erecta. Chem Pharm Bull 2001, 49, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Segraves, NL; Crews, P. Investigation of brominated tryptophan alkaloids from two Thorectidae sponges: Thorectandra and. Smenospongia J Nat Prod 2005, 68, 1484–1488. [Google Scholar] [CrossRef]
- Fattorusso, E; Lanzotti, V; Magno, S; Novellino, E. Tryptophan derivatives from a Mediterranean anthozoan Astroides calycularis. J Nat Prod 1985, 48, 924–927. [Google Scholar] [CrossRef]
- Guella, G; Mancini, I; Zibrowius, H; Pietra, F. Novel aplysinopsin-type alkaloids from scleractinian corals of the family Dendrophylliidae of the Mediterranean and the Philippines. Configurational-assignment criteria, stereospecific synthesis, and photoisomerization. Helv Chim Acta 1988, 71, 773–782. [Google Scholar] [CrossRef]
- Okuda, RK; Klein, D; Kinnel, RB; Li, M; Scheuer, PJ. Marine natural products: the past twenty years and beyond. Pure Appl Chem 1982, 54, 1907–1914. [Google Scholar] [CrossRef]
- Fusetani, N; Asano, M; Matsunaga, S; Hashimoto, K. Bioactive marine metabolites - XV. Isolation of aplysinopsin from the scleractinian coral Tubastrea aurea as an inhibitor of development of fertilized sea urchin eggs. Comp Biochem Physiol 1986, 85B, 845–846. [Google Scholar]
- Guella, G; Mancini, I; Zibrowius, H; Pietra, F. Aplysinopsin-type alkaloids from Dendrophyllia sp., a scleractinian coral of the family Dendrophylliidae of the Philippines. Facile photochemical (Z/E) photoisomerization and thermal reversal. Helv Chim Acta 1989, 72, 1444–1450. [Google Scholar] [CrossRef]
- Koh, EGL; Sweatman, H. Chemical warfare among scleractinians: bioactive natural products from Tubastraea faulkneri Wells kill larvae of potential competitors. J Exp Mar Biol 2000, 251, 141–160. [Google Scholar] [CrossRef]
- Iwagawa, T; Miyazaki, M; Okumara, H; Nakatani, M; Doe, M; Takemura, K. Three novel bis(indole) alkaloids from a stony coral Tubastraea sp. Tetrahedron Lett 2003, 44, 2533–2535. [Google Scholar] [CrossRef]
- Murata, M; Miyagawa-Kohshima, K; Nakanishi, K; Naya, Y. Characterization of compounds that induce symbiosis between sea anemone and anemone fish. Science 1986, 234, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Gribble, GW. The diversity of naturally occurring organobromine compounds. Chem Soc Rev 1999, 28, 335–346. [Google Scholar] [CrossRef]
- Molina, P; Almendros, P; Fresneda, PM. Iminophosphorane-mediated imidazole ring formation: A new and general entry to aplysinopsin-type alkaloids of marine origin. Tetrahedron 1994, 50, 2241–2254. [Google Scholar]
- Delmes, G; Deplat, P; Chezal, JM; Chavignon, O; Gueiffier, A; Blache, Y; Chabord, JL; Dauphin, G; Teulade, JC. Preparation of aza-analogs of aplysinopsins and zwitterionic by reactions of heterocumulenes in naphthyridine’s series. Heterocycles 1996, 43, 1229–1241. [Google Scholar] [CrossRef]
- Selič, L; Jakše, R; Lapmi, K; Golič, L; Golič-Grdadolnik, S; Stanovnik, B. A simple stereoselective synthesis of aplysinopsin analogs. Helv Chim Acta 2000, 83, 2802–2811. [Google Scholar] [CrossRef]
- Jakše, R; Rečnik, S; Svete, J; Golobi, A; Golič, L; Stanovnik, B. A simple synthesis of aplysinopsin analogues by dimethylamine substitution in N,N-(dimethylamino)methylidene derivatives of five-membered heterocycles. Tetrahedron 2001, 57, 8395–8403. [Google Scholar] [CrossRef]
- Selič, L; Rečnik, S; Stanovnik, B. A synthesis of some novel 2-phenyl- and 5-bromo-substituted aplysinopsin analogues. Heterocycles 2002, 58, 577–585. [Google Scholar] [CrossRef]
- Mancini, I; Guella, G; Zibrowius, H; Pietra, F. On the origin of quasi-racemic aplysinopsin cycloadducts, (bis)indole alkaloids isolated from scleractinian corals of the family Dendrophylliidae. Involvement of enantiodefective Diels–Alderases or asymmetric induction in artifact processes involving adventitious catalysts. Tetrahedron 2003, 59, 8757–8762. [Google Scholar] [CrossRef]
- Stanovnik, B; Svete, J. The synthesis aplysinopsins, meridianines, and related compounds. Mini-Rev Org Chem 2005, 2, 211–224. [Google Scholar] [CrossRef]
- Wells, KM; Murphy, PT. 5-(Indol-3-ylmethylene)-1,3-dimethyl-2-methylamino-4-imidazolidinone US Patent 4, 195, 179. 1980.
- Baird-Lambert, J; Davis, PA; Taylor, KM. Methylaplysinopsin: a natural product of marine origin with effects on serotonergic neurotransmission. Clin Exp Pharmacol Physiol 1982, 9, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Roth, BL. The Serotonin Receptors: From Molecular Pharmacology to Human Terapeutics; Humana Press: Totowa, New Jersey, 2006; p. 618. [Google Scholar]
- Leysen, JE. 5-HT2 Receptors. Curr Drug Targets-CNS Neurol Disord 2004, 3, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig-Lipson, S; Zhang, J; Mazandarani, H; Harrison, BL; Sabb, A; Sabalski, J; Stack, G; Welmaker, G; Barrett, JE; Dunlop, J. Antiobesity-like effects of the 5-HT2C receptor agonist WAY-161503. Brain Res 2006. [Google Scholar]
- Gul, W; Hamann, MT. Indole alkaloid marine natural products: An established source of cancer drug leads with considerable promise for the control of parasitic, neurological and other diseases. Life Sci 2005, 78, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Gulati, D; Chauhan, PMS; Bhakuni, RP; Bhakuni, DS. A new synthesis of aplysinopsin, a marine alkaloid and its analogues and their biological activities. Indian J Chem 1993, 33B, 4–9. [Google Scholar]
- Lindel, T; Hoffmann, H; Hochgürtel, M; Pawlik, JR. Structure-activity relationship of inhibition of fish feeding by sponge-derived and synthetic pyrrole-imidazole alkaloids. J Chem Ecol 2000, 26, 1477–1496. [Google Scholar] [CrossRef]
- Santini, S; Polacco, G. Finding Nemo: Molecular phylogeny and evolution of the unusual life style of anemonefish. Gene 2006, 385, 19–27. [Google Scholar] [CrossRef] [PubMed]
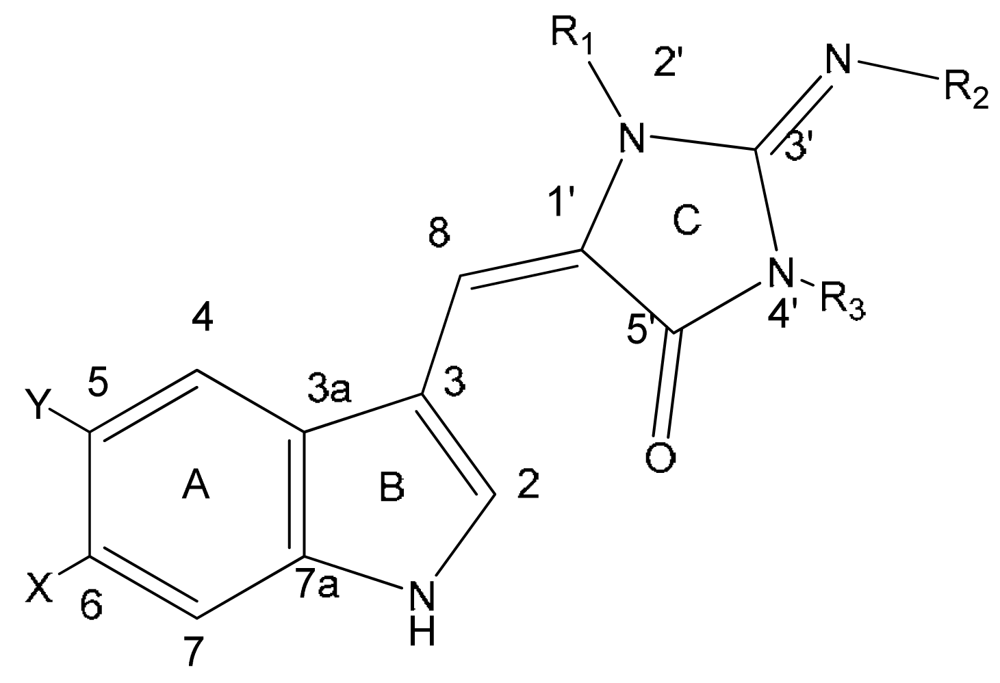
| Compound | X | Y | R1 | R2 | R3 | |
|---|---|---|---|---|---|---|
| 1 | aplysinopsin | H | H | CH3 | H | CH3 |
| 2 | isoplysin A | H | H | CH3 | CH3 | H |
| 3 | 2′-de-N-methyl-aplysinopsin | H | H | H | H | CH3 |
| 4 | 6-bromo-2′-de-N-methylaplysinopsin | Br | H | H | H | CH3 |
| 5 | 6-bromoaplysinopsin | Br | H | CH3 | H | CH3 |
| 6 | 6-bromo-4′-de-N-methylaplysinopsin | Br | H | CH3 | H | H |
| 7 | methylaplysinopsin | H | H | CH3 | CH3 | CH3 |
| 8 | 4′-demethyl-3′-N-methylaplysinopsin | H | H | H | CH3 | CH3 |
| 9 | 6-bromo-4′-demethyl-3′-N-methyl-aplysinopsin | Br | H | H | CH3 | CH3 |
| 10 | 5,6-dibromo-2′-demethylaplysinopsin | Br | Br | H | H | CH3 |
| 11 | N-3′-ethylaplysinopsin | H | H | CH3 | CH2CH3 | CH3 |
| Compound | X | Y | R1 | R2 | R3 | |
|---|---|---|---|---|---|---|
| 1 | aplysinopsin | H | H | CH3 | H | CH3 |
| 2 | isoplysin A | H | H | CH3 | CH3 | H |
| 3 | 2′-de-N-methyl-aplysinopsin | H | H | H | H | CH3 |
| 4 | 6-bromo-2′-de-N-methylaplysinopsin | Br | H | H | H | CH3 |
| 5 | 6-bromoaplysinopsin | Br | H | CH3 | H | CH3 |
| 6 | 6-bromo-4′-de-N-methylaplysinopsin | Br | H | CH3 | H | H |
| 7 | methylaplysinopsin | H | H | CH3 | CH3 | CH3 |
| 8 | 4′-demethyl-3′-N-methylaplysinopsin | H | H | H | CH3 | CH3 |
| 9 | 6-bromo-4′-demethyl-3′-N-methyl-aplysinopsin | Br | H | H | CH3 | CH3 |
| 10 | 5,6-dibromo-2′-demethylaplysinopsin | Br | Br | H | H | CH3 |
| 11 | N-3′-ethylaplysinopsin | H | H | CH3 | CH2CH3 | CH3 |
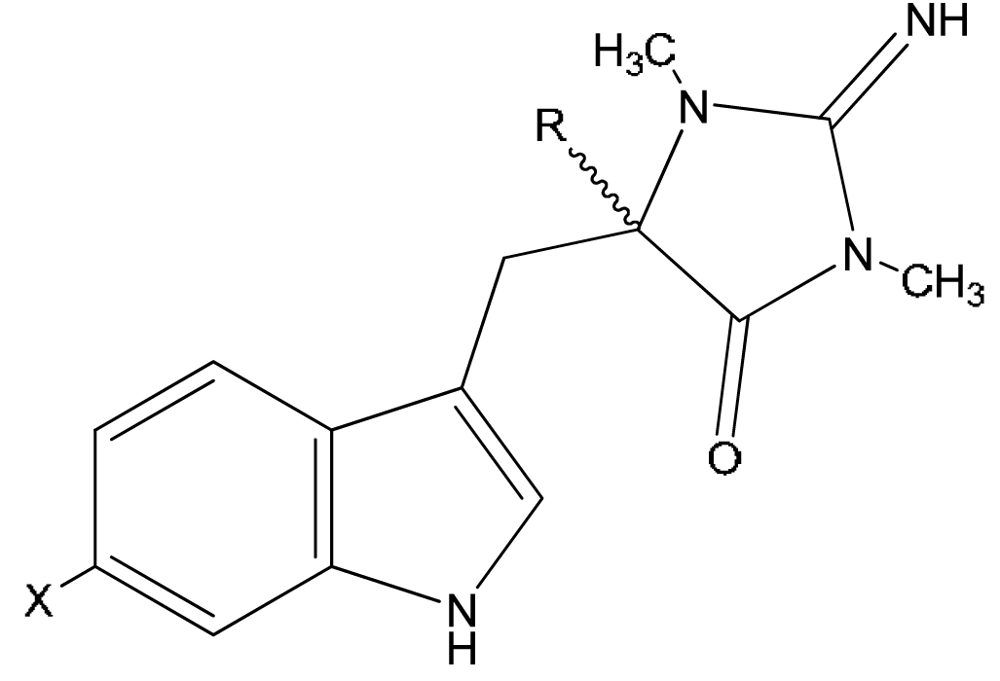
| Compound | X | R | |
|---|---|---|---|
| 12 | 1′,8-dihydroaplysinopsin | H | H |
| 13 | 6-bromo-1′,8-dihydro-aplysinopsin | Br | H |
| 14 | 6-bromo-1′-hydroxy-1′,8-dihydroaplysinopsin | Br | OH |
| 15 | 6-bromo-1′-methoxy-1′,8-dihydroxyaplysinopsin | Br | OCH3 |
| 16 | 6-bromo-1′-ethoxy-1′,8-dihydroxyaplysinopsin | Br | OCH2CH3 |
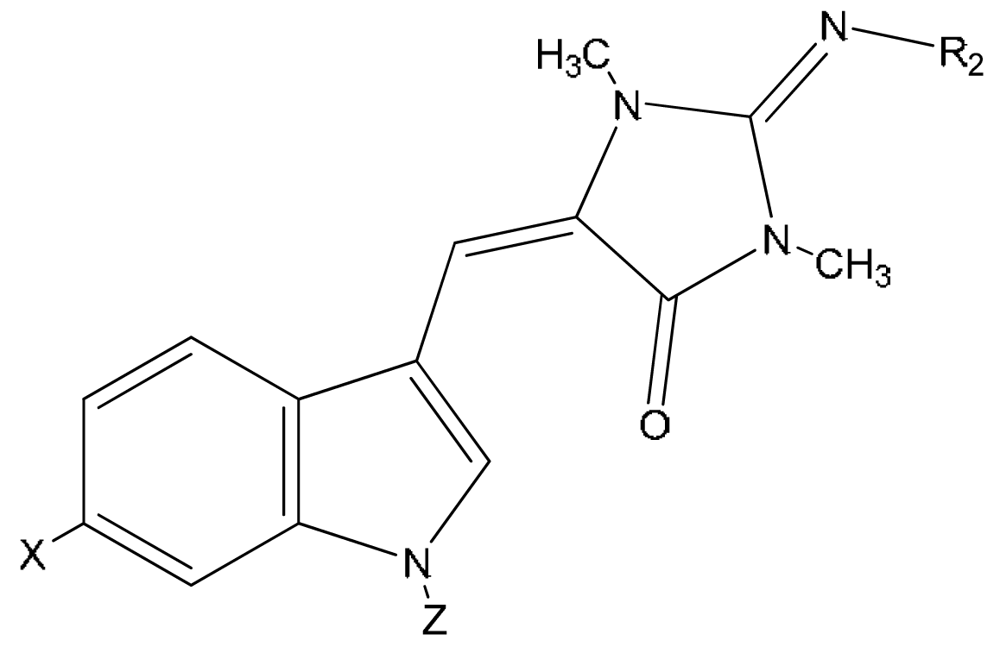
| Compound | X | R1 | R3 | |
|---|---|---|---|---|
| 17 | 3′-deimino-3′-oxo-aplysinopsin | H | CH3 | CH3 |
| 18 | 6-bromo-3′-deimino-3′-oxoaplysinopsin | Br | CH3 | CH3 |
| 19 | 3′-deimino-2′,4′-bis(demethyl)-3′-oxo-aplysinopsin | H | H | H |
| 20 | 6-bromo-3′-deimino-2′,4′-bis(demethyl)-3′-oxoaplysinopsin | Br | H | H |
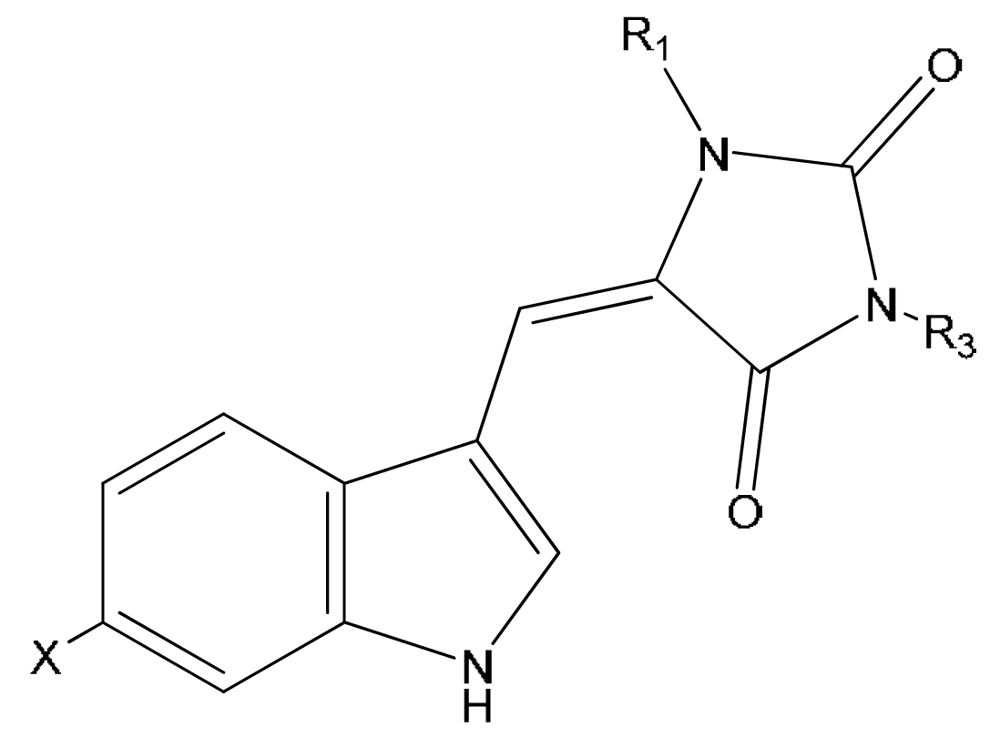
| Compound | X | Z | R2 | |
|---|---|---|---|---|
| 21 | N-propionylaplysinopsin | H | OCCH2CH3 | H |
| 22 | 6-bromo-N-propionylaplysinopsin | Br | OCCH2CH3 | H |
| 23 | N-methylaplysinopsin | H | CH3 | H |
| Source | Activity | |
|---|---|---|
| 1 | Thorecta sp. sponge Great Barrier Reef Australia [1]
Verongia spengelli sponge Florida Keys [2] Dercitus sp. sponge Caribbean [3] Smenospongia aurea sponge Caribbean [5] Dictyoceratida sponges [8] Astroides calycularis anthozoan Mediterranean [13] Tubastraea aurea Japan scleractinian coral [16] Tubastraea sp. scleractinian coral Philippines [14] Radianthus kuekenthali sea anemone Japan [20] Aplysina sp. sponge Japan [10] Tubastraea faulkneri scleractinian coral Australia [18] Tubastraea sp. scleractinian Japane [19] Smenospongia sp. sponge Indo-Pacific reefs [12] Verongula rigida sponge Florida Keys [7] | Anticancer [2,10]
Antimicrobial [12] Inhibitor of development of fertilized sea urchin eggs [16] Induces symbiosis between sea anemone and anemone fish [20] |
| 2 | Aplysina sp. sponge Japan [10]
Smenospongia aurea sponge Jamaica [6] | Anticancer [10]
Antiplasmodial [6] |
| 3 | Dercitus sp. sponge Caribbean [3]
Tubastrea coccinea coral Hawaii [15] Phestilla melanobrachia mollusc [15] Dendrophyllia sp. scleractinian coral Philippines [17] Smenospongia aurea sponge Jamaica [6] Verongula rigida sponge Florida Keys [7] | |
| 4 | Dercitus sp. sponge Caribbean [3]
Tubastrea coccinea coral Hawaii [15] Phestilla melanobrachia mollusc [15] Dendrophyllia sp. scleractinian coral Philippines [17] Tubastraea faulkneri scleractinian coral Australia [18] Smenospongia aurea sponge Jamaica [6] Hyrtios erecta sponge Japan [11] | Serotonin receptors modulator [6]
Antiplasmodial [6] Inhibitor of nitric oxide synthase (nNOS) [11] |
| 5 | Tubastrea coccinea coral Hawaii [15]
Smenospongia aurea sponge Caribbean [5] Astroides calycularis anthozoan Mediterranean [13] Radianthus kuekenthali sea anemone Japan [20] Tubastraea faulkneri scleractinian coral Australia [18] Smenospongia aurea sponge Jamaica [6] Smenospongia aurea sponge Florida Keys [7] | Serotonin receptors modulator [6]
Antiplasmodial [6] Induces symbiosis between sea anemone and anemone fish [20] |
| 6 | Smenospongia aurea sponge Caribbean [5] | |
| 7 | Aplysinopsis reticulata sponge Australia [9]
Smenospongia aurea sponge Jamaica [6] | Antidepressant – inhibitor of monoamine oxidase [9,29,30] |
| 8 | Dendrophyllia sp. scleractinian coral Philippines [17]
Smenospongia aurea sponge Jamaica [6] | |
| 9 | Dendrophyllia sp. scleractinian coral Philippines17 | |
| 10 | Hyrtios erecta sponge Japan [11] | Inhibitor of nitric oxide synthase (nNOS) [11] |
| 11 | Smenospongia aurea sponge Jamaica [6] | Serotonin receptors modulator [6] |
| 12 | Tubastrea coccinea coral Hawaii [15]
Radianthus kuekenthali sea anemone Japan [20] Thorectandra sp. sponge Indo-Pacific reefs [12] | Induces symbiosis between sea anemone and anemone fish [20] |
| 13 | Tubastrea coccinea coral Hawaii [15]
Radianthus kuekenthali sea anemone Japan [20] Thorectandra sp. sponge Indo-Pacific reefs [12] | Antimicrobial [12] |
| 14 | Thorectandra sp. sponge Indo-Pacific reefs [12] | Antimicrobial [12] |
| 15 | Thorectandra sp. sponge Indo-Pacific reefs [12] | Antimicrobial [12] |
| 16 | Smenospongia sp. sponge Indo-Pacific reefs [12] | Antimicrobial [12] |
| 17 | Thorecta sp. sponge Great Barrier Reef Australia [1]
Tubastraea sp. scleractinian coral Philippines [14] | |
| 18 | Astroides calycularis anthozoan Mediterranean [13]
Tubastraea sp. scleractinian coral Philippines [14] | |
| 19 | Leptopsammia pruvoti scleractinian coral France [14] | |
| 20 | Smenospongia aurea sponge Caribbean [4]
Leptopsammia pruvoti scleractinian coral France [14] | |
| 21 | Astroides calycularis anthozoan Mediterranean [13] | |
| 22 | Astroides calycularis anthozoan Mediterranean [13] | |
| 23 | Aplysina sp. sponge Japan [10] | |
| 24 | Tubastraea faulkneri scleractinian coral Australia [18] | Antimicrobial [18] |
| 25 | Tubastraea sp. stony coral Japan [19] | |
| 26 | Tubastraea sp. stony coral Japan [19] | |
| 27 | Tubastraea sp. stony coral Japan [19] |
| aplysinopsin | 5-HT2A (Ki, μM) | 5-HT2C (Ki, μM) | Selectivity towards 5-HT2C |
|---|---|---|---|
| isoplysin A (2) | NA | NA | -- |
| 2′-de-N-methylaplysinopsin (2) | NA | NA | -- |
| 6-bromo-2′-de-N-methyl-aplysinopsin (6) | >100 | 2.3 | >43 |
| 6-bromoaplysinopsin (5) | 2.0 | 0.33 | 6 |
| methylaplysinopsin (7) | NA | NA | -- |
| N-3′-ethylaplysinopsin (11) | 1.7 | 3.5 | 0.5 |
| serotonin | 0.32 | 0.13 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bialonska, D.; Zjawiony, J.K. Aplysinopsins - Marine Indole Alkaloids: Chemistry, Bioactivity and Ecological Significance. Mar. Drugs 2009, 7, 166-183. https://doi.org/10.3390/md7020166
Bialonska D, Zjawiony JK. Aplysinopsins - Marine Indole Alkaloids: Chemistry, Bioactivity and Ecological Significance. Marine Drugs. 2009; 7(2):166-183. https://doi.org/10.3390/md7020166
Chicago/Turabian StyleBialonska, Dobroslawa, and Jordan K. Zjawiony. 2009. "Aplysinopsins - Marine Indole Alkaloids: Chemistry, Bioactivity and Ecological Significance" Marine Drugs 7, no. 2: 166-183. https://doi.org/10.3390/md7020166
APA StyleBialonska, D., & Zjawiony, J. K. (2009). Aplysinopsins - Marine Indole Alkaloids: Chemistry, Bioactivity and Ecological Significance. Marine Drugs, 7(2), 166-183. https://doi.org/10.3390/md7020166




