Ciguatera Fish Poisoning: Treatment, Prevention and Management
Abstract
:1. Introduction
1.1. Epidemiology
1.2. Pharmacology
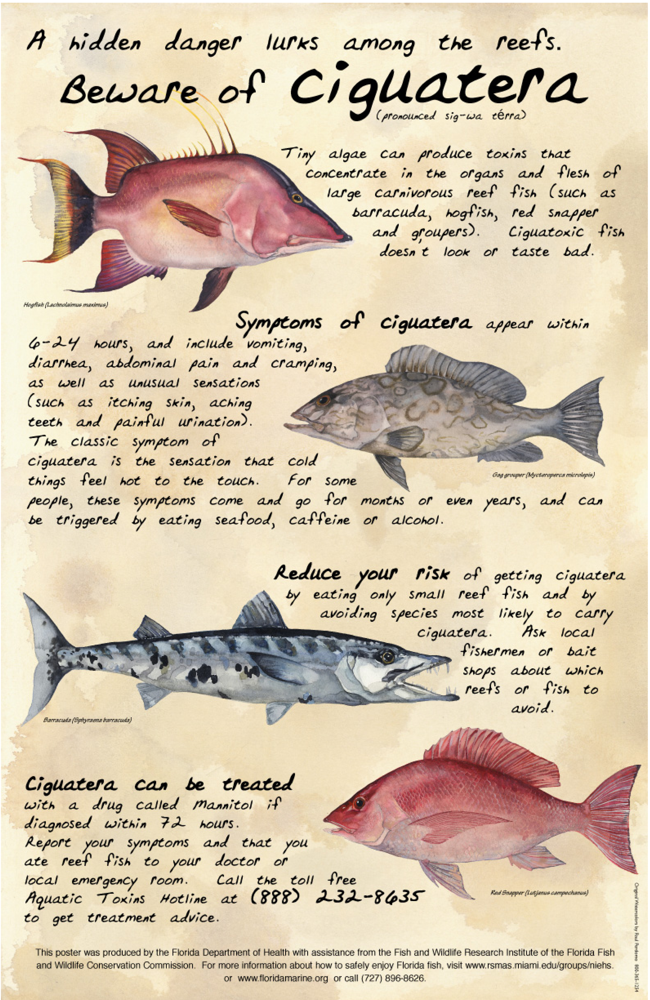
1.3. Symptoms and Course
1.4. Chronicity
1.5. Symptom recurrence and sensitization
1.6. Diagnosis
1.7. Detection in Fish
2. Treatments
2.1. Mannitol
2.2. Symptomatic and Supportive Treatments
2.3. Food and Dehydration Avoidance
3. Prevention
3.1. Avoiding Ciguateric Fish
3.2. Surveillance and Reporting
3.3. Education and Outreach
4. Future Directions
5. Recommendations
- Use of IV mannitol: If a patient meets the criteria for a diagnosis of CFP, consumed the implicated species within the past 48–72 hours, and there are no contra-indications to its use, then treatment with IV mannitol is recommended. Repeat treatments may be necessary if symptoms return. After 72 hours, IV mannitol treatment may be considered on a case by case basis, though it is not routinely recommended. Prior to treatment, as part of the process of informing patients about treatment options, patients should be informed about the inconsistency of research findings on the effectiveness of IV mannitol for CFP, the limitations of knowledge about its effects, as well as the fact that insurance will often not pay for this treatment. In all cases, the decision to proceed with mannitol treatment should be based upon the risk-benefit analysis, discussing such risks and benefits with the patient, and ultimately, the personal preference of the patient after being informed that it may not work.
- Supportive and symptomatic medical treatments: Supportive and symptomatic medical treatments for CFP symptoms should be determined on a case by case basis, according to the patient’s health situation, with the caveat that there are no randomized controlled studies investigating the effectiveness of any medical treatments other than mannitol for CFP. Caution is warranted in the use of medications with addictive potential for treating CFP symptoms, especially given the lack of research evidence supporting the safety or effectiveness of such medications for that purpose.
- For patients with chronic complaints that are not clearly caused by CFP, it is recommended that they receive a full evaluation by a neurologist, internist, and psychologist and/or psychiatrist, who can provide joint input on the diagnosis and a recommended plan of care.
- For patients with chronic complaints that do appear to be caused by CFP, based on experience at a local South Florida care center, patients may benefit from a low dose of selective serotonin reuptake inhibitor (SSRI), as well as a combination of assessment and care including a physical/occupational therapist, psychologist, and group psychotherapy sessions and/or family counseling, as needed, on a case by case basis.
- Fish avoidance: Avoidance of fish reported to be associated with CFP, as well as any large reef fish, large fish portions, head and viscera (e.g. liver, gonads), and fish obtained from regions known to be associated with CFP, is recommended (Table 4).
- Avoiding symptom recurrence: Avoidance of dehydration and certain foods is recommended for 3–6 months after initial CFP intoxication or until the patient is symptom-free (Table 3). As an alternative to food and alcohol avoidance, patients may opt to try certain of the listed items but should be advised to do so cautiously and watch for recurrent symptoms. These recommendations are made with the following caveats: a) This is based on anecdotal reports; b) There are no empirical studies verifying the utility of such interventions, and c) This may not be helpful to all CFP patients.
- Report CFP: CFP should be reported to state or local health authorities. In some states (e.g. Florida), licensed physicians, laboratories and certain other practitioners are required by law to report suspected cases of CFP. Also, the Aquatic Toxins Hotline of the Florida Poison Information Center in Miami is a 24-hour resource available to people nationwide (888-232-8635; from outside of the US but not toll-free: 305-585-5250).
- Submit fish and clinical samples for testing: Upon determining that a patient has an illness caused by seafood consumption, physicians are encouraged to record time of meal consumption, amount of time between meal consumption and symptom onset, and obtain a detailed profile of patient symptoms. Physicians, other health care providers, or public health officials should also request from patients remnants of the fish that was actually consumed. Obtaining related information about the fish such as the kind of fish (e.g. grouper, snapper, amberjack, etc.), where it was purchased or caught, and other people consuming the same fish (symptomatic or not) is also helpful.In the USA, implicated remnants of the consumed fish and the case-related information should be sent to the FDA laboratory for analysis of ciguatoxicity by public health authorities or poison control centers responding to the report, or by the Emergency Department physicians. In addition, in cases where fish remnants are being submitted for analysis, clinical samples of the patient’s urine and whole blood, taken as close as possible to the time of symptom onset, may be solicited for submission to the FDA. For instructions on submitting fish and clinical samples, the FDA contact person is Ray Granade (hudson.granade@fda.hhs.gov), phone: (251) 690–3379.
- Randomized clinical trials of sufficient size are needed to document the occurrence of CFP, and the efficacy of IV mannitol and other common treatments.
- Creation of messages is recommended, to inform the public, fishermen, fish vendors, and restaurants about where to obtain good information on ciguatera.
- Educational modules targeted at medical students, emergency department personnel, poison information center personnel, and other health care providers should be developed and implemented.
Acknowledgements
References
- Pearn, J. Neurology of ciguatera. J Neurol Neurosurg & Psych 2001, 70, 4–8. [Google Scholar]
- Crump, JA; McLay, CL; Chambers, ST. Ciguatera fish poisoning. Postgrad Med J 1999, 75, 678–679. [Google Scholar]
- Swift, AE; Swift, TR. Ciguatera. J Toxicol - Clin Toxicol 1993, 31, 1–29. [Google Scholar]
- Gillespie, NC; Lewis, RJ; Pearn, JH; Bourke, AT; Holmes, MJ; Bourke, JB; Shields, WJ. Ciguatera in Australia. Occurrence, clinical features, pathophysiology and management. Med J Austr 1986, 145, 584–590. [Google Scholar]
- Lehane, L; Lewis, RJ. Ciguatera: recent advances but the risk remains. Int J Food Microbiol 2000, 61, 91–125. [Google Scholar]
- Fleming, LE; Blythe, DG; Baden, DG. ciguatera fish poisoning. Shoreman’s Travel Medicine Monthly 1997, 1, 1–5. [Google Scholar]
- Baden, D; Fleming, LE; Bean, J. deWolff, FA, Ed.; Marine toxins; Elsevier Press: Amsterdam, 1995; Volume 21. [Google Scholar]
- Lewis, RJ. The changing face of ciguatera. Toxicon 2001, 39, 97–106. [Google Scholar]
- Lawrence, DN; Enriquez, MB; Lumish, RM; Maceo, A. Ciguatera fish poisoning in Miami. J Am Med Assoc 1980, 244, 254–258. [Google Scholar]
- McKee, D; Fleming, LE; Tamer, R; Weisman, R; Blythe, DG. Physician diagnosis and reporting of ciguatera fish poisoning in an endemic area. Harmful Algal Blooms 2001, 451–453. [Google Scholar]
- Begier, E; Weisman, R; Hammond, R; Fleming, LE; Blythe, DG; Backer, L. Outbreak bias in illness reporting and case confirmation in ciguatera fish poisoning Surveillance in South Florida. Pub Health Rep 2006, 121, 658–665. [Google Scholar]
- Nicholson, GM; Lewis, RJ. Ciguatoxins: cyclic polyether modulators of voltage-gated ion channel function. Mar Drugs 2006, 4, 88–118. [Google Scholar]
- Arias, HR. Marine toxins targeting ion channels. Mar Drugs 2006, 4, 37–69. [Google Scholar]
- Cameron, J; Flowers, AE; Capra, MF. Electrophysiological studies on ciguatera poisoning in man (Part II). J Neurol Sci 1991, 101, 93–97. [Google Scholar]
- Bagnis, R. Clinical aspects of ciguatera (fish poisoning) in French Polynesia. Hawaii Med J 1968, 28, 25–28. [Google Scholar]
- Bagnis, R; Kuberski, T; Laugier, S. Clinical observations on 3,009 cases of ciguatera (fish poisoning) in the South Pacific. Am J Trop Med Hyg 1979, 28, 1067–1073. [Google Scholar]
- Withers, NW. Ciguatera fish poisoning. Ann Rev Med 1982, 33, 97–111. [Google Scholar]
- Yasumoto, T; Raj, U; Bagnis, R. Symposium on Seafood Toxins from Tropical Regions; Tohoku University Japan, 1984; p. 74. [Google Scholar]
- Steidinger, K; Baden, DG. Toxic marine dinoflagellates; Academic Press: Orlando, 1985. [Google Scholar]
- Calvert, GM; Hryhorczuk, DO; Leikin, JB. Treatment of ciguatera fish poisoning with amitriptyline and nifedipine. J Toxicol - Clin Toxicol 1987, 25, 423–428. [Google Scholar]
- Naar, JP; Flewelling, LJ; Lenzi, A; Abbott, JP; Granholm, A; Jacocks, HM; Gannon, D; Henry, M; Pierce, R; Baden, DG; Wolny, J; Landsberg, JH. Brevetoxins, like ciguatoxins, are potent ichthyotoxic neurotoxins that accumulate in fish. Toxicon 2007, 50, 707–723. [Google Scholar]
- Flewelling, l; Naar, J; Abbott, J; Baden, DG; Barros, NB; Bottein Dechraoui, M-Y; Hammond, D; Haubold, E; Heil, C; Henry, M; Jacocks, H; Leighfield, T; Pierce, R; Pitchford, T; Rommel, S; Rowles, T; Scott, P; Steidinger, KA; Truby, E; Van Dolah, F; Landsberg, J. Red tide and marine mammal mortalities. Nature 2005, 435, 755–756. [Google Scholar]
- Friedman, MA; Arena, P; Levin, B; Fleming, L; Fernandez, M; Weisman, R; Bernstein, J; Schrank, K; Blythe, D; Backer, L; Reich, A. Neuropsychological study of ciguatera fish poisoning: A longitudinal case-control study. Arch Clin Neuropsychol 2007, 22, 545–553. [Google Scholar]
- Arena, P; Levin, B; Fleming, LE; Friedman, MA; Blythe, DG. A pilot study of the cognitive and psychological correlates of chronic ciguatera poisoning. Harmful Algae 2004, 3, 51–60. [Google Scholar]
- Quod, JP; Turquet, J. Ciguatera in Reunion Island (SW Indian Ocean): epidemiology and clinical patterns. Toxicon 1996, 34, 779–785. [Google Scholar]
- Bagnis, R; Legrand, AM. Gopalakrishnakone, P, Tan, CK, Eds.; Progress in venom and toxin research; National University of Singapore: Singapore, 1987; pp. 372–384. [Google Scholar]
- Lewis, R. Ciguatera management. SPC Live Reef Fish Information Bulletin 2000, 7, 11–13. [Google Scholar]
- Bagnis, R. Falconer, I, Ed.; Algal toxins in seafood and drinking water; Academic Press: London, 1993; pp. 105–115. [Google Scholar]
- Chateau-Degat, ML; Huin-Blondey, MO; Chinain, M; Darius, T; Legrand, AM; Nguyen, NL; Laudon, F; Chansin, R; Dewailly, E. Prevalence of chronic symptoms of ciguatera disease in French Polynesian adults. Am J Trop Med Hyg 2007, 77, 842–846. [Google Scholar]
- Kodama, AM; Hokama, Y. Variations in symptomatology of ciguatera poisoning. Toxicon 1989, 27, 593–595. [Google Scholar]
- Chan, TY; Kwok, TC. Chronicity of neurological features in ciguatera fish poisoning. Hum Exp Toxicol 2001, 20, 426–428. [Google Scholar]
- Blythe, DG; de Sylva, DP; Fleming, LE; Ayyar, RA; Baden, DG; Shrank, K. Clinical experience with i.v. Mannitol in the treatment of ciguatera. Bull Soc Pathol Exotique 1992, 85, 425–426. [Google Scholar]
- Benoit, E; Laurent, D; Mattei, C; Legrand, AM; Molgo, J. Reversal of Pacific ciguatoxin-1B effects on myelinated axons by agents used in ciguatera treatment. Cybium 2000, 24, 33–40. [Google Scholar]
- Chan, TY; Wang, AY. Life-threatening bradycardia and hypotension in a patient with ciguatera fish poisoning. Trans Roy Soc Trop Med Hyg 1993, 87, 71. [Google Scholar]
- Ruff, RL; Lewis, J. Clinical aspects of ciguatera: an overview. Memoirs of the Queensland Museum 1994, 609–619. [Google Scholar]
- Glaziou, P; Martin, PM. Study of factors that influence the clinical response to ciguatera fish poisoning. Bull Soc Pathol Exotique 1992, 85, 419–420. [Google Scholar]
- Ting, JY; Brown, AF. Ciguatera poisoning: a global issue with common management problems. Europ J Emerg Med 2001, 8, 295–300. [Google Scholar]
- Bagnis, RA; Bronstein, JA; Jouffe, G; Forestier, R; Meunier, JL; Lejan, J; Brulefer, D; Parc, F; Tetaria, C. [Neurologic complications of ciguatera]. Bull Soc Pathol Exotique 1977, 70, 89–93. [Google Scholar]
- Bottein Dechraoui, M-Y; Wang, Z; Turquet, J; Chinain, M; Darius, T; Cruchet, P; Radwan, FFY; Dickey, RW; Ramsdell, JS. Biomonitoring of ciguatoxin exposure in mice using blood collection cards. Toxicon 2005, 46, 243–251. [Google Scholar]
- Bottein Dechraoui, MY; Wang, Z; Ramsdell, JS. Optimization of ciguatoxin extraction method from blood for Pacific ciguatoxin (P-CTX-1). Toxicon 2007, 49, 100–105. [Google Scholar]
- Ryan, JC; Bottein Dechraoui, MY; Morey, JS; Rezvani, A; Levin, ED; Gordon, CJ; Ramsdell, JS; Van Dolah, FM. Transcriptional profiling of whole blood and serum protein analysis of mice exposed to the neurotoxin Pacific Ciguatoxin-1. NeuroToxicol 2007, 28, 1099–1109. [Google Scholar]
- Stinn, JF; de Sylva, DP; Fleming, LE; Hack, E. GIS in public health, 3rd National Conference, San Diego, CA, 2000.
- Dickey, R; Jester, E; Granade, R; Mowdy, D; Moncreiff, C; Rebarchik, D; Robl, M; Musser, S; Poli, M. Monitoring brevetoxins during a Gymnodinium breve red tide: comparison of sodium channel specific cytotoxicity assay and mouse bioassay for determination of neurotoxic shellfish toxins in shellfish extracts. Nat Toxins 1999, 7, 157–165. [Google Scholar]
- Manger, RL; Leja, LS; Lee, SY; Hungerford, JM; Hokama, Y; Dickey, RW; Granade, HR; Lewis, R; Yasumoto, T; Wekell, MM. Detection of sodium channel toxins: directed cytotoxicity assays of purified ciguatoxins, brevetoxins, saxitoxins, and seafood extracts. J AOAC Int 1995, 78, 521–527. [Google Scholar]
- Dickey, R. Botana, LM, Ed.; Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 2; Taylor and Francis (CRC Press), 2008; pp. 479–500. [Google Scholar]
- Dechraoui, MY; Naar, J; Pauillac, S; Legrand, AM. Ciguatoxins and brevetoxins, neurotoxic polyether compounds active on sodium channels. Toxicon 1999, 37, 125–143. [Google Scholar]
- Bottein Dechraoui, MY; Tiedeken, J; Persad, R; Wang, Z; Granade, HR; Dickey, R; Ramsdell, JS. Use of two detection methods for discriminating ciguatoxins from brevetoxins: application to Great Barracuda from Florida. Toxicon 2005, 46, 261–270. [Google Scholar]
- Pottier, I; Hamilton, B; Jones, A; Lewis, R; Vernoux, JP. Identification of slow and fast-acting toxins in highly ciguatoxic barracuda (Sphyraene barracuda) by HPLC/MS and radiolabelled ligand binding. Toxicon 2003, 42, 663–672. [Google Scholar]
- Hamilton, B; Hurbungs, M; Jones, A; Lewis, RJ. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar]
- Lombet, A; Bidard, JN; Lazdunski, M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett 1987, 219, 355–359. [Google Scholar]
- Darius, HT; Ponton, D; Revel, T; Cruchet, P; Ung, A; Tchou Fouc, M; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar]
- Lewis, RJ; Jones, A; Vernoux, JP. HPLC/tandem electrospray mass spectrometry for the determination of Sub-ppb levels of Pacific and Caribbean ciguatoxins in crude extracts of fish. Anal Chem 1999, 71, 247–250. [Google Scholar]
- Hamilton, B; Hurbungs, M; Vernoux, JP; Jones, A; Lewis, RJ. Isolation and characterisation of Indian Ocean ciguatoxin. Toxicon 2002, 40, 685–693. [Google Scholar]
- Lewis, J. Hallegraeff, GM, Anderson, DM, Cembella, AD, Eds.; Manual on harmful marine microalgae; UNESCO: Paris, 2004; IOC Manuals and Guides No. 33; pp. 1–22. [Google Scholar]
- Hokama, Y; Nishimura, K; Takenaka, W; Ebesu, JS. Simplified solid-phase membrane immunobead assay (MIA) with monoclonal anti-ciguatoxin antibody (MAb-CTX) for detection of ciguatoxin and related polyether toxins. J Nat Tox 1998, 7, 1–21. [Google Scholar]
- Wong, CK; Hung, P; Lee, KL; Kam, KM. Study of an outbreak of ciguatera fish poisoning in Hong Kong. Toxicon 2005, 46, 563–571. [Google Scholar]
- Dickey, R; Granade, HR; McClure, F. Evaluation of a solid-phase immunobead assay for detection of ciguatera-related biotoxins in Caribbean finfish. Memoirs of the Queensland Museum 1994, 34, 481–488. [Google Scholar]
- Hirama, M; Oishi, T; Uehara, H; Inoue, M; Maruyama, M; Guri, H; Satake, M. Total synthesis of ciguatoxin CTX3C. Science 2001, 294, 1904–1907. [Google Scholar]
- Murata, M; Legrand, AM; Ishibashi, Y; Fukui, M; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J Am Chem Soc 1990, 112, 4380–4386. [Google Scholar]
- Murata, M; Legrand, AM; Ishibashi, Y; Yasumoto, T. Structures of ciguatoxin and its congener. J Am Chem Soc 1989, 111, 8929–8931. [Google Scholar]
- Crouch, RC; Martin, GE; Musser, SM; Grenade, HR; Dickey, RW. Improvements in the Sensitivity of Inverse-Detected Heteronuclear Correlation Spectra Using Micro Inverse Probes and Micro Cells: HMQC and HMBC Spectra of Caribbean Ciguatoxin -- Preliminary Structural Inferences. Tetrahedron Lett 1995, 36, 6827–6830. [Google Scholar]
- Vernoux, JP; Lewis, RJ. Isolation and characterisation of Caribbean ciguatoxins from the horse-eye jack (Caranx latus). Toxicon 1997, 35, 889–900. [Google Scholar]
- Lewis, RJ; Sellin, M; Poli, MA; Norton, RS; MacLeod, JK; Sheil, MM. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar]
- Schnorf, H; Taurarii, M; Cundy, T. Ciguatera fish poisoning: a double-blind randomized trial of mannitol therapy. Neurology 2002, 58, 873–880. [Google Scholar]
- Bagnis, R; Spiegel, A; Boutin, JP; Burucoa, C; Nguyen, L; Cartel, JL; Capdevielle, P; Imbert, P; Prigent, D; Gras, C. [Evaluation of the efficacy of mannitol in the treatment of ciguatera in French Polynesia]. Med Tropicale 1992, 52, 67–73. [Google Scholar]
- Blythe, DG; Hack, E; Washington, G; Fleming, LE. Hui, YH, Kitts, D, Stanfield, PS, Eds.; Foodborne Disease Handbook; Marcel Dekker: New York, NY, 2001; pp. 311–320. [Google Scholar]
- Palafox, NA; Jain, LG; Pinano, AZ; Gulick, TM; Williams, RK; Schatz, IJ. Successful treatment of ciguatera fish poisoning with intravenous mannitol. J Am Med Assoc 1988, 259, 2740–2742. [Google Scholar]
- Blythe, DG; Fleming, LE; Ayyar, RA; de Sylva, DP; Baden, DG; Shrank, K. Mannitol Therapy for acute and chronic ciguatera fish poisoning. Memoirs of the Queensland Museum 1994, 34. [Google Scholar]
- Birinyi-Strachan, LC; Davies, MJ; Lewis, RJ; Nicholson, GM. Neuroprotectant effects of iso-osmolar d-mannitol to prevent Pacific ciguatoxin-1 induced alterations in neuronal excitability: A comparison with other osmotic agents and free radical scavengers. Neuropharmacol 2005, 49, 669–686. [Google Scholar]
- Lewis, R; King, G. Williamson, JA, Fenner, PJ, Burnett, JW, Rifkin, JF, Eds.; Ciguatera (fish poisoning); University of New South Wales Press: Sydney, 1996. [Google Scholar]
- Pearn, JH; Lewis, RJ; Ruff, T; Tait, M; Quinn, J; Murtha, W; King, G; Mallett, A; Gillespie, NC. Ciguatera and mannitol: experience with a new treatment regimen. Medical Journal of Australia 1989, 151, 77–80. [Google Scholar]
- Watters, MR. Botana, LM, Ed.; Marine neurotoxins as a starting point to drugs; Taylor and Francis (CRC Press), 2007. [Google Scholar]
- Berlin, RM; King, SL; Blythe, DG. Symptomatic improvement of chronic fatigue with fluoxetine in ciguatera fish poisoning. Med J Austr 1992, 157, 567. [Google Scholar]
- Lange, WR; Snyder, FR; Fudala, PJ. Travel and ciguatera fish poisoning. Arch Int Med 1992, 152, 2049–2053. [Google Scholar]
- Davis, RT; Villar, LA. Symptomatic improvement with amitriptyline in ciguatera fish poisoning. New Engl J Med 1986, 315, 65. [Google Scholar]
- Perez, CM; Vasquez, PA; Perret, CF. Treatment of ciguatera poisoning with gabapentin. New Engl J Med 2001, 344, 692–693. [Google Scholar]
- Bourdy, G; Cabalion, P; Amade, P; Laurent, D. Traditional remedies used in the western Pacific for the treatment of ciguatera poisoning. J Ethnopharmacol 1992, 36, 163–74. [Google Scholar]
- Blythe, DG; de Sylva, DP. Mother’s milk turns toxic following fish feast. J Am Med Assoc 1990, 264, 2074. [Google Scholar]
- Lange, WR; Lipkin, KM; Yang, GC. Can ciguatera be a sexually transmitted disease? J Toxicol - Clin Toxicol 1989, 27, 193–197. [Google Scholar]
- Lange, WR. Ciguatera toxicity. Am Fam Phys 1987, 35, 177–182. [Google Scholar]
- Lewis, R; Jones, A; Vernoux, J. 5th Indo-Pacific Fish conference, Noumea, New Caledonia, 1999; pp. 739–744.
- Ebesu, J. Isolation and Characterization of Novel Ciguateric Compounds from Acanthurus Triostegus (Manini); Ph.D. Thesis; University of Hawaii, 1998. [Google Scholar]
- Vernoux, J-P; Lejeune, J. Ciguatera in the French West Indies. Memoires of the Queensland Museum 1993, 34, 631–638. [Google Scholar]
- Tosteson, T; Ballantine, DL; Winter, A. Reguera, B, Blanco, J, Fernandez, ML, Wyatt, T, Eds.; Sea surface temperature, benthic dinoflagellate toxicity and toxin transmission in the ciguatera food web; Xunta de Galicia and IOC, 1998. [Google Scholar]
- Villareal, TA; Hanson, S; Qualia, S; Jester, ELE; Granade, HR; Dickey, RW. Petroleum production platforms as sites for the expansion of ciguatera in the northwestern Gulf of Mexico. Harmful Algae 2007, 6, 253–259. [Google Scholar]
- Davin, WT; Kohler, CC. Ciguatera toxins adversely affect piscivorous fishes. Trans Am Fish Soc 1988, 117, 374–384. [Google Scholar]
- Davin, WT; Kohler, CC. Effects of ciguatera toxins on the bluehead. Trans Am Fish Soc 1986, 115, 908–912. [Google Scholar]
- Fleming, LE; Baden, DG; Bean, JA; Weisman, R; Blythe, DG. B Reguera, JB, Fernandez, ML, Wyatt, T, Eds.; Harmful Algae; Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO: Santiago de Compostela (Spain), 1998; pp. 245–248. [Google Scholar]
- Kirkpatrick, B; Fleming, LE; Stephan, W; Backer, L; Reich, A; Dalpra, D; Weisman, R; Van De Bogart, G. International Conference on Harmful Algal bloom, Cape Town, South Africa, Nov 2004.
- Fleming, LE; Jerez, E; Stephan, W; Cassedy, A; Bean, JA; Reich, A; Kirkpatrick, B; Backer, L; Nierenberg, K; Watkins, S; Weisman, R. Florida poison information center; University of Miami; Florida Department of Health; CDC; Childrens Hospital Cincinnati; Mote Marine Laboratory: Miami, FL, 2007. [Google Scholar]
- Fleming, LE; Broad, K; Clement, A; Dewailly, E; Elmir, S; Knap, A; Pomponi, SA; Smith, S; Solo Gabriele, H; Walsh, P. Oceans and human health: Emerging public health risks in the marine environment. Mar Poll Bull 2006, 53, 545–560. [Google Scholar]
- Dewailly, E; Pereg, D; Knap, A; Rouja, P; Galvin, J; Owen, R. Walsh, PJ, Smith, SL, Fleming, LE, Solo Gabriele, H, Gerwick, WH, Eds.; Oceans and Human Health: Risks and Remedies from the Sea; Elsevier Science Publishers: New York, NY, in press.
- Fleming, LE; Jerez, E; Stephan, W; Cassedy, A; Bean, J; Reich, A; Kirkpatrick, B; Backer, L; Nierenberg, K; Watkins, S; Hollenbeck, J; Weisman, R. Evaluation of Harmful Algal Bloom Outreach Activities. Mar Drugs 2007, 5, 208–219. [Google Scholar]
- Tsumuraya, T; Fujii, I; Inoue, M; Tatami, A; Miyazaki, K; Hirama, M. Production of monoclonal antibodies for sandwich immunoassay detection of ciguatoxin 51-hydroxyCTX3C. Toxicon 2006, 48, 287–294. [Google Scholar]
- Abraham, WM; Bourdelais, AJ; Ahmed, A; Serebriakov, I; Baden, DG. Effects of inhaled brevetoxins in allergic airways: toxin-allergen interactions and pharmacologic intervention. Environ Health Perspect 2005, 113, 632–637. [Google Scholar]
- Anderson, BS; Sims, JK; Wiebenga, NH; Sugi, M. The epidemiology of ciguatera fish poisoning in Hawaii, 1975–1981. Hawaii Med J 1983, 42, 326–334. [Google Scholar]
- Morris, JG, Jr; Lewin, P; Hargrett, NT; Smith, CW; Blake, PA; Schneider, R. Clinical features of ciguatera fish poisoning: a study of the disease in the US Virgin Islands. Arch Int Med 1982, 142, 1090–1092. [Google Scholar]
- Czernichow, P; Droy, JM; Ezelin, F; Leroy, J. [Epidemiology of Ciguatera in the Iles Saintes (Guadeloupe)]. Rev Epidemiol Sante Pub 1984, 32, 315–321. [Google Scholar]
- Lewis, ND. Disease and development: ciguatera fish poisoning. Soc Sci Med 1986, 23, 983–993. [Google Scholar]
- Luber, G; Azziz-Baumgartner, E; Latka, R; Monteihl, C; Conklin, L; Backer, L. Surveillance for ciguatera fish poisoning in Culebra, Puerto Rico. (in press).
- McMillan, J; Granade, HF; Hoffman, P. Ciguatera fish poisoning in the United States Virgin Islands: preliminary studies. J Coll Virgin Is 1980, 6, 84–107. [Google Scholar]
- Holt, RJ; Miro, G; Del Valle, A. An analysis of poison control center reports of ciguatera toxicity in Puerto Rico for one year. J Toxicol - Clin Toxicol 1984, 22, 177–185. [Google Scholar]
- Frennette, C; Maclean, JD; Gyorkos, TW. A large common-source outbreak of ciguatera fish poisoning. J Infect Dis 1988, 158, 1128–1131. [Google Scholar]
- Engleberg, NC; Morris, JG, Jr; Lewis, J; McMillan, JP; Pollard, RA; Blake, PA. Ciguatera fish poisoning: a major common-source outbreak in the U.S. Virgin Islands. Ann Int Med 1983, 98, 336–337. [Google Scholar]
- Escalona de Motta, G; De la Noceda, GG. Proc. 5th Intl Coral Reef Congress, Tahiti (French Polynesia), 1985; 4, pp. 415–416.
- Chateau-Degat, ML; Beuter, A; Vauterin, G; Nguyen, NL; Chinain, M; Darius, T; Legrand, AM; Chansin, R; Dewailly, E. Neurologic signs of ciguatera disease: evidence of their persistence. Am J Trop Med Hyg 2007, 77, 1170–1175. [Google Scholar]
| Geographic Region | Incidence/10,000/year | Data collection time period | Reference |
|---|---|---|---|
| Reunion Island | 0.78 | 1986–1994 | Quod 1996 [25] |
| Queensland, Australia | 3 | 1965–1984 | Gillespie 1986 [4] |
| Hawaii | 0.3 | 1975–1981 | Anderson 1983 [96] |
| US Virgin Islands | 7.6 | 1982 | Morris 1982 [97] |
| Guadeloupe | 30 | 1984 | Czernichow 1984 [98] |
| South Pacific Region | 970 | 1973–1983 | Lewis 1986 [99] |
| Marshall Islands | 2,820 | 1982–1983 | Lewis 1986 [99] |
| French Polynesia | 5,850 | 1979–1983 | Lewis 1986 [99] |
| Dade County, FL | 5 | 1974–1976 | Lawrence 1980 |
| Culebra, Puerto Rico | 73.6–169.5 | 2005–2006 | Luber, In prep [100] |
| Geographic Region | Prevalence (%) | Time range | Citation |
| St. Thomas (US Virgin Islands) | 4.4 | Annual (1980) | McMillan 1980 [101] |
| Puerto Rico | 7 | Lifetime | Holt 1984 [102] |
| Tahiti | 8.45 | Annual (1966) | Bagnis 1979 [16] |
| Hao (Tuamotos) | 43 | Annual (1978) | Lewis 1986 [99] |
| Polynesian Islands | 70 | Lifetime | Lewis 1986 [99] |
| Region of Study Author Number of Cases | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oceans | Caribbean | Atlantic | Pacific | Indian | |||||||||
| Reported Symptoms | Friedman [23] | Arena [24] | Stinn [42] | Frennette [103] | Engleberg [104] | Escalona [105] | Lawrence [9] | Bagnis [26] | Schnorf [64] | Chateau-Degat [106] | Gillespie [4] | Bagnis [16] | Quod [25] |
| N=12 | N=12 | N=442 | N=57 | N=47 | N=80 | N=129 | N=12,890 | N=50 | N=47 | N=527 | N=3009 | N=167 | |
| Gastrointestinal: | |||||||||||||
| Diarrhea | 67 | 75 | 79 | 77 | 81 | 83 | 76 | 73 | 50 | 64 | 71 | 49 | |
| Vomiting | 42 | 43 | 37 | 40 | 69 | 68 | 39 | 35 | 38 | 50 | |||
| Nausea | 42 | 82 | 69 | 44 | 26 | 55 | 43 | 50 | |||||
| Abdominal Pain | 42 | 75 | 65 | 58 | 30 | 74 | 43 | 52 | 52 | 46 | 29 | ||
| Neurologic: | |||||||||||||
| Extremity Paresthesia | 67 | 100 | 81 | 79 | 36 | 71 | 89 | 72 | 93 | 64–71 | 89 | 82 | |
| Circumoral | 58 | 70 | 79 | 38 | 38 | 54 | 88 | 91 | 66 | 89 | 82 | ||
| Paresthesia | |||||||||||||
| Temperature | 58 | 92 | 64 | 77 | 23 | 48 | 87 | 94 | 34 | 76 | 88 | 65 | |
| Dysesthesia | |||||||||||||
| Myalgia | 67 | 75 | 79 | 75 | 34 | 56 | 86 | 85 | 56 | 80 | 83 | 82 | 38 |
| Arthralgia | 42 | 83 | 79 | 75 | 34 | 60 | 86 | 62 | 80 | 79 | 86 | 29 | |
| Pruritis | 67 | 67 | 77 | 66 | 45 | 48 | 44 | 42 | 76 | 45 | 5 | ||
| Headache | 56 | 45 | 39 | 47 | 60 | 50 | 62 | 59 | 25 | ||||
| Vertigo | 25 | 58 | 50 | 33 | 47 | 62 | 45 | 42 | |||||
| Weakness (Asthenia) | 92 | 100 | 84 | 65 | 30 | 60 | 80 | 60 | 70 | ||||
| Dental Pain/Feeling like teeth are loose or falling out | 33 | 32 | 23 | 13 | 11 | 21 | 37 | 25 | |||||
| Dysuria | 8 | 33 | 25 | 13 | 26 | 22 | 19 | ||||||
| Chills/Sweating | 36 | 24 | 60 | 49 | 59 | ||||||||
| Neuropsychiatric: | |||||||||||||
| Hallucinations | 8 | 17 | <5 | 16 | |||||||||
| Depression | 25 | 17 | 16 | ||||||||||
| Memory/concentration problems | 17 | 58 | |||||||||||
| Multi-tasking problems | 25 | ||||||||||||
| Giddiness | 29 | 30 | |||||||||||
| Cardiovascular: | |||||||||||||
| Arrhythmia | 33 | ||||||||||||
| Hypertension | 12 | 12 | |||||||||||
| Bradycardia | 16 | 16 | |||||||||||
| Alcohol [4,6,27,74] |
| Nuts [6,8] |
| Caffeine [6] |
| Pork [8] |
| Chicken [4,8] |
| Any kind of fish [4,6,27] |
| Physical activity/exertion [74] |
| Some Common Ciguatoxic Fish [4,32,52,81–84] |
|---|
| Moray eel |
| Barracuda |
| Grouper |
| Kingfish |
| Jacks |
| Snapper |
| Surgeonfish |
| Parrot fish |
| Wrasses |
| Hogfish |
| Narrow barred Spanish mackerel |
| Coral trout |
| Flowery cod |
| Red emperor |
| The following are also associated with CFP |
| Eating fish viscera or roe [29,70] |
| Large, predatory reef fish* [37,70,80] |
| Reef fish from areas known to be associated with CFP occurrence [70] |
| Note that eating small portions (i.e. <50 grams or <0.11 pounds) of different fish may be safer [27] than eating larger portions (i.e. >200 grams) of any one potentially ciguatoxic fish [70]. |
Share and Cite
Friedman, M.A.; Fleming, L.E.; Fernandez, M.; Bienfang, P.; Schrank, K.; Dickey, R.; Bottein, M.-Y.; Backer, L.; Ayyar, R.; Weisman, R.; et al. Ciguatera Fish Poisoning: Treatment, Prevention and Management. Mar. Drugs 2008, 6, 456-479. https://doi.org/10.3390/md6030456
Friedman MA, Fleming LE, Fernandez M, Bienfang P, Schrank K, Dickey R, Bottein M-Y, Backer L, Ayyar R, Weisman R, et al. Ciguatera Fish Poisoning: Treatment, Prevention and Management. Marine Drugs. 2008; 6(3):456-479. https://doi.org/10.3390/md6030456
Chicago/Turabian StyleFriedman, Melissa A., Lora E. Fleming, Mercedes Fernandez, Paul Bienfang, Kathleen Schrank, Robert Dickey, Marie-Yasmine Bottein, Lorraine Backer, Ram Ayyar, Richard Weisman, and et al. 2008. "Ciguatera Fish Poisoning: Treatment, Prevention and Management" Marine Drugs 6, no. 3: 456-479. https://doi.org/10.3390/md6030456
APA StyleFriedman, M. A., Fleming, L. E., Fernandez, M., Bienfang, P., Schrank, K., Dickey, R., Bottein, M.-Y., Backer, L., Ayyar, R., Weisman, R., Watkins, S., Granade, R., & Reich, A. (2008). Ciguatera Fish Poisoning: Treatment, Prevention and Management. Marine Drugs, 6(3), 456-479. https://doi.org/10.3390/md6030456





