Purification, Characterization and in vitro Anti-Tumor Activity of Proteins from Arca subcrenata Lischke
Abstract
:1. Introduction
2. Materials and Methods
2.1 Materials
2.2 Extraction of total proteins
2.3 Purification of proteins
Anion exchange chromatography (DEAE Sepharose Fast Flow)
Gel filtration chromatography (Sephadex G-50)
2.4 Cytotoxicity assay
Cell lines and culture
Evaluation of cytotoxicity
2.5 Protein assay
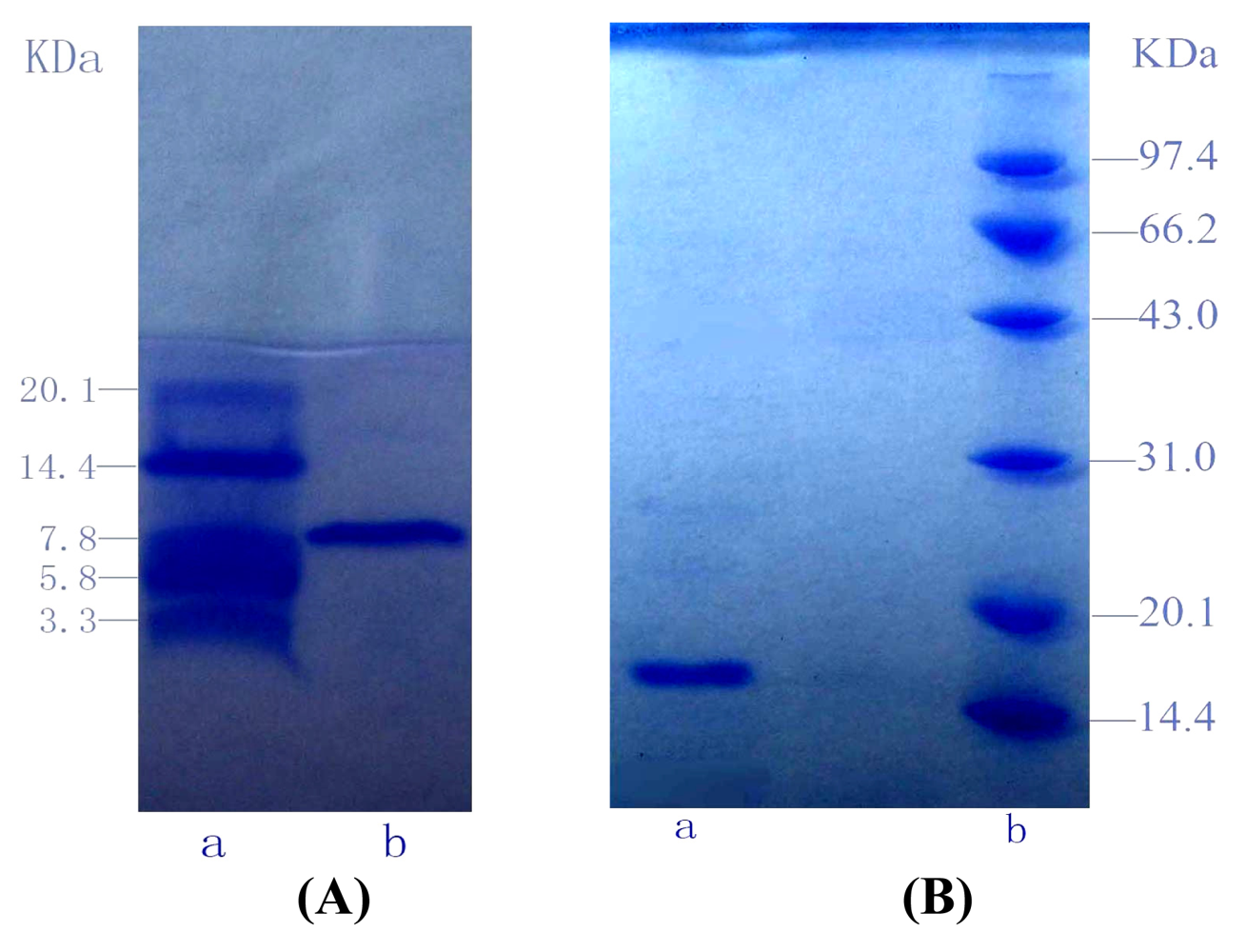
2.6 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
2.7 Isoelectric focusing-polyacrylamide gel electrophoresis (IEF)
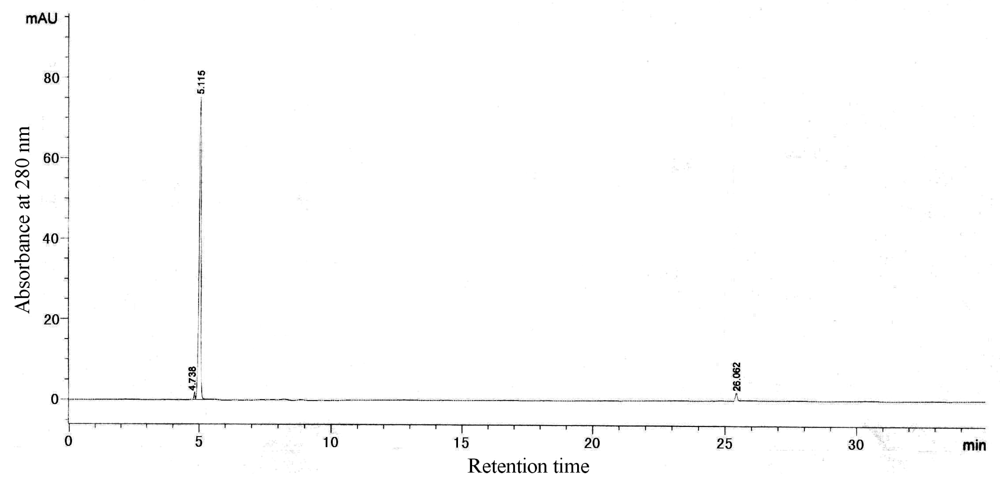
2.8 Reversed-phase high performance liquid chromatography (HPLC)
2.9 Analysis of saccharides
2.10 Analysis of amino acid components
3. Results and Discussion
3.1 In vitro bioactivity-guided fractionation
3.2 Purification of cytotoxic proteins from A. subcrenata
3.3 Characterization of purified proteins
4. Conclusions
Acknowledgments
References
- Belarbi, EH; Contreras Gómez, A; Chisti, Y; García Camacho, F; Molina Grima, E. Producing drugs from marine sponges. Biotechnol Adv 2003, 21, 585–598. [Google Scholar]
- Schwartsmann, G; da Rocha, AB; Berlinck, R; Jimeno, J. Marine organisms as a source of new anticancer agents. Lancet Oncol 2001, 2, 221–225. [Google Scholar]
- Mayer, AMS. Marine pharmacology in 1998: antitumor and cytotoxic compounds. Pharmacologist 1999, 41, 159–164. [Google Scholar]
- Mayer, AMS; Lehmann, VKB. Marine pharmacology in 1999: antitumor and cytotoxic compounds. Anticancer Res 2001, 21, 2489–2500. [Google Scholar]
- Mayer, AMS; Gustafson, KR. Marine pharmacology in 2000: antitumor and cytotoxic compounds. Int J Cancer 2003, 105, 291–299. [Google Scholar]
- Mayer, AMS; Gustafson, KR. Marine pharmacology in 2001–2: antitumour and cytotoxic compounds. Eur J Cancer 2004, 40, 2676–2704. [Google Scholar]
- Mayer, AMS; Gustafson, KR. Marine pharmacology in 2003–2004: anti-tumour and cytotoxic compounds. Eur J Cancer 2006, 42, 2241–2270. [Google Scholar]
- Zhang, YP; Xu, F; Wang, M. Progress on Marine Anticancer Drugs in R&D. Prog Pharmaceut Sci 2006, 30, 433–442. [Google Scholar]
- Suárez, Y; González, L; Cuadrado, A; Berciano, M; Lafarga, M; Muñoz AKahalalide, F. A new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol Cancer Ther 2003, 2, 863–872. [Google Scholar]
- Riely, GJ; Gadgeel, S; Rothman, I; Saidman, B; Sabbath, K; Feit, K; Kris, MG; Rizvi, NA. A phase 2 study of TZT-1027, administered weekly to patients with advanced non-small cell lung cancer following treatment with platinum-based chemotherapy. Lung Cancer 2007, 55, 181–185. [Google Scholar]
- Kerbrat, P; Dieras, V; Pavlidis, N; Ravaud, A; Wanders, J; Fumoleau, P. For the EORTC Early Clinical Studies Group/New Drug Development Office. Phase II study of LU 103793 (dolastatin analogue) in patients with metastatic breast cancer. Eur J Cancer 2003, 39, 317–320. [Google Scholar]
- Guo, XS; Li, Y. Marine Chinese Traditional Medicines; Sciences Press: Beijing, P.R. China, 2003; pp. 138–141. [Google Scholar]
- Li, Q; Li, TM; Wang, XQ; Huang, Q; Wu, WJ. Biochemical properties analysis of Arca subcrenata extractive. Pharm Biotechnol 1998, 5, 245–247. [Google Scholar]
- Dou, CG; Yan, YQ; Zhang, Z. Experimental studies on hypoglycemia and hypolipid effects of hydrolysate of Arca subcrenata. Chin J Mar Drug 1996, 15(1), 13–15. [Google Scholar]
- Dou, CG; Yan, YQ; Zhang, Z. The protective action of hydrolysate of Arca Subcrenata on liver injuries in mice. Chin J Mar Drug 1996, 15(3), 17–19. [Google Scholar]
- He, YM; Chen, YX; Liu, CH; Xi, T; Shen, ZL; Yao, QS. Isolation, purification and immunological activity assay of polysaccharide from Arca subcrenata Lischke. Chin J Mar Drug 2007, 26(2), 23–26. [Google Scholar]
- Murphy, EJ; Edmondson, RD; Russell, DH; Colles, S; Schroeder, F. Isolation and characterization of two distinct forms of livers fatty acid binding protein from the rat. Biochim Biophys Acta 1999, 1436, 413–425. [Google Scholar]
- Fatope, MO; Zeng, L; Ohayaga, JE; Shi, G; Mclaughlin, JL. Selectively cytotoxic diterpenes from Euphorbia poisonii. J Med Chem 1996, 39, 1005–1008. [Google Scholar]
- Bradford, MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976, 72, 248–254. [Google Scholar]
- Laemmli, UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar]
- Schägger, H; von Jagow, G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem 1987, 166, 368–379. [Google Scholar]
- Stephano, JL; Gould, M; Rojas-Galicia, L. Advantages of picrate fixation for staining polyproteins in polyacrylamide gels. Anal Biochem 1986, 152, 308–313. [Google Scholar]
- Thompson, PE; Hearn, MT. A simple and inexpensive sample-handling method for the semi-preparative RP-HPLC of polyproteins and non-polar peptide derivatives: pre-adsorption of sample. J Biochem Biophys Methods 1995, 30, 153–161. [Google Scholar]
- Lee, RP; Doughty, SW; Ashman, K; Walker, J. Purification of hydrophobic integral membrane proteins from Mycoplasma hyopneumoniae by reversed-phase high-performance liquid chromatography. J Chromatogr A 1996, 737, 273–279. [Google Scholar]
- Fujinari, EM; Manes, JD; Bizanek, R. Peptide content determination of crude synthetic proteins by reversed-phase liquid chromatography and nitrogen-specific detection with a chemiluminescent nitrogen detector. J Chromatogr A 1996, 743, 85–89. [Google Scholar]
- Venkateshwaran, TG; Stewart, JT; de Haseth, JA; Bartlett, MG. Solution conformation of model polyproteins with the use of particle beam LC/FT-IR spectrometry and electrospray mass spectrometry. J Pharm Biomed Anal 1999, 19, 709–723. [Google Scholar]
- Zolla, L; Bianchetti, M. High-performance liquid chromatography coupled on-line with electrospray ionization mass spectrometry for the simultaneous separation and identification of the Synechocystis PCC 6803 phycobilisome proteins. J Chromatogr A 2001, 912, 269–279. [Google Scholar]
- Pavli, V; Kmetec, V. Optimization of HPLC method for stability testing of bacitracin. J Pharm Biomed Anal 2001, 24, 977–982. [Google Scholar]
- Saha, SK; Brewer, EF. Determination of the concentration of oligosaccharides: complex type carbohydrates and glycoproteins using the phenol-sulfric acid method. Carbohydr Res 1994, 254, 157–167. [Google Scholar]
- Dong, Q; Zheng, LY; Fang, JY. Modified phenol-sulfuric acid method for determination of the content of oligosaccharides and polysaccharides. Chin Pharm J 1996, 31, 550–553. [Google Scholar]
- Bao, XF; Fang, JN. Studies on difference between sporoderm-broken and nonbroken spores of gan derma lucidum (leyss. Ex fr.) karst. by polysaccharide analysis. China J Chin Mat Med 2001, 26, 326–328. [Google Scholar]
- Suffness, M; Pezzuto, JM. Methods in Plant Biochemistry; Academic Press: New York; NY, USA; Volume 1, 1991. [Google Scholar]
- Xu, SY; Bian, RL; Chen, X. Methodology of Pharmacological Experiment, 3 ed; People’s Medical Publishing House: Beijing, P.R. China, 1991. [Google Scholar]
- da Rocha, AB; Lopes, RM; Schwartsmann, G. Natural products in anticancer therapy. Curr Opin Pharmacol 2001, 1, 364–369. [Google Scholar]
- Huang, DC; Li, QF; Li, P; Li, XQ; Song, YZ. Effects of oyster low molecular weight bioactive substance on the human lung adenocarcinoma A549 cells. J Xiamen Univ (Nat Sci) 2002, 41, 614–617. [Google Scholar]
- Wang, Y; Ma, AL; Zhang, HZ; Xue, BH; Zhao, ZJ; Fu, FH; Zhou, GY. Experimental studies on the antitumor effect of oyster extract. Chin J Mar Drug 1997, 16, 18–22. [Google Scholar]
- Li, B-H; Zhou, Y-B; Guo, S-B; Wang, C-B. Protein from Chlamys farreri inhibits UVB-induced HaCaT cells apoptosis via inhibition CD95 pathway and reactive oxygen species. Free Rad Res 2007, 41, 1224–1232. [Google Scholar]
- Kim, JY; Yoon, MY; Cha, MR; Hwang, JH; Park, E; Choi, SU; Park, H-R; Hwang, Y-I. Methanolic extracts of Plocamium telfairiae induce cytotoxicity and caspase-dependent apoptosis in HT-29 human colon carcinoma cells. J Med Food 2007, 10, 587–593. [Google Scholar]



| Samples | Cell lines | ||||||
|---|---|---|---|---|---|---|---|
| A549 | Hela | PC-3 | HL-60 | KB | BEL-7404 | CNE | |
| Total protein extract | 306.1±28.4 | 35.6±2.9 | >500 | 106.0±14.6 | >500 | >500 | 145.9±25.9 |
| Fraction-I | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Fraction-II | >500 | >500 | >500 | >500 | >500 | >500 | >500 |
| Fraction-III | 351.7±40.6 | 6.7±0.8 | >500 | 14.7±1.7 | 76.5±4.9 | >500 | >500 |
| G-6 | >500 | >500 | >500 | 123.2±11.3 | >500 | >500 | >500 |
| G-4 | >500 | 38.2±2.7 | >500 | 67.8±7.1 | 78.1±9.0 | >500 | >500 |
| G-4-2 | >500 | 22.9±2.4 | >500 | 46.1±3.5 | 57.7±7.2 | >500 | >500 |
| Amino acid | The content of amino acid(mg/ml) | The mass percentage of amino acid (%) |
|---|---|---|
| Asp | 0.0352 | 11.72 |
| Thr | 0.0160 | 5.33 |
| Ser | 0.0000 | 0.00 |
| Glu | 0.0426 | 14.18 |
| Gly | 0.0102 | 3.39 |
| Ala | 0.0178 | 5.93 |
| Cys | 0.0483 | 16.08 |
| Val | 0.0123 | 4.09 |
| Met | 0.0056 | 1.86 |
| Ile | 0.0079 | 2.63 |
| Leu | 0.0142 | 4.73 |
| Tyr | 0.0157 | 5.23 |
| Phe | 0.0039 | 1.30 |
| Lys | 0.0251 | 8.36 |
| His | 0.0214 | 7.12 |
| Arg | 0.0197 | 6.56 |
| Pro | 0.0000 | 0.00 |
| Amino acid | The content of amino acid(mg/ml) | The mass percentage of amino acid (%) |
|---|---|---|
| Asp | 0.2563 | 15.64 |
| Thr | 0.1005 | 6.13 |
| Ser | 0.0633 | 3.86 |
| Glu | 0.2364 | 14.43 |
| Gly | 0.0741 | 4.52 |
| Ala | 0.0747 | 4.56 |
| Cys | 0.0440 | 2.69 |
| Val | 0.0657 | 4.01 |
| Met | 0.0613 | 3.74 |
| Ile | 0.1035 | 6.32 |
| Leu | 0.0412 | 2.51 |
| Tyr | 0.0758 | 4.63 |
| Phe | 0.1056 | 6.45 |
| Lys | 0.2100 | 12.82 |
| His | 0.0335 | 2.04 |
| Arg | 0.0426 | 2.60 |
| Pro | 0.0000 | 0.00 |
Share and Cite
Song, L.; Ren, S.; Yu, R.; Yan, C.; Li, T.; Zhao, Y. Purification, Characterization and in vitro Anti-Tumor Activity of Proteins from Arca subcrenata Lischke. Mar. Drugs 2008, 6, 418-430. https://doi.org/10.3390/md6030418
Song L, Ren S, Yu R, Yan C, Li T, Zhao Y. Purification, Characterization and in vitro Anti-Tumor Activity of Proteins from Arca subcrenata Lischke. Marine Drugs. 2008; 6(3):418-430. https://doi.org/10.3390/md6030418
Chicago/Turabian StyleSong, Liyan, Shengfang Ren, Rongmin Yu, Chunyan Yan, Tingfei Li, and Yu Zhao. 2008. "Purification, Characterization and in vitro Anti-Tumor Activity of Proteins from Arca subcrenata Lischke" Marine Drugs 6, no. 3: 418-430. https://doi.org/10.3390/md6030418
APA StyleSong, L., Ren, S., Yu, R., Yan, C., Li, T., & Zhao, Y. (2008). Purification, Characterization and in vitro Anti-Tumor Activity of Proteins from Arca subcrenata Lischke. Marine Drugs, 6(3), 418-430. https://doi.org/10.3390/md6030418




