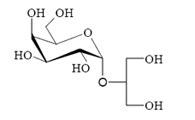
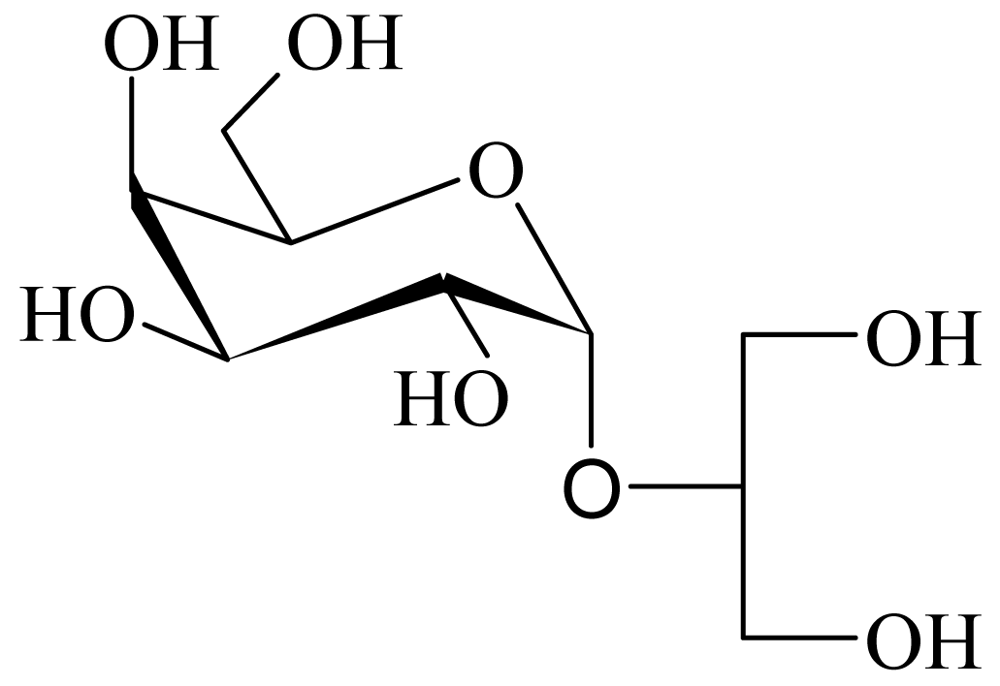
Floridoside Extracted from the Red Alga Mastocarpus stellatus Is a Potent Activator of the Classical Complement Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction, purification and chemical structure
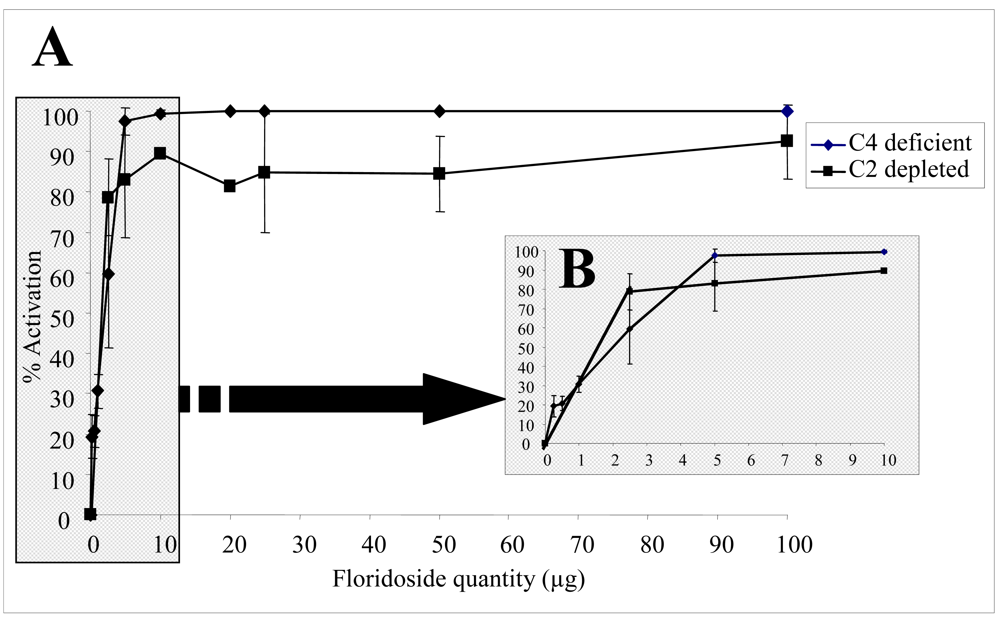
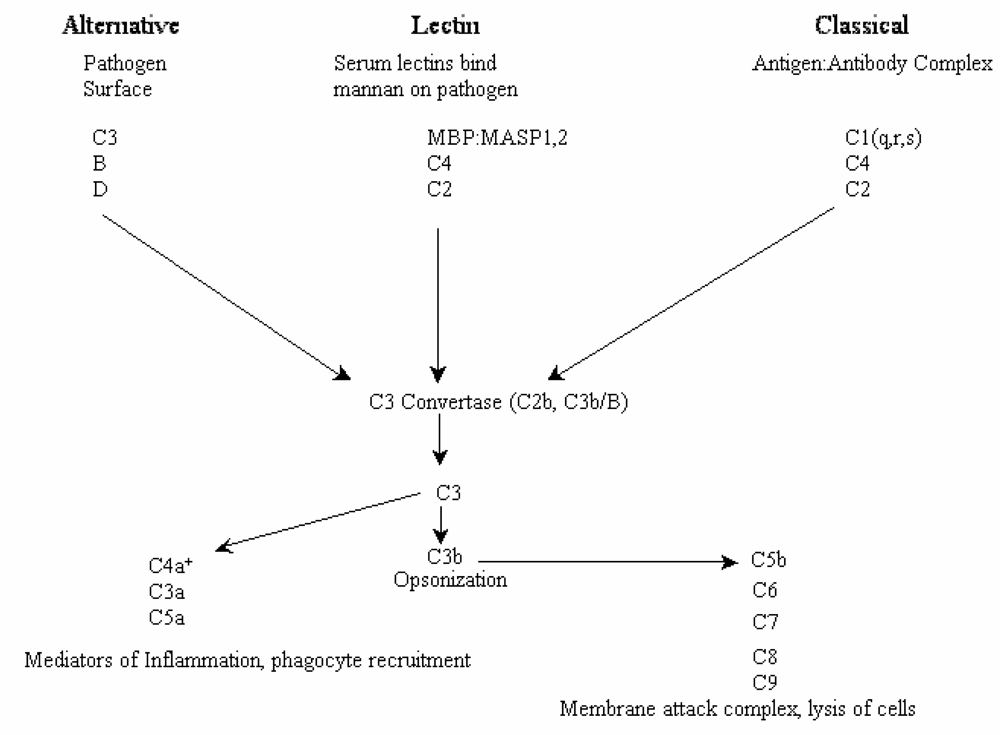
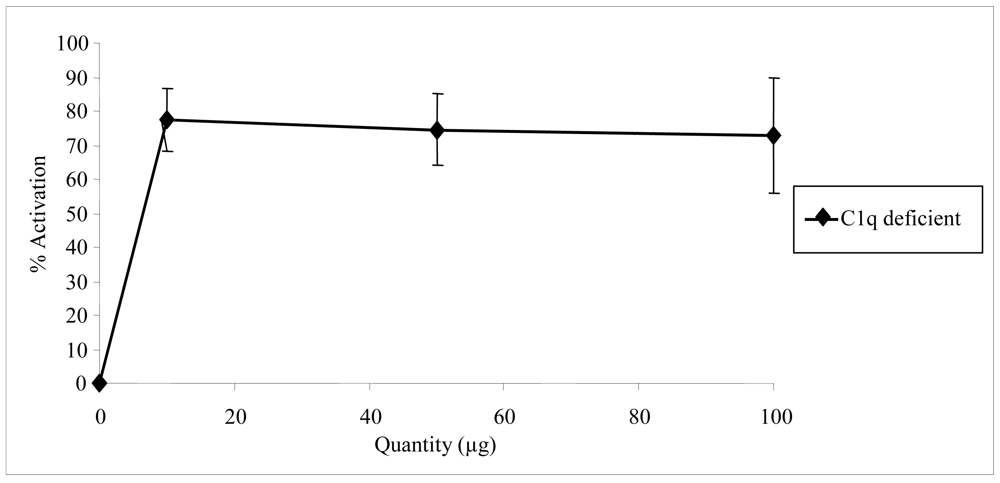
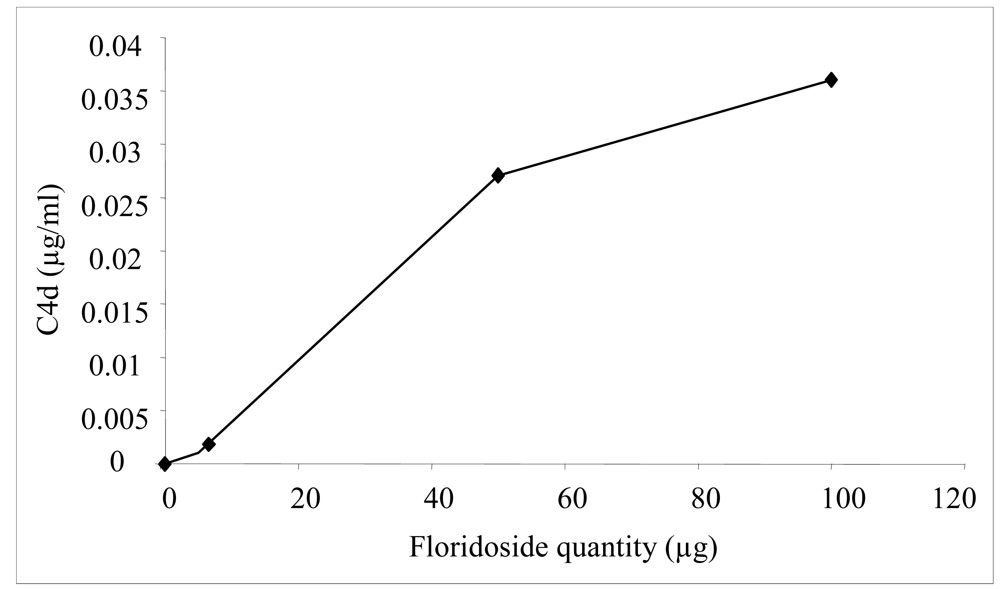
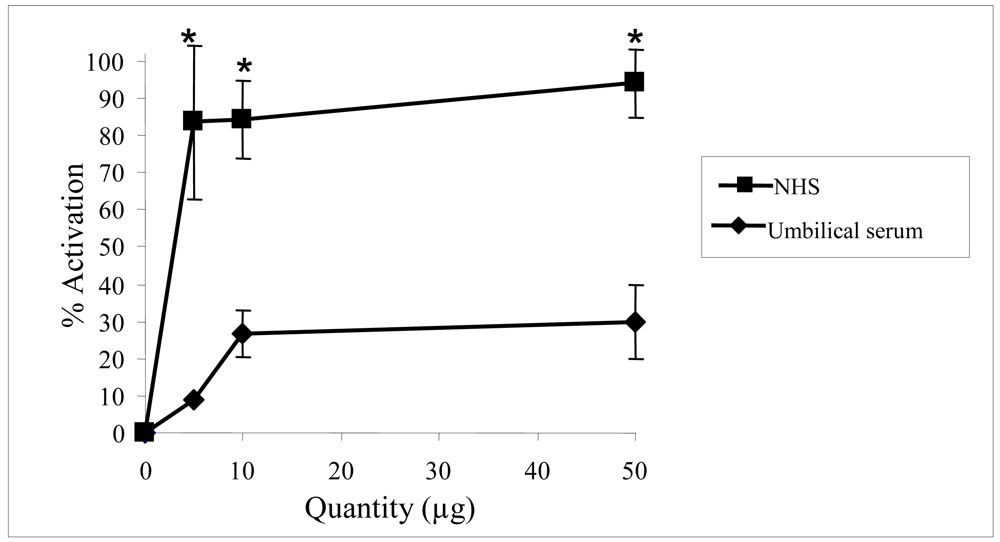
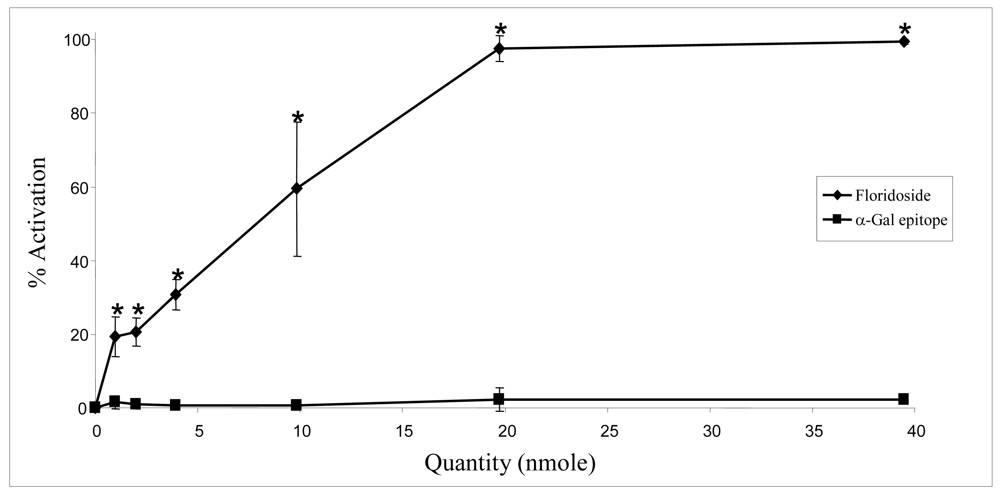
2.2. Biological activities of floridoside
3. Experimental
3.1. Materials
3.2. Buffers
3.3. Extraction and isolation of floridoside
3.4. Capacity of normal human serum to lyse 50% of sensitized erythrocytes through the classical pathway (CH50 assay)
3.5. Hemolytic assay for evaluation of the classical complement pathway activation
3.6. ELISA for the detection of complement activation products: Quantification of the C4d protein
3.7. Statistical analysis
- Sample Availability: Available from the authors
References
- Simon-Colin, C; Kervarec, N; Pichon, R; Deslandes, E. Complete 1H and 13C spectral assignment of floridoside. Carbohydr Res 2002, 337(3), 279–80. [Google Scholar]
- Simon-Colin, C; Michaud, F; Leger, JM; Deslandes, E. Crystal structure and chirality of natural floridoside. Carbohydr Res 2003, 338(22), 2413–6. [Google Scholar]
- Putman, EW; Hassid, WZ. Structure of galactosylglycerol from Iridea laminaroides. Biochem J 1954, 79, 7–12. [Google Scholar]
- Galili, U; Shohet, SB; Kobrin, E; Stults, CL; Macher, BA. Man, apes, and Old World monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J Biol Chem 1988, 263(33), 17755–62. [Google Scholar]
- Walport, MJ. Complement. First of two parts. N Engl J Med 2001, 344(14), 1058–66. [Google Scholar]
- Walport, MJ. Complement. Second of two parts. N Engl J Med 2001, 344(15), 1140–4. [Google Scholar]
- Lutz, HU; Stammler, P; Jelezarova, E; Nater, M; Spath, PJ. High doses of immunoglobulin G attenuate immune aggregate-mediated complement activation by enhancing physiologic cleavage of C3b in C3bn-IgG complexes. Blood 1996, 88(1), 184–93. [Google Scholar]
- Brasher, GW; Hartley, TF. Quantitation of IgA and IgM in umbilical cord serum of normal newborn infants. J Pediatr 1969, 74(5), 784–8. [Google Scholar]
- Galili, U; Basbaum, CB; Shohet, SB; Buehler, J; Macher, BA. Identification of erythrocyte Gal alpha 1–3Gal glycosphingolipids with a mouse monoclonal antibody, Gal-13. J Biol Chem 1987, 262(10), 4683–8. [Google Scholar]
- Thall, A; Etienne-Decerf, J; Winand, RJ; Galili, U. The alpha-galactosyl epitope on human normal and autoimmune thyroid cells. Autoimmunity 1991, 10(2), 81–7. [Google Scholar]
- Galili, U. The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy. Immunol Cell Biol 2005, 83(6), 674–86. [Google Scholar]
- Galili, U; Rachmilewitz, EA; Peleg, A; Flechner, I. A unique natural human IgG antibody with anti-alpha-galactosyl specificity. J Exp Med 1984, 160(5), 1519–31. [Google Scholar]
- Galili, U; Macher, BA; Buehler, J; Shohet, SB. Human natural anti-alpha-galactosyl IgG. II. The specific recognition of alpha (1→3)-linked galactose residues. J Exp Med 1985, 162(2), 573–82. [Google Scholar]
- Galili, U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today 1993, 14(10), 480–2. [Google Scholar]
- Rieben, R; Bovin, NV; Korchagina, EY; Oriol, R; Nifant'ev, NE; Tsvetkov, DE; Daha, MR; Mohacsi, PJ; Joziasse, DH. Xenotransplantation: in vitro analysis of synthetic alpha-galactosyl inhibitors of human anti-Galalpha1→3Gal IgM and IgG antibodies. Glycobiol 2000, 10(2), 141–8. [Google Scholar]
- Morgan, BP; Harris, CL. Complement therapeutics; history and current progress. Mol Immunol 2003, 40(2–4), 159–70. [Google Scholar]
- Edens, RE; Linhardt, RJ; Bell, CS; Weiler, JM. Heparin and derivatized heparin inhibit zymosan and cobra venom factor activation of complement in serum. Immunopharmacol 1994, 27(2), 145–53. [Google Scholar]
- Trail, PA; Bianchi, AB. Monoclonal antibody drug conjugates in the treatment of cancer. Curr Opin Immunol 1999, 11(5), 584–8. [Google Scholar]
- Kazatchkine, M; Hauptmann, G; Nydegger, U. Dosages hémolytiques des composants du Complément; Société française d’Immunologie: Paris, France, 1985; pp. 15–44. [Google Scholar]
- Blondin, C; Chaubet, F; Nardella, A; Sinquin, C; Jozefonvicz, J. Relationships between chemical characteristics and anticomplementary activity of fucans. Biomaterials 1996, 17(6), 597–603. [Google Scholar]
Share and Cite
Courtois, A.; Simon-Colin, C.; Boisset, C.; Berthou, C.; Deslandes, E.; Guézennec, J.; Bordron, A. Floridoside Extracted from the Red Alga Mastocarpus stellatus Is a Potent Activator of the Classical Complement Pathway. Mar. Drugs 2008, 6, 407-417. https://doi.org/10.3390/md6030407
Courtois A, Simon-Colin C, Boisset C, Berthou C, Deslandes E, Guézennec J, Bordron A. Floridoside Extracted from the Red Alga Mastocarpus stellatus Is a Potent Activator of the Classical Complement Pathway. Marine Drugs. 2008; 6(3):407-417. https://doi.org/10.3390/md6030407
Chicago/Turabian StyleCourtois, Anthony, Christelle Simon-Colin, Claire Boisset, Christian Berthou, Eric Deslandes, Jean Guézennec, and Anne Bordron. 2008. "Floridoside Extracted from the Red Alga Mastocarpus stellatus Is a Potent Activator of the Classical Complement Pathway" Marine Drugs 6, no. 3: 407-417. https://doi.org/10.3390/md6030407
APA StyleCourtois, A., Simon-Colin, C., Boisset, C., Berthou, C., Deslandes, E., Guézennec, J., & Bordron, A. (2008). Floridoside Extracted from the Red Alga Mastocarpus stellatus Is a Potent Activator of the Classical Complement Pathway. Marine Drugs, 6(3), 407-417. https://doi.org/10.3390/md6030407