The Nemertine Toxin Anabaseine and Its Derivative DMXBA (GTS-21): Chemical and Pharmacological Properties
Abstract
:Introduction
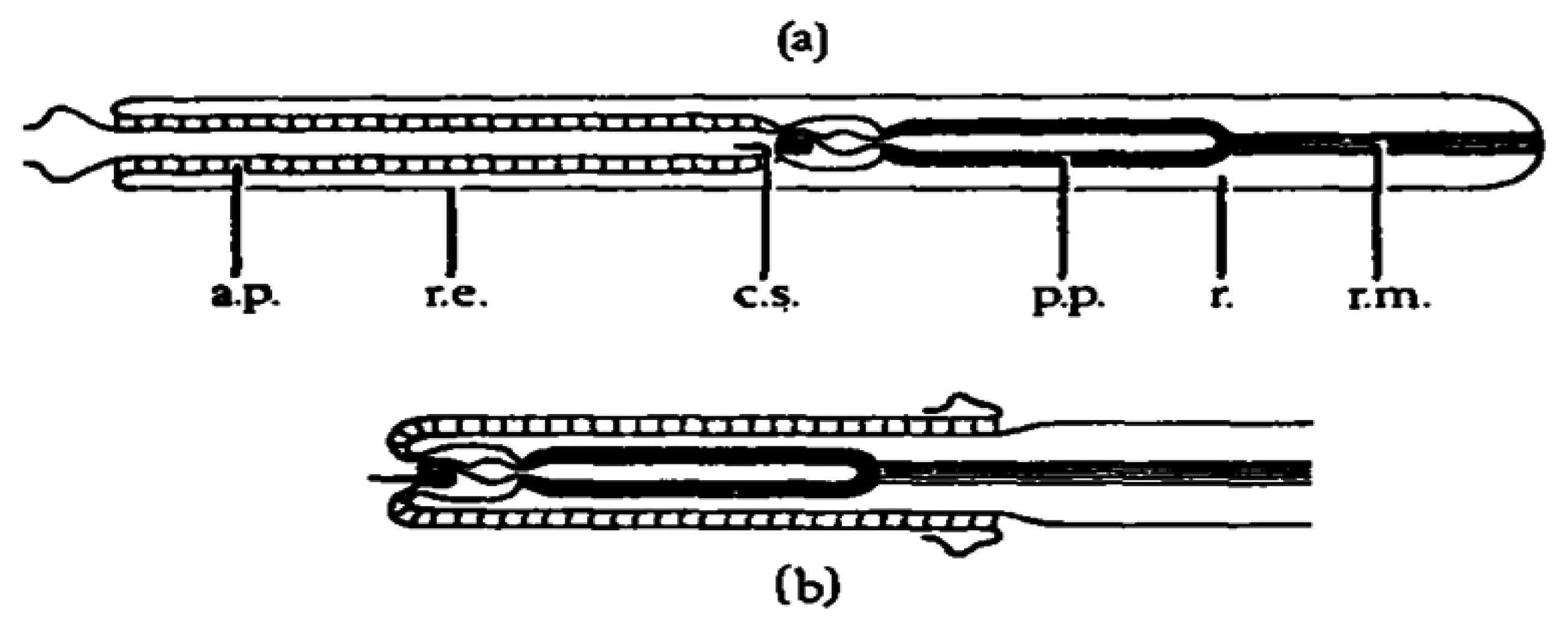
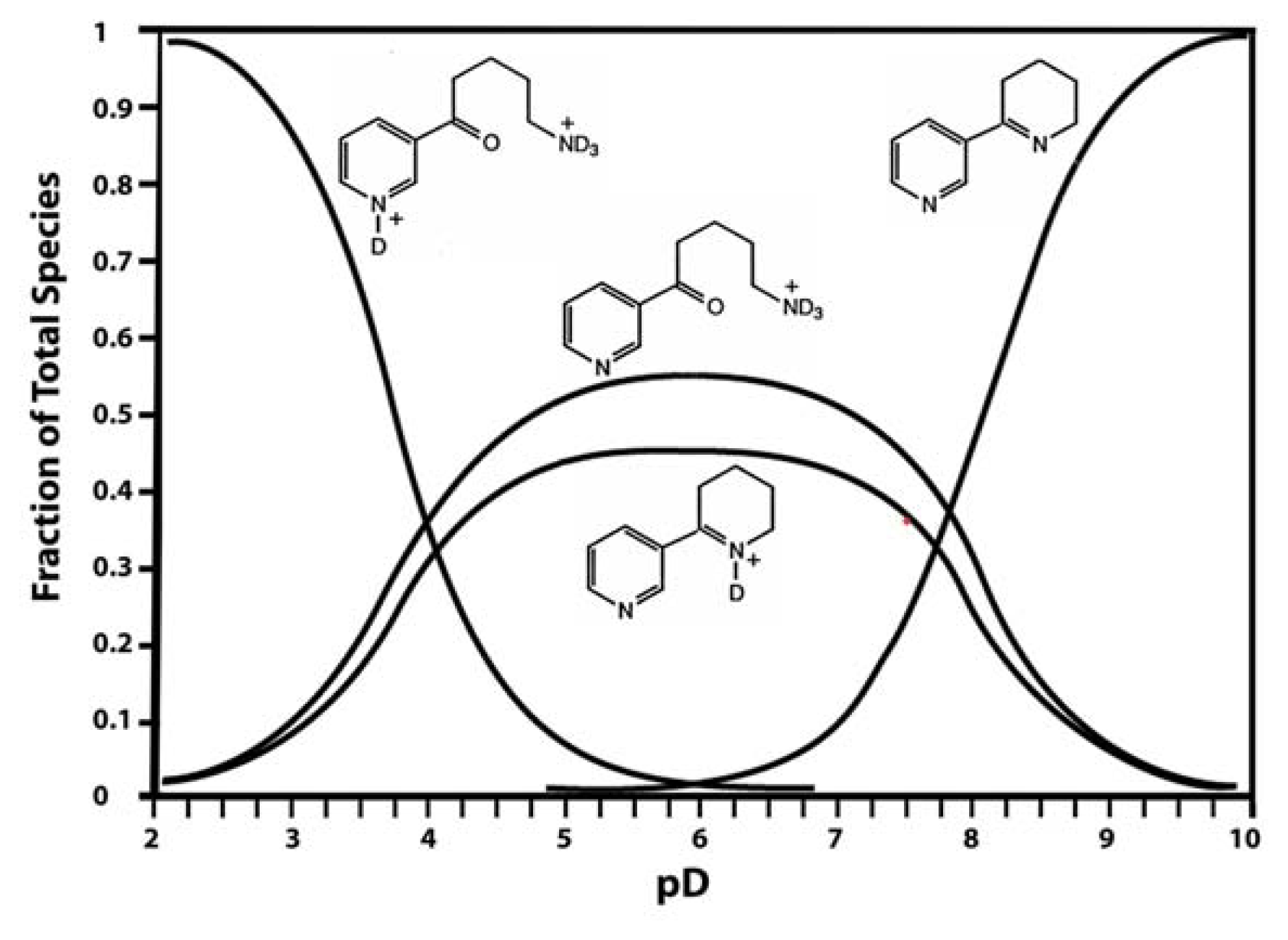
Anabaseine
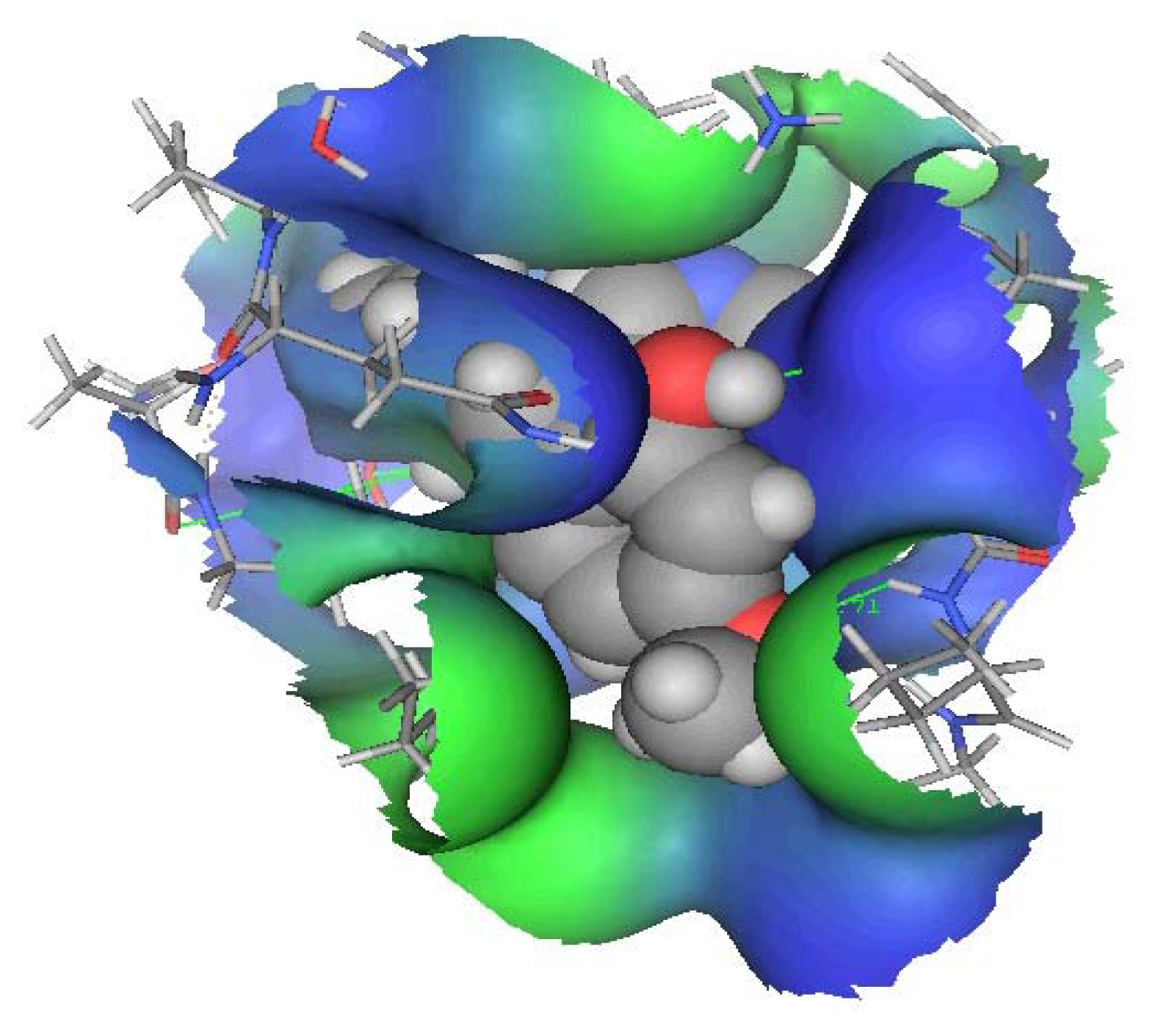
DMXB-Anabaseine (DMXBA), A Synthetic Anabaseine Derivative
Future Directions of Research
Acknowledgements
Abbreviations
| ACh | acetylcholine |
| AChR | nicotinic acetylcholine receptor |
| α-BTx | α-bungarotoxin |
| DMXBA (or GTS-21) | 3-(2,4-dimethoxybenzylidene)-anabaseine |
| LTP | long-term potentiation |
| NMDA | N-methyl-D-aspartate |
| CNS | central nervous system |
| 5-HT | 5-hydroxytryptamine or serotonin |
| AD | Alzheimer’s disease |
| Aβ | β-amyloid |
| PTHP | 2- (3,4,5,6-tetrahydropyrimidinyl)-3-pyridine |
| PCP | phencyclidine |
| Ki | inhibition constant |
- Samples Availability: Available from the authors.
References
- Adams, C. E.; Stevens, K. E.; Kem, W. R.; Freedman, R. Inhibition of nitric oxide synthase prevents α7 nicotinic receptor-mediated restoration of inhibitory auditory gating in rat hippocampus. Brain Res 2000, 877, 235–244. [Google Scholar]
- Arendash, G. W.; Sengstock, G.J; Sanberg, R.; Kem, W. R. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res 1995, 674, 252–259. [Google Scholar]
- Arias, H. R. Localization of agonist and competitive antagonist binding sites on nicotinic acetylcholine receptors. Neurochem. Int 2000, 36, 595–645. [Google Scholar]
- Arias, H. R. Biological and Biophysical Aspects of Ligand-Gated Ion Channel Receptor Superfamilies; Arias, H. R., Ed.; Research Signpost: India, 2006; Volume Chapter 1, pp. 1–25. [Google Scholar]
- Arias, H. R.; Bhumireddy, P. Anesthetics as chemical tools to study the structure and function of nicotinic acetylcholine receptors. Curr. Protein Pept. Sci 2005, 6, 451–472. [Google Scholar]
- Arias, H. R.; Bhumireddy, P.; Bouzat, C. Molecular mechanisms and binding site locations for noncompetitive antagonists of nicotinic acetylcholine receptors. Int. J. Biochem. Cell Biol. 2006, in press. [Google Scholar]
- Arias, H. R.; Bhumireddy, P.; Soti, F.; Blanton, M. P.; Kem, W. R. Characterization of the noncompetitive binding site for anabaseine analogs on the Torpedonicotinic acetylcholine receptor. 2006. submitted. [Google Scholar]
- Arias, H. R.; Bhumireddy, P.; Spitzmaul, G.; Trudell, J. R.; Bouzat, C. Molecular mechanisms and binding site location for the noncompetitive antagonist crystal violet on nicotinic acetylcholine receptors. Biochemistry 2006, 45, 2014–2026. [Google Scholar]
- Arias, H. R.; Blanton, M. P.; Kem, W. R. Modulation of nicotinic acetylcholine receptors by anabaseine analogs. Biophys. J. 2004, 86, 545a, (Abstr. 2824). [Google Scholar]
- Arias, H. R.; Kem, W. R.; Trudell, J. R.; Blanton, M. P. Unique general anesthetic binding sites within distinct conformational states of the nicotinic acetylcholine receptor. Int. Rev. Neurobiol 2002, 54, 1–50. [Google Scholar]
- Arias, H. R.; McCardy, E. A.; Bayer, E. Z.; Gallagher, M. J.; Blanton, M. B. Allosterically linked noncompetitive antagonist binding sites in the resting nicotinic acetylcholine receptor ion channel. Arch. Biochem. Biophys 2002, 403, 121–131. [Google Scholar]
- Arias, H. R.; McCardy, E. A.; Gallagher, M. J.; Blanton, M. B. Interaction of barbiturate analogs with the Torpedo nicotinic acetylcholine receptor ion channel. Mol. Pharmacol 2001, 60, 497–506. [Google Scholar]
- Arias, H. R.; Trudell, J. R.; Bayer, E. Z.; Hester, B.; McCardy, E. A.; Blanton, M. B. Noncompetitive antagonist binding sites in the Torpedo nicotinic acetylcholine receptor ion channel. Structureactivity relationship studies using adamantane derivatives. Biochemistry 2003, 42, 7358–7370. [Google Scholar]
- Azuma, R.; Komuro, M.; Rorsch, B. H.; Andre, J. C.; Onnagawa, O.; Black, S. R.; Mathews, J. M. Metabolism and disposition of GTS-21, a novel drug for Alzheimer’s disease. Xenobiotica 1999, 7, 747–762. [Google Scholar]
- Bacq, Z. M. Les poisons des nemertiens. Bull. Cl. Sci. Acad. Roy. Belg (S) 1936, 22, 1072–1079. [Google Scholar]
- Bacq, Z. M. L’”amphiporine” et la “nemertine,” poisons des vers nemertiens. Arch. Int. Physiol 1937, 44, 190–204. [Google Scholar]
- Bjugstad, K. B.; Mahnir, V. M.; Kem, W. R.; Arendash, G. W. Long-term treatment with GTS-21 or nicotine enhances water maze performance in aged rats without affecting the density of nicotinic receptor subtypes in neocortex. Drug Devel. Res 1996, 39, 19–28. [Google Scholar]
- Bloom, L. B. Influence of solvent on the ring-chain hydrolysis equilibrium of anabaseine and synthesis of anabaseine and nicotine analogues. Ph.D. Dissertation, Department of Chemistry, University of Florida, FL, USA, 1990. [Google Scholar]
- Brejc, K.; van Dijk, W. J.; Klaassen, R.V.; Schuurmans, M.; van der Oost, J.; Smit, A. B.; Sixma, T. S. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 2001, 411, 269–276. [Google Scholar]
- Briggs, C. A.; Anderson, D. J.; Brioni, J. D.; Buccafusco, J. J.; Buckley, M. J.; Campbell, J. E.; Decker, W.; Donnelly-Roberts, D.; Elliott, R. L.; Gopalakrishnan, M.; Holladay, M. W.; Hui, Y-H.; Jackson, W. J.; Kim, D. J. B.; Marsh, K. C.; O’Neill, A.; Predergast, M. A.; Ryther, K. B.; Sullivan, J. P.; Arneric, S. P. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro. Pharmacol. Biochem. Behav 1997, 57, 231–241. [Google Scholar]
- Buccafusco, J. J.; Letchworth, S. R.; Bencherif, M.; Lippiello, P. M. Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic–pharmacodynamic discordance. Trends Pharmacol. Sci 2005, 26, 352–360. [Google Scholar]
- Chou, K-C. Structural bioinformatics and its impact to biomedical science. Curr. Med. Chem. 2004, 11, 2105–2134. [Google Scholar]
- Chou, K-C. Insights from modelling the 3D structure of the extracellular domain of α7 nicotinic acetylcholine receptor. Biochem. Biophys. Res. Commun. 2004, 319, 433–438. [Google Scholar]
- Dajas-Bailador, F.; Wonnacott, S. Nicotinic acetylcholine receptors and the regulation of neuronal signaling. Trends Pharmacol. Sci 2004, 25, 317–324. [Google Scholar]
- de Fiebre, N. C.; de Fiebre, C. M. α7 nicotinic acetylcholine receptor knockout selectively enhances ethanol-, but not β-amyloid-induced neurotoxicity. Neurosci. Lett 2005, 373, 42–47. [Google Scholar]
- de Fiebre, C. M.; Meyer, E. M.; Henry, J. C.; Muraskin, S. I.; Kem, W. R.; Papke, R. L. Characterization of a series of anabaseine-derived compounds reveals that the 3-(4)- Dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic α7/125I-α-bungarotoxin receptor subtypes. Mol. Pharmacol 1995, 47, 164–171. [Google Scholar]
- Dineley, K. T.; Bell, K. A.; Bui, D.; Seatt, J. D. β-Amyloid peptide activates α7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J. Biol. Chem 2002, 277, 25056–25061. [Google Scholar]
- Fabian-Fine, R.; Skehel, P.; Errington, M. L.; Davies, H. A.; Sher, E.; Stewart, M. G.; Fine, A. Ultrastructural distribution of the α7 nicotinic acetylcholine receptor subunit in rat hippocampus. J. Neurosci 2001, 21, 7993–8003. [Google Scholar]
- Freedman, R. F.; Adler, L. E.; Bickford, P.; Byerley, W.; Coon, H.; Cullum, C. M.; Griffith, J. M.; Harris, J. G.; Leonard, S; Miller, C.; Myles-Worsley, M.; Nagamoto, H. T.; Rose, G.; Waldo, M. Schizophrenia and nicotinic receptors. Harvard Rev. Psychiatry 1994, 2, 179–192. [Google Scholar]
- Fu, W.; Jhamandas, J. H. β-Amyloid peptide activates non-α7 nicotinic acetylcholine receptors in rat basal forebrain neurons. J. Neurophysiol 2003, 90, 3130–3136. [Google Scholar]
- Gallagher, M. J.; Cohen, J. B. Identification of amino acids of the Torpedo nicotinic acetylcholine receptor contributing to the binding site for the noncompetitive antagonist [3H]tetracaine. Mol. Pharmacol 1999, 56, 300–307. [Google Scholar]
- Gibson, R. Nemerteans; Hutchinson: University Library, London, 1972; p. pp 224. [Google Scholar]
- Grassi, F.; Palma, E.; Tonini, R.; Amic, M.; Ballivet, M.; Eusebi, F. Amyloid β1–42 peptide alters the gating of human and mouse α-bungarotoxin-sensitive nicotinic receptors. J. Physiol 2003, 547, 147–157. [Google Scholar]
- Grottick, A. J.; Trub, G.; Corrigall, W. A.; Huwyler, J.; Malherbe, P.; Wyler, R.; Higgins, G. A. Evidence that nicotinic α7 receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J. Pharmacol. Exper. Ther 2000, 294, 1112–1119. [Google Scholar]
- Hatt, H.; Schmiedel-Jacob, I. Electrophysiological studies of pyridine-sensitive units on the crayfish walking leg. I. Characteristics of stimulatory molecules. J. Comp. Physiol 1984, 154A, 855–863. [Google Scholar]
- Heeschen, C.; Weis, M.; Aicher, A.; Dimmeler, S.; Cooke, J. P. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J. Clin. Invest 2002, 110, 527–536. [Google Scholar]
- Hunter, B. E.; de Fiebre, C. M.; Papke, R. L.; Kem, W. R.; Meyer, E. M. A novel nicotinic agonist facilitates induction of long-term potentiation in the rat hippocampus. Neurosci. Lett 1994, 168, 130–134. [Google Scholar]
- Jacobi, J.; Jang, J. J.; Sundram, U.; Dayoub, H.; Fajardo, L. F.; Cooke, J. P. Nicotine accelerates angiogenesis and wound healing in genetically diabetic mice. Amer. J. Pathol 2002, 161, 97–104. [Google Scholar]
- Kem, W. R. A study of the occurrence of anabaseine in Paranemertes and other nemertines. Toxicon 1971, 9, 23–32. [Google Scholar]
- Kem, W. R. Biochemistry of Nemertine Toxins. In Marine Pharmacognosy: Action of Marine Biotoxins at the Cellular Level; Martin, D., Padilla, G., Eds.; Academic Press: New York, 1973; pp. 37–84. [Google Scholar]
- Kem, W. R. Purification and characterization of a new family of polypeptide neurotoxins from the heteronemertine Cerebratulus lacteus (Leidy). J. Biol. Chem 1976, 251, 4184–4192. [Google Scholar]
- Kem, W. R. Pyridine distribution in the hoplonemertines. Hydrobiol 1985, 156, 145–151. [Google Scholar]
- Kem, W. R. Worm Toxins. In Handbook of Natural Toxins; Tu, A. T., Ed.; Marcel Dekker: New York, 1988; Volume Chapter 15, pp. 353–378. [Google Scholar]
- Kem, W. R. The brain α7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: Studies with DMXBA (GTS-21). Behav. Brain Res 2000, 113, 169–183. [Google Scholar]
- Kem, W. R. Nemertine Neurotoxins. In Neurotoxicology Handbook: Natural Toxins of Animal Origin; Harvey, A., Ed.; Humana Press: Totowa, NJ, 2001; pp. 573–594. [Google Scholar]
- Kem, W. R.; Abbott, B. C.; Coates, R. M. Isolation and structure of a hoplonemertine toxin. Toxicon 1971, 9, 15–22. [Google Scholar]
- Kem, W. R.; Scott, K. N.; Duncan, J. H. Hoplonemertine worms – a new source of pyridine neurotoxins. Exper 1976, 32, 684–686. [Google Scholar]
- Kem, W. R.; Mahnir, V. M.; Lin, B.; Prokai-Tatrai, K. Two primary GTS-21 metabolites are potent partial agonists at α7 nicotinic receptors expressed in the Xenopus oocyte. Neurosci 1996, 22, 268, (Abstr. 110.7).. [Google Scholar]
- Kem, W. R.; Mahnir, V. M.; Papke, R.; Lingle, C. Anabaseine is a potent agonist upon muscle and neuronal α-bungarotoxin sensitive nicotinic receptors. J. Pharmacol. Exp. Ther 1997, 283, 979–992. [Google Scholar]
- Kem, W. R.; Soti, F. Amphiporus alkaloid multiplicity implies functional diversity: Intial studies on crustacean pyridyl receptors. Hydrobiol 2001, 456, 221–231. [Google Scholar]
- Kem, W. R.; Mahnir, V. M.; Prokai, L.; Papke, R. M.; Cao, X. F.; LeFrancois, S.; Wildeboer, K.; Porter-Papke, J.; Prokai-Tatrai, K.; Soti, F. Hydroxy metabolites of the Alzheimer’s drug candidate DMXBA (GTS-21): Their interactions with brain nicotinic receptors, and brain penetration. Mol. Pharmacol 2004, 65, 56–67. [Google Scholar]
- King, H. Amphiporine, an active base from the marine worm Amphiporus lactifloreus. J. Chem. Soc. London 1939, 1365, (Abstr.). [Google Scholar]
- Kitagawa, H.; Takenouchi, T.; Azuma, R.; Wesnes, K. A.; Dramer, W. G.; Clody, D. E. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology 2003, 28, 542–551. [Google Scholar]
- Lee, S. T.; Wildeboer, K.; Panter, K. E.; Kem, W. R.; Gardner, D. R.; Molyneux, R. J.; Chang, C-W. T.; Soti, F.; Pfister, J. A. Relative toxicities and neuromuscular nicotinic receptor agonistic potencies of anabasine enantiomers and anabaseine. Neurotoxicol. Teratol. 2006, in press. [Google Scholar]
- Levin, E. D.; Bettegowda, C.; Blosser, J.; Gordon, J. AR-R17779, an α7 nicotinic agonist, improves learning and memory in rats. Behav. Pharmacol 1999, 10, 675–680. [Google Scholar]
- Li, Y.; King, M. A.; Grimes, J.; Smith, N.; de Fiebre, C. M.; Meyer, W. M. α7 Nicotinic receptor mediated protection against ethanol-induced cytotoxicity in PC12 cells. Brain Res 1999, 816, 225–228. [Google Scholar]
- Liu, Y-S.; Kawai, H.; Berg, D. K. β-Amyloid peptide blocks the response of α7-containing nicotinic receptors on hippocampal neurons. Proc. Nat. Acad. Sci. USA 2001, 98, 4734–4739. [Google Scholar]
- Machu, T. K.; Hamilton, M. E.; Frye, T. F.; Shanklin, C. L.; Harris, M. C.; Sun, H.; Tenner, T. E., Jr; Soti, F.; Kem, W. R. Benzylidene analogs of anabaseine display partial agonist and antagonist properties at the mouse 5-hydroxytryptamine3A receptor. J. Pharmacol. Exp. Ther 2001, 299, 1112–1119. [Google Scholar]
- Mahnir, V. M.; Lin, B.; Prokai-Tatrai, K.; Kem, W. R. Pharmacokinetics and urinary excretion of DMXBA (GTS-21), a compound enhancing cognition. Biopharm. Drug Dispos 1998, 19, 147–151. [Google Scholar]
- Mansvelder, H. D.; McGehee, D. S. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol 2002, 53, 606–617. [Google Scholar]
- Martin, E. J.; Panickar, K. S.; King, M. A.; Deyrup, M.; Hunter, B. E.; Wang, G.; Meyer, E. M. Cytoprotective actions of 2,4-dimethoxybenzylidene anabaseine in differentiated PC12 cells and septal cholinergic neurons. Drug Dev. Res 1994, 31, 135–141. [Google Scholar]
- Martin, L. F.; Kem, W. R.; Freedman, R. α7-Nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacol 2004, 174, 54–64. [Google Scholar]
- Marutle, A.; Unger, C.; Hellstrom-Lindahl, E.; Wang, J.; Puolivali, J.; Tanila, H.; Nordberg, A.; Zhang, X. Elevated levels of Aβ1–40 and Aβ1–42 do not alter the binding sites of nicotinic receptor subtypes in the brain of APPswe and PS1 double transgenic mice. Neurosci. Lett 2002, 328, 269–272. [Google Scholar]
- Matsuyama, S.; Matsumoto, A.; Enomoto, T.; Nishizaki, T. Activation of nicotinic acetylcholine receptors induces long-term potentiation in vivo in the intact mouse dentate gyrus. Eur. J. Neurosci 2000, 12, 3741–3747. [Google Scholar]
- Meyer, E. M.; de Fiebre, C. M.; Hunter, B. E.; Simpkins, C. E.; de Fiebre, N. E. Effects of anabaseine related analogs on rat brain nicotinic receptor binding and on avoidance behavior. Drug Dev. Res 1994, 31, 135–141. [Google Scholar]
- Meyer, E. M.; Tay, E. T.; Papke, R. L.; Meyers, C.; Huang, G-L.; de Fiebre, C. M. 3-[2,4-Dimethoxybenzylidene]anabaseine (DMXB) selectively activates rat α7 receptors and improves memory-related behaviors in a mecamylamine-sensitive manner. Brain Res. 1997, 768, 49–56. [Google Scholar]
- Middleton, R. E.; Strnad, N. P.; Cohen, J. B. Photoaffinity labeling the Torpedo nicotinic acetylcholine receptor with [3H]tetracaine, a nondesensitizing noncompetitive antagonist. Mol. Pharmacol 1999, 56, 290–299. [Google Scholar]
- Nanri, M.; Yamamoto, J.; Miyake, H.; Watanabe, H. Protective effect of GTS-21, a novel nicotinic receptor agonist, on delayed neuronal death induced by ischemia in gerbils. Jpn. J. Pharmacol 1998a, 76, 23–29. [Google Scholar]
- Nanri, M.; Miyake, H.; Murakami, Y.; Matsumoto, K.; Watanabe, H. GTS-21, a nicotinic agonist, attenuates multiple infarctions and cognitive deficit caused by permanent occlusion of bilateral common carotid arteries in rats. Jpn. J. Pharmacol 1998, 78, 463–469. [Google Scholar]
- Oddo, S.; Caccamo, A.; Green, K. N.; Liang, K.; Tran, L.; Chen, Y.; Leslie, F. M.; LaFerla, F. M. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proc. Nat. Acad. Sci. USA 2005, 102, 3046–3051. [Google Scholar]
- Olincy, A.; Harris, J. G.; Johnson, L. L.; Pender, V.; Kongs, S.; Allensworth, D.; Ellis, J.; Zerbe, GO; Leonard, S.; Stevens, K. E.; Stevens, J. O.; Martin, L.; Adler, L. E.; Soti, F.; Kem, W. R.; Freedman, R. An α7-nicotinic cholinergic agonist enhances cognitive function in schizophrenia. Arch. Gen. Psychiat.
- Papke, R. L.; Meyer, E. M.; Lavieri, S.; Bollampally, S. R.; Papke, T. A. S.; Horenstein, N. A.; Itoh, Y.; Porter Papke, J. K. Effects at a distance in α7 nAChR selective agonists: benzylidene substitutions that regulate potency and efficacy. Neuropharmacology 2004, 46, 1023–1038. [Google Scholar]
- Pratt, M. B.; Pedersen, S. E.; Cohen, J. B. Identification of the sites of incorporation of [3H]ethidium diazide within the Torpedo nicotinic acetylcholine receptor ion channel. Biochemistry 2000, 39, 11452–11462. [Google Scholar]
- Ren, K.; Puig, V.; Papke, R. L.; Itoh, Y.; Hughes, J. A.; Meyer, E. M. Multiple calcium channels and kinases mediate α7 nicotinic receptor neuroprotection in PC12 cells. J. Neurochem 2005, 94, 926–933. [Google Scholar]
- Sher, E.; Chen, Y.; Sharples, T. J. W.; Broad, L. M.; Benedetti, G.; Zwart, R.; McPhie, G. I.; Pearson, K. H.; Baldwinson, T.; De Filippi, G. Physiological roles of neuronal nicotinc receptor subtypes: New insights on the nicotinc modulation of neurotransmitter release, synaptic transmission and plasticity. Curr. Op. Med. Chem 2004, 4, 283–297. [Google Scholar]
- Shimohama, K. T.; Sawada, H.; Kimura, J.; Kume, T.; Kochiyama, H.; Maeda, T.; Akaike, A. Nicotinic receptor stimulation protects neurons against β-amyloid toxicity. Ann. Neurol 1997, 42, 159–163. [Google Scholar]
- Spath, E.; Mamoli, L. Eine neue synthese des D,L-anabasins. Chem. Ber 1936, 69, 1082–1085. [Google Scholar]
- Stevens, K. E.; Kem, W. R.; Freedman, R. Selective α7 nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology 1998, 136, 320–327. [Google Scholar]
- Stevens, K. E.; Kem, W. R.; Freedman, R. Selective α7 nicotinic receptor stimulation normalizes chronic cocaine-induced loss of hippocampal sensory inhibition in C3H mice. Biol. Psychiat 1999, 46, 1443–1450. [Google Scholar]
- Summers, K.; Cuadra, G.; Naritoku, D.; Giacobini, E. Effects of nicotine on levels of acetylcholine and biogenic amines in rat cortex. Drug Devel. Res 1994, 31, 108–119. [Google Scholar]
- Summers, K.; Giacobini, E. Effects of local and repeated systemic administration of (-)nicotine on extracellular levels of acetylcholine, norepinephrine, dopamine, and serotonin in rat cortex. Neurochem. Res 1995, 20, 753–759. [Google Scholar]
- Summers, K.; Kem, W. R.; Giacobini, E. Nicotinic agonist modulation of neurotransmitter levels in the rat frontoparietal cortex. Jap. J. Pharmacol 1997, 74, 139–146. [Google Scholar]
- Talley, T. T.; Yalda, S.; Ho, K-Y.; Soti, F.; Kem, W. R.; Taylor, P. Spectroscopic analysis of benzylidene anabaseine complexes with acetylcholine binding proteins as models for ligandnicotinic recptor interactions. Biochemistry 2006, in press. [Google Scholar]
- Tani, Y.; Saito, K.; Imoto, M.; Ohno, T. Pharmacological characterization of nicotinic-receptormediated acetylcholine release in rat brain—an in vivo microdialysis study. Eur. J. Pharmacol 1998, 351, 181–188. [Google Scholar]
- Unger, C.; Hedberg, M. M.; Mustafiz, T.; Svedberg, M. M.; Nordberg, A. E. Early changes in Aβ levels in the brain of APPswe transgenic mice—implication on synaptic density, α7 neuronal nicotinic acetylcholine- and N-methyl-D-aspartate receptor levels. Mol. Cell. Neurosci 2005, 30, 218–227. [Google Scholar]
- Van Haaren, F.; Anderson, K. G.; Haworth, S. C.; Kem, W. R. GTS-21, a mixed nicotinic receptor agonist/antagonist, does not affect the nicotine cue. Pharmacol. Biochem. Behav 1999, 64, 439–444. [Google Scholar]
- Wang, H-Y.; Lee, D. H. S.; Davis, C. B.; Shank, R. P. Amyloid peptide Aβ1–42 binds selectively and with picomolar affinity to α7 nicotinic receptors. J. Neurochem. 2000, 75, 1155–1161. [Google Scholar]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C. A.; Tanovic, M.; Susaria, S.; Li, J. H.; Wang, H.; Yang, H.; Ulloa, L.; Al-Abed, Y.; Czura, C. J.; Tracey, K. J. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature 2002, 421, 384–388. [Google Scholar]
- Wang, Y.; Sherwood, J. L.; Miles, C. P.; Whiffin, G.; Lodge, D. TC-2559 excites dopaminergic neurons in the ventral tegmental area by stimulating α4β2-like nicotinc acetylcholine receptors in anaesthetized rats. Brit. J. Pharmacol 2006, 147, 379–390. [Google Scholar]
- Wei, D.; Sirois, S.; Du, Q-S.; Arias, H. R.; Chou, K-C. Theoretical studies of Alzheimer’s disease drug candidate 3-[(2,4-dimethoxy)benzylidene]-anabaseine (GTS-21) and its derivatives. Biochem. Biophys. Res. Commun. 2005, 338, 1059–1064. [Google Scholar]
- Wheeler, J. W.; Olubajo, O.; Storm, C. B.; Duffield, R. M. Anabaseine: venom alkaloid of Aphaenogaster ants. Science 1981, 211, 1051–1052. [Google Scholar]
- Whitehouse, R. J.; Price, D. L.; Clark, A. W.; Coyle, J. T.; DeLong, M. R. Nicotinic acetylcholine binding in Alzheimer’s disease. Brain Res 1986, 371, 146–151. [Google Scholar]
- Woodruff-Pak, D. S.; Li, Y-T.; Kem, W. R. A nicotinic receptor agonist (GTS-21), eyeblink classical conditioning, and nicotinic receptor binding in rabbit brain. Brain Res. 1994, 645, 309–317. [Google Scholar]
- Zhang, R.; White, N. A.; Soti, F. S.; Kem, W. R.; Machu, T. K. N-terminal domains in mouse and human 5-hydroxytryptamine3Areceptors confer partial agonist and antagonist properties to benzylidene analogs of anabaseine. Mol. Pharmacol.
- Zoltewicz, J. A.; Bloom, L. B.; Kem, W. R. Quantitative determination of the ring-chain hydrolysis equilibrium constant for anabaseine and related tobacco alkaloids. J. Org. Chem 1989, 54, 4462–4468. [Google Scholar]
- Zoltewicz, J. A.; Bloom, L. B.; Kem, W. R. Hydrolysis of cholinergic anabaseine and Nmethylanabaseine: Influence of cosolvents on the position of the ring-chain equilibrium– compensatory changes. Bioorgan. Chem 1990, 18, 395–412. [Google Scholar]
- Zoltewicz, J. A.; Prokai-Tatrai, K.; Bloom, L. B.; Kem, W. R. Long range transmission of polar effects of cholinergic 3-arylideneanabaseines. Conformations calculated by molecular modelling. Heterocycles 1993, 35, 171–179. [Google Scholar]

| Receptor Type | Anabaseine | Nicotine | DMXBA |
|---|---|---|---|
| - | |||
| CNS | |||
| α7 | Full Agonist | Weak Partial Agonist | Partial Agonist |
| α4β2 | Weak Partial Agonist | Strong Partial Agonist | Competitive Antagonist |
| Sympathetic | |||
| PC12 Cell | Partial Agonist | Full Agonist | Noncompetitive Antagonist |
| α3β4 (oocyte) | Partial Agonist | Full Agonist | Noncompetitive Antagonist |
| Muscle-type | |||
| α1β1ɛδ | Full Agonist | Full Agonist | 1Competitive Antagonist |
| α1β1γδ (Torpedo) | Full Agonist | Full Agonist | 2Noncompetitive Antagonist |
| Peak Effect (% Increase) | |||
|---|---|---|---|
| Receptor Type | 1Nicotine | 2Anabaseine | 2DMXBA |
| Acetylcholine | 106 | 50 | NE3 |
| Dopamine | NE3 | 85 | 96 |
| Norepinephrine | 86 | 62 | 83 |
| Serotonin | NE3 | NE3 | NE3 |
© 2006 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Kem, W.; Soti, F.; Wildeboer, K.; LeFrancois, S.; MacDougall, K.; Wei, D.-Q.; Chou, K.-C.; Arias, H.R. The Nemertine Toxin Anabaseine and Its Derivative DMXBA (GTS-21): Chemical and Pharmacological Properties. Mar. Drugs 2006, 4, 255-273. https://doi.org/10.3390/md403255
Kem W, Soti F, Wildeboer K, LeFrancois S, MacDougall K, Wei D-Q, Chou K-C, Arias HR. The Nemertine Toxin Anabaseine and Its Derivative DMXBA (GTS-21): Chemical and Pharmacological Properties. Marine Drugs. 2006; 4(3):255-273. https://doi.org/10.3390/md403255
Chicago/Turabian StyleKem, William, Ferenc Soti, Kristin Wildeboer, Susan LeFrancois, Kelly MacDougall, Dong-Qing Wei, Kuo-Chen Chou, and Hugo R. Arias. 2006. "The Nemertine Toxin Anabaseine and Its Derivative DMXBA (GTS-21): Chemical and Pharmacological Properties" Marine Drugs 4, no. 3: 255-273. https://doi.org/10.3390/md403255
APA StyleKem, W., Soti, F., Wildeboer, K., LeFrancois, S., MacDougall, K., Wei, D.-Q., Chou, K.-C., & Arias, H. R. (2006). The Nemertine Toxin Anabaseine and Its Derivative DMXBA (GTS-21): Chemical and Pharmacological Properties. Marine Drugs, 4(3), 255-273. https://doi.org/10.3390/md403255




