Effects of Polybrominated Diphenol Ethers from a Marine Sponge Phyllospongia dendyi on IL-8 Production in a PMAstimulated Promyelocytic Leukemia Cell Line
Abstract
:Introduction
Materials and Methods
Materials
Cell lines and culture conditions
Relative Plating Efficiency
Detection of human IL-8 by ELISA
Determination of cell proliferation
Results and Discussion
The effects of polybrominated diphenol ethers 1–5 on relative plating efficiencies of Chinese hamster V79 cells
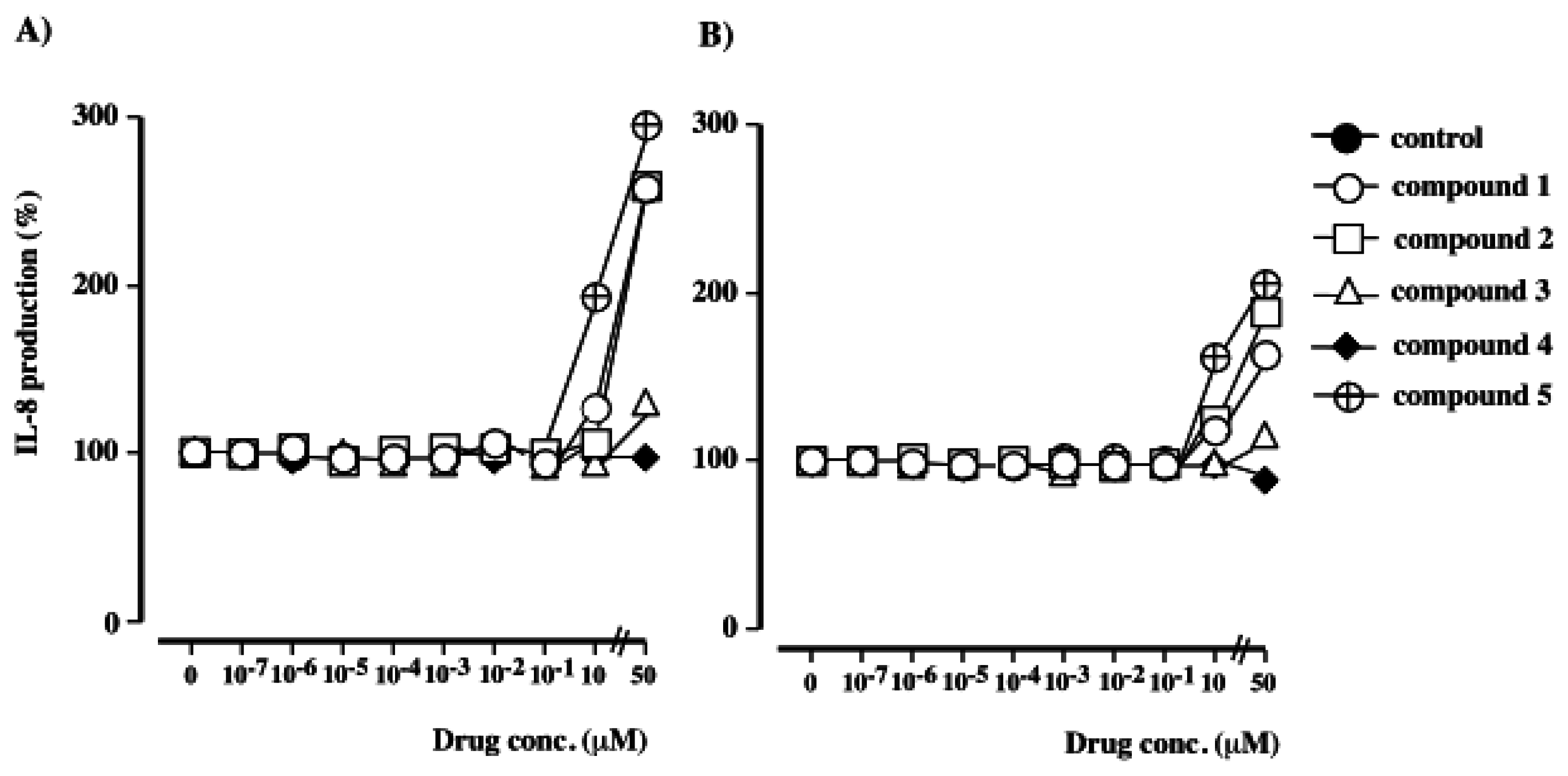
The effects of polybrominated diphenol ethers 1–5 on IL-8 production by PMA-stimulated HL-60 cells


Acknowledgments
- Sample availability: Not available.
References and Notes
- Faulkner, D.J. Marine natural products. Nat. Prod. Rep 19, 1–48.2000, 18, 1–49.1999, 17, 7–55.1998, 16, 155–198.1997, 15, 113–158.1996, 14, 259–302.1995, 13, 75–125.1994, 12, 223–269.1993, 11, 355–394.1992, 10, 497–539.1991, 9, 323–364.1990, 8, 97–147.1988, 7, 269–309.1987, 5, 613–576.1986, 4, 539–576.1984, 3, 1–33.1.
- Blunt, J. W.; Copp, B. R.; Munro, M. H. G.; Northcote, P. T.; Prinsep, M. R. Marine natural products. Nat. Prod. Rep 22, 15–61.2003, 21, 1–49.20, 1–48.
- Liu, H.; Namikoshi, M.; Meguro, S.; Nagai, S.; Kobayashi, H.; Yao, X. Isolation and Characterization of Polybrominated Diphenyl Ethers as Inhibitors of Microtubule Assembly from a Marine Sponge Phyllospongia dendyi Collected at Palau. J. Nat. Prod 2004, 67, 472–474. [Google Scholar]
- Graves, D.T.; Jiang, Y. Molecular cloning and functional analysis of the promoter of the human squalene synthase gene. Crit. Rev. Oral Biol. Med 1995, 6, 109–118. [Google Scholar]
- Zachariae, C.O.; Thestrup-Pedersen, K.; Matsushima, K. Expression and secretion of leukocyte chemotactic cytokines by normal human melanocytes and melanoma cells. J. Invest. Dermatol 1991, 97, 593–599. [Google Scholar]
- Di Celle, P.F.; Carbone, A.; Marchis, D.; Zhou, D.; Sozzani, S.; Zupo, S.; Pini, M.; Mantovani, A.; Foa, R. Cytokine gene expression in B-cell chronic lymphocytic leukemia: evidence of constitutive interleukin-8 (IL-8) mRNA expression and secretion of biologically active IL-8 protein. Blood 1994, 84, 220–228. [Google Scholar]
- Morita, M.; Kasahara, T.; Mukaida, N.; Matsushima, K.; Nagashima, T.; Nishizawa, M.; Yoshida, M. Induction and regulation of IL-8 and MCAF production in human brain tumor cell lines and brain tumor tissues. Eur. Cytokine. Netw 1993, 4, 351–358. [Google Scholar]
- Green, A.R.; Green, V.L.; White, M.C.; Speirs, V. Expression of Cytokine messenger RNA in normal and neoplastic human breast tissue: identification of interleukin-8 as a potential regulatory factor in breast tumours. Int. J. Cancer 1997, 72, 937–941. [Google Scholar]
- Mizuno, K.; Sone, S.; Orino, E.; Mukaida, N.; Matsushima, K.; Ogura, T. Spontaneous production of interleukin-8 by human lung cancer cells and its augmentation by tumor necrosis factor alpha and interleukin-1 at protein and mRNA levels. Oncology 1994, 51, 467–471. [Google Scholar]
- Suliman, M.E.; Royds, J.A.; Baxter, L.; Timperley, W.R.; Cullen, D.R.; Jones, T.H. IL-8 mRNA expression by in situ hybridisation in human pituitary adenomas. Eur. J. Endocrinol 1999, 140, 155–158. [Google Scholar]
- Konig, B.; Steinbach, F.; Janocha, B.; Drynda, A.; Stumm, M.; Philipp, C.; Allhoff, E.P.; Konig, W. The differential expression of proinflammatory cytokines IL-6, IL-8 and TNF-alpha in renal cell carcinoma. Anticancer Res 1999, 19, 1519–1524. [Google Scholar]
- Yoshida, M.; Matsuzaki, H.; Sakata, K.; Takeya, M.; Kato, K.; Mizushima, S.; Kawakita, M.; Takatsuki, K. Neutrophil chemotactic factors produced by a cell line from thyroid carcinoma. Cancer Res 1992, 52, 464–469. [Google Scholar]
- Brew, R.; Erikson, J.S.; West, D.C.; Flanagan, B.F.; Christmas, S.E. Interleukin-8 as a growth factor for human colorectal carcinoma cells in vitro. Biochem. Soc. Trans 1997, 25, 264S–268S. [Google Scholar]
- Galffy, G.; Mohammed, K.A.; Dowling, P.A.; Nasreen, N.; Ward, M.J. Antony, V.B. Interleukin 8: an autocrine growth factor for malignant mesothelioma. Cancer Res 1999, 59, 367–371. [Google Scholar]
- Carté, B.; Faulkner, D. J. Polybrominated diphenyl ethers from Dysidia herbaces, Dysidia chlorea and Phyllospongia foliascens. Tetrahedron 1981, 37, 2335–2339. [Google Scholar]
- Fu, X.; Schmitz, F. J.; Govindan, M.; Abbas, S. A.; Hanson, K. M.; Horton, P. A.; Crews, P.; Laney, M.; Schatzman, R. C. Enzyme inhibitors: new and known polybrominated phenols and diphenyl ethers from four Indo-Pacific Dysidia sponges. J. Nat. Prod 1995, 58, 1384–1391. [Google Scholar]
- Sato, Y.; Sakakibara, Y.; Oda, T.; Aizu-yokota, E.; Ichinoseki, I. Effects of estradiol and ethynylestradiol on microtubule distribution in Chinese hamster V79 cells. Chem. Pharm. Bull 1992, 40, 182–184. [Google Scholar]
- Kasahara, T.; Oda, T.; Hatake, K.; Akiyama, M.; Mukaida, N.; Matsushima, K. Interleukin-8 and monocyte chemotactic protein-1 production by a human glioblastoma cell line, T98G in coculture with monocytes: involvement of monocyte-derived interleukin-1alpha. Eur. Cytokine Netw 1998, 9, 47–55. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 1987, 47, 939–942. [Google Scholar]
© 2005 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Oda, T.; Liu, H.; Namikoshi, M. Effects of Polybrominated Diphenol Ethers from a Marine Sponge Phyllospongia dendyi on IL-8 Production in a PMAstimulated Promyelocytic Leukemia Cell Line. Mar. Drugs 2005, 3, 119-125. https://doi.org/10.3390/md304119
Oda T, Liu H, Namikoshi M. Effects of Polybrominated Diphenol Ethers from a Marine Sponge Phyllospongia dendyi on IL-8 Production in a PMAstimulated Promyelocytic Leukemia Cell Line. Marine Drugs. 2005; 3(4):119-125. https://doi.org/10.3390/md304119
Chicago/Turabian StyleOda, Taiko, Hongwei Liu, and Michio Namikoshi. 2005. "Effects of Polybrominated Diphenol Ethers from a Marine Sponge Phyllospongia dendyi on IL-8 Production in a PMAstimulated Promyelocytic Leukemia Cell Line" Marine Drugs 3, no. 4: 119-125. https://doi.org/10.3390/md304119
APA StyleOda, T., Liu, H., & Namikoshi, M. (2005). Effects of Polybrominated Diphenol Ethers from a Marine Sponge Phyllospongia dendyi on IL-8 Production in a PMAstimulated Promyelocytic Leukemia Cell Line. Marine Drugs, 3(4), 119-125. https://doi.org/10.3390/md304119



