Co-Culture of Auxenochlorella protothecoides and Serratia liquefaciens Promotes Lutein Accumulation
Abstract
1. Introduction
2. Results
2.1. Screening of Bacterial Strains
2.2. Strain Characterization and Identification
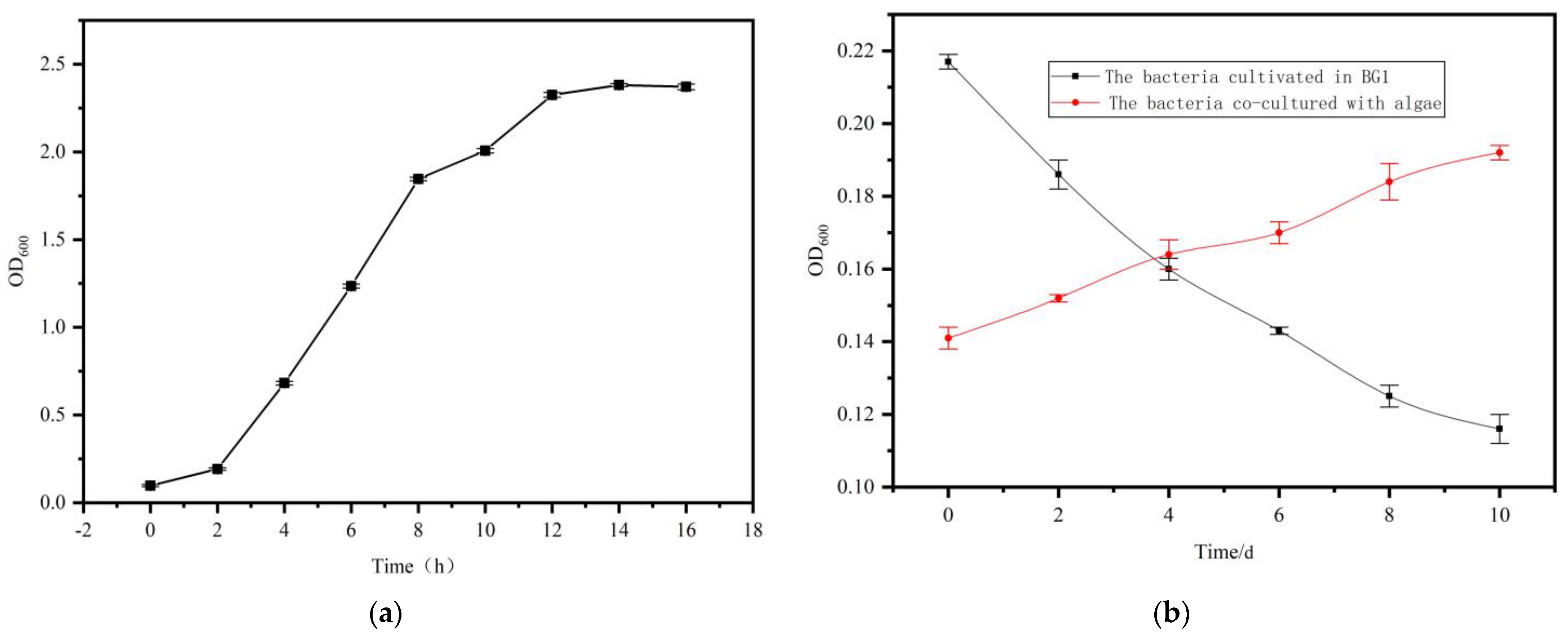
2.3. Growth Analysis of S. liquefaciens LZ03
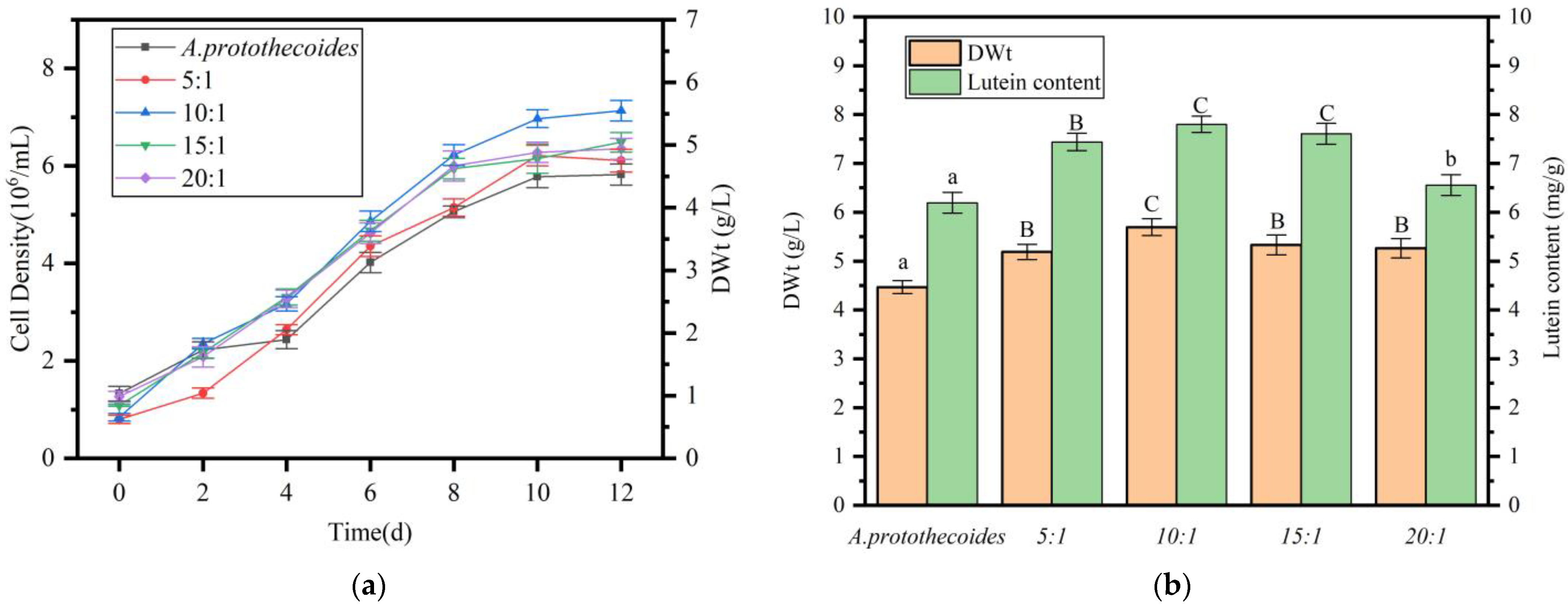
2.4. Effect of Algal:Bacterial Ratio on Growth and Lutein Accumulation in A. protothecoides Co-Cultures
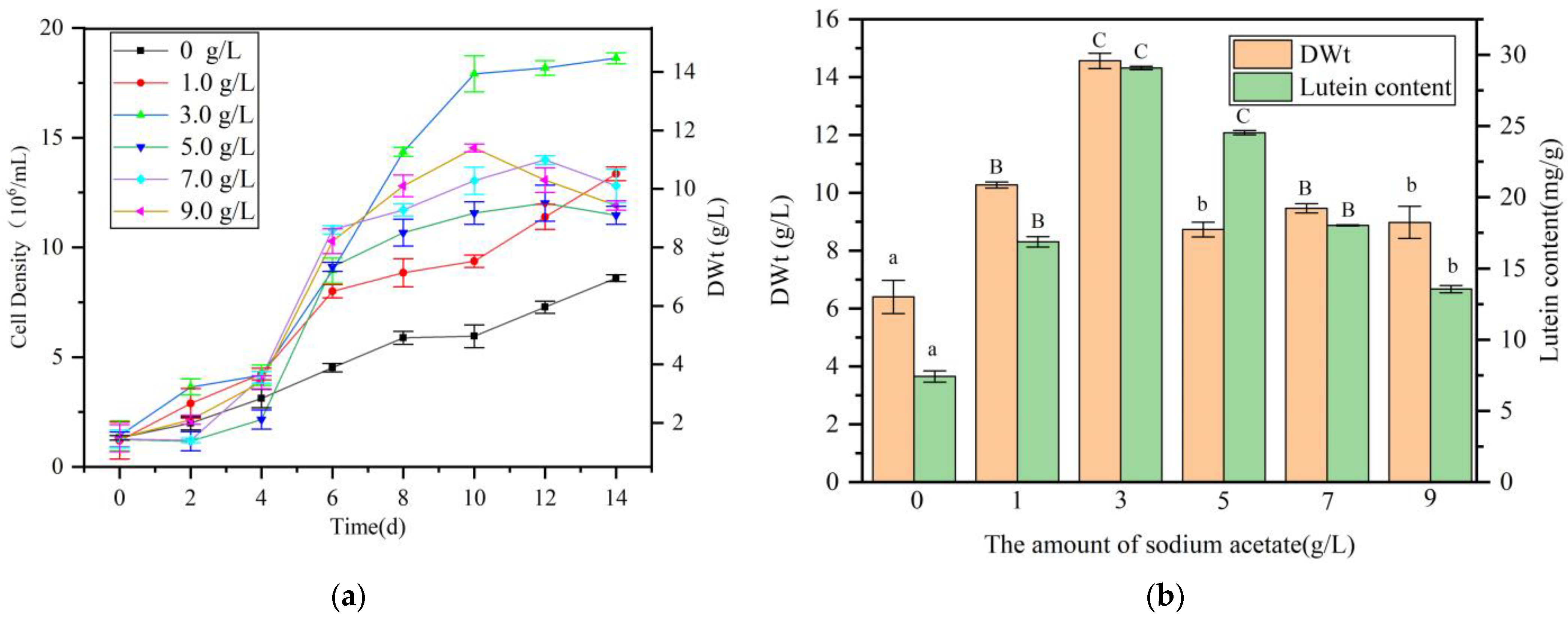
2.5. Impact of Carbon Source on the Performance of A. protothecoides in Co-Culture with S. liquefaciens LZ03
2.6. Impact of Nitrogen Source Supplement on the Growth of A. protothecoides and the Accumulation of Lutein in Algal-Bacterial Co-Culture
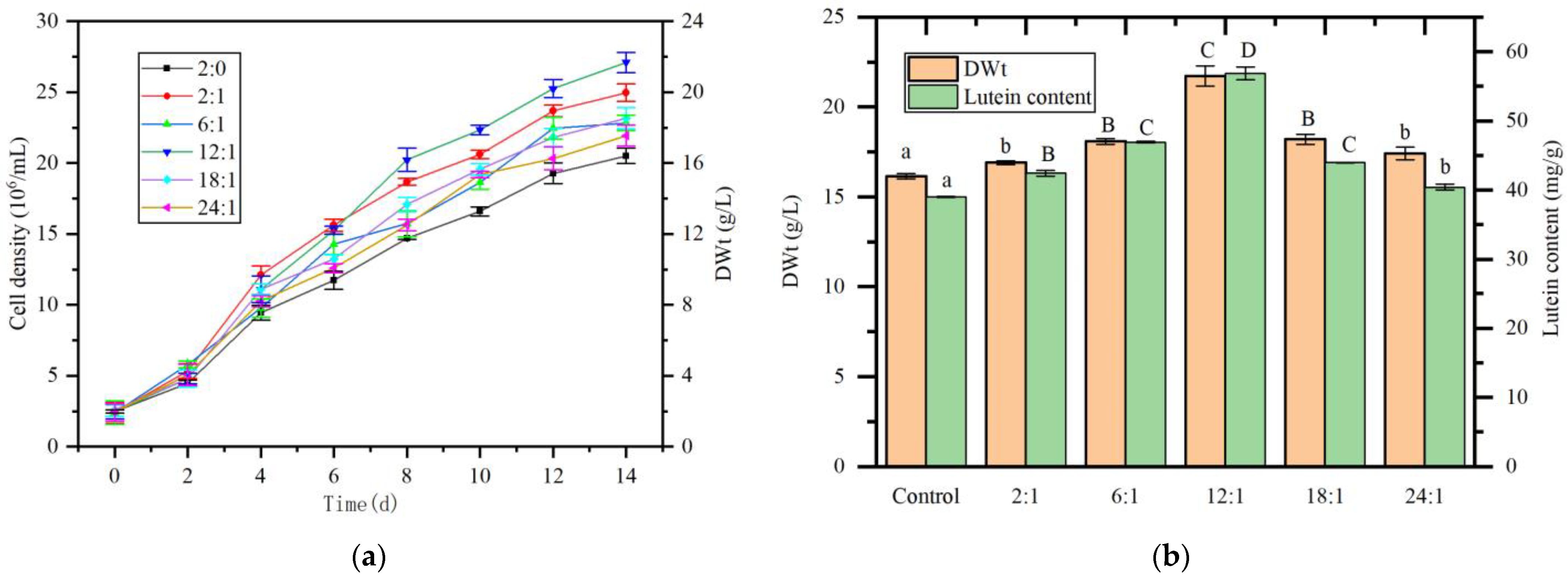
2.7. Impact of N:P Ratio on the Growth of A. protothecoides and Lutein Content in Co-Culture with S. liquefaciens LZ03
3. Discussion
3.1. Algal-Bacterial Co-Culture Significantly Enhances Microalgal Growth and Metabolite Accumulation
3.2. The Precise Metabolic Division of Labor, Signal Communication, and Microenvironment Regulation Between Algae and Bacteria Enable the Growth of Microalgae and the Accumulation of Metabolites
3.3. The Limitations and Future Research Directions
4. Materials and Methods
4.1. The Source and Cultivation of Algal Strains
4.2. Bacterial Isolation, Co-Culture, and Screening
4.3. Biomass Quantification of A. protothecoides
4.4. Detection of Lutein Content
- Column: C18 column, 4.6 mm × 250 mm, 5 μm
- Column temperature: 30 °C
- Mobile phase: Methanol:water:acetonitrile = 10:10:80
- Flow rate: 1.0 mL/min
- Injection volume: 10 μL
- Detection wavelength: 445 nm
4.5. Strain Characterization
4.6. Bacterial Growth Curve Analysis
4.7. Analysis of Bacterial Growth in Co-Culture
4.8. Effect of Bacterial Inoculum Size on A. protothecoides Growth and Lutein Accumulation
4.9. Effect of Carbon Source Addition on Lutein Production in the Algal-Bacterial Co-Culture
4.10. Effect of Nitrogen Source on Lutein Production in the Algal-Bacterial Co-Culture
4.11. Effect of Nitrogen-to-Phosphorus (N:P) Ratio on Lutein Production in the Algal-Bacterial Co-Culture
4.12. Data Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.L.; Satriano, A.; Marchesini, G. The effect of lutein on eye and extra-eye health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H. Lutein as a modulator of oxidative stress-mediated inflammatory diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Muhammad, G.; Butler, T.O.; Chen, B.; Lv, Y.; Xiong, W.; Zhao, X.; Solovchenko, A.E.; Zhao, A.; Mofijur, M.; Xu, J.; et al. Sustainable production of lutein—An underexplored commercially relevant pigment from microalgae. Biomass Convers. Biorefin. 2022, 14, 7255–7276. [Google Scholar] [CrossRef]
- Lin, J.-H.; Lee, D.-J.; Chang, J.-S. Lutein production from biomass: Marigold flowers versus microalgae. Bioresour. Technol. 2015, 184, 421–428. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Y.; Li, S.; Nagarajan, D.; Varjani, S.; Lee, D.-J.; Chang, J.-S. Recent advances in lutein production from microalgae. Renew. Sustain. Energy Rev. 2022, 153, 111795. [Google Scholar] [CrossRef]
- Montuori, E.; Lima, S.; Marchese, A.; Scargiali, F.; Lauritano, C. Lutein production and extraction from microalgae: Recent insights and bioactive potential. Int. J. Mol. Sci. 2024, 25, 2892. [Google Scholar] [CrossRef] [PubMed]
- Camarena-Bernard, C.; Pozzobon, V. Evolving perspectives on lutein production from microalgae—A focus on productivity and heterotrophic culture. Biotechnol. Adv. 2024, 73, 108375. [Google Scholar] [CrossRef]
- Wu, K.; Lai, J.; Zhang, Q.; Wang, Y.; Cui, X.; Liu, Y.; Wu, X.; Yu, Z.; Ruan, R. Optimizing Chlorella vulgaris cultivation to enhance biomass and lutein production. Foods 2024, 13, 2514. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, W.; Ma, R.; Ho, S.-H.; Xie, Y.; Chen, J. Two-stage trophic strategy coupled with fed-batch operation for simultaneous enhancement of cell growth and lutein synthesis in Chlorella sorokiniana. Biochem. Eng. J. 2025, 215, 109632. [Google Scholar] [CrossRef]
- Kamal, I.; Besombes, C.; Allaf, K. One-step processes for in situ transesterification to biodiesel and lutein extraction from microalgae Phaeodactylum using instant controlled pressure drop (DIC). Green Process. Synth. 2014, 3, 431–440. [Google Scholar] [CrossRef]
- McQuillan, J.L.; Cutolo, E.A.; Evans, C.; Pandhal, J. Proteomic characterization of a lutein-hyperaccumulating Chlamydomonas reinhardtii mutant reveals photoprotection-related factors as targets for increasing cellular carotenoid content. Biotechnol. Biofuels Bioprod. 2023, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.; Vereecke, E.; Van Laere, K.; Novoveska, L.; Robbens, J. Genetic engineering and innovative cultivation strategies for enhancing the lutein production in microalgae. Mar. Drugs 2024, 22, 329. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, R.; Tang, Y.; Wang, Z.; Feng, B.; Li, Y. The growth and lutein accumulation in heterotrophic Chlorella protothecoides provoked by waste monascus fermentation broth feeding. Appl. Microbiol. Biotechnol. 2019, 103, 8863–8874. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yi, L.; Yang, S.; Lu, X.; Liu, B.; Chen, F.; Huang, J.; Cheng, K.; Sun, H.; Wu, X. Light induction of seed culture accelerates lutein accumulation in heterotrophic fermentation of Chlorella protothecoides cs-41. Fermentation 2023, 9, 768. [Google Scholar] [CrossRef]
- Udaypal, U.; Goswami, R.K.; Verma, P. Strategies for improvement of bioactive compounds production using microalgal consortia: An emerging concept for current and future perspective. Algal Res. 2024, 82, 103664. [Google Scholar] [CrossRef]
- Dong, H.; Liu, W.; Zhang, H.; Wang, Z.; Feng, F.; Zhou, L.; Duan, H.; Xu, T.; Li, X.; Ma, J. Enhanced biomass production and wastewater treatment in attached co-culture of Chlorella pyrenoidosa with nitrogen-fixing bacteria Azotobacter beijerinckii. Bioprocess Biosyst. Eng. 2023, 46, 707–716. [Google Scholar] [CrossRef]
- Borges Lopes, L.D.; Soares, R.C.; de Freitas, R.M.; Amarante, D.O.; de Carvalho, F.C.T.; Cavalcante, K.M.d.S.P.; de Sousa, O.V.; de Menezes, F.G.R. Co-culture of microalgae and bacteria for the production of bioactive compounds. Ann. Microbiol. 2025, 75, 16. [Google Scholar] [CrossRef]
- Makaranga, A.; Jutur, P.P. Dynamic metabolomic crosstalk between Chlorella saccharophila and its new symbiotic bacteria enhances lutein production in microalga without compromising its biomass. Enzym. Microb. Technol. 2023, 170, 110291. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Wang, Z.; Fu, M.; Zhao, H.; Chen, C.; Sun, F.; Jiao, W. Improvement of photosynthesis efficiency of Chlorella vulgaris by co culture with Bacillus licheniformis. Food Biosci. 2025, 63, 105629. [Google Scholar] [CrossRef]
- Majed Tavakol, F.N. Single and co-cultivation of Chlorella vulgaris and Pseudomonas aeruginosa: Efficient nutrient removal and lipid/starch formation by photoperiodic co-culture. Bioresour. Technol. 2025, 436, 132983. [Google Scholar]
- Liu, J. Metabolomic of the Interaction Between Pseudomonas putida, Staphylococcus sp. and Chlorella vulgaris. Master’s Thesis, Northwest A&F University, Xianyang, China, 2023. [Google Scholar]
- Wei, Z.; Wang, H.; Li, X.; Zhao, Q.; Yin, Y.; Xi, L.; Ge, B.; Qin, S. Enhanced biomass and lipid production by co-cultivation of Chlorella vulgaris with Mesorhizobium sangaii under nitrogen limitation. J. Appl. Phycol. 2020, 32, 233–242. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, Z.; Xu, S.; Wei, T.; Song, L.; Wang, G.; Zhang, J.; Yang, X. Improved carotenoid productivity and cod removal efficiency by co-culture of Rhodotorula glutinis and Chlorella vulgaris using starch wastewaters as raw material. Appl. Biochem. Biotechnol. 2019, 189, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, X.; Wang, H.; Yin, Y.; Xi, L.; Ge, B. Enhanced biomass production and lipid accumulation by co-cultivation of Chlorella vulgaris with azotobacter Mesorhizobium sp. China biotechnology 2019, 39, 56–64. China Biotechnol. 2019, 39, 56–64. [Google Scholar]
- Choix, F.J.; Palacios, O.A.; Contreras, C.A.; Espinoza-Hicks, J.C.; Mondragón-Cortez, P.; Torres, J.R. Growth and metabolism enhancement in microalgae co-cultured in suspension with the bacterium Azospirillum brasilense under heterotrophic conditions. J. Appl. Phycol. 2023, 35, 57–71. [Google Scholar] [CrossRef]
- Li, C.; Ping, W.; Ge, J.; Lin, Y. Advances in the co-culture of microalgae with other microorganisms and applications. Sheng Wu Gong Cheng Xue Bao 2022, 38, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Deniz, B.; Boyacı, İ.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Lin, G.; Shi, X.M.; Lin, L.; Hu, S.Q.; Ling, C. Determination of lutein in Chlorella pyrenoidosa by high performance liquid chromatography. Food Ferment. Ind. 2005, 31, 95–97. [Google Scholar]
- Sampathkumar, S.J.; Srivastava, P.; Ramachandran, S.; Sivashanmugam, K.; Gothandam, K.M. Lutein: A potential antibiofilm and antiquorum sensing molecule from green microalga Chlorella pyrenoidosa. Microb. Pathog. 2019, 135, 103658. [Google Scholar] [CrossRef]
- Machado, S.G.; da Silva, F.L.; Bazzolli, D.M.S.; Heyndrickx, M.; Costa, P.M.d.A.; Vanetti, M.C.D. Pseudomonas spp. and Serratia liquefaciens as predominant spoilers in cold raw milk. J. Food Sci. 2015, 80, 1842–1849. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, W.; Li, Z.; Qiu, Y.; Ma, Y.; Xue, Y.; Zhang, Z.; Liu, C. Co-Culture of Auxenochlorella protothecoides and Serratia liquefaciens Promotes Lutein Accumulation. Mar. Drugs 2025, 23, 360. https://doi.org/10.3390/md23090360
Xue W, Li Z, Qiu Y, Ma Y, Xue Y, Zhang Z, Liu C. Co-Culture of Auxenochlorella protothecoides and Serratia liquefaciens Promotes Lutein Accumulation. Marine Drugs. 2025; 23(9):360. https://doi.org/10.3390/md23090360
Chicago/Turabian StyleXue, Weiwei, Zhen Li, Yanhong Qiu, Yong Ma, Yongchang Xue, Zongshen Zhang, and Changbin Liu. 2025. "Co-Culture of Auxenochlorella protothecoides and Serratia liquefaciens Promotes Lutein Accumulation" Marine Drugs 23, no. 9: 360. https://doi.org/10.3390/md23090360
APA StyleXue, W., Li, Z., Qiu, Y., Ma, Y., Xue, Y., Zhang, Z., & Liu, C. (2025). Co-Culture of Auxenochlorella protothecoides and Serratia liquefaciens Promotes Lutein Accumulation. Marine Drugs, 23(9), 360. https://doi.org/10.3390/md23090360





