Structural Diversity and Bioactivities of Marine Fungal Terpenoids (2020–2024)
Abstract
1. Introduction
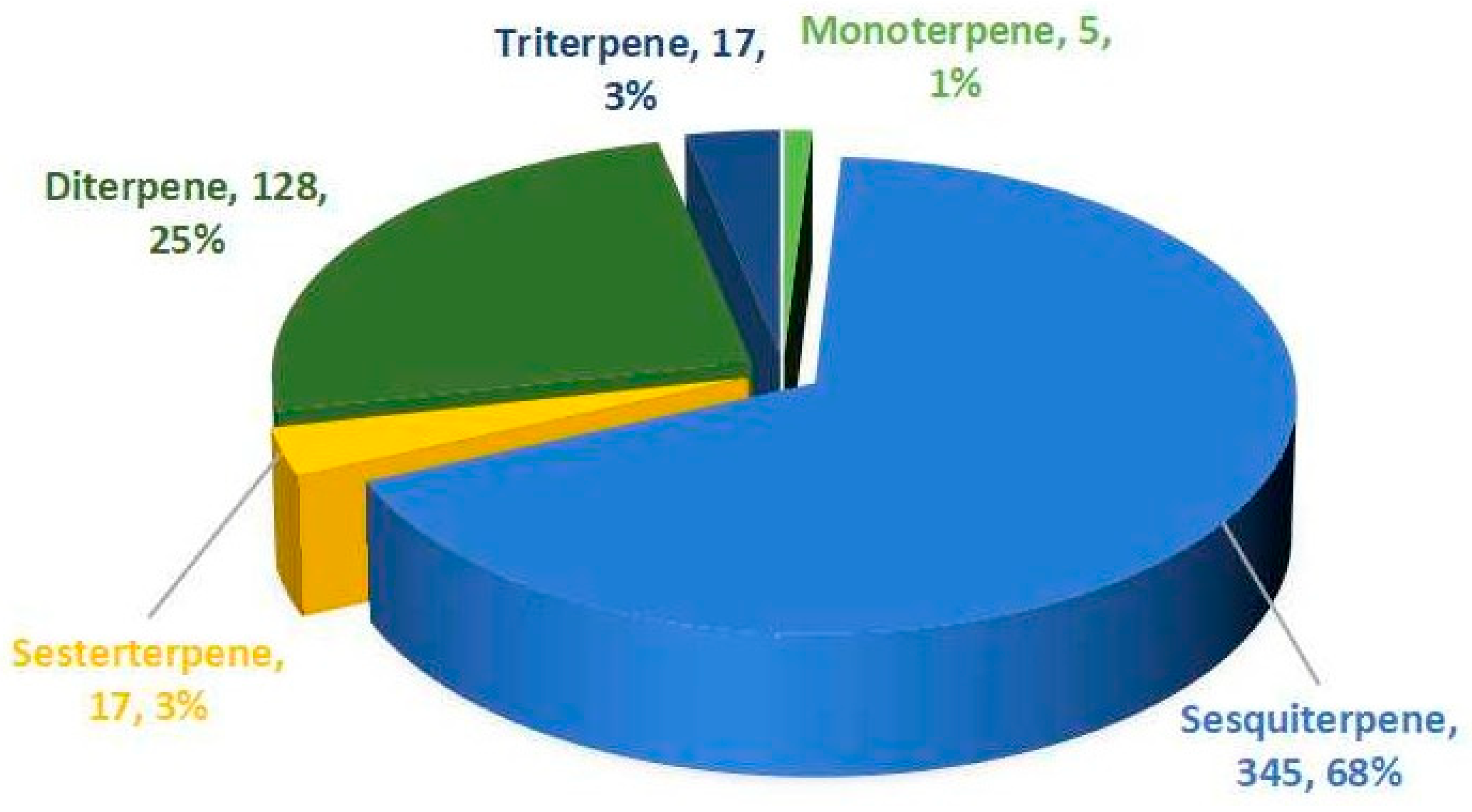
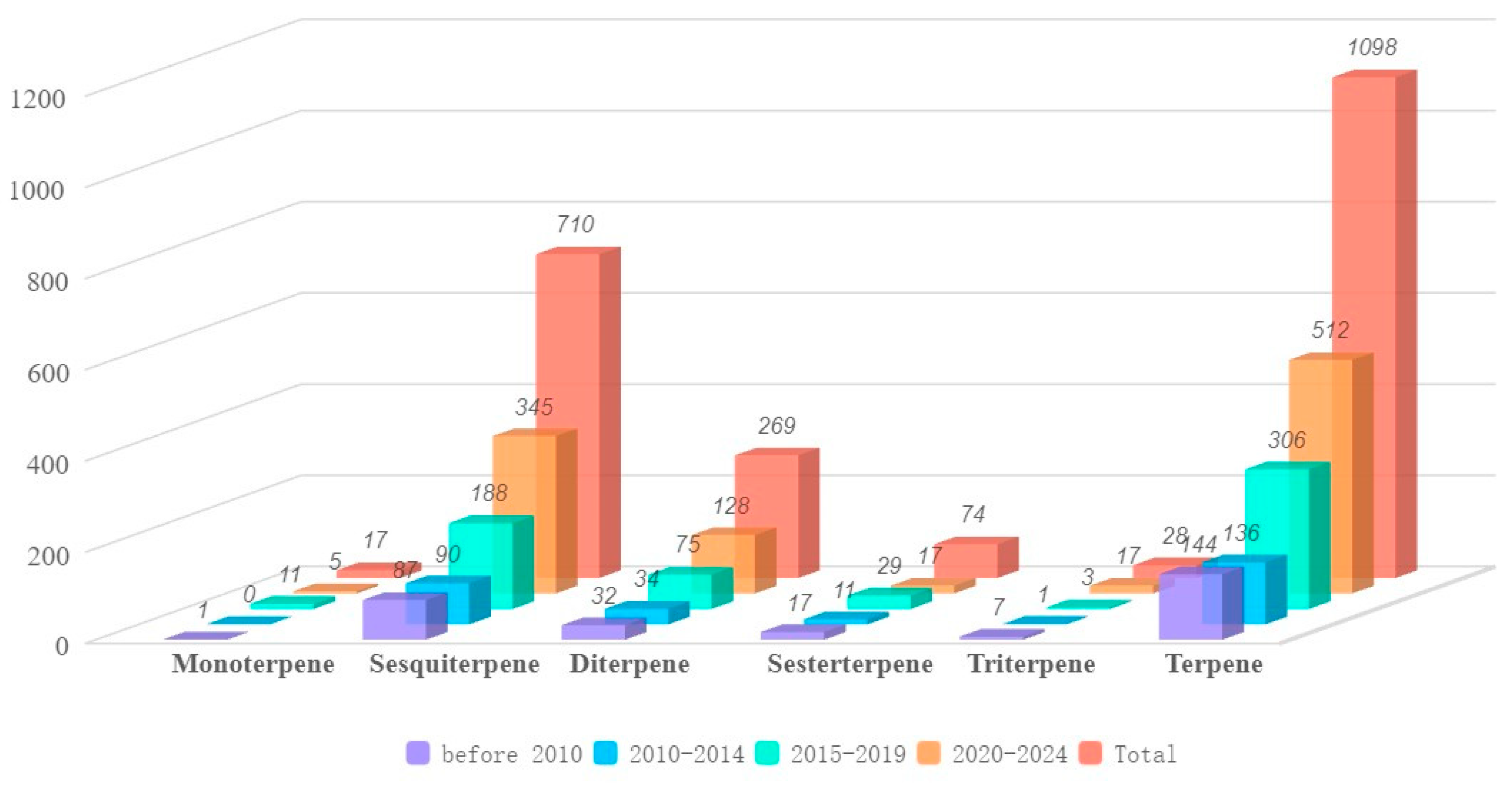
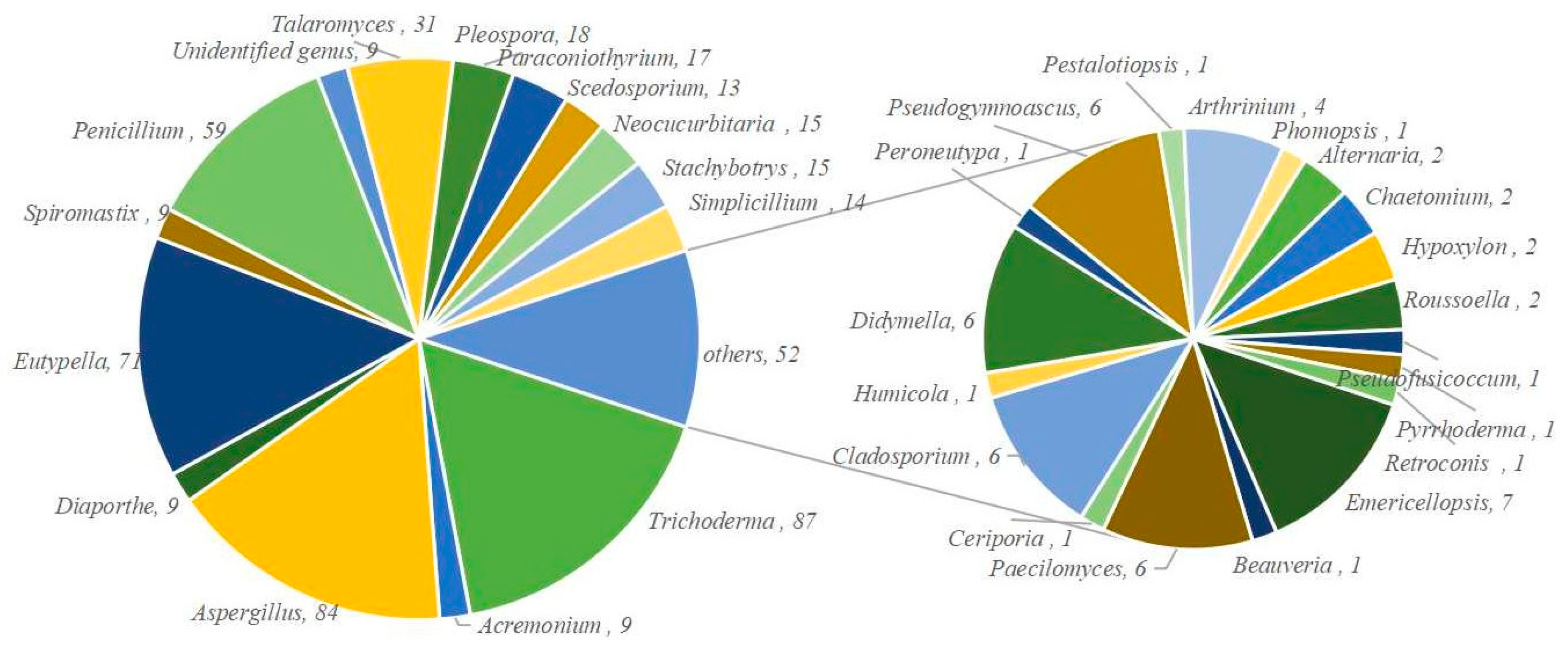
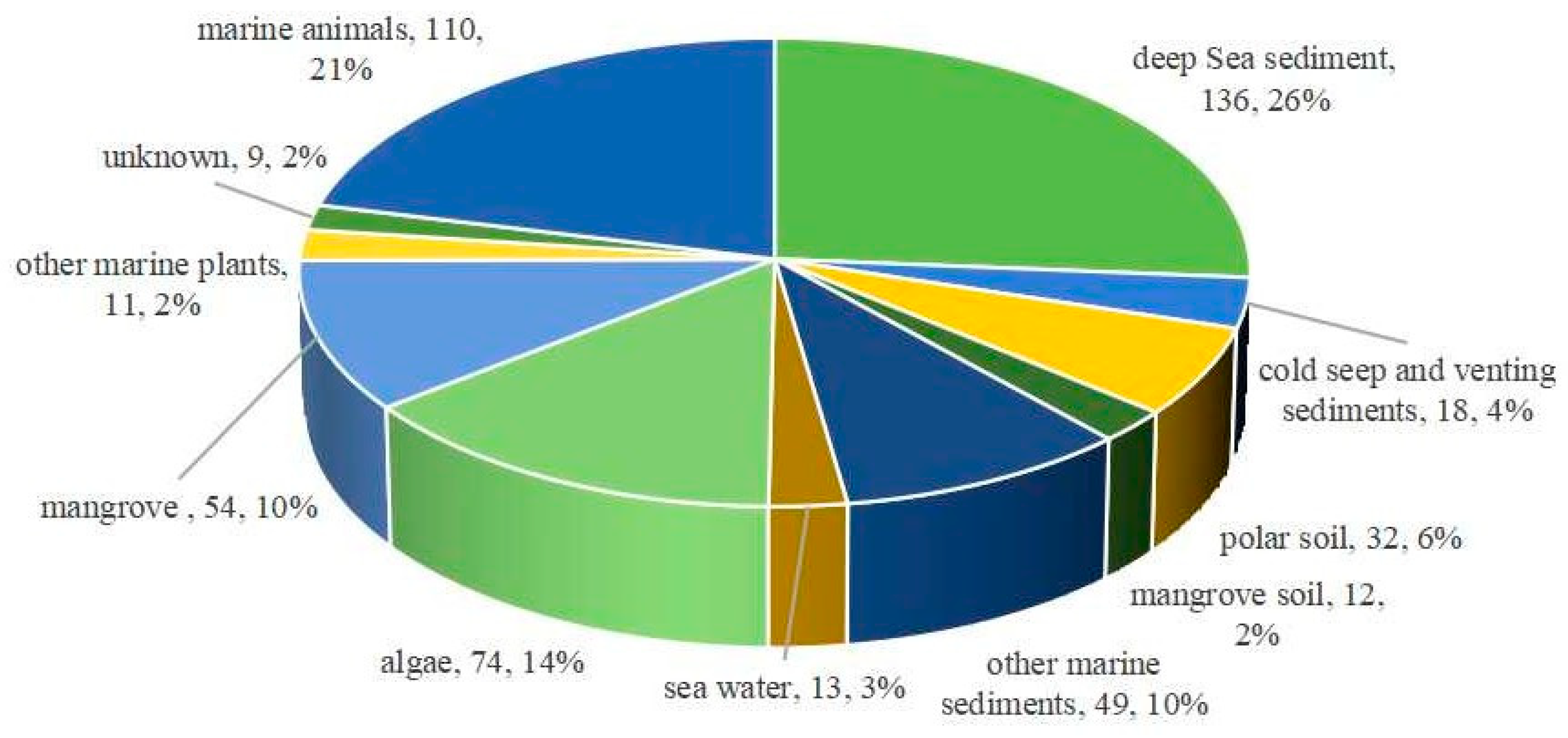
2. The Characteristics of Marine Fungal Terpenoids
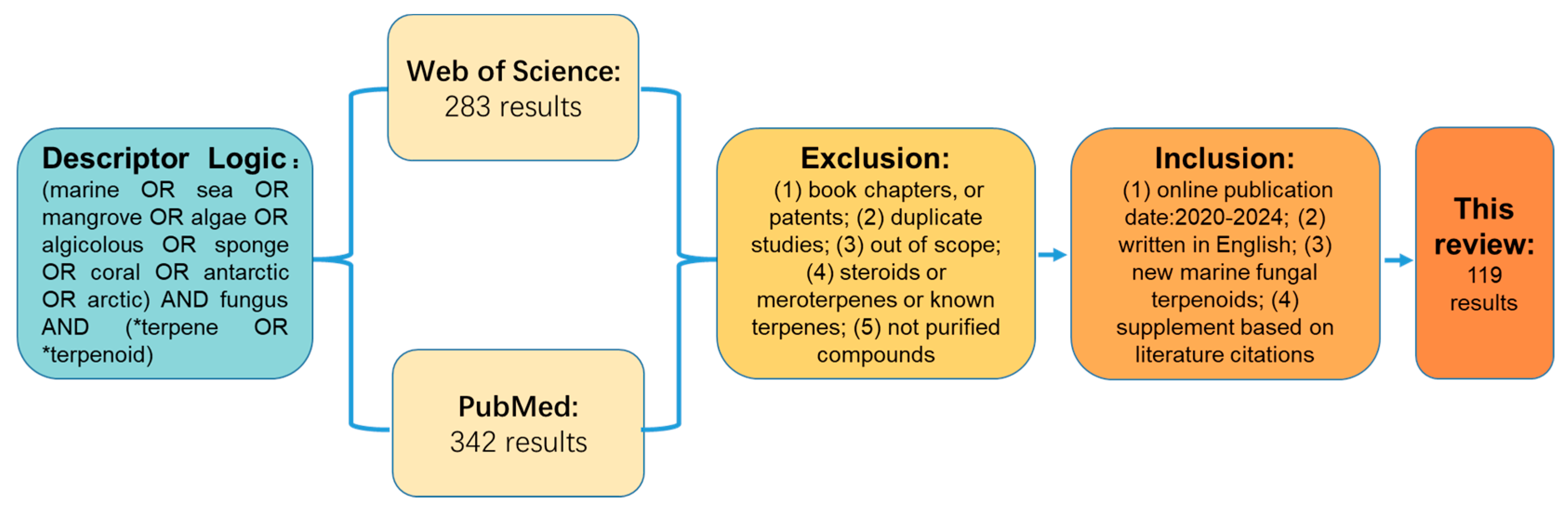
3. Materials and Methods
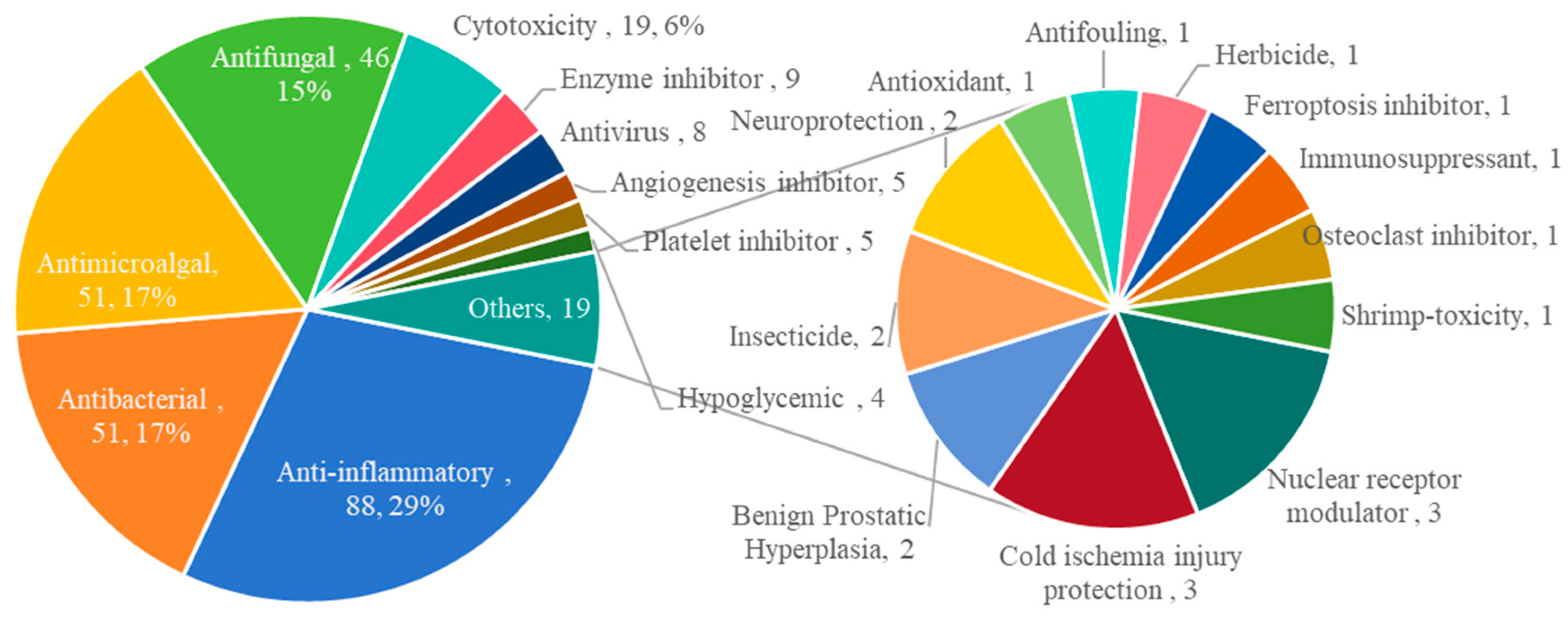
4. Isolation, Structure, and Bioactivities of Marine Fungal Terpenoids
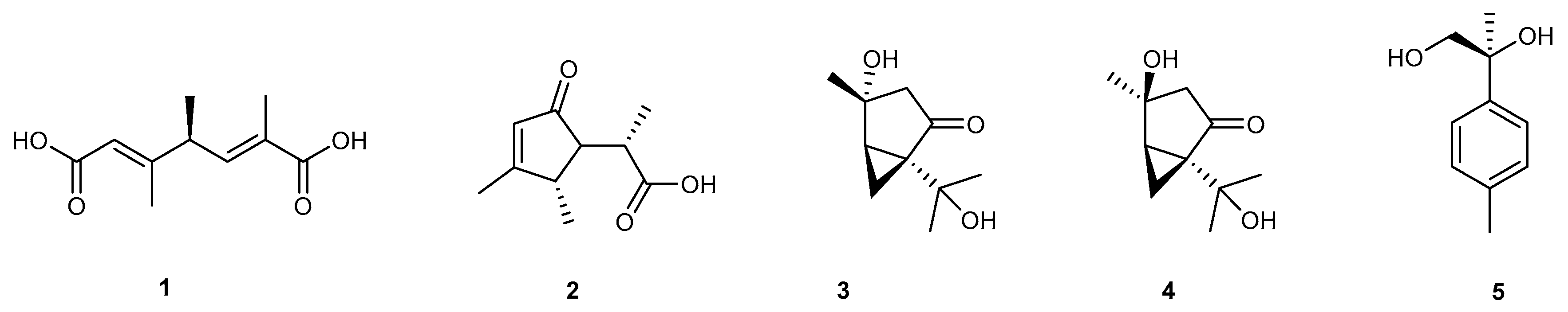
4.1. Monoterpenes Five Compounds (1–5)
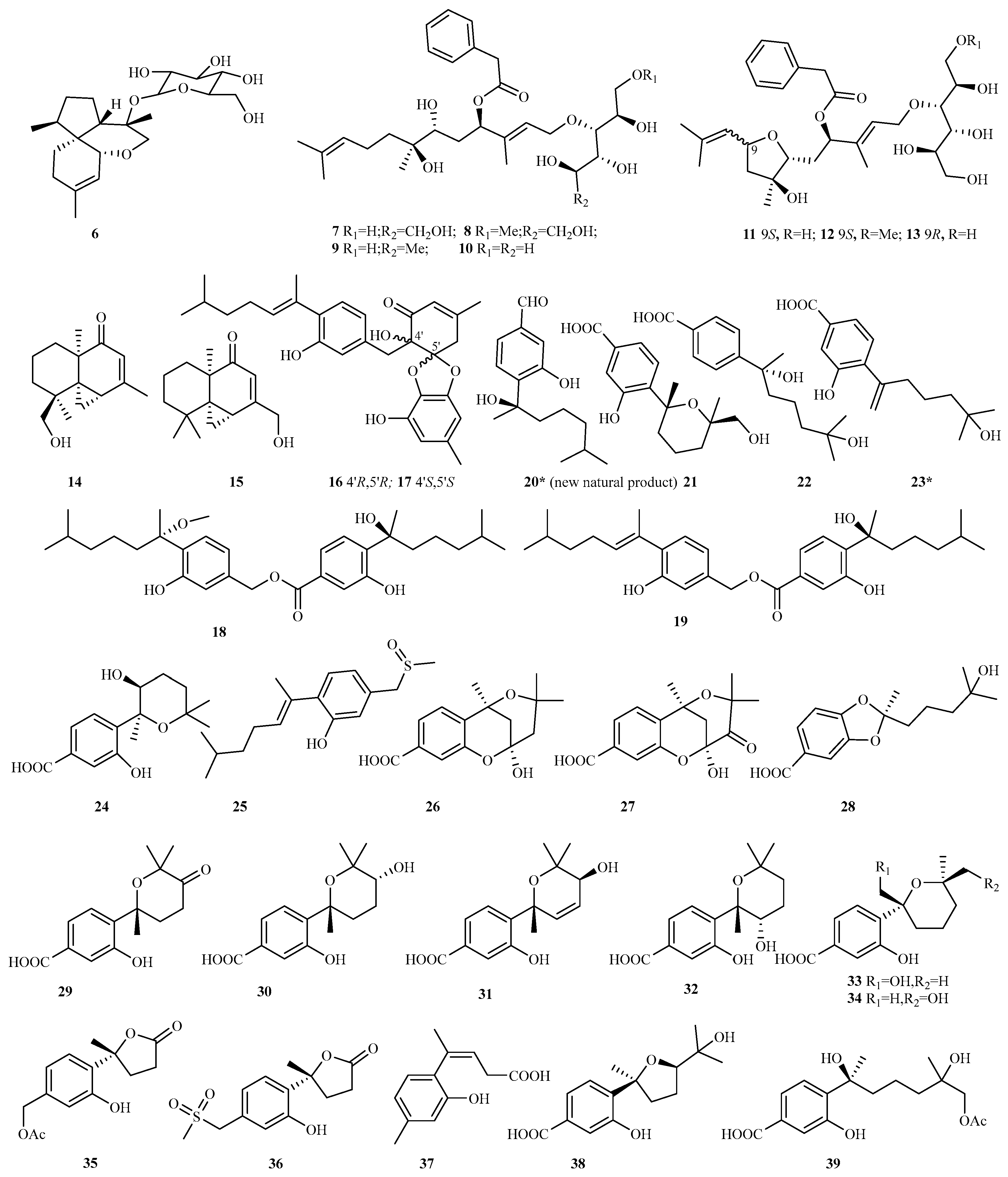
4.2. Sesquiterpenes 345 Compounds (6–350)
4.2.1. Acremonium sp. 8 (6–13)
4.2.2. Alternaria sp. 2 (14–15)
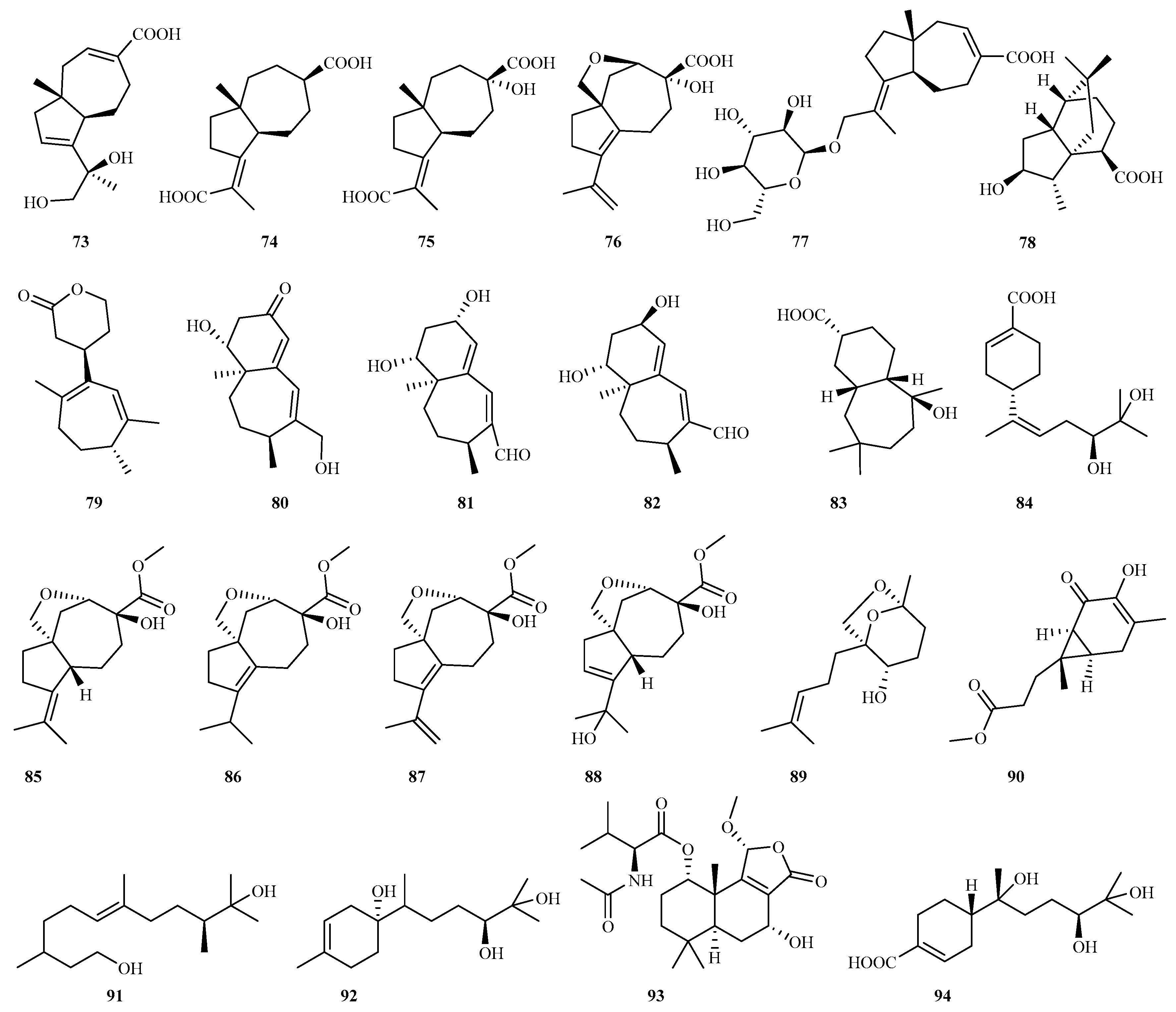
4.2.3. Aspergillus sp. 69 (16–84)
4.2.4. Byssochlamys (Paecilomyces) sp. 6 (85–90)
4.2.5. Cladosporium sp. 3 (91–93)
4.2.6. Colletotrichum sp. 1 (94)
4.2.7. Diaporthe sp. 3 (95–97)
4.2.8. Emericellopsis sp. 7 (98–104)
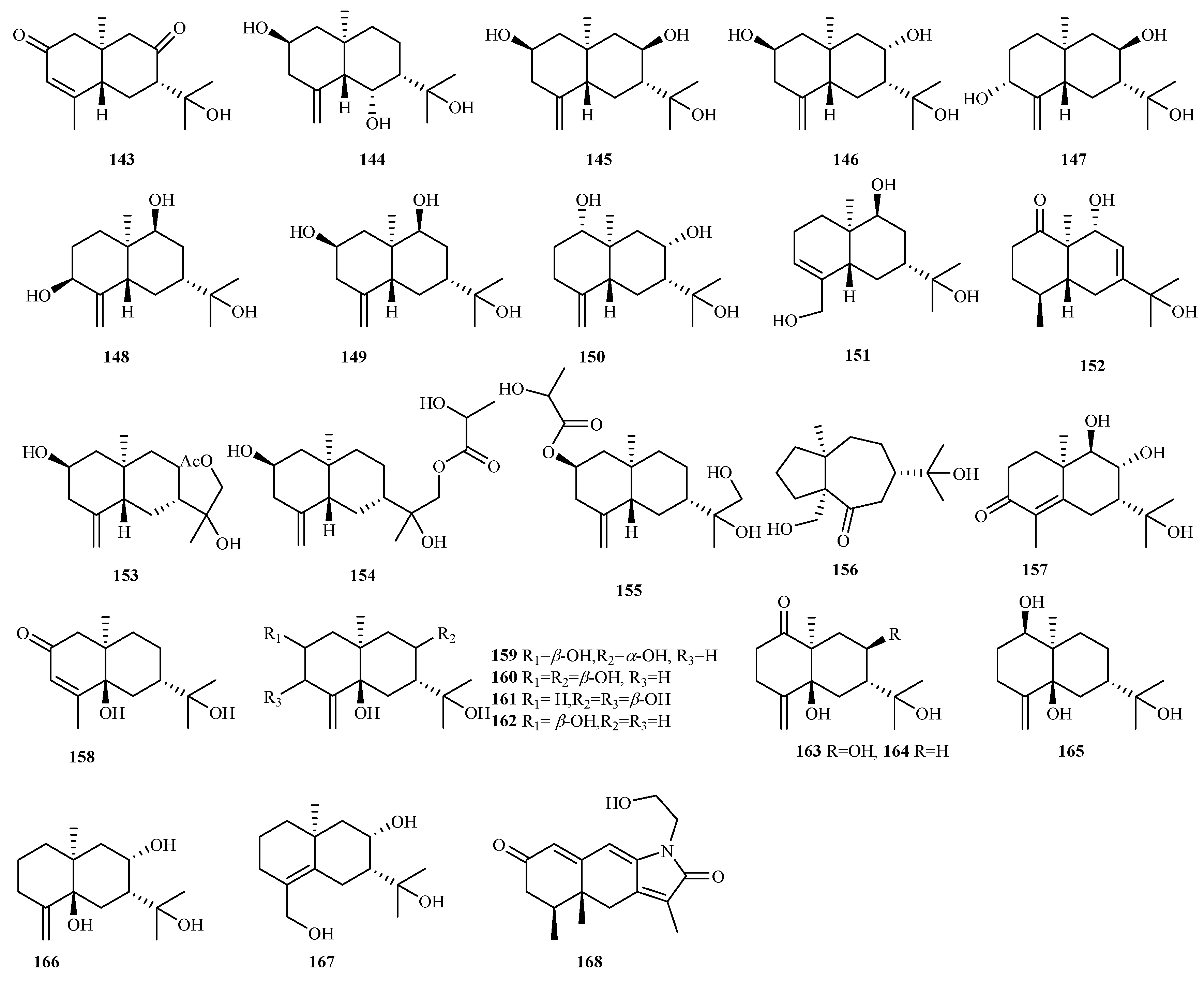
4.2.9. Eutypella sp. 63 (105–167)
4.2.10. Humicola sp. 1 (168)
4.2.11. Penicillium sp. 50 (169–218)
4.2.12. Phoma (Didymella) sp. 1 (219)
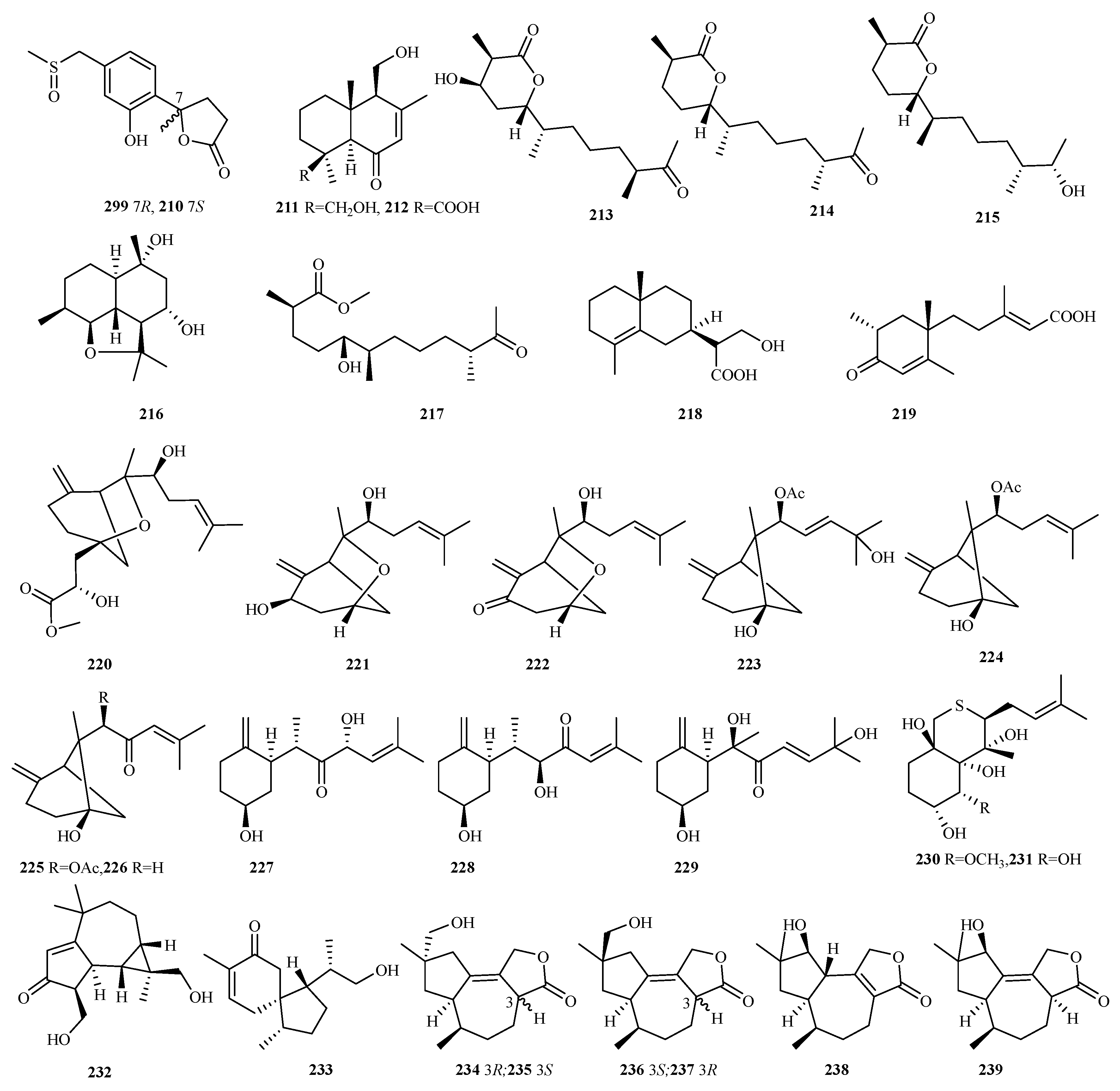
4.2.13. Pseudallescheria (Scedosporium) sp. 13 (220–232)
4.2.14. Pseudofusicoccum sp. 1 (233)
4.2.15. Pseudogymnoascus sp. 6 (234–239)
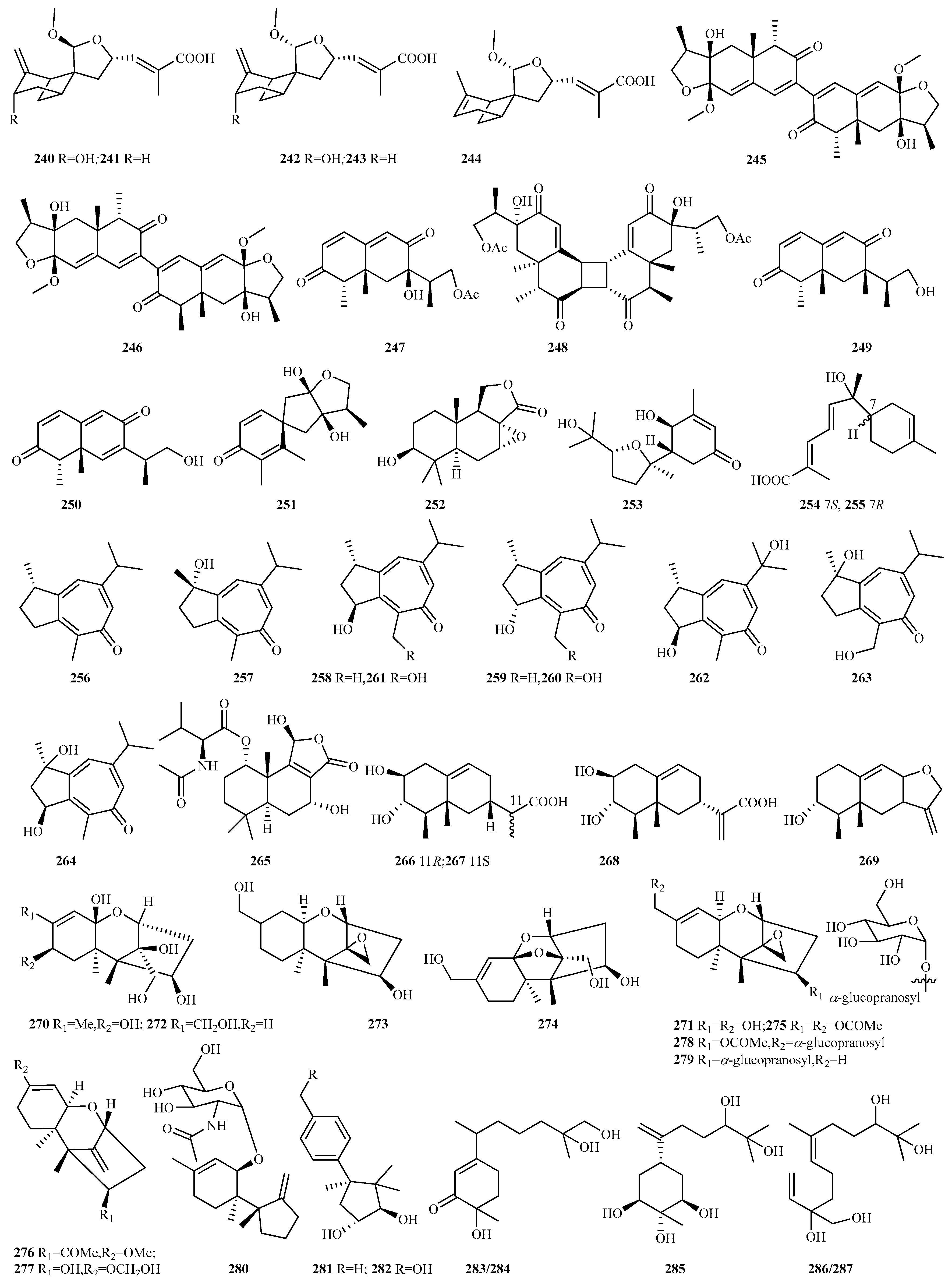
4.2.16. Paraconiothyrium sp. 12 (240–251)
4.2.17. Pyrrhoderma sp. 1 (252)
4.2.18. Retroconis sp. 1 (253)
4.2.19. Roussoella sp. 2 (254 and 255)
4.2.20. Spiromastix sp. 9 (256–264)
4.2.21. Talaromyces sp. 5 (265–269)
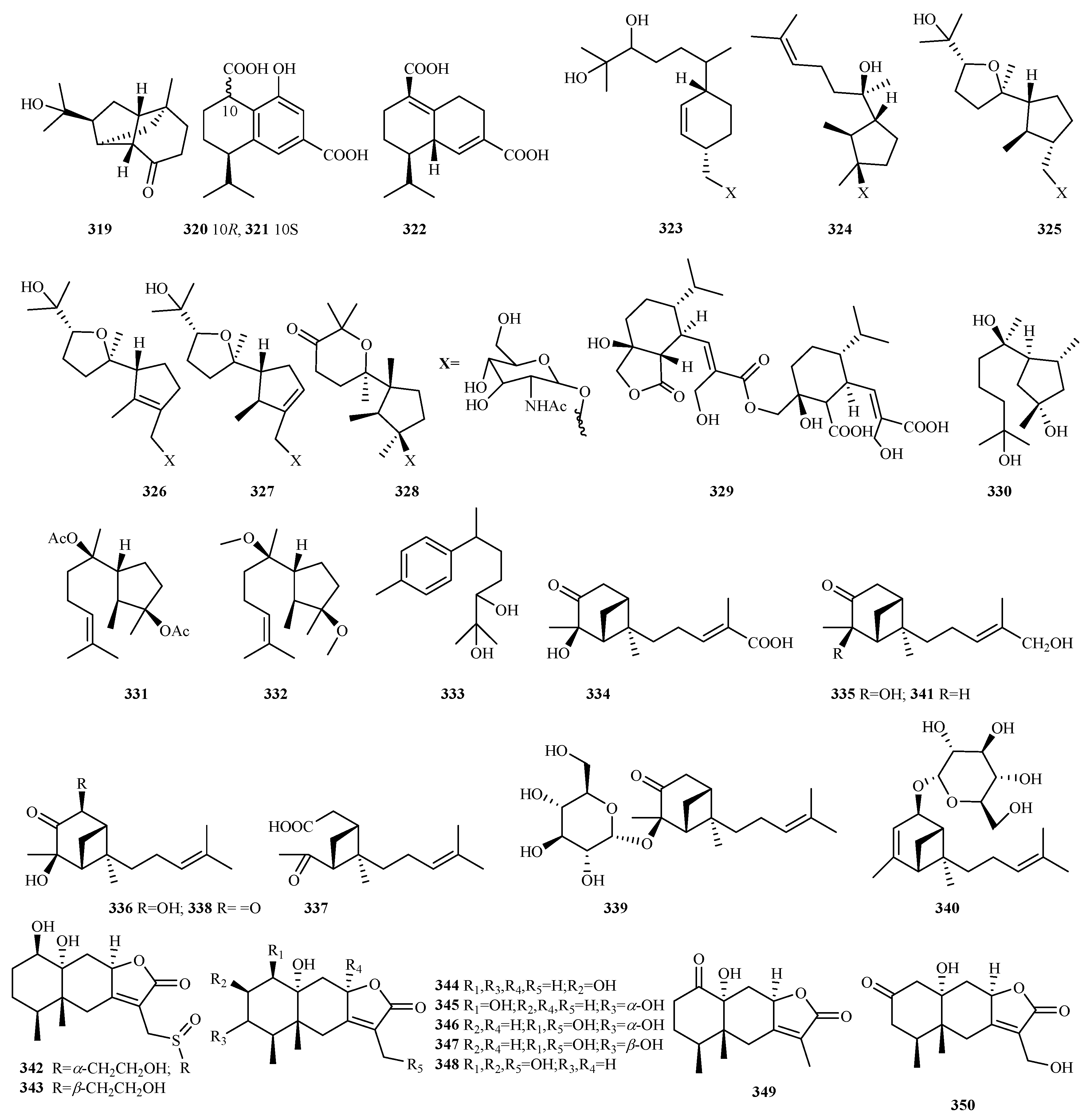
4.2.22. Trichoderma sp. 72 (270–341)
4.2.23. Unidentified Fungal Species 9 (342–350)
4.3. Diterpenes 128 Compounds (351–478)
4.3.1. Acremonium sp. 1 (351)
4.3.2. Aspergillus sp. 2 (352–353)
4.3.3. Beauveria sp. 1 (354)
4.3.4. Cladosporium sp. 2 (355–356)
4.3.5. Diaporthe sp. 3 (357–359)
4.3.6. Eutypella sp. 8 (360–367)
4.3.7. Hypoxylon sp. 2 (368 and 369)
4.3.8. Neocucurbitaria sp. 15 (370–384)
4.3.9. Paraconiothyrium sp. 5 (385–389)
4.3.10. Penicillium sp. 9 (390–398)
4.3.11. Peroneutypa sp. 1 (399)
4.3.12. Pestalotiopsis sp. 1 (400)
4.3.13. Phoma (Didymella) sp. 5 (401–405)
4.3.14. Pleospora sp. 18 (406–423)
4.3.15. Stachybotrys sp. 15 (424–438)
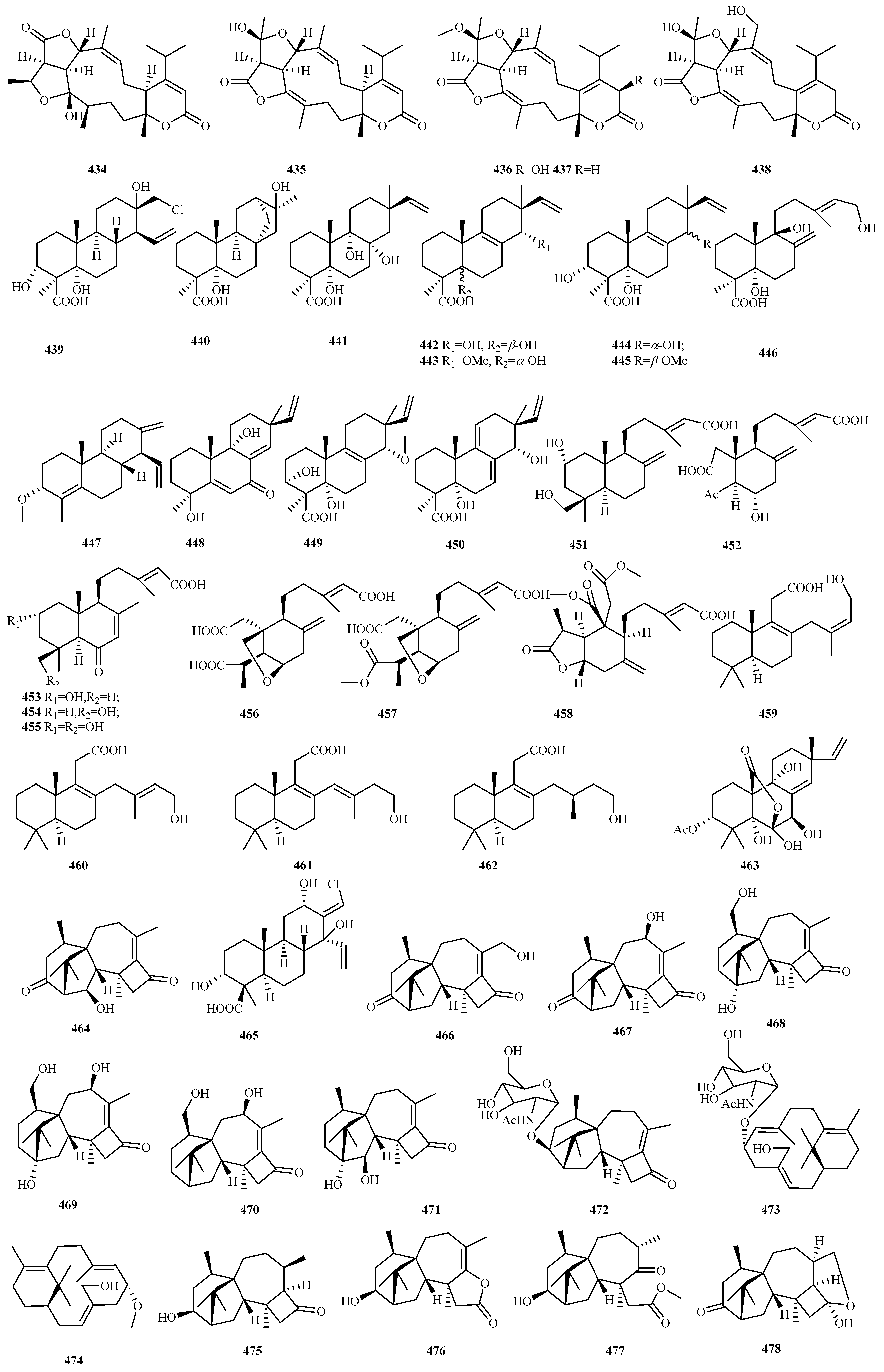
4.3.16. Talaromyces sp. 25 (439–463)
4.3.17. Trichoderma sp. 15 (464–478)
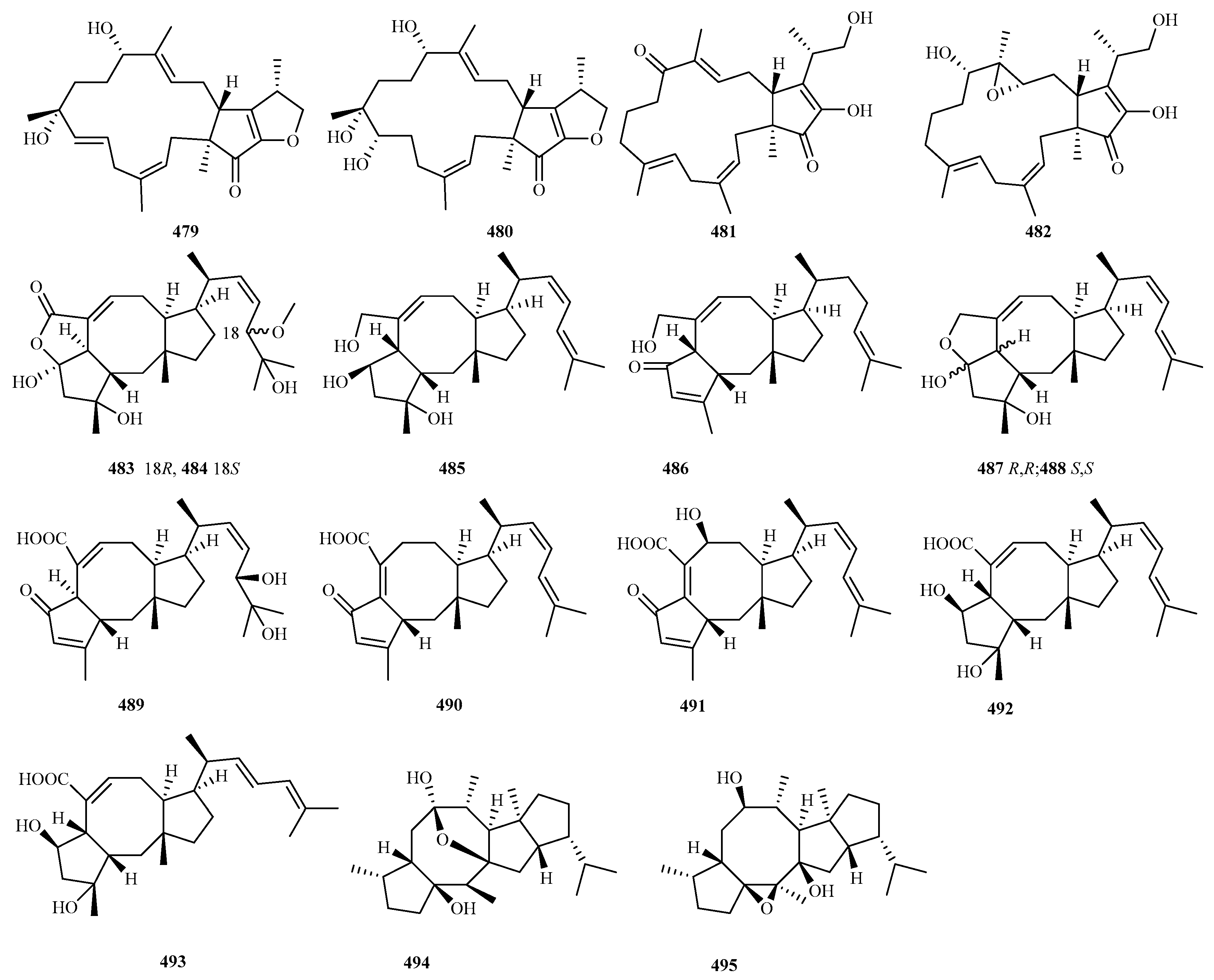
4.4. Sesterterpenes 17 Compounds (479–495)
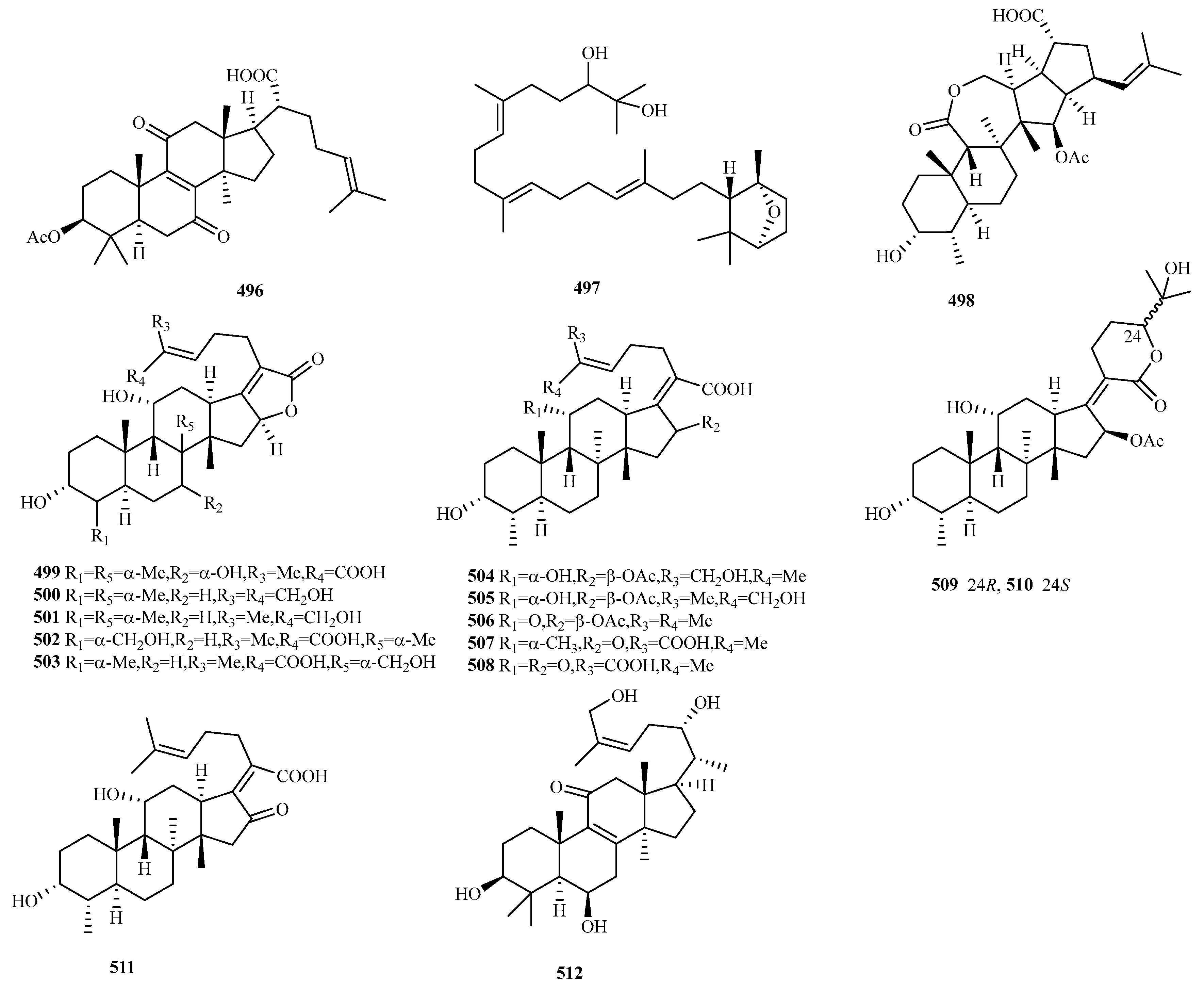
4.5. Triterpenes 17 Compounds (496–512)
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Imhoff, J.F. Natural products from marine fungi—Still an underrepresented resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Proksch, P. Bioactive secondary metabolites from endophytes and associated marine derived fungi. Fungal Divers. 2011, 49, 1–12. [Google Scholar] [CrossRef]
- Bugni, T.S.; Ireland, C.M. Marine-derived fungi: A chemically and biologically diverse group of microorganisms. Nat. Prod. Rep. 2004, 21, 143–163. [Google Scholar] [CrossRef]
- Galindo-Solis, J.M.; Fernandez, F.J. Endophytic fungal terpenoids: Natural role and bioactivities. Microorganisms 2022, 10, 339. [Google Scholar] [CrossRef]
- Amirzakariya, B.Z.; Shakeri, A. Bioactive terpenoids derived from plant endophytic fungi: An. updated review (2011–2020). Phytochemistry 2022, 197, 113130. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2025, 42, 257–297. [Google Scholar] [CrossRef]
- Ebel, R. Terpenes from marine-derived fungi. Mar. Drugs 2010, 8, 2340–2368. [Google Scholar] [CrossRef]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Guo, H.; Liu, L.; Chen, S. A review of terpenes from marine-derived fungi: 2015–2019. Mar. Drugs 2020, 18, 321. [Google Scholar] [CrossRef]
- Ma, X.Y.; Wang, H.N.; Sun, L.X.; Sun, J.; Jin, S.H.; Dai, F.X.; Sai, C.M.; Zhang, Z. Bioactive steroids from marine-derived fungi: A review (2015–2023). J. Asian Nat. Prod. Res. 2025; in press. [Google Scholar] [CrossRef]
- Jiang, M.; Wu, Z.; Liu, L.; Chen, S. The chemistry and biology of fungal meroterpenoids (2009–2019). Org. Biomol. Chem. 2021, 19, 1644–1704. [Google Scholar] [CrossRef]
- Luo, P.; Huang, J.H.; Lv, J.M.; Wang, G.Q.; Hu, D.; Gao, H. Biosynthesis of fungal terpenoids. Nat. Prod. Rep. 2024, 41, 748–783. [Google Scholar] [CrossRef]
- Niu, S.; Yang, L.; Chen, T.; Hong, B.; Pei, S.; Shao, Z.; Zhang, G. New monoterpenoids and polyketides from the deep-sea sediment-derived fungus Aspergillus sydowii MCCC 3A00324. Mar. Drugs 2020, 18, 561. [Google Scholar] [CrossRef]
- Zhai, G.; Chen, S.; Shen, H.; Guo, H.; Jiang, M.; Liu, L. Bioactive monoterpenes and polyketides from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 553. [Google Scholar] [CrossRef]
- Kim, H.J.; Li, X.J.; Kim, D.C.; Kim, T.K.; Sohn, J.H.; Kwon, H.; Lee, D.; Kim, Y.C.; Yim, J.H.; Oh, H. PTP1B inhibitory secondary metabolites from an antarctic fungal strain Acremonium sp. SF-7394. Molecules 2021, 26, 5505. [Google Scholar] [CrossRef]
- Hao, X.; Li, S.; Li, J.; Wang, G.; Li, J.; Peng, Z.; Gan, M. Acremosides A-G, sugar alcohol-conjugated acyclic sesquiterpenes from a sponge-derived Acremonium Species. J. Nat. Prod. 2024, 87, 1059–1066. [Google Scholar] [CrossRef]
- Chen, S.; Deng, Y.; Yan, C.; Wu, Z.; Guo, H.; Liu, L.; Liu, H. Secondary metabolites with Nitric Oxide inhibition from marine-derived fungus Alternaria sp. 5102. Mar. Drugs 2020, 18, 426. [Google Scholar] [CrossRef]
- Weng, H.Z.; Zhu, J.Y.; Yuan, F.Y.; Tang, Z.Y.; Tian, X.Q.; Chen, Y.; Fan, C.Q.; Tang, G.H.; Yin, S. Homo/hetero-dimers of aromatic bisabolane sesquiterpenoids with neuroprotective activity from the fungus Aspergillus versicolor A18 from South China Sea. Mar. Drugs 2022, 20, 322. [Google Scholar] [CrossRef]
- Pang, X.; Lin, X.; Zhou, X.; Yang, B.; Tian, X.; Wang, J.; Xu, S.; Liu, Y. New quinoline alkaloid and bisabolane-type sesquiterpenoid derivatives from the deep-sea-derived fungus Aspergillus sp. SCSIO06786. Fitoterapia 2020, 140, 104406. [Google Scholar] [CrossRef]
- Hu, X.; Li, X.; Meng, L.; Wang, B. Antioxidant bisabolane-type sesquiterpenoids from algal-derived fungus Aspergillus sydowii EN-434. J. Oceanol. Limnol. 2020, 38, 1532–1536. [Google Scholar] [CrossRef]
- Yang, X.; Yu, H.; Ren, J.; Cai, L.; Xu, L.; Liu, L. Sulfoxide-containing bisabolane sesquiterpenoids with antimicrobial and nematicidal activities from the marine-derived fungus Aspergillus sydowii LW09. J. Fungi 2023, 9, 347. [Google Scholar] [CrossRef]
- Niu, S.; Yang, L.; Zhang, G.; Chen, T.; Hong, B.; Pei, S.; Shao, Z. Phenolic bisabolane and cuparene sesquiterpenoids with anti-inflammatory activities from the deep-sea-derived Aspergillus sydowii MCCC 3A00324 fungus. Bioorg. Chem. 2020, 105, 104420. [Google Scholar] [CrossRef]
- Wu, J.S.; Yao, G.S.; Shi, X.H.; Rehman, S.U.; Xu, Y.; Fu, X.M.; Zhang, X.L.; Liu, Y.; Wang, C.Y. Epigenetic agents trigger the production of bioactive nucleoside derivatives and bisabolane sesquiterpenes from the marine-derived fungus Aspergillus versicolor. Front. Microbiol. 2020, 11, 85. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.; Liu, R.; Liu, Y.; Liu, R.; Wu, Z.; Xu, W.; Ma, H.; Luo, H.B.; Cheng, Z. Structure revisions of phenolic bisabolane sesquiterpenes and a ferroptosis inhibitor from the marine-derived fungus Aspergillus versicolor YPH93. J. Nat. Prod. 2023, 86, 830–841. [Google Scholar] [CrossRef]
- Yu, H.Y.; Chen, Y.S.; Wang, Y.; Zou, Z.B.; Xie, M.M.; Li, Y.; Li, L.S.; Meng, D.L.; Wu, L.Q.; Yang, X.W. Anti-necroptosis and anti-ferroptosis compounds from the deep-sea-derived fungus Aspergillus sp. MCCC 3A00392. Bioorg Chem. 2024, 144, 107175. [Google Scholar] [CrossRef]
- Gui, P.; Fan, J.; Zhu, T.; Fu, P.; Hong, K.; Zhu, W. Sesquiterpenoids from the mangrove-derived Aspergillus ustus 094102. Mar. Drugs 2022, 20, 408. [Google Scholar] [CrossRef]
- Fang, S.T.; Liu, X.H.; Yan, B.F.; Miao, F.P.; Yin, X.L.; Li, W.Z.; Ji, N.Y. Terpenoids from the marine-derived fungus Aspergillus sp. RR-YLW-12, associated with the red alga Rhodomela confervoides. J. Nat. Prod. 2021, 84, 1763–1771. [Google Scholar] [CrossRef]
- Fang, S.T.; Shi, Z.Z.; Song, Y.P.; Yin, X.L.; Ji, N.Y. New ophiobolin sesterterpenoid and drimane sesquiterpenoids from a marine-alga-derived fungus Aspergillus sp. Fitoterapia 2023, 170, 105659. Fitoterapia 2023, 170, 105659. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.; Liu, Q.; Wu, Q.; Chen, S.; Wang, J.; Li, J.; Liu, L.; Gao, Z. Cyclohexenone derivative and drimane sesquiterpenes from the seagrass-derived fungus Aspergillus insuetus. Chem. Biodivers. 2023, 20, e202300424. [Google Scholar] [CrossRef]
- Zhuravleva, O.I.; Belousova, E.B.; Oleinikova, G.K.; Antonov, A.S.; Khudyakova, Y.V.; Rasin, A.B.; Popov, R.S.; Menchinskaya, E.S.; Trinh, P.T.H.; Yurchenko, A.N.; et al. Cytotoxic drimane-type sesquiterpenes from co-culture of the marine-derived fungi Aspergillus carneus KMM 4638 and Beauveria felina (=Isaria felina) KMM 4639. Mar. Drugs 2022, 20, 584. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Sun, N.; Zhang, X.; Shah, M.; Zhang, G.; Che, Q.; Zhu, T.; Li, J.; Li, D. Cytotoxic nitrobenzoyl sesquiterpenoids from an antarctica sponge-derived Aspergillus insulicola. J. Nat. Prod. 2022, 85, 987–996. [Google Scholar] [CrossRef]
- Hu, Z.; Zhu, Y.; Chen, J.; Chen, J.; Li, C.; Gao, Z.; Li, J.; Liu, L. Sesquiterpenoids with phytotoxic and antifungal activities from a pathogenic fungus Aspergillus alabamensis. J. Agric. Food Chem. 2022, 70, 12065–12073. [Google Scholar] [CrossRef]
- Shang, R.-Y.; Cui, J.; Li, J.-X.; Miao, X.-X.; Zhang, L.; Xie, D.-D.; Zhang, L.; Lin, H.-W.; Jiao, W.-H. Nigerin and ochracenes J−L, new sesquiterpenoids from the marine sponge symbiotic fungus Aspergillus niger. Tetrahedron 2022, 104, 132599. [Google Scholar] [CrossRef]
- Niu, S.; Huang, S.; Wang, J.; He, J.; Chen, M.; Zhang, G.; Hong, B. Malfilanol C, a new sesquiterpenoid isolated from the deep-sea-derived Aspergillus puniceus A2 fungus. Nat. Prod. Res. 2024, 38, 1362–1368. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, W.; Hao, L.; Qin, X.; Yang, R.; Li, J.; Huang, X. A new sesquiterpene from mangrove endophytic fungus Aspergillus sp. GXNU-MA1. Nat. Prod. Res. 2022, 36, 1857–1863. [Google Scholar] [CrossRef]
- Liu, X.H.; Song, Y.P.; Yin, X.L.; Ji, N.Y. Antimicrobial terpenoids and polyketides from the algicolous fungus Byssochlamys spectabilis RR-dl-2-13. J. Agric. Food Chem. 2022, 70, 4658–4666. [Google Scholar] [CrossRef]
- Li, D.-D.; Luo, X.; Ying, W.; La Kim, E.; Hong, J.; Lee, J.-H.; Jung, J.H. Peroxisome proliferator activated receptor-γ agonistic compounds from the jellyfish-derived fungus Cladosporium oxysporum. Chem. Biodivers. 2023, 20, e202300851. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, Y.; Pang, X.; Liu, Y.; Zhang, X.; Liu, Q.; Wang, J. Structurally diverse secondary metabolites from a deep-sea derived Cladosporium sp. SCSIO 41318 and their biological evaluation. Chem. Biodivers. 2024, 21, e202401751. [Google Scholar] [CrossRef]
- Li, K.L.; Dai, Y.; She, J.L.; Zeng, Y.B.; Dai, H.F.; Ou, S.L.; Zhou, X.F.; Liu, Y.H. Bisabolanoic acid A, a new polychiral sesquiterpene with AChE inhibitory activity from a mangrove-derived fungus Colletotrichum sp. J. Asian Nat. Prod. Res. 2022, 24, 88–95. [Google Scholar] [CrossRef]
- Luo, X.W.; Chen, C.M.; Li, K.L.; Lin, X.P.; Gao, C.H.; Zhou, X.F.; Liu, Y.H. Sesquiterpenoids and meroterpenoids from a mangrove derived fungus Diaporthe sp. SCSIO 41011. Nat. Prod. Res. 2021, 35, 282–288. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, G.; Yang, W.; Zhao, Y.; Tan, Q.; Chen, L.; Wang, J.; Ma, C.; Kang, W.; She, Z. Metabolites with anti-inflammatory activity from the mangrove endophytic fungus Diaporthe sp. QYM12. Mar. Drugs 2021, 19, 56. [Google Scholar] [CrossRef]
- Virués-Segovia, J.R.; Millán, C.; Pinedo, C.; González-Rodríguez, V.E.; Papaspyrou, S.; Zorrilla, D.; Mackenzie, T.A.; Ramos, M.C.; de la Cruz, M.; Aleu, J.; et al. New eremophilane-type sesquiterpenes from the marine sediment-derived fungus Emericellopsis maritima BC17 and their cytotoxic and antimicrobial activities. Mar. Drugs 2023, 21, 634. [Google Scholar] [CrossRef]
- Virués-Segovia, J.R.; Pinedo, C.; Zorrilla, D.; Sánchez-Márquez, J.; Sánchez, P.; Ramos, M.C.; de la Cruz, M.; Aleu, J.; Durán-Patrón, R. Discovery of new eremophilanes from the marine-derived fungus Emericellopsis maritima BC17 by culture conditions changes: Evaluation of cytotoxic and antimicrobial activities. Front. Mar. Sci. 2024, 11, 1386175. [Google Scholar] [CrossRef]
- Ning, Z.; Hu, B.; Sun, Y.Y.; Ding, J.F.; Han, X.Y.; Lu, X.L.; Yin, Z.F.; He, Y.; Jiao, B.H.; Yu, H.B.; et al. Eutypellaolides A-J, sesquiterpene diversity expansion of the polar fungus Eutypella sp. D-1. Front. Microbiol. 2024, 15, 1349151. [Google Scholar] [CrossRef]
- Ning, Y.; Qu, Y.; Fu, Y.; Zhang, S.; Xu, Y.; Jiao, B.; Lu, X. Discovery of bioactive terpenes derived from a polar fungus. Chem. Biodivers. 2024, 21, e202401750. [Google Scholar] [CrossRef]
- Ning, Y.; Gu, Q.; Zheng, T.; Xu, Y.; Li, S.; Zhu, Y.; Hu, B.; Yu, H.; Liu, X.; Zhang, Y.; et al. Genome mining leads to diverse sesquiterpenes with anti-inflammatory activity from an arctic-derived fungus. J. Nat. Prod. 2024, 87, 1426–1440. [Google Scholar] [CrossRef]
- Niu, S.; Liu, D.; Shao, Z.; Liu, J.; Fan, A.; Lin, W. Chemical epigenetic manipulation triggers the production of sesquiterpenes from the deep-sea derived Eutypella fungus. Phytochemistry 2021, 192, 112978. [Google Scholar] [CrossRef]
- Jiang, Z.P.; Su, R.; Chen, M.T.; Li, J.Y.; Chen, H.Y.; Yang, L.; Liu, F.F.; Liu, J.; Xu, C.J.; Li, W.S.; et al. Ent-eudesmane sesquiterpenoids with anti-neuroinflammatory activity from the marine-derived fungus Eutypella sp. F0219. Phytochemistry 2024, 223, 114121. [Google Scholar] [CrossRef]
- Li, W.-S.; Lei, X.-P.; Li, Z.; He, Y.; Chen, M.; Jiang, Z.-P.; Chen, G.-Y. Discovery of marine ent-eudesmane sesquiterpenoids as angiogenic inhibitors via suppressing VEGF-A/VEGFR2 signaling pathway. Bioorg Chem. 2025, 154, 108055. [Google Scholar] [CrossRef]
- Teng, Q.; Nong, Q.; Jin, X.; Gao, C.; Zhang, A.; Xing, N.; Chen, X. New eremophilane-type sesquiterpenoid and new chromanone isolated from marine derived fungus Humicola sp. GXIMD02070. Phytochem. Lett. 2025, 65, 48–52. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, D.; Fan, A.; Huang, J.; Lin, W. Eremophilane-type sesquiterpenes from a marine-derived fungus Penicillium copticola with antitumor and neuroprotective activities. Mar. Drugs 2022, 20, 712. [Google Scholar] [CrossRef]
- Wu, Q.; Chang, Y.; Che, Q.; Li, D.; Zhang, G.; Zhu, T. Citreobenzofuran D-F and phomenone A-B: Five novel sesquiterpenoids from the mangrove-derived fungus Penicillium sp. HDN13-494. Mar. Drugs 2022, 20, 137. [Google Scholar] [CrossRef]
- Zhang, W.; Meng, Q.; Wu, J.; Cheng, W.; Liu, D.; Huang, J.; Fan, A.; Xu, J.; Lin, W. Acorane sesquiterpenes from the deep-sea derived Penicillium bilaiae fungus with anti-neuroinflammatory effects. Front. Chem. 2022, 10, 1036212. [Google Scholar] [CrossRef]
- Yang, L.-J.; Lv, L.; Han, Z.; Gu, Y.-C.; Li, X.; Shao, C.-L.; Liu, Z.-Q.; Wang, C.-Y. Penijanacoranes A-F, acorane-type sesquiterpenes from a deep sea-derived fungus Penicillium janthinellum SH0301. Chin. J. Chem. 2024, 43, 268–274. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Chi, X.; Liu, C.; Zhang, J. A new drimane sesquiterpene ester from the marine-derived fungus Penicillium chrysogenum LD-201810. Chem. Nat. Compd. 2022, 58, 1042–1044. [Google Scholar] [CrossRef]
- Ge, Y.; Tang, W.L.; Huang, Q.R.; Wei, M.L.; Li, Y.Z.; Jiang, L.L.; Li, C.L.; Yu, X.; Zhu, H.W.; Chen, G.Z.; et al. New enantiomers of a nor-bisabolane derivative and two new phthalides produced by the marine-derived fungus Penicillium chrysogenum LD-201810. Front. Microbiol. 2021, 12, 727670. [Google Scholar] [CrossRef]
- Gou, X.; Tian, D.; Wei, J.; Ma, Y.; Zhang, Y.; Chen, M.; Ding, W.; Wu, B.; Tang, J. New drimane sesquiterpenes and polyketides from marine-derived fungus Penicillium sp. TW58-16 and their anti-inflammatory and alpha-Glucosidase inhibitory effects. Mar. Drugs 2021, 19, 416. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, X.M.; Yang, S.Q.; Liu, H.; Meng, L.H.; Wang, B.G. Three new sesquiterpenoids from the algal-derived fungus Penicillium chermesinum EN-480. Mar. Drugs 2020, 18, 194. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Antonov, A.S.; Pivkin, M.V.; Denisenko, V.A.; Popov, R.S.; Ngoc, N.T.D.; Leshchenko, E.V. New cadinane-type sesquiterpene from marine isolate of the fungus Penicillium oxalicum KMM 4683. Chem. Nat. Compd. 2021, 57, 187–189. [Google Scholar] [CrossRef]
- Ying, Z.; Li, X.M.; Yang, S.Q.; Wang, B.G.; Li, H.L.; Meng, L.H. New polyketide and sesquiterpenoid derivatives from the magellan seamount-derived fungus Penicillium rubens AS-130. Chem. Biodivers. 2023, 20, e202300229. [Google Scholar] [CrossRef]
- Ji, R.-N.; Wu, J.-T.; Hao, B.-C.; Zhu, X.-H.; Xue, J.-C.; Zheng, C.-J.; Chen, M. A new eudesmane sesquiterpenoid from the marine-derived fungus Penicillium sp. HK1-22. Chem. Nat. Compd. 2023, 59, 285–287. [Google Scholar] [CrossRef]
- Pan, D.; Lin, S.; Huang, X.; Lv, D.; Lai, Q.; Xia, J.; Shao, Z.; Wang, W. New polyketides and sesquiterpenoids from the deep-sea sulphide-derived fungus Phoma sp. 3A00413. Fitoterapia 2023, 168, 105546. [Google Scholar] [CrossRef]
- Ying, Z.; Li, X.M.; Yang, S.Q.; Li, H.L.; Li, X.; Wang, B.G.; Meng, L.H. Antifungal pseuboyenes A-J, bergamotene-derived sesquiterpenoids from a cold-seep-derived Pseudallescheria boydii. J. Nat. Prod. 2024, 87, 1347–1357. [Google Scholar] [CrossRef]
- Ying, Z.; Li, X.M.; Yang, S.Q.; Li, H.L.; Li, X.; Wang, B.G.; Meng, L.H. Pseudallenes A and B, new sulfur-containing ovalicin sesquiterpenoid derivatives with antimicrobial activity from the deep-sea cold seep sediment-derived fungus Pseudallescheria boydii CS-793. Beilstein J. Org. Chem. 2024, 20, 470–478. [Google Scholar] [CrossRef]
- Yuan, M.-X.; Guo, Q.; Ran, Y.-Q.; Qiu, Y.; Lan, W.-J.; Li, H.-J. New aromadendrane sesquiterpenoid pseuboydone f from the marine-derived fungus Pseudallescheria boydii F44-1. Rec. Nat. Prod. 2020, 14, 166–170. [Google Scholar] [CrossRef]
- Jia, S.; Su, X.; Yan, W.; Wu, M.; Wu, Y.; Lu, J.; He, X.; Ding, X.; Xue, Y. Acorenone C: A new spiro-sesquiterpene from a mangrove-associated fungus, Pseudofusicoccum sp. J003. Front. Chem. 2021, 9, 780304. [Google Scholar] [CrossRef]
- Shi, T.; Li, X.Q.; Zheng, L.; Zhang, Y.H.; Dai, J.J.; Shang, E.L.; Yu, Y.Y.; Zhang, Y.T.; Hu, W.P.; Shi, D.Y. Sesquiterpenoids from the antarctic fungus Pseudogymnoascus sp. HSX2#-11. Front. Microbiol. 2021, 12, 688202. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Liu, Y.; Zhang, Y.; Wu, J.; Zhang, G.; Che, Q.; Zhu, T.; Li, M.; Li, D. Brasilterpenes A-E, bergamotane sesquiterpenoid derivatives with hypoglycemic activity from the deep sea-derived fungus Paraconiothyrium brasiliense HDN15-135. Mar. Drugs 2022, 20, 338. [Google Scholar] [CrossRef]
- Sun, B.; Wang, D.; Ren, J.; Wang, C.; Yan, P.; Gustafson, K.R.; Jiang, W. Paraconulones A-G: Eremophilane sesquiterpenoids from the marine-derived fungus Paraconiothyrium sporulosum DL-16. J. Nat. Prod. 2023, 86, 1360–1369. [Google Scholar] [CrossRef]
- Wang, J.W.; Liu, D.Y.; Zhang, H.Z.; Tan, Z.; Zheng, C.J.; Chen, G.Y.; Nong, X.H. Drimane-type sesquiterpenoids and their anti-inflammatory evaluation from Pyrrhoderma noxium HNNU0524. Nat. Prod. Res. 2024, 38, 1711–1718. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Y.; He, J.; Hong, B.; Shao, Z.; Yu, M.; Niu, S. Retrobisabolane A, a novel bisabolane-derived sesquiterpenoid isolated from deep-sea-derived fungus Retroconis fusiformis MCCC 3A00792. Chem. Biodivers. 2024, 21, e202400805. [Google Scholar] [CrossRef]
- Xiao, Z.; Cai, J.; Chen, T.; Wang, Y.; Chen, Y.; Zhu, Y.; Chen, C.; Yang, B.; Zhou, X.; Tao, H. Two new sesquiterpenoids and a new shikimic acid metabolite from mangrove sediment-derived fungus Roussoella sp. SCSIO 41427. Mar. Drugs 2024, 22, 103. [Google Scholar] [CrossRef]
- Guo, X.; Meng, Q.; Niu, S.; Liu, J.; Guo, X.; Sun, Z.; Liu, D.; Gu, Y.; Huang, J.; Fan, A.; et al. Epigenetic manipulation to trigger production of guaiane-type sesquiterpenes from a marine-derived Spiromastix sp. fungus with antineuroinflammatory effects. J. Nat. Prod. 2021, 84, 1993–2003. [Google Scholar] [CrossRef]
- Yang, S.Q.; Song, Q.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. Antimicrobial polyketides and sesquiterpene lactones from the deep-sea cold-seep-derived fungus Talaromyces minioluteus CS-113 triggered by the histone deacetylase inhibitor SAHA. Org. Biomol. Chem. 2023, 21, 2575–2585. [Google Scholar] [CrossRef]
- Gu, T.; Cai, J.; Xie, D.; She, J.; Liu, Y.; Zhou, X.; Tang, L. New sesquiterpenoids from the mangrove-derived fungus Talaromyces sp. as modulators of nuclear receptors. Mar. Drugs 2024, 22, 403. [Google Scholar] [CrossRef]
- Safwan, S.; Wang, S.W.; Hsiao, G.; Hsiao, S.W.; Hsu, S.J.; Lee, T.H.; Lee, C.K. New trichothecenes isolated from the marine algicolous fungus Trichoderma brevicompactum. Mar. Drugs 2022, 20, 80. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Liu, X.H.; Li, X.N.; Ji, N.Y. Antifungal and antimicroalgal trichothecene sesquiterpenes from the marine algicolous fungus Trichoderma brevicompactum A-DL-9-2. J. Agric. Food Chem. 2020, 68, 15440–15448. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Liu, X.H.; Song, Y.P.; Yin, X.L.; Ji, N.Y. Sesquiterpenoids and a steroid from the algicolous Trichoderma brevicompactum. Fitoterapia 2021, 153, 104983. [Google Scholar] [CrossRef]
- Liu, X.-H.; Song, Y.-P.; Wang, B.-G.; Ji, N.-Y. Sesquiterpenes and lipids from the algicolous fungus Trichoderma atroviride RR-dl-3-9. Phytochem. Lett. 2021, 45, 6–12. [Google Scholar] [CrossRef]
- Zou, J.X.; Song, Y.P.; Liu, X.H.; Li, X.N.; Ji, N.Y. Bisabolane, cadinane, and cyclonerane sesquiterpenes from an algicolous strain of Trichoderma asperelloides. Bioorg Chem. 2021, 115, 105223. [Google Scholar] [CrossRef]
- Li, H.L.; Li, X.M.; Ying, Z.; Li, Y.H.; Wang, B.G. Bisabolane sesquiterpene and cyclopentene derivatives from the marine algal-derived endophytic fungus Trichoderma asperellum EN-764. Phytochemistry 2023, 210, 113644. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.M.; Yang, S.Q.; Zhang, F.Z.; Wang, B.G.; Li, H.L.; Meng, L.H. Sesquiterpene and sorbicillinoid glycosides from the endophytic fungus Trichoderma longibrachiatum EN-586 derived from the marine red alga Laurencia obtusa. Mar. Drugs 2022, 20, 177. [Google Scholar] [CrossRef]
- Liu, K.; Shi, D.-L.; Gao, W.; Sun, C.; Wang, B.-C. A new norsesquiterpene, nor-bisabolan-1,11-diol, from marine- derived fungus Trichoderma atroviride TD-8. Rec. Nat. Prod. 2023, 17, 1069–1073. [Google Scholar]
- Liu, Y.; Qi, L.; Xu, M.; Li, W.; Liu, N.; He, X.; Zhang, Y. Anti-agrobacterium tumefactions sesquiterpene derivatives from the marine-derived fungus Trichoderma effusum. Front. Microbiol. 2024, 15, 1446283. [Google Scholar] [CrossRef]
- Qin, C.; Hu, Z.; Xiong, Y.; Chen, M.; Li, C.; Ding, W. A new sesquiterpene derivative from the mangrove endophytic fungus Trichoderma harzianum (strain No. R1). Chem. Nat. Compd. 2021, 57, 312–314. [Google Scholar] [CrossRef]
- Ma, X.-Y.; Song, Y.-P.; Shi, Z.-Z.; Ji, N.-Y. Three sesquiterpenes from the marine-alga-epiphytic fungus Trichoderma hamatum Z36-7. Phytochem. Lett. 2021, 43, 98–102. [Google Scholar] [CrossRef]
- Du, F.Y.; Ju, G.L.; Xiao, L.; Zhou, Y.M.; Wu, X. Sesquiterpenes and cyclodepsipeptides from marine-derived fungus Trichoderma longibrachiatum and their antagonistic activities against soil-borne pathogens. Mar. Drugs 2020, 18, 165. [Google Scholar] [CrossRef]
- Cui, J.; Shang, R.Y.; Sun, M.; Li, Y.X.; Liu, H.Y.; Lin, H.W.; Jiao, W.H. Trichodermaloids A-C, cadinane sesquiterpenes from a marine sponge symbiotic Trichoderma sp. SM16 fungus. Chem. Biodivers. 2020, 17, e2000036. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Hu, Z.; Wang, L. Novel sesquiterpene and diterpene aminoglycosides from the deep-sea-sediment fungus Trichoderma sp. SCSIOW21. Mar. Drugs 2023, 21, 7. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.P.; Wang, R.Q.; Jiao, W.H.; Zhang, X.X. A new sesquiterpenoid from Trichoderma virens, a marine sponge symbiotic Trichoderma sp. fungus. Chin. Trad. Herb. Drug 2023, 54, 5840–5845. [Google Scholar]
- Shi, T.; Shao, C.L.; Liu, Y.; Zhao, D.L.; Cao, F.; Fu, X.M.; Yu, J.Y.; Wu, J.S.; Zhang, Z.K.; Wang, C.Y. Terpenoids from the coral-derived fungus Trichoderma harzianum (XS-20090075) induced by chemical epigenetic manipulation. Front. Microbiol. 2020, 11, 572. [Google Scholar] [CrossRef]
- Guo, Y.W.; Gong, B.Q.; Yuan, J.; Li, H.J.; Mahmud, T.; Huang, Y.; Li, J.F.; Yang, D.P.; Lan, W.J. L-Phenylalanine alters the privileged secondary metabolite production in the marine-derived fungus Trichoderma erinaceum F1-1. J. Nat. Prod. 2020, 83, 79–87. [Google Scholar] [CrossRef]
- Yang, W.; Tian, S.; Du, Y.F.; Zeng, X.L.; Liang, J.J.; Lan, W.J.; Li, H. Genome mining of the marine-derived fungus Trichoderma erinaceum F1-1 unearths bergamotene-type sesquiterpenoids. J. Nat. Prod. 2024, 87, 2746–2756. [Google Scholar] [CrossRef]
- Yoiprommarat, S.; Pruksatrakul, T.; Surawatanawong, P.; Srichomthong, K.; Unagul, P.; Klaysuban, A.; Suetrong, S.; Bunyapaiboonsri, T. Eremophilanolide sulfoxides and eremophilanolides from the mangrove fungus TBRC-BCC 64093. Tetrahedron 2023, 136, 133354. [Google Scholar] [CrossRef]
- Qiu, P.; Xia, J.; Zhang, H.; Lin, D.; Shao, Z. A review of diterpenes from marine-derived fungi: 2009–2021. Molecules 2022, 27, 8303. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; He, L.M.; Xue, Y.Q.; Jia, J.; Wang, S.B.; Zhu, K.K.; Hong, K.; Cai, Y.S. A new fusicoccane-type norditerpene and a new indone from the marine-derived fungus Aspergillus aculeatinus WHUF0198. Chem. Biodivers. 2021, 18, e2100562. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, G.; Heard, S.C.; Niu, C.; Bell, S.A.; Li, F.; Ye, Y.; Zhang, Y.; Winter, J.M. Identification and characterization of a cryptic bifunctional type I diterpene synthase involved in talaronoid biosynthesis from a marine-derived fungus. Org. Lett. 2022, 24, 7037–7041. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Sun, Z.C.; Meng, L.H.; Wang, B.G. Secondary metabolites with agricultural antagonistic potentials from Beauveria felina, a marine-derived entomopathogenic fungus. J. Agric. Food Chem. 2020, 68, 14824–14831. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Y.; Li, S.; Wang, Q.; Hu, C.; Liu, H.; Zhang, W. Bioactive metabolites from the deep-sea-derived fungus Diaporthe longicolla FS429. Mar. Drugs 2020, 18, 381. [Google Scholar] [CrossRef]
- Ning, Y.; Zhang, S.; Zheng, T.; Xu, Y.; Li, S.; Zhang, J.; Jiao, B.; Zhang, Y.; Ma, Z.; Lu, X. Pimarane-type diterpenes with anti-inflammatory activity from arctic-derived fungus Eutypella sp. D-1. Mar. Drugs 2023, 21, 541. [Google Scholar] [CrossRef]
- He, J.; Zou, Q.; Deng, H.; He, S.; Yan, D.; Pan, K.; Zhou, Y.; Zhao, Z.; Cui, H.; Liu, Y. Novel 6/7/6 ring system diterpenoids and cytochalasins from the fungus Eutypella scoparia GZU-4-19Y and their anti-inflammatory activity. Fitoterapia 2024, 173, 105804. [Google Scholar] [CrossRef]
- Hou, B.; Liu, S.; Huo, R.; Li, Y.; Ren, J.; Wang, W.; Wei, T.; Jiang, X.; Yin, W.; Liu, H.; et al. New diterpenoids and isocoumarin derivatives from the mangrove-derived fungus Hypoxylon sp. Mar. Drugs 2021, 19, 362. [Google Scholar]
- Hu, J.; Zhang, W.; Tan, H.; Li, S.; Gao, X.; Liu, Z.; Wang, Y.; Liu, H.; Zhang, W. Neocucurbins A-G, novel macrocyclic diterpenes and their derivatives from Neocucurbitaria unguis-hominis FS685. Org. Biomol. Chem. 2022, 20, 4376–4384. [Google Scholar] [CrossRef]
- Hu, J.; Zou, Z.; Chen, Y.; Li, S.; Gao, X.; Liu, Z.; Wang, Y.; Liu, H.; Zhang, W. Neocucurbols A-H, phomactin diterpene derivatives from the marine-derived fungus Neocucurbitaria unguis-hominis FS685. J. Nat. Prod. 2022, 85, 1967–1975. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Li, S.; Chen, Y.; Ye, W.; Li, H.; Tan, H.; Li, D.; Liu, Z.; Zhang, W. Hawanoids A–E, unprecedented diterpenoids with PAF-induced platelet aggregation inhibitory activities from the deep-sea-derived fungus Paraconiothyrium hawaiiense. Chinese Chem. Lett. 2023, 34, 107513. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, W.; Fan, R.; Han, S.; Li, Y.; Cui, X.; Zhang, J.; Wu, Y.; Lv, X.; Zhang, Y.; et al. Terpenoids from the deep-sea-derived fungus Penicillium thomii YPGA3 and their bioactivities. Mar. Drugs 2020, 18, 164. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Han, S.; Zhang, J.; Xu, W.; Li, Q.; Cheng, Z. Penitholabene, a rare 19-nor labdane-type diterpenoid from the deep-sea-derived fungus Penicillium thomii YPGA3. Fitoterapia 2020, 146, 104691. [Google Scholar] [CrossRef]
- Li, F.; Sun, W.; Zhang, S.; Gao, W.; Lin, S.; Yang, B.; Chai, C.; Li, H.; Wang, J.; Hu, Z.; et al. New cyclopiane diterpenes with anti-inflammatory activity from the sea sediment-derived fungus Penicillium sp. TJ403-2. Chinese Chem. Lett. 2020, 31, 197–201. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, W.; Xu, Z.; Bai, Q.; Zhou, X.; Zheng, C.; Bai, M.; Chen, G. Biological secondary metabolites from the Lumnitzera littorea-derived fungus Penicillium oxalicum HLLG-13. Mar. Drugs 2022, 21, 22. [Google Scholar] [CrossRef]
- Zhao, M.; Ruan, Q.; Pan, W.; Tang, Y.; Zhao, Z.; Cui, H. New polyketides and diterpenoid derivatives from the fungus Penicillium sclerotiorum GZU-XW03-2 and their anti-inflammatory activity. Fitoterapia 2020, 143, 104561. [Google Scholar] [CrossRef]
- de Amorim, M.R.; Barbosa, C.S.; Paz, T.A.; Ioca, L.P.; Nicacio, K.J.; de Oliveira, L.F.P.; Goulart, M.O.; Paulino, J.M.; da Cruz, M.O.; Ferreira, A.G.; et al. Polyketide- and terpenoid-derived metabolites produced by a marine-derived fungus, Peroneutypa sp. J. Nat. Prod. 2023, 86, 1476–1486. [Google Scholar] [CrossRef]
- Wang, T.; Feng, Y.; Huang, J.; Wu, S.; Hu, K.; Wu, J.; Naman, C.B.; Wang, H.; Lin, W.; He, S. Pestanoid A, a rearranged pimarane diterpenoid osteoclastogenesis inhibitor from a marine mesophotic zone chalinidae sponge-associated fungus, Pestalotiopsis sp. NBUF145. J. Nat. Prod. 2024, 87, 160–165. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, B.; Zhu, A.; Liu, S.H.; Wu, R.; Zhang, X.; Xu, Z.; Tan, R.X.; Ge, H.M. Biosynthesis of phomactin platelet activating factor antagonist requires a two-enzyme cascade. Angew. Chem. Int. Ed. Engl. 2023, 62, e202312996. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, Y.; Li, Z.; Liu, X.; Chu, Y.; She, Z.; Kang, W.; Chen, Y. Pleosmaranes A-R, isopimarane and 20-nor isopimarane diterpenoids with anti-inflammatory activities from the mangrove endophytic fungus Pleosporales sp. HNQQJ-1. J. Nat. Prod. 2024, 87, 304–314. [Google Scholar] [CrossRef]
- Lin, S.; Chai, Z.; Zeng, H.; Yang, B.; Chi, J.; Zhang, Y.; Hu, Z. Atranones and dolabellanes with cardiomyocyte protective activity against cold ischemic injury from a coral-associated fungus Stachybotrys chartarum. Phytochemistry 2024, 225, 114199. [Google Scholar] [CrossRef]
- Lin, S.; Zeng, H.; Wang, C.; Chai, Z.; Zhang, X.; Yang, B.; Chi, J.; Zhang, Y.; Hu, Z. Discovery of novel natural cardiomyocyte protectants from a toxigenic fungus Stachybotrys chartarum. Bioorg Chem. 2024, 148, 107461. [Google Scholar] [CrossRef]
- Meng, L.H.; Li, X.M.; Zhang, F.Z.; Wang, Y.N.; Wang, B.G. Talascortenes A-G, highly oxygenated diterpenoid acids from the sea-anemone-derived endozoic fungus Talaromyces scorteus AS-242. J. Nat. Prod. 2020, 83, 2528–2536. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.M.; Song, N.; Wang, B.G.; Li, H.L.; Meng, L.H. Secondary metabolites with fungicide potentials from the deep-sea seamount-derived fungus Talaromyces scorteus AS-242. Bioorg Chem. 2024, 147, 107417. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, C.; Che, Q.; Zhu, T.; Zhang, G.; Li, D. Extending the structural diversity of labdane diterpenoids from marine-derived fungus Talaromyces sp. HDN151403 using heterologous expression. Mar. Drugs 2023, 21, 628. [Google Scholar] [CrossRef]
- Peng, Z.H.; Jia, H.; Luo, Y.L.; Zhang, L.J.; Zhou, J.T.; Xie, Y.H.; Wang, L.J.; Qin, J.K.; Li, J.; Zhang, G.H.; et al. Talaroterpenoids A-F: Six new seco-terpenoids from the marine-derived fungus Talaromyces aurantiacus. Mar. Drugs 2024, 22, 475. [Google Scholar] [CrossRef]
- Wang, G.; Wu, J.; Li, Z.; Chen, T.; Liu, Y.; Wang, B.; Chen, Y.; She, Z. Talaroacids A-D and talaromarane A, diterpenoids with anti-inflammatory activities from mangrove endophytic fungus Talaromyces sp. JNQQJ-4. Int. J. Mol. Sci. 2024, 25, 6691. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, X.; Hu, Z.; Wang, L. Novel harziane diterpenes from deep-sea sediment fungus Trichoderma sp. SCSIOW21 and their potential anti-inflammatory effects. Mar. Drugs 2021, 19, 689. [Google Scholar] [CrossRef]
- Zou, J.X.; Song, Y.P.; Zeng, Z.Q.; Ji, N.Y. Proharziane and harziane derivatives from the marine algicolous fungus Trichoderma asperelloides RR-dl-6-11. J. Nat. Prod. 2021, 84, 1414–1419. [Google Scholar] [CrossRef]
- Shi, Z.Z.; Yin, X.L.; Song, Y.P.; Ji, N.Y. A new harziane diterpene, harziaketal A, and a new sterol, trichosterol A, from the marine-alga-epiphytic Trichoderma sp. Z43. Chem. Biodivers. 2023, 20, e202301099. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Chen, Y.; He, Y.; She, J.; Zhou, X.; Tao, H.; Peng, B. Arthproliferins A-D, four new sesterterpenes from the mangrove-sediment-derived fungus Arthrinium sp. SCSIO41221. Molecules 2023, 28, 7246. [Google Scholar] [CrossRef]
- Chi, L.P.; Li, X.M.; Wan, Y.P.; Li, X.; Wang, B.G. Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512. J. Nat. Prod. 2020, 83, 3652–3660. [Google Scholar] [CrossRef]
- Ding, W.; Uvarani, C.; Wang, F.; Xue, Y.; Wu, N.; He, L.; Tian, D.; Chen, M.; Zhang, Y.; Hong, K.; et al. New ophiobolins from the deep-sea derived fungus Aspergillus sp. WHU0154 and their anti-inflammatory effects. Mar. Drugs 2020, 18, 575. [Google Scholar] [CrossRef]
- Li, X.D.; Li, X.M.; Wang, B.G.; Li, X. Antimicrobial sesterterpenoids with a unique 5/8/6/5 tetracyclic carbon-ring-system and diepoxide polyketides from a deep sea-sediment-sourced fungus Chaetomium globosum SD-347. Org. Biomol. Chem. 2024, 22, 3979–3985. [Google Scholar] [CrossRef]
- Fang, S.T.; Miao, F.P.; Yin, X.L.; Ji, N.Y. A new lanostane-type triterpenoid from the marine shellfish symbiotic fungus Ceriporia lacerata CD7-5. Nat. Prod. Res. 2024, 38, 1510–1516. [Google Scholar] [CrossRef]
- Hu, J.; Wang, N.; Liu, H.; Li, S.; Liu, Z.; Zhang, W.; Gao, X. Secondary metabolites from a deep-sea derived fungal strain of Phomopsis lithocarpus FS508. Nat. Prod. Res. 2023, 37, 2351–2358. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, X.; Yao, F.H.; Liu, X.B.; Qi, S.H. Fusidane-type antibiotics from the marine-derived fungus Simplicillium sp. SCSIO 41513. J. Nat. Prod. 2021, 84, 2945–2952. [Google Scholar] [CrossRef]
- Kwon, H.; Jin, B.R.; Yoo, S.; Kim, H.J.; Hwang, B.Y.; Guo, Y.; Yim, J.H.; Kim, I.C.; Shim, S.H.; An, H.J.; et al. New fusidane-type nortriterpenoids from the Arctic marine-derived fungus Simplicillium lamellicola culture medium with their inhibitory effect on benign prostatic hyperplasia. Bioorg Chem. 2024, 143, 107070. [Google Scholar] [CrossRef]
- Li, T.; Jiang, S.; Dai, Y.; Wu, X.; Guo, H.; Shi, L.; Sang, X.; Ren, L.; Wang, J.; Shi, L.; et al. Total synthesis and target identification of marine cyclopiane diterpenes. Nat. Commun. 2024, 15, 10851. [Google Scholar] [CrossRef]
- Wang, D.G.; Hu, J.Q.; Wang, C.Y.; Liu, T.; Li, Y.Z.; Wu, C. Exploring microbial natural products through NMR-based metabolomics. Nat. Prod. Rep. 2025, in press. [CrossRef]
- Jiang, M.; Chen, S.; Lu, X.; Guo, H.; Chen, S.; Yin, X.; Li, H.; Dai, G.; Liu, L. Integrating genomics and metabolomics for the targeted discovery of new cyclopeptides with antifungal activity from a marine-derived fungus Beauveria felina. J. Agric. Food Chem. 2023, 71, 9782–9795. [Google Scholar] [CrossRef]
- Caesar, L.K.; Montaser, R.; Keller, N.P.; Kelleher, N.L. Metabolomics and genomics in natural products research: Complementary tools for targeting new chemical entities. Nat. Prod. Rep. 2021, 38, 2041–2065. [Google Scholar] [CrossRef]


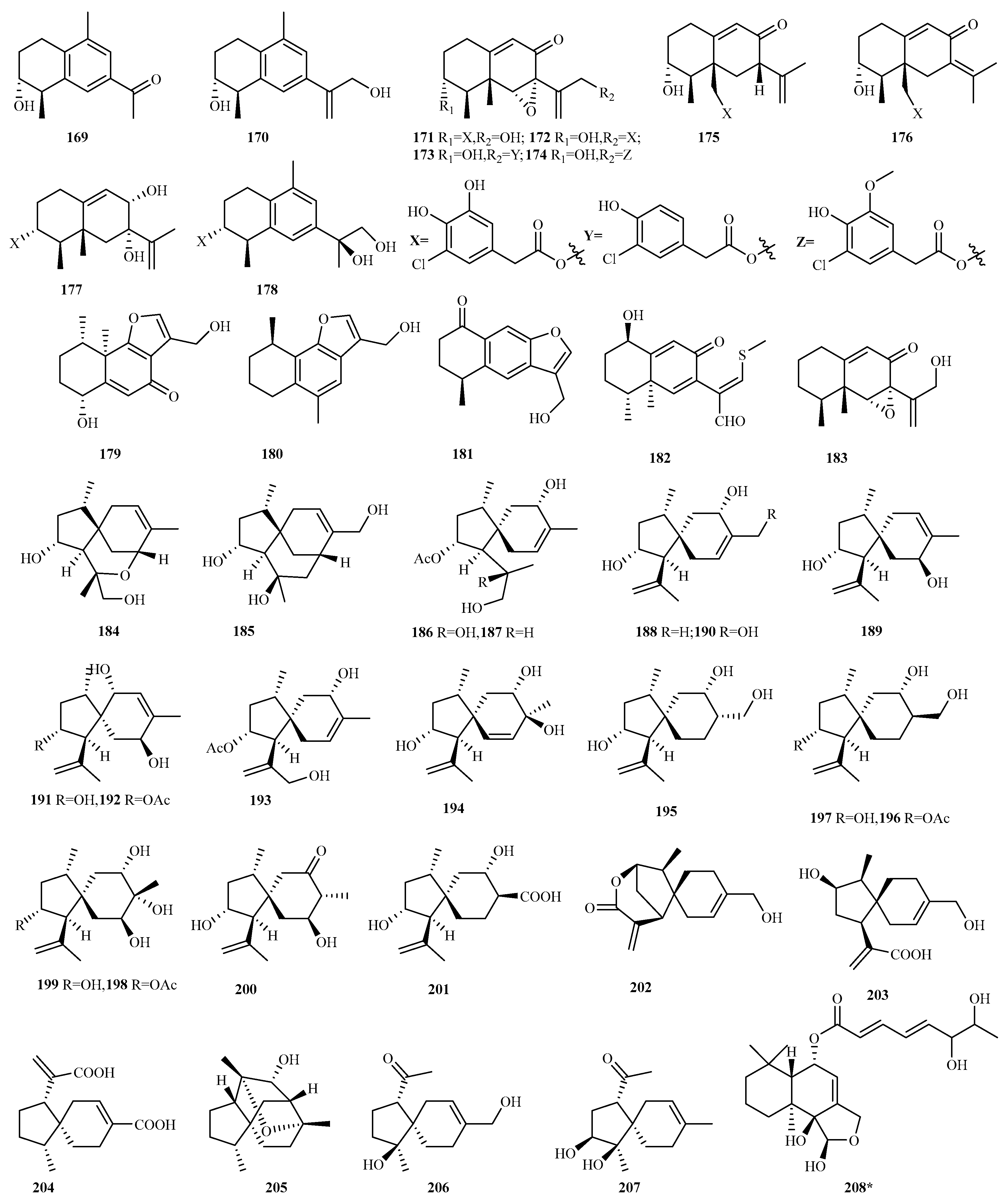



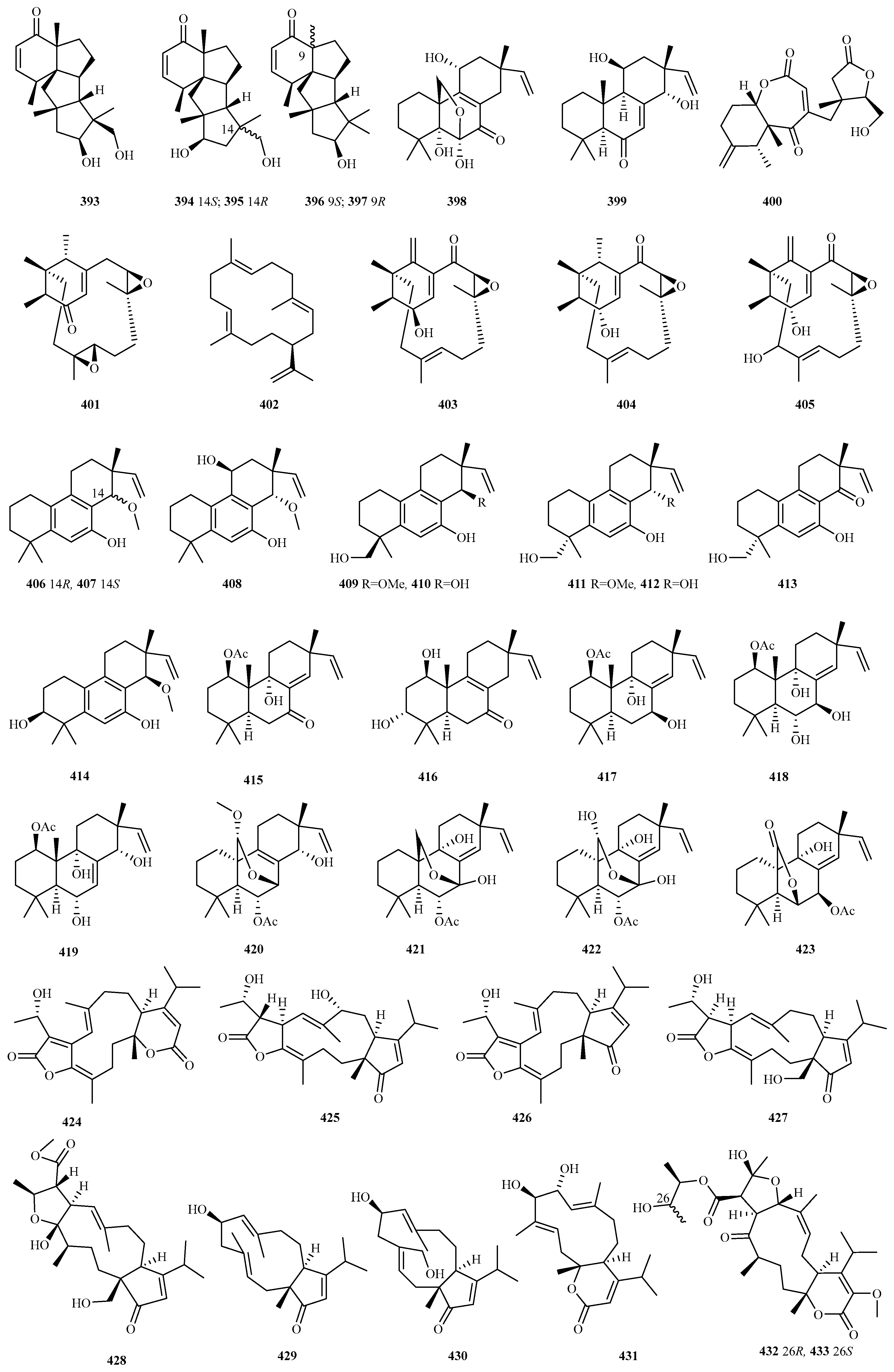
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Chen, S.; Zhang, Z.; Xiao, Y.; Zhu, D.; Liu, L. Structural Diversity and Bioactivities of Marine Fungal Terpenoids (2020–2024). Mar. Drugs 2025, 23, 300. https://doi.org/10.3390/md23080300
Jiang M, Chen S, Zhang Z, Xiao Y, Zhu D, Liu L. Structural Diversity and Bioactivities of Marine Fungal Terpenoids (2020–2024). Marine Drugs. 2025; 23(8):300. https://doi.org/10.3390/md23080300
Chicago/Turabian StyleJiang, Minghua, Senhua Chen, Zhibin Zhang, Yiwen Xiao, Du Zhu, and Lan Liu. 2025. "Structural Diversity and Bioactivities of Marine Fungal Terpenoids (2020–2024)" Marine Drugs 23, no. 8: 300. https://doi.org/10.3390/md23080300
APA StyleJiang, M., Chen, S., Zhang, Z., Xiao, Y., Zhu, D., & Liu, L. (2025). Structural Diversity and Bioactivities of Marine Fungal Terpenoids (2020–2024). Marine Drugs, 23(8), 300. https://doi.org/10.3390/md23080300






