Anthelmintic Potential of Agelasine Alkaloids from the Australian Marine Sponge Agelas axifera
Abstract
1. Introduction
2. Results and Discussion
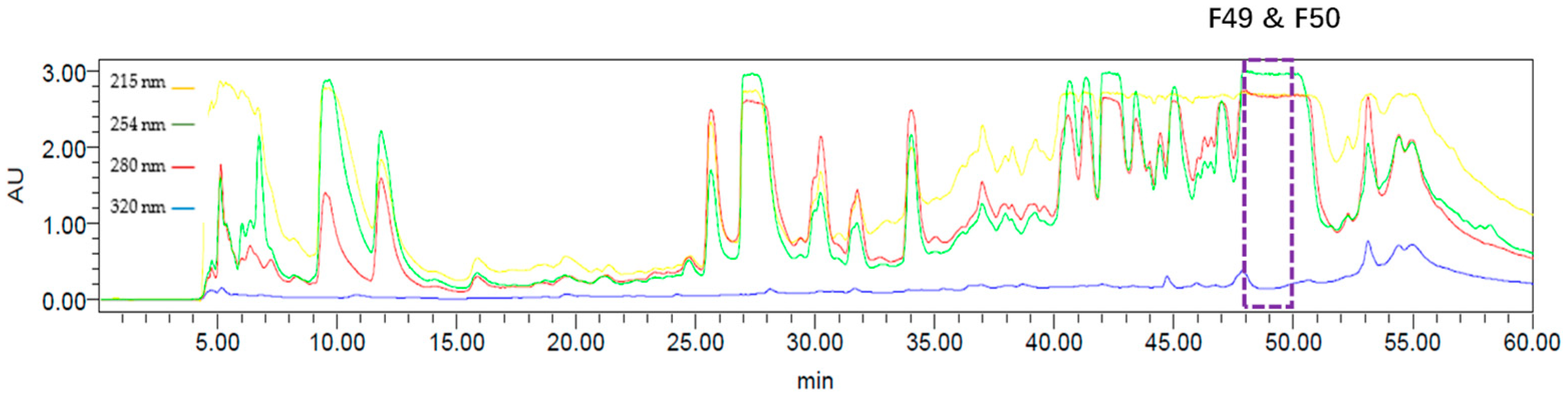
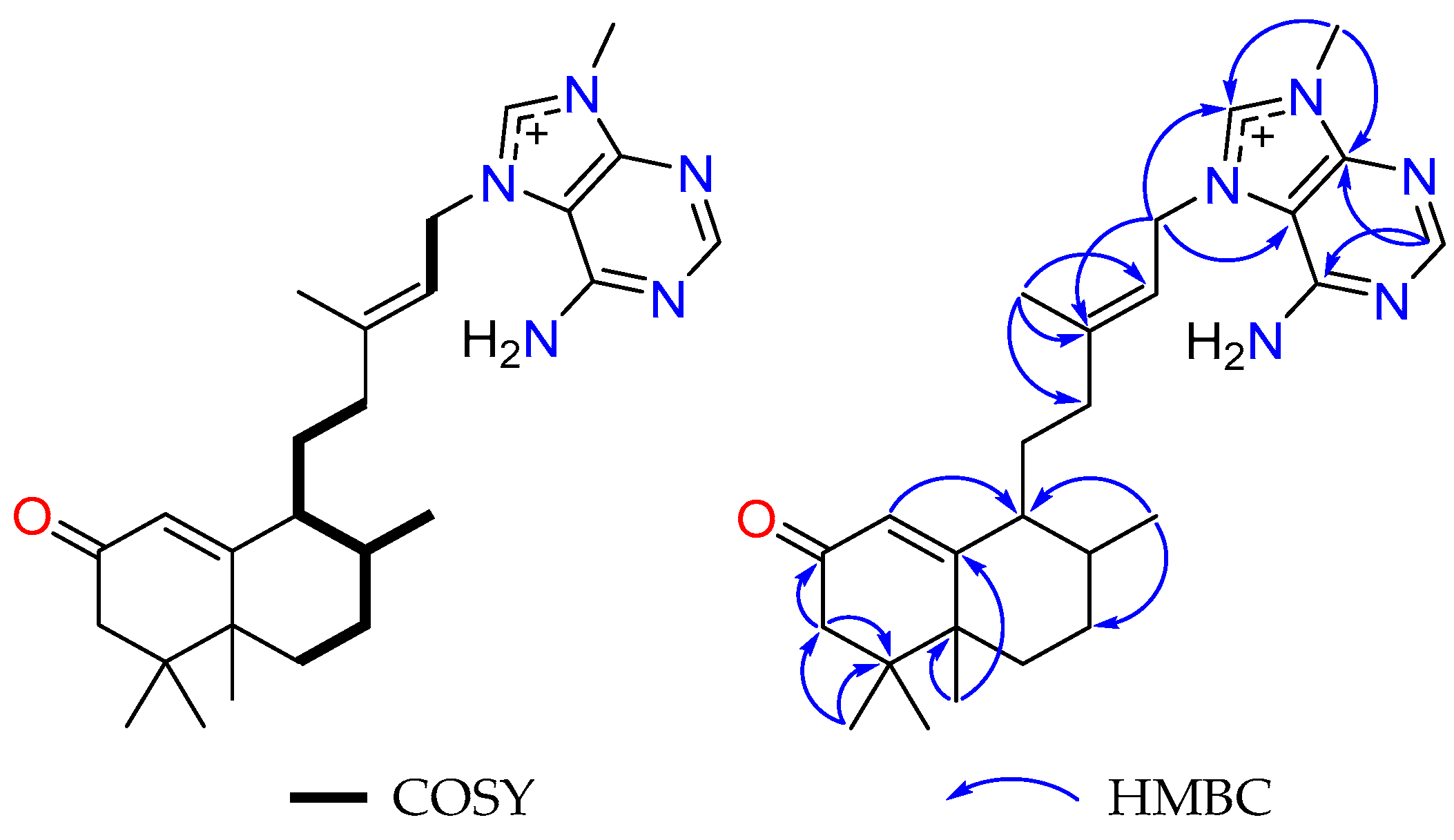
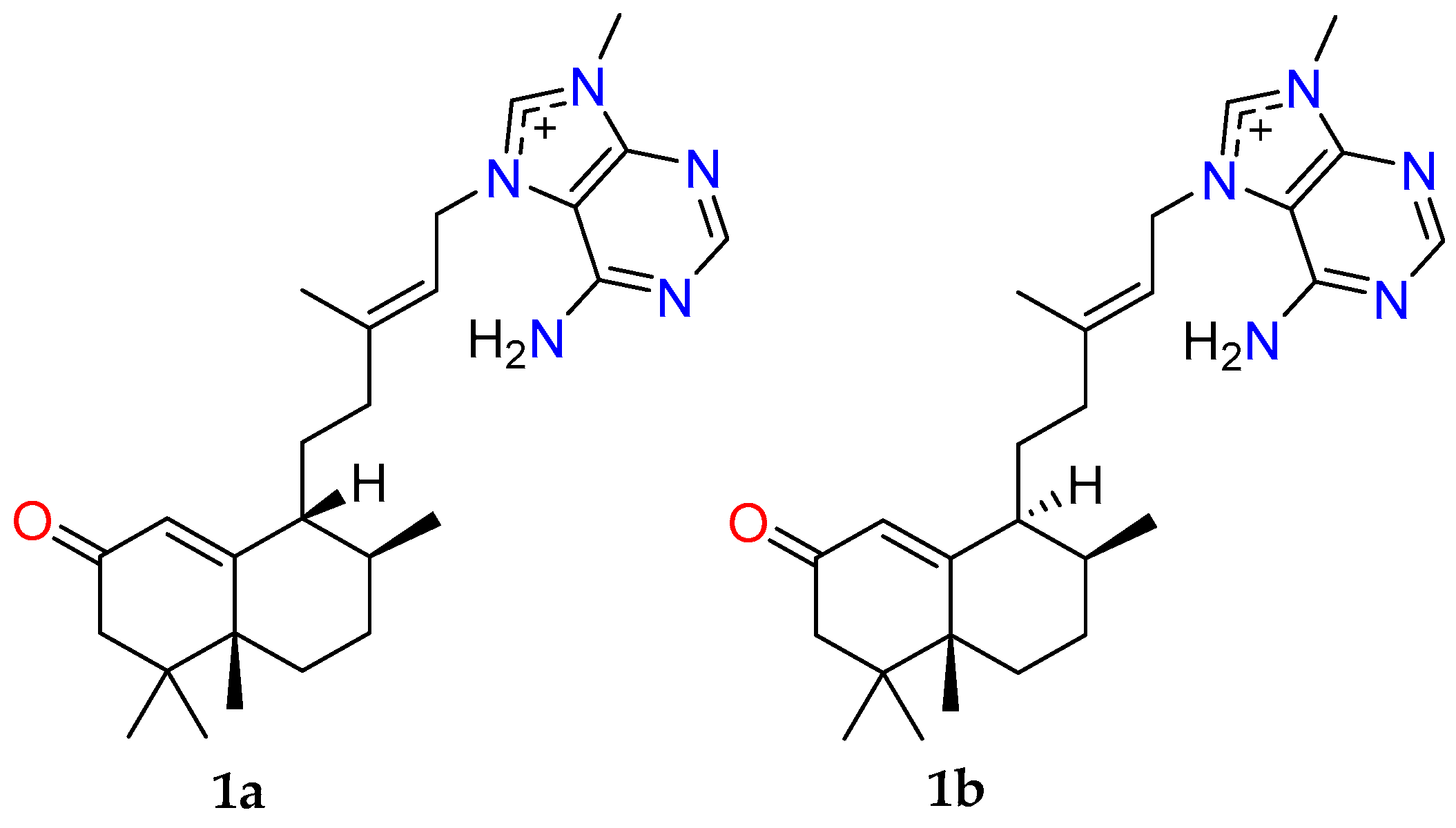
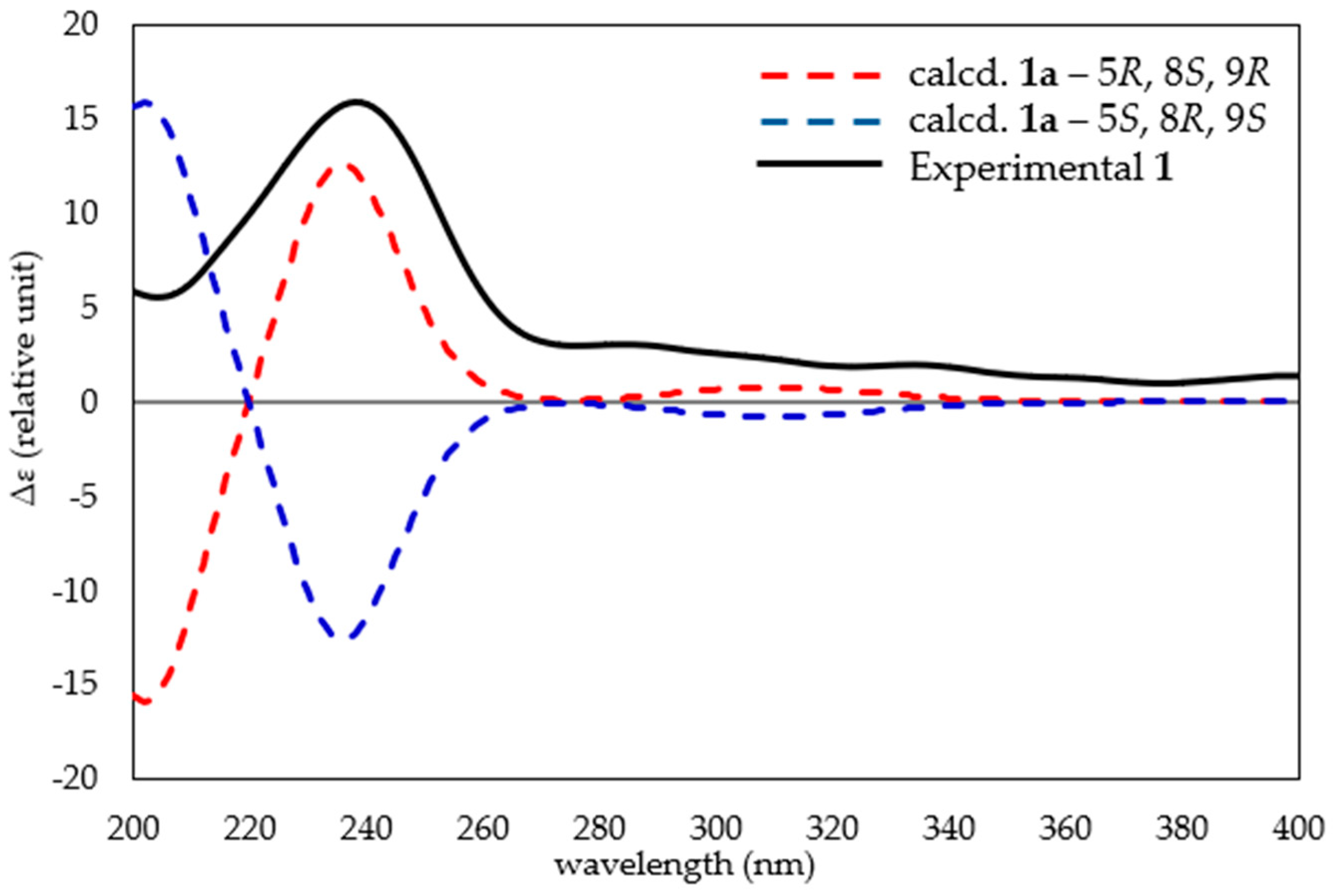
2.1. Bioassay-Guided Fractionation of Compounds from A. axifera
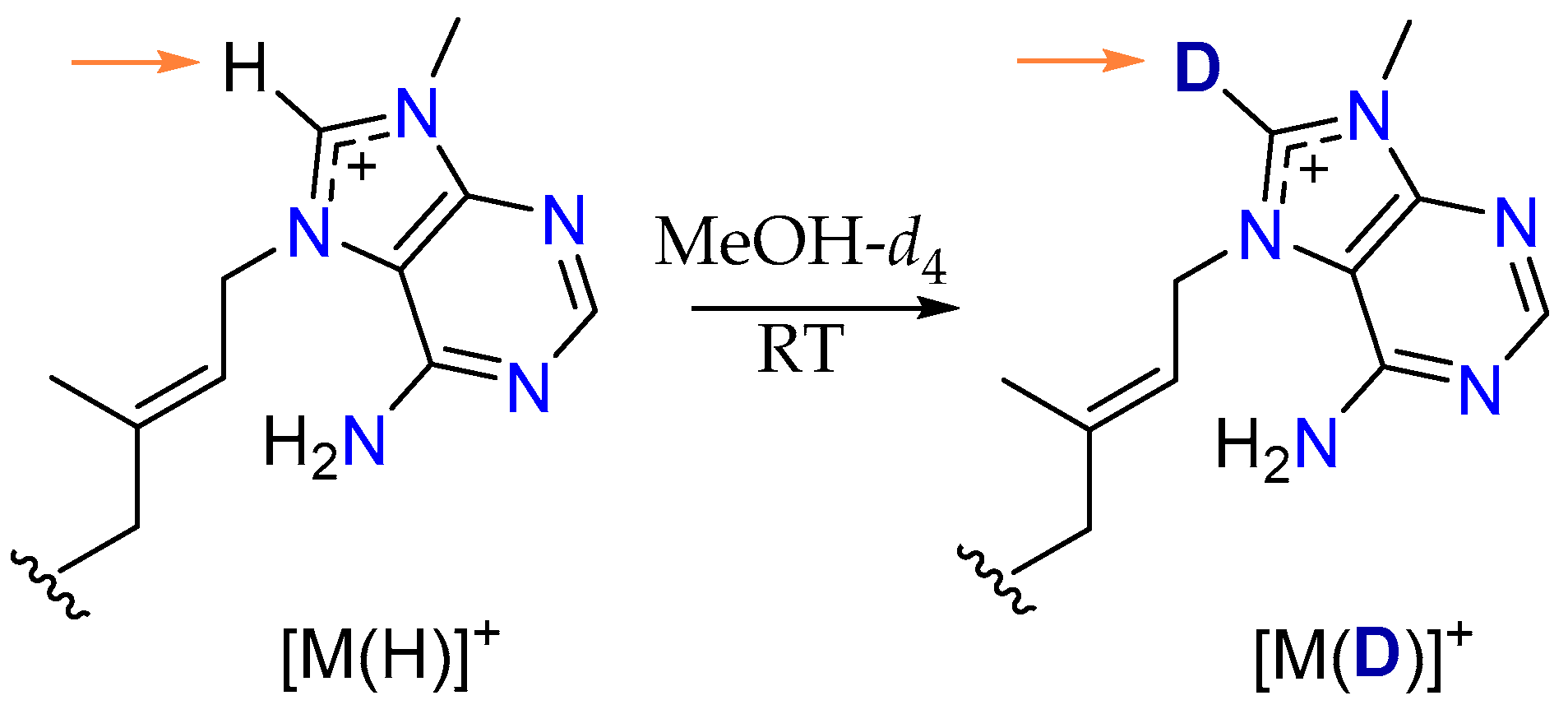
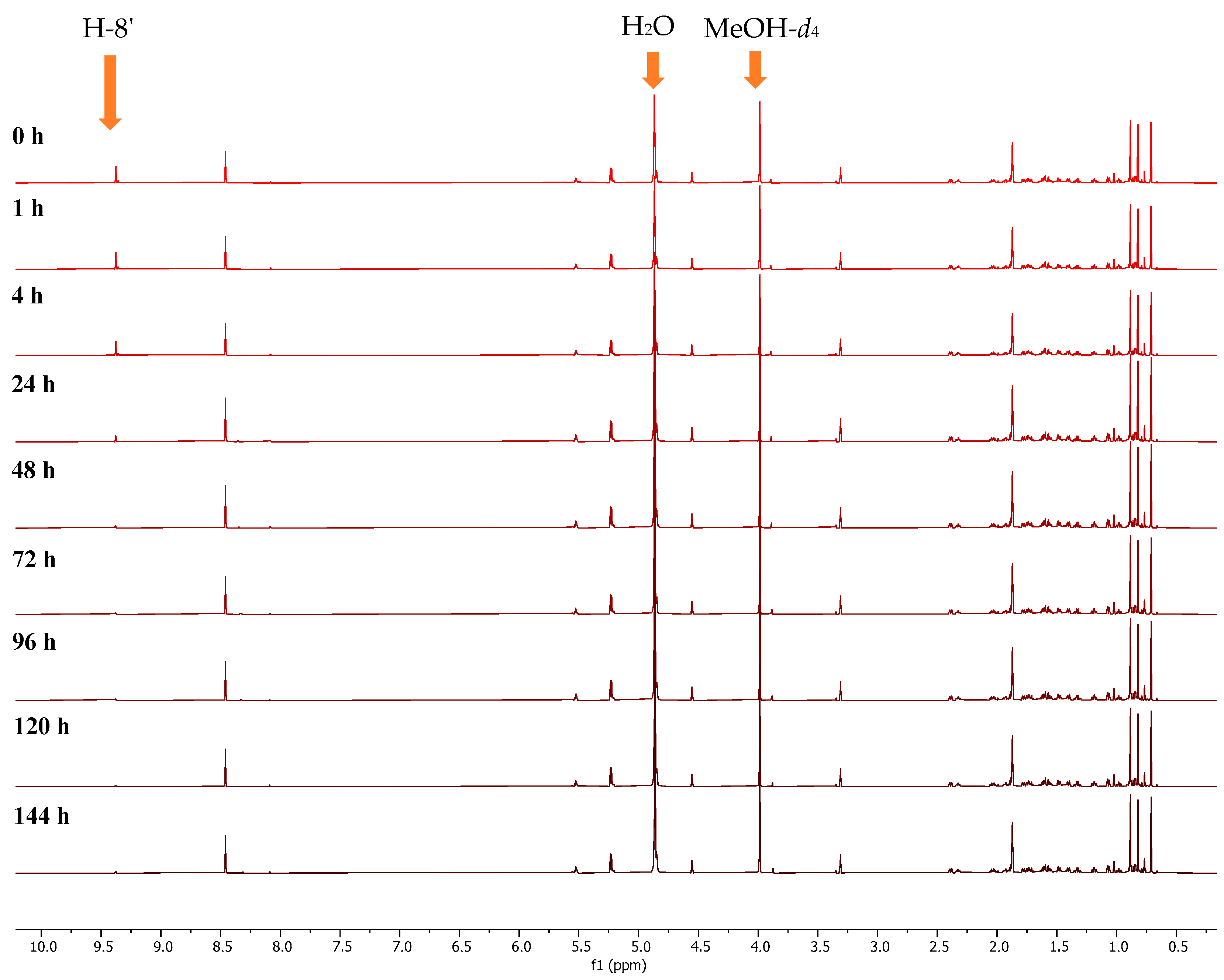
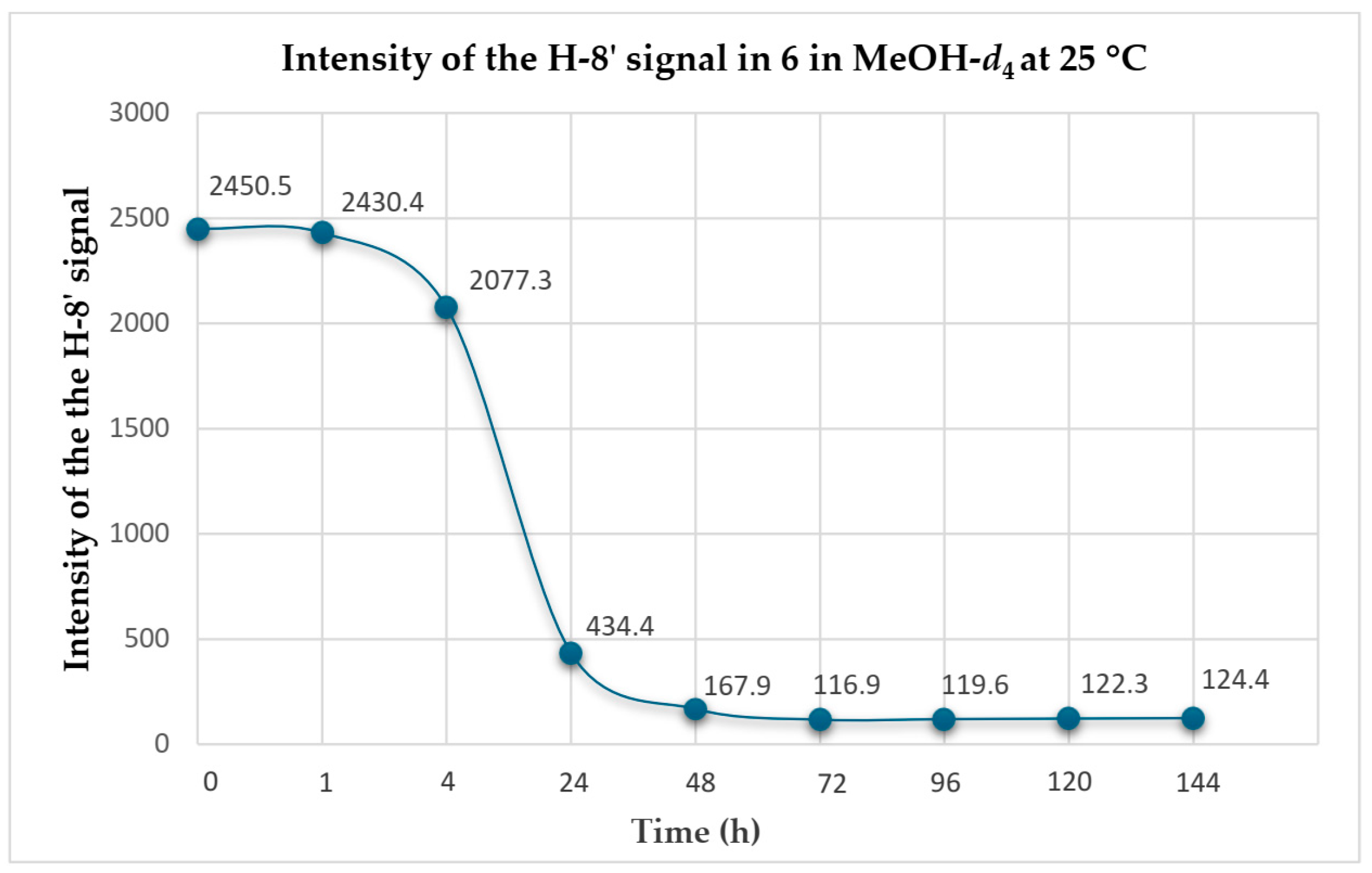
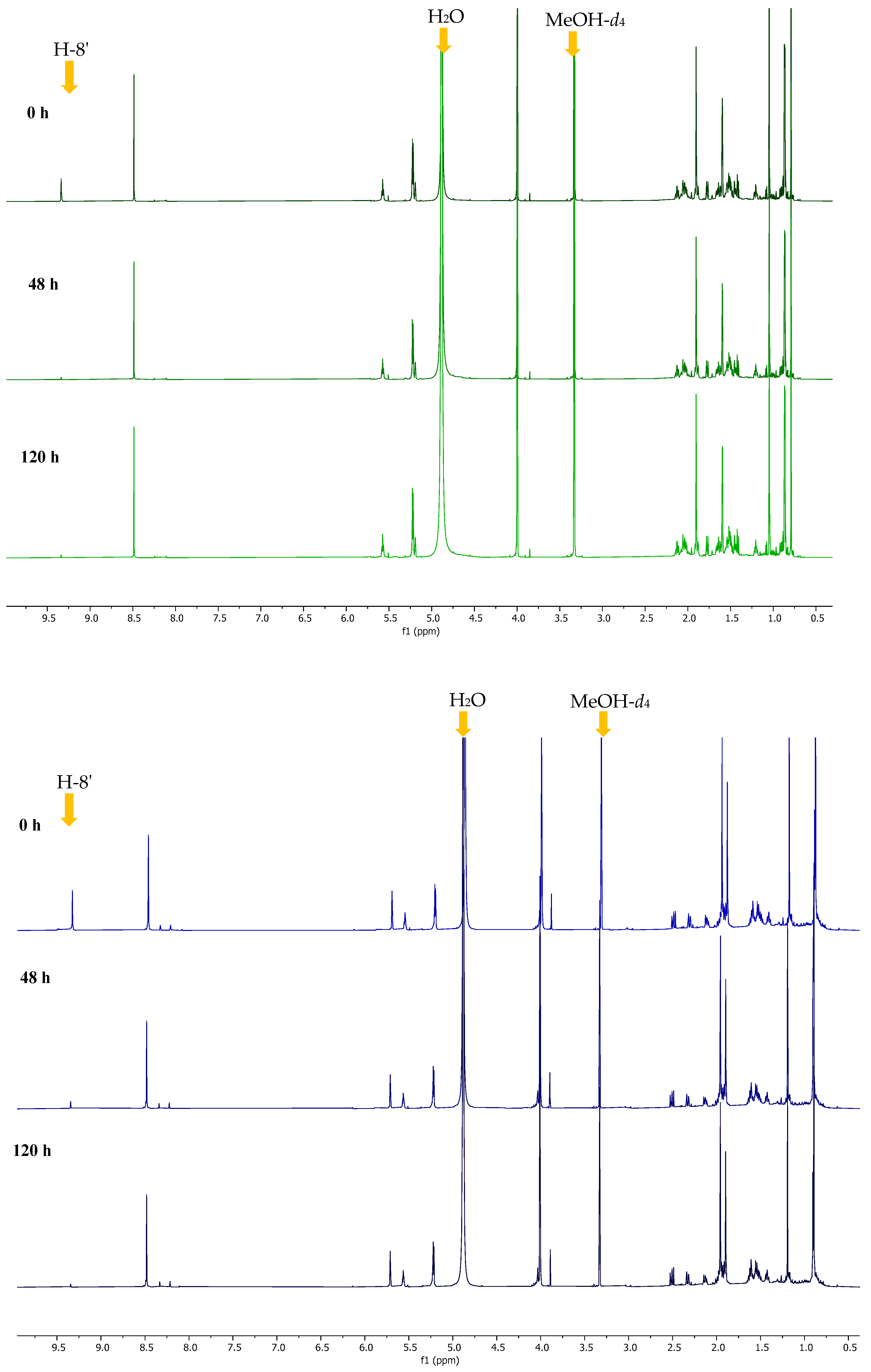
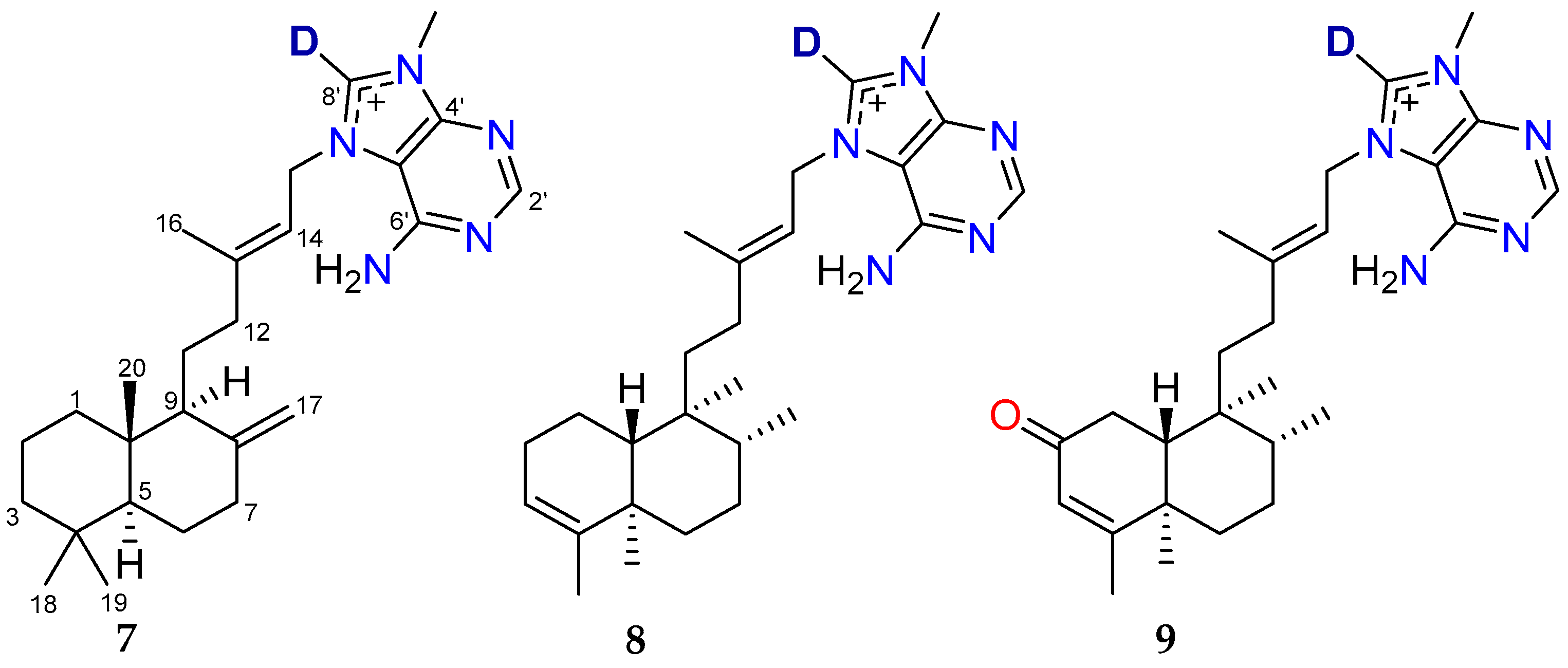
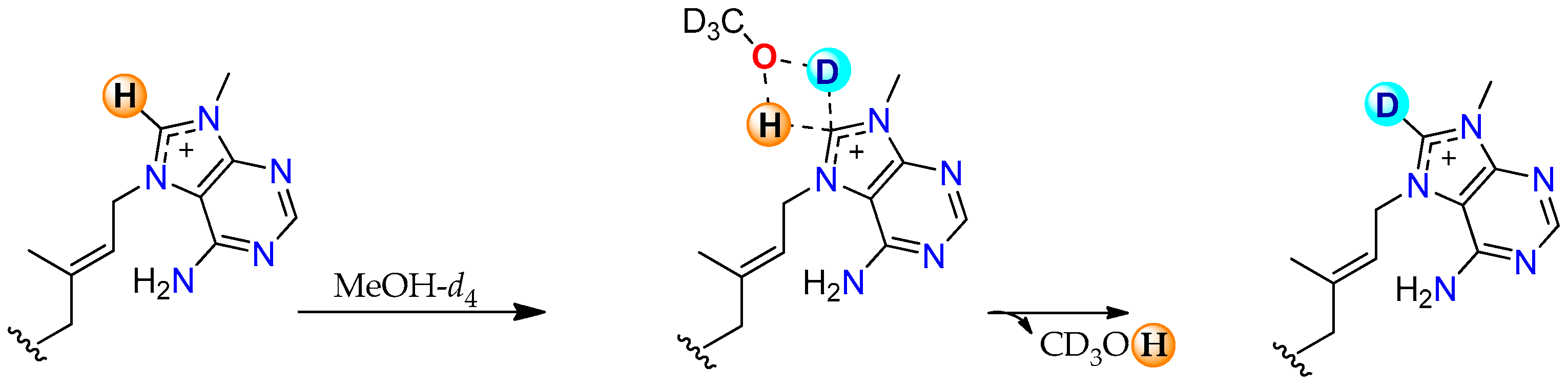
2.2. Conversion of H-8’ to D-8’ of Agelasines in MeOH-d4
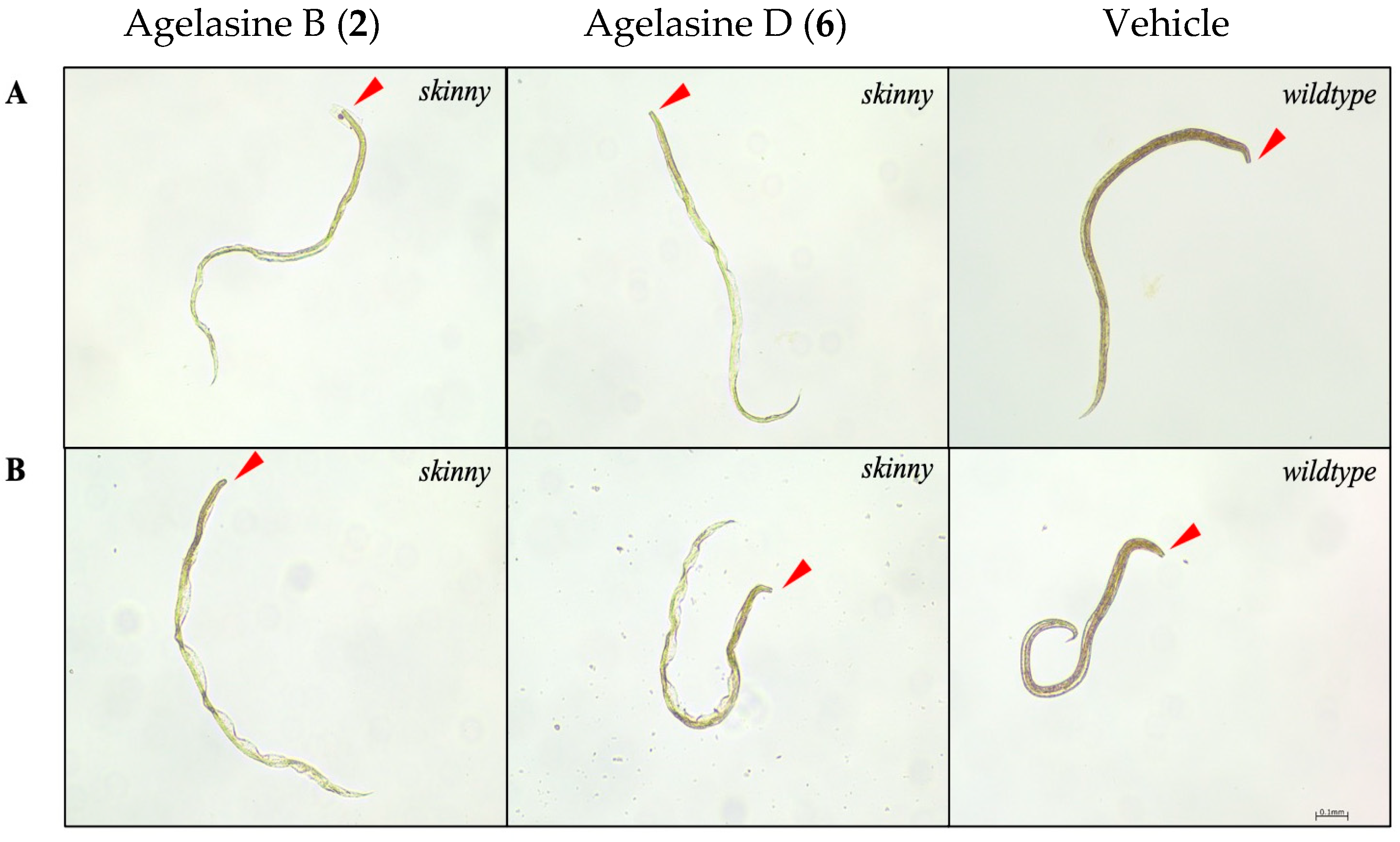
2.3. Anthelmintic Activity of Compounds 1–6
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Production and Maintenance of H. contortus for Bioassays
3.5. Production and Synchronization of C. elegans for Bioassays
3.6. Bioassay for Assessment of Anthelmintic Activity of A. axifera-Derived HPLC Fractions
3.7. Bioassay for Evaluation of Anthelmintic Activity of Pure Compounds Using Larvae of H. contortus or C. elegans
3.8. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charlier, J.; Bartley, D.; Sotiraki, S.; Martinez-Valladares, M.; Claerebout, E.; von Samson-Himmelstjerna, G.; Thamsborg, S.; Hoste, H.; Morgan, E.; Rinaldi, L. Anthelmintic resistance in ruminants: Challenges and solutions. Adv. Parasitol. 2022, 115, 171–227. [Google Scholar] [PubMed]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef]
- Sangster, N.C.; Cowling, A.; Woodgate, R.G. Ten events that defined anthelmintic resistance research. Trends Parasitol. 2018, 34, 553–563. [Google Scholar] [CrossRef]
- Kotze, A.; Prichard, R. Anthelmintic resistance in Haemonchus contortus: History, mechanisms and diagnosis. Adv. Parasitol. 2016, 93, 397–428. [Google Scholar] [PubMed]
- Herath, H.D.; Preston, S.; Jabbar, A.; Garcia-Bustos, J.; Taki, A.C.; Addison, R.S.; Hayes, S.; Beattie, K.D.; McGee, S.L.; Martin, S.D. Identification of fromiamycalin and halaminol A from Australian marine sponge extracts with anthelmintic activity against Haemonchus contortus. Mar. Drugs 2019, 17, 598. [Google Scholar] [CrossRef]
- Taki, A.C.; Brkljača, R.; Wang, T.; Koehler, A.V.; Ma, G.; Danne, J.; Ellis, S.; Hofmann, A.; Chang, B.C.H.; Jabbar, A.; et al. Natural Compounds from the Marine Brown Alga Caulocystis cephalornithos with Potent In Vitro-Activity against the Parasitic Nematode Haemonchus contortus. Pathogens 2020, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Herath, H.D.; Taki, A.C.; Sleebs, B.E.; Hofmann, A.; Nguyen, N.; Preston, S.; Davis, R.A.; Jabbar, A.; Gasser, R.B. Advances in the discovery and development of anthelmintics by harnessing natural product scaffolds. Adv. Parasitol. 2021, 111, 203–251. [Google Scholar]
- NatureBank. Available online: https://www.griffith.edu.au/institute-drug-discovery/unique-resources/naturebank (accessed on 31 March 2025).
- Taki, A.C.; Byrne, J.J.; Jabbar, A.; Lum, K.Y.; Hayes, S.; Addison, R.S.; Ramage, K.S.; Hofmann, A.; Ekins, M.G.; Wang, T. High Throughput Screening of the NatureBank ‘Marine Collection’ in a Haemonchus Bioassay Identifies Anthelmintic Activity in Extracts from a Range of Sponges from Australian Waters. Molecules 2021, 26, 5846. [Google Scholar] [CrossRef]
- Hayes, S.; Taki, A.C.; Lum, K.Y.; Byrne, J.J.; White, J.M.; Ekins, M.G.; Gasser, R.B.; Davis, R.A. Identification of Anthelmintic Bishomoscalarane Sesterterpenes from the Australian Marine Sponge Phyllospongia bergquistae and Structure Revision of Phyllolactones A–D. J. Nat. Prod. 2022, 85, 1723–1729. [Google Scholar] [CrossRef]
- Ramage, K.S.; Taki, A.C.; Lum, K.Y.; Hayes, S.; Byrne, J.J.; Wang, T.; Hofmann, A.; Ekins, M.G.; White, J.M.; Jabbar, A. Dysidenin from the Marine Sponge Citronia sp. Affects the Motility and Morphology of Haemonchus contortus Larvae In Vitro. Mar. Drugs 2021, 19, 698. [Google Scholar] [CrossRef]
- Chu, M.-J.; Li, M.; Ma, H.; Li, P.-L.; Li, G.-Q. Secondary metabolites from marine sponges of the genus Agelas: A comprehensive update insight on structural diversity and bioactivity. RSC Adv. 2022, 12, 7789–7820. [Google Scholar] [CrossRef]
- Chu, M.J.; Tang, X.L.; Qin, G.F.; Sun, Y.T.; Li, L.; de Voogd, N.J.; Li, P.L.; Li, G.Q. Pyrrole Derivatives and Diterpene Alkaloids from the South China Sea Sponge Agelas nakamurai. Chem. Biodivers. 2017, 14, e1600446. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A, -B, -C and -D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on Na, K-ATPase from the Okinawa sea sponge Agelas sp. Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar] [CrossRef]
- Abdjul, D.B.; Yamazaki, H.; Kanno, S.-i.; Takahashi, O.; Kirikoshi, R.; Ukai, K.; Namikoshi, M. Structures and biological evaluations of agelasines isolated from the Okinawan marine sponge Agelas nakamurai. J. Nat. Prod. 2015, 78, 1428–1433. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chao, C.-H.; Fu, C.-W.; Chiou, S.-F.; Huang, T.-Y.; Yang, Y.-J.; Wu, S.-H.; Chen, S.-L.; Wang, H.-C.; Yu, M.-C. Computationally assisted structure elucidation of new 2-guanidinoethanesulfonyl sesquiterpenoid alkaloids: Agelasidines G–I from the marine sponge Agelas nakamurai. Tetrahedron 2022, 126, 133077. [Google Scholar] [CrossRef]
- Regalado, E.L.; Laguna, A.; Mendiola, J.; Thomas, O.P.; Nogueiras, C. Bromopyrrole alkaloids from the Caribbean sponge Agelas cerebrum. Quím. Nova 2011, 34, 289–291. [Google Scholar] [CrossRef]
- Jiao, Y.; Preston, S.; Garcia-Bustos, J.F.; Baell, J.B.; Ventura, S.; Le, T.; McNamara, N.; Nguyen, N.; Botteon, A.; Skinner, C. Tetrahydroquinoxalines induce a lethal evisceration phenotype in Haemonchus contortus in vitro. Int. J. Parasitol. Drugs Drug Resist. 2019, 9, 59–71. [Google Scholar] [CrossRef]
- Gundersen, L.-L. Synthesis and biological activities of marine terpene-adenine hybrids and synthetic analogs. Phytochem. Rev. 2013, 12, 467–486. [Google Scholar] [CrossRef]
- Fu, C.-W.; Chiang, L.; Chao, C.-H.; Huang, Y.-L.; Chiou, S.-F.; Wang, L.-C.; Chang, H.-W.; Chen, S.-L.; Wang, H.-C.; Yu, M.-C.; et al. Nakamusines A−C, new 9-methyladeninium diterpenoid alkaloids from a Formosan marine sponge Agelas nakamurai. Tetrahedron 2023, 149, 133745. [Google Scholar] [CrossRef]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, M.M.; Biglione, F.A.; Sortino, M.A.; Sarotti, A.M. General quantum-based NMR method for the assignment of absolute configuration by single or double derivatization: Scope and limitations. J. Org. Chem. 2018, 83, 11839–11849. [Google Scholar] [CrossRef]
- Holland, D.C.; Carroll, A.R. Structure Revision of Formyl Phloroglucinol Meroterpenoids: A Unified Approach Using NMR Fingerprinting and DFT NMR and ECD Analyses. Molecules 2024, 29, 594. [Google Scholar] [CrossRef]
- Pech-Puch, D.; Forero, A.M.; Fuentes-Monteverde, J.C.; Lasarte-Monterrubio, C.; Martinez-Guitian, M.; González-Salas, C.; Guillén-Hernández, S.; Villegas-Hernández, H.; Beceiro, A.; Griesinger, C. Antimicrobial Diterpene Alkaloids from an Agelas citrina Sponge Collected in the Yucatán Peninsula. Mar. Drugs 2022, 20, 298. [Google Scholar] [CrossRef]
- Atzrodt, J.; Derdau, V.; Kerr, W.J.; Reid, M. Deuterium and tritium labelled compounds: Applications in the life sciences. Angew. Chem. Int. Ed. 2018, 57, 1758–1784. [Google Scholar] [CrossRef]
- Yang, H.; Hesk, D. Base metal catalyzed hydrogen isotope exchange. J. Labelled Compd. Radiopharm. 2020, 63, 296–307. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Chen, J.; Luo, Y.; Xia, Y. Solvent as photoreductant for dehalogenation of α-haloketones under catalyst-free conditions. Tetrahedron Lett. 2022, 98, 153835. [Google Scholar] [CrossRef]
- Kumar, V.; Rai, R.; Pandey, S. Controlling excited-state prototropism via the acidity of ionic liquids. RSC Adv. 2013, 3, 11621–11627. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Köcher, C. N-Heterocyclic carbenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 2162–2187. [Google Scholar] [CrossRef]
- Rico del Cerro, D.; Mera-Adasme, R.; King, A.W.; Perea-Buceta, J.E.; Heikkinen, S.; Hase, T.; Sundholm, D.; Wähälä, K. On the Mechanism of the Reactivity of 1, 3-Dialkylimidazolium Salts under Basic to Acidic Conditions: A Combined Kinetic and Computational Study. Angew. Chem. Int. Ed. 2018, 57, 11613–11617. [Google Scholar] [CrossRef]
- Wijesekera, K.; Taki, A.C.; Byrne, J.J.; White, J.M.; Carroll, A.R.; Gasser, R.B.; Davis, R.A. Anthelmintic activity of selected neolignans and semisynthetic derivatives from Styrax suberifolius. Tetrahedron 2025, 169, 134366. [Google Scholar] [CrossRef]
- Taki, A.C.; Byrne, J.J.; Wang, T.; Sleebs, B.E.; Nguyen, N.; Hall, R.S.; Korhonen, P.K.; Chang, B.C.; Jackson, P.; Jabbar, A. High-throughput phenotypic assay to screen for anthelmintic activity on Haemonchus contortus. Pharmaceuticals 2021, 14, 616. [Google Scholar] [CrossRef]
- Preston, S.; Jabbar, A.; Nowell, C.; Joachim, A.; Ruttkowski, B.; Baell, J.; Cardno, T.; Korhonen, P.K.; Piedrafita, D.; Ansell, B.R. Low cost whole-organism screening of compounds for anthelmintic activity. Int. J. Parasitol. 2015, 45, 333–343. [Google Scholar] [CrossRef]
- Kusama, T.; Tanaka, N.; Takahashi-Nakaguchi, A.; Gonoi, T.; Fromont, J.; Kobayashi, J.i. Bromopyrrole Alkaloids from a Marine Sponge Agelas sp. Chem. Pharm. Bull. 2014, 62, 499–503. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Alqarni, A.S.; Almohammadi, A.M.; Abujamel, T.; Shaala, L.A. Marmaricines A-C: Antimicrobial Brominated Pyrrole Alkaloids from the Red Sea Marine Sponge Agelas sp. aff. marmarica. Mar. Drugs 2025, 23, 80. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tang, Y.; Zhang, Q.; Bourne, G.T.; Arm, C.A.; Leet, J.E.; Knight, J.C.; Pettit, R.K.; Chapuis, J.-C.; Doubek, D.L. Isolation and structures of axistatins 1–3 from the Republic of Palau marine sponge Agelas axifera Hentschel. J. Nat. Prod. 2013, 76, 420–424. [Google Scholar] [CrossRef]
- Appenzeller, J.; Mihci, G.; Martin, M.T.; Gallard, J.F.; Menou, J.L.; Boury-Esnault, N.; Hooper, J.; Petek, S.; Chevalley, S.; Valentin, A. Agelasines J, K, and L from the Solomon Islands marine sponge Agelas cf. mauritiana. J. Nat. Prod. 2008, 71, 1451–1454. [Google Scholar] [CrossRef]
- Ueoka, R.; Nakao, Y.; Kawatsu, S.; Yaegashi, J.; Matsumoto, Y.; Matsunaga, S.; Furihata, K.; Van Soest, R.W.; Fusetani, N. Gracilioethers A− C, antimalarial metabolites from the marine sponge Agelas gracilis. J. Org. Chem. 2009, 74, 4203–4207. [Google Scholar] [CrossRef]
- Gordaliza, M. Terpenyl-Purines from the Sea. Mar. Drugs 2009, 7, 833–849. [Google Scholar] [CrossRef]
- Vik, A.; Proszenyák, Á.; Vermeersch, M.; Cos, P.; Maes, L.; Gundersen, L.L. Screening of Agelasine D and Analogs for Inhibitory Activity against Pathogenic Protozoa; Identification of Hits for Visceral Leishmaniasis and Chagas Disease. Molecules 2009, 14, 279–288. [Google Scholar] [CrossRef]
- Risi, G.; Liu, M.; Vairoletti, F.; Quinn, R.J.; Salinas, G. A Screening of 10,240 NatureBank Fractions Identifies Nematicidal Activity in Agelasine-Containing Extracts from Sponges. J. Nat. Prod. 2024, 87, 1532–1539. [Google Scholar] [CrossRef]
- Schwarz, E.M.; Korhonen, P.K.; Campbell, B.E.; Young, N.D.; Jex, A.R.; Jabbar, A.; Hall, R.S.; Mondal, A.; Howe, A.C.; Pell, J. The genome and developmental transcriptome of the strongylid nematode Haemonchus contortus. Genome Biol. 2013, 14, R89. [Google Scholar] [CrossRef] [PubMed]
- Taki, A.C.; Byrne, J.J.; Boag, P.R.; Jabbar, A.; Gasser, R.B. Practical high-throughput method to screen compounds for anthelmintic activity against Caenorhabditis elegans. Molecules 2021, 26, 4156. [Google Scholar] [CrossRef] [PubMed]
- Bertani, G. Studies on lysogenesis I: The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook: The Online Review of C. elegans Biology; The C. elegans Research Community: Pasadena, CA, USA, 2006. [Google Scholar]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans methods: Synchronization and observation. J. Vis. Exp. 2012, 64, e4019. [Google Scholar]
- Le, T.G.; Kundu, A.; Ghoshal, A.; Nguyen, N.H.; Preston, S.; Jiao, Y.; Ruan, B.; Xue, L.; Huang, F.; Keiser, J. Optimization of novel 1-methyl-1H-pyrazole-5-carboxamides leads to high potency larval development inhibitors of the barber’s pole worm. J. Med. Chem. 2018, 61, 10875–10894. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
- Willoughby, P.H.; Jansma, M.J.; Hoye, T.R. A guide to small-molecule structure assignment through computation of (1H and 13C) NMR chemical shifts. Nat. Protoc. 2014, 9, 643–660. [Google Scholar] [CrossRef]
- Bulcock, B.W.; Chooi, Y.-H.; Flematti, G.R. SpectroIBIS: Automated Data Processing for Multiconformer Quantum Chemical Spectroscopic Calculations. J. Nat. Prod. 2025, 88, 495–501. [Google Scholar] [CrossRef]
- Nothias, L.-F.; Nothias-Esposito, M.; da Silva, R.; Wang, M.; Protsyuk, I.; Zhang, Z.; Sarvepalli, A.; Leyssen, P.; Touboul, D.; Costa, J.; et al. Bioactivity-Based Molecular Networking for the Discovery of Drug Leads in Natural Product Bioassay-Guided Fractionation. J. Nat. Prod. 2018, 81, 758–767. [Google Scholar] [CrossRef] [PubMed]


| Position | δC, Type | δH, mult., (J in Hz) |
|---|---|---|
| 1 | 122.6, CH | 5.87, s |
| 2 | 202.7, C | - |
| 3a | 47.8, CH2 | 2.42, d (16.1) |
| 3b | 1.98, d (16.1) | |
| 4 | 35.4, C | - |
| 5 | 46.1, C | - |
| 6a | 31.0, CH2 | 2.11, m |
| 6b | 1.43, m | |
| 7a | 31.6, CH2 | 1.64, m |
| 7b | ||
| 8 | 47.5, CH | 1.48, m |
| 9 | 47.2, CH | 2.19, dd (12.8, 3.7) |
| 10 | 176.4, C | - |
| 11a | 31.0, CH2 | 2.08, m |
| 11b | 1.50, m | |
| 12a | 35.2, CH2 | 2.10, m |
| 12b | 1.78, m | |
| 13 | 149.1, C | - |
| 14 | 115.8, CH | 5.55, t (7.0) |
| 15a | 48.6, CH2 | 5.21, d (7.0) |
| 15b | ||
| 16 | 17.0, CH3 | 1.88, s |
| 17 | 16.4, CH3 | 0.98, d (6.8) |
| 18 | 27.8, CH3 | 1.01, s |
| 19 | 28.8, CH3 | 1.04, s |
| 20 | 23.2, CH3 | 1.20, s |
| 2’ | 157.1, CH | 8.47, s |
| 4’ | 150.9, C | - |
| 5’ | 111.2, C | - |
| 6’ | 154.1, C | - |
| 8’ | 141.9, CH | 9.33, s |
| NH2 | - | n.d. |
| 9-NCH3 | 32.0, CH3 | 3.98, s |
| Position | δC, Type | δH, mult., (J in Hz) |
|---|---|---|
| 1a | 40.3, CH2 | 1.78, m |
| 1b | 0.98, td (12.8, 3.5) | |
| 2a | 20.4, CH2 | 1.74, m |
| 2b | 1.57, m | |
| 3a | 43.3, CH2 | 1.40, m |
| 3b | 1.19, td (13.5, 3.7) | |
| 4 | 34.5, C | - |
| 5 | 57.4, CH | 1.06, dd (12.6, 2.8) |
| 6a | 25.6, CH2 | 1.75, m |
| 6b | 1.33, m | |
| 7a | 39.4, CH2 | 2.39, m |
| 7b | 1.93, m | |
| 8 | 149.7, C | - |
| 9 | 56.9, CH | 1.60, m |
| 10 | 40.7, C | - |
| 11a | 22.6, CH2 | 1.71, m |
| 11b | 1.55, m | |
| 12a | 39.4, CH2 | 2.33, m |
| 12b | 2.04, m | |
| 13 | 149.5, C | - |
| 14 | 115.7, CH | 5.52, t (7.2) |
| 15a | 48.6, CH2 | 5.23, d (7.2) |
| 15b | ||
| 16 | 17.0, CH3 | 1.87, s |
| 17a | 107.0, CH2 | 4.88, m |
| 17b | 4.59, brs | |
| 18 | 34.1, CH3 | 0.87, s |
| 19 | 22.1, CH3 | 0.82, s |
| 20 | 15.0, CH3 | 0.71, s |
| 2’ | 157.2, CH | 8.47, s |
| 4’ | 150.9, C | - |
| 5’ | 111.1, C | - |
| 6’ | 145.4, C | - |
| 8’ | 142.0, CD | - |
| NH2 | - | n.d. |
| 9-NCH3 | 32.0, CH3 | 3.98, s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijesekera, K.; Taki, A.C.; Byrne, J.J.; Holland, D.C.; Jenkins, I.D.; Ekins, M.G.; Carroll, A.R.; Gasser, R.B.; Davis, R.A. Anthelmintic Potential of Agelasine Alkaloids from the Australian Marine Sponge Agelas axifera. Mar. Drugs 2025, 23, 276. https://doi.org/10.3390/md23070276
Wijesekera K, Taki AC, Byrne JJ, Holland DC, Jenkins ID, Ekins MG, Carroll AR, Gasser RB, Davis RA. Anthelmintic Potential of Agelasine Alkaloids from the Australian Marine Sponge Agelas axifera. Marine Drugs. 2025; 23(7):276. https://doi.org/10.3390/md23070276
Chicago/Turabian StyleWijesekera, Kanchana, Aya C. Taki, Joseph J. Byrne, Darren C. Holland, Ian D. Jenkins, Merrick G. Ekins, Anthony R. Carroll, Robin B. Gasser, and Rohan A. Davis. 2025. "Anthelmintic Potential of Agelasine Alkaloids from the Australian Marine Sponge Agelas axifera" Marine Drugs 23, no. 7: 276. https://doi.org/10.3390/md23070276
APA StyleWijesekera, K., Taki, A. C., Byrne, J. J., Holland, D. C., Jenkins, I. D., Ekins, M. G., Carroll, A. R., Gasser, R. B., & Davis, R. A. (2025). Anthelmintic Potential of Agelasine Alkaloids from the Australian Marine Sponge Agelas axifera. Marine Drugs, 23(7), 276. https://doi.org/10.3390/md23070276







