Neuroprotective Mechanisms of Red Algae-Derived Bioactive Compounds in Alzheimer’s Disease: An Overview of Novel Insights
Abstract
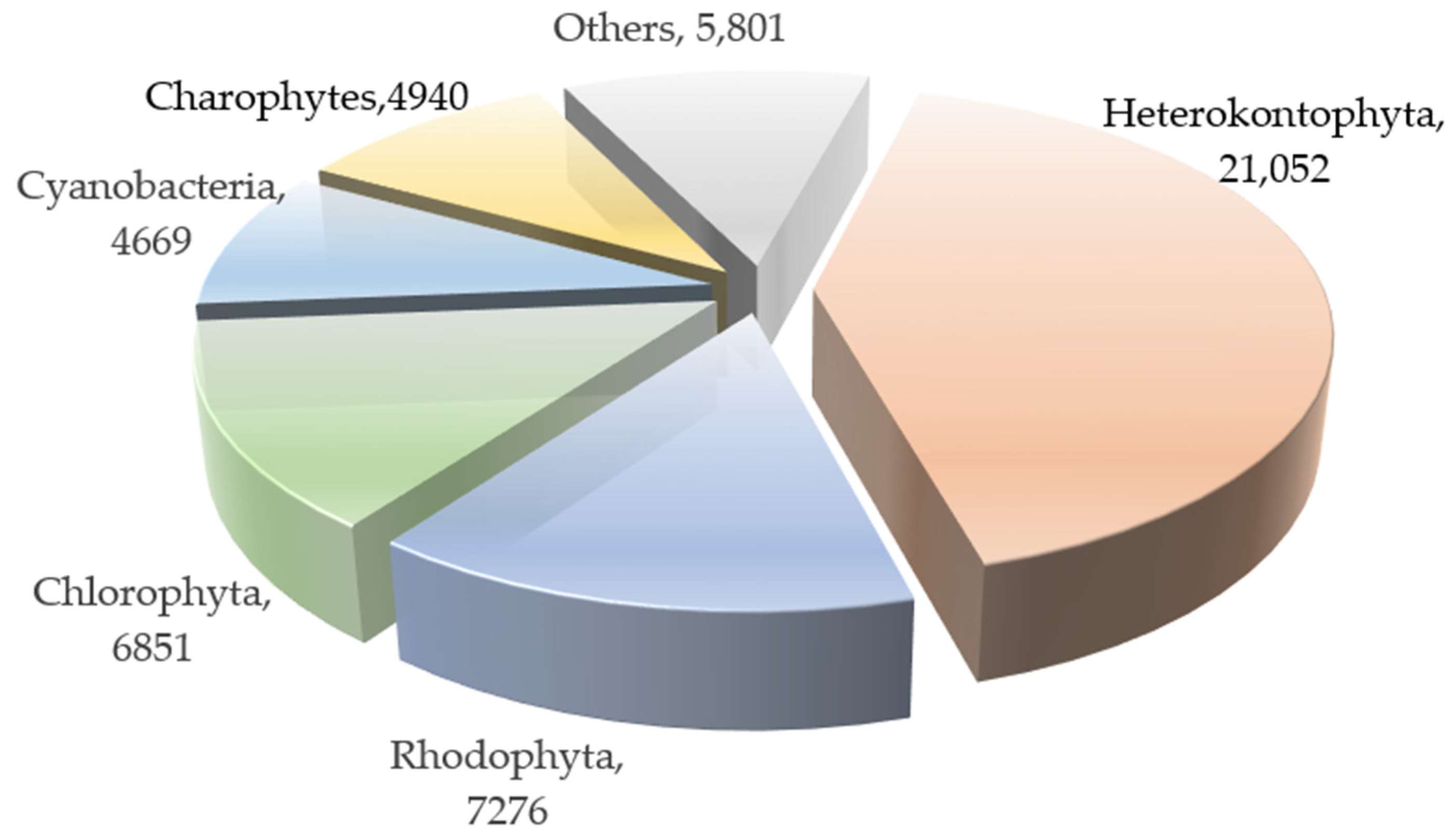
1. Introduction
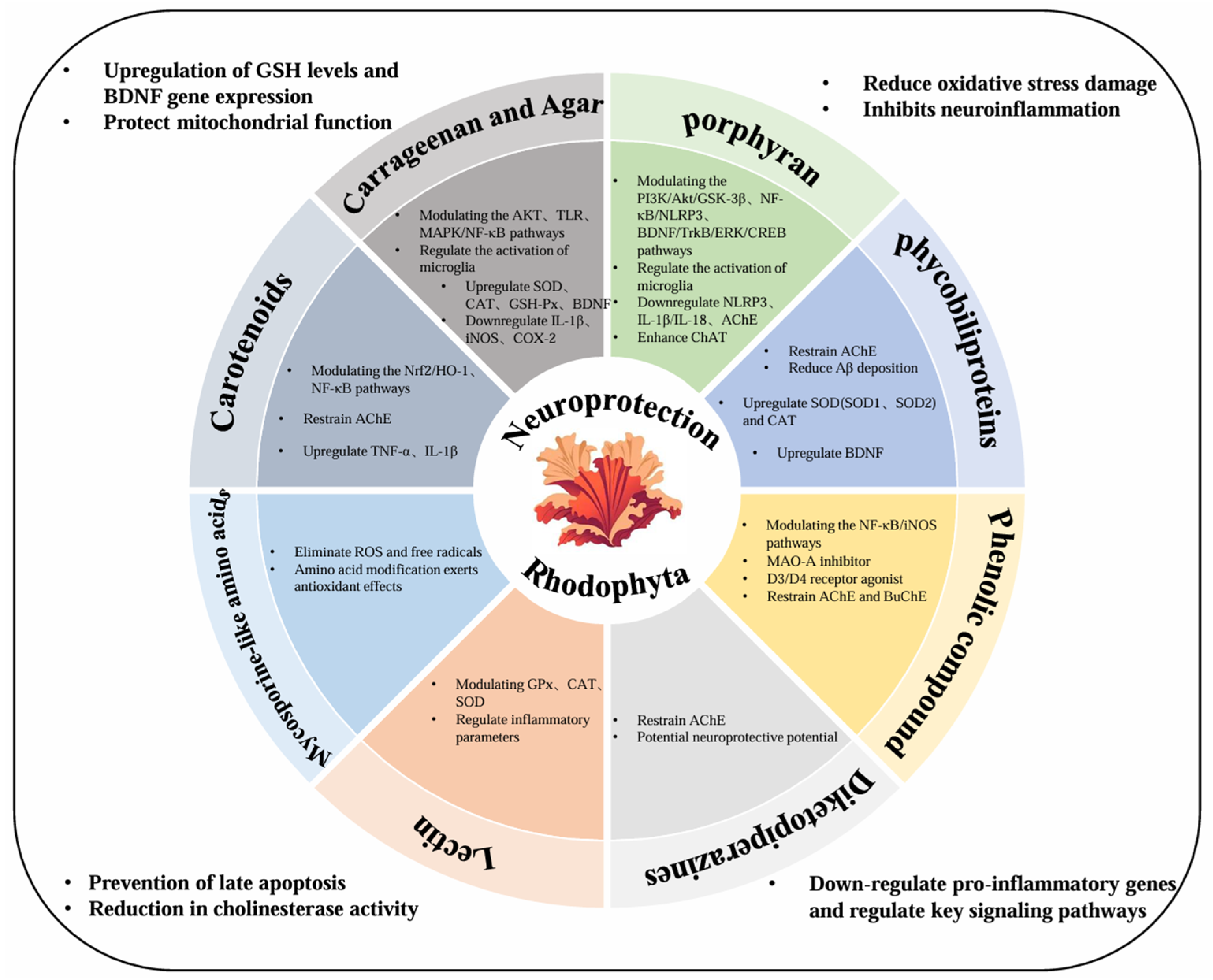
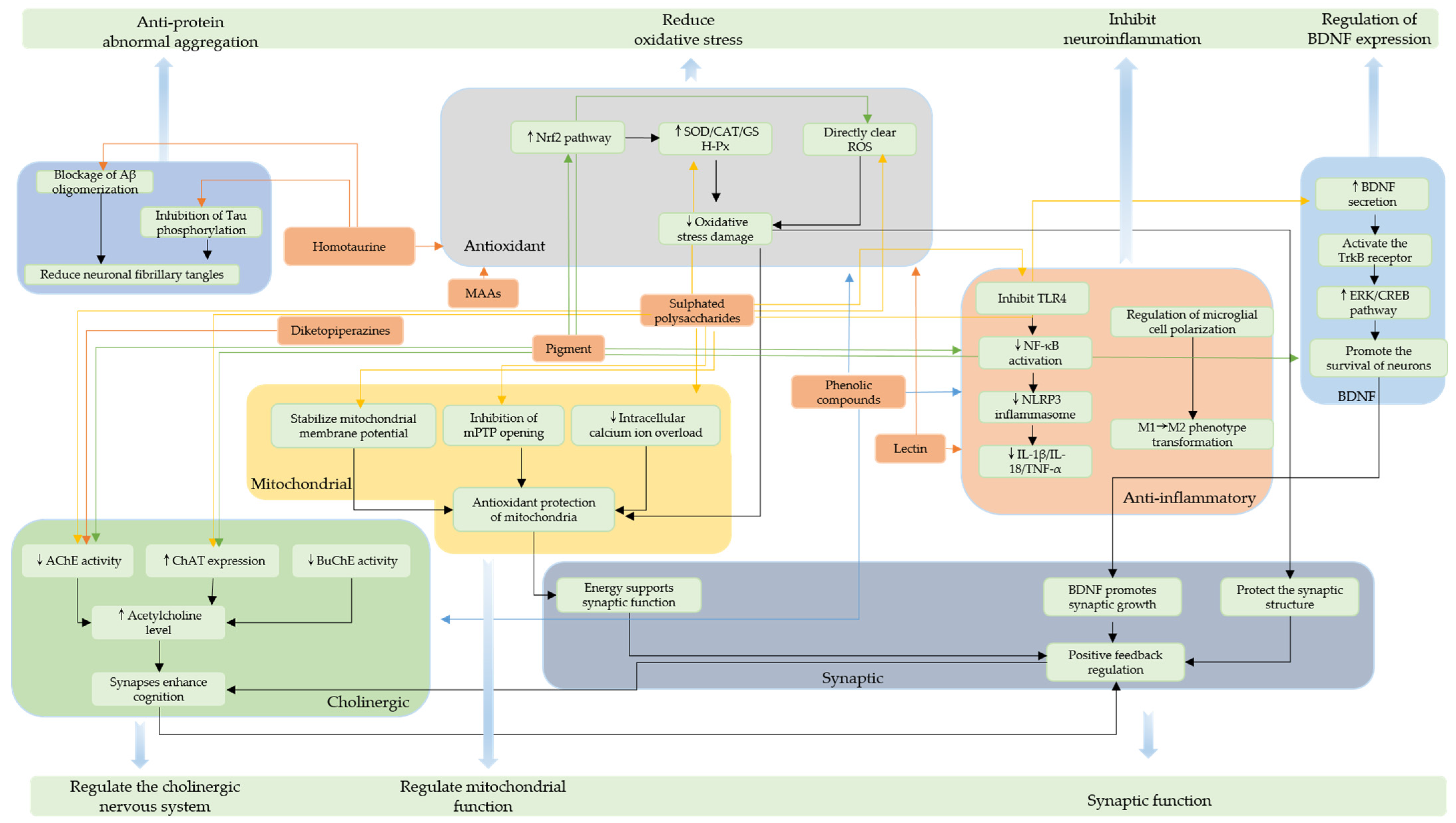
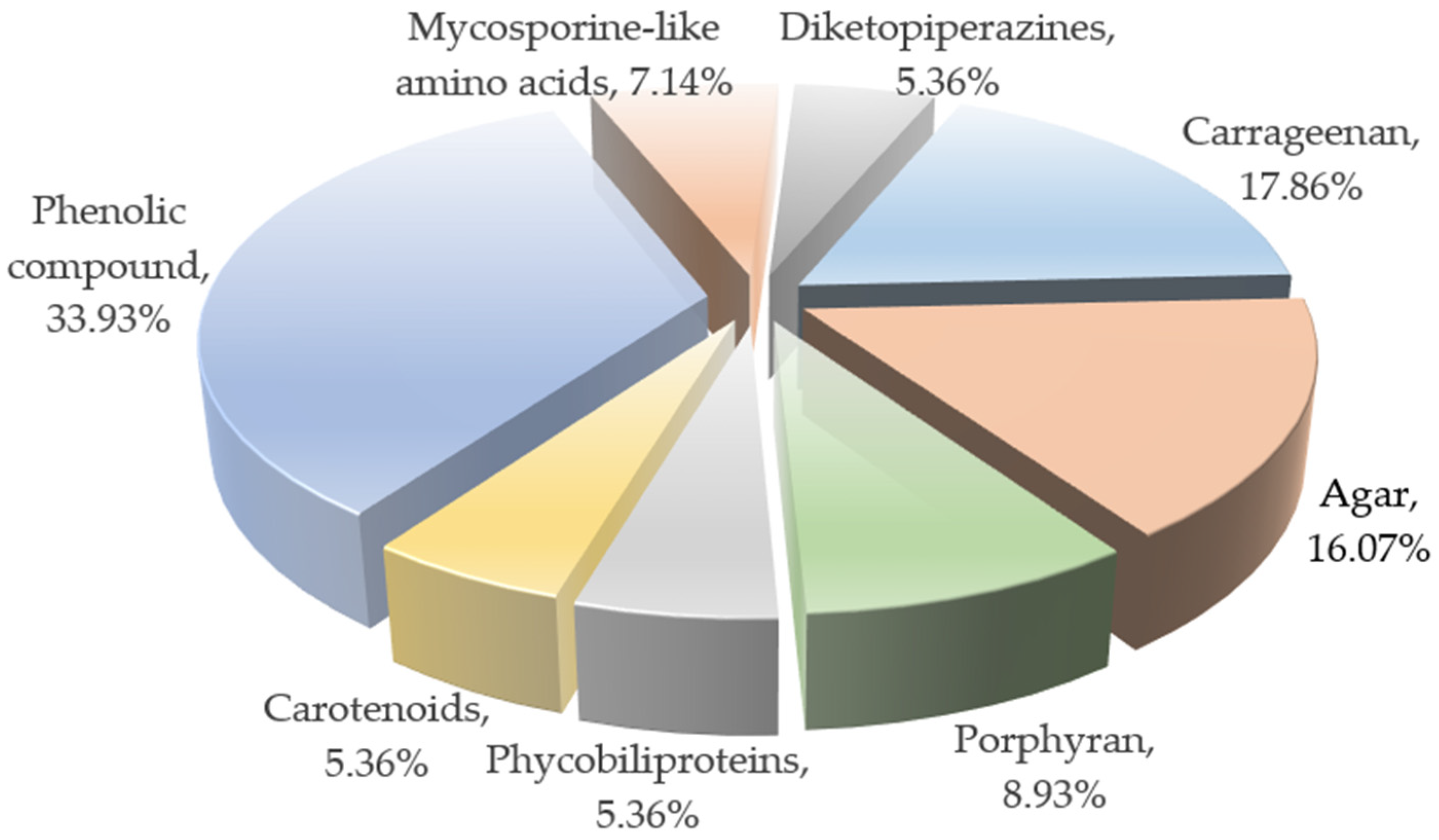
2. The Primary Neuroprotective Compounds of Red Algae and Their Characteristics
2.1. Sulphated Polysaccharides (SPs)
2.1.1. Carrageenan and Agar
| Activities | Source | Test Models/Cell Lines | Pathway/Mechanism | Effect on Test Models/Cell Lines | Author’s Conclusions | References |
|---|---|---|---|---|---|---|
| Antioxidant activity | Gracilaria birdiae | Mice induced by CCL4; 3T3-L1 cell | Glutathione and catalase levels | Increased antioxidant capacity and effects on the levels of glutathione reductase and catalase. | It has antioxidant activity and protective effect. | [35] |
| Neuroprotectant activity | Gracilaria gracilis | HT-22 cell line | Inhibition of apoptosis, oxidative damage, and acetylcholinesterase activity | Increased the contents of antioxidant enzymes and glutathione; the activity of acetylcholinesterase in cells decreased after zinc treatment. | These polysaccharides are good therapeutic agents for protecting neuronal cells from zinc-induced Alzheimer’s disease. | [36] |
| κ-Carrageenan | APP/PS1 transgenic mice | Inhibition of the excessive activation of microglia, thereby demonstrating neuroprotective effects | Alleviated clinical symptoms in AD mice, decreased the expression of inflammatory factors and inflammation-related proteins in brain tissue. | KOS can be used as a therapeutic drug for neurodegenerative diseases. | [25] | |
| Hypnea musciformis | SH-SY5Y cells | Antioxidant and neuroprotective activities | Neuroprotective effect on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. | Hm-SP shows neuroprotective activities. | [37] | |
| Anti-inflammatory activity | Spyrida Species Red Seaweed | Bovine serum albumin (BSA) | Significantly inhibited the denaturation of bovine serum albumin (BSA) | Increasing the concentration of the extract from 25 to 100 µg/mL led to an increase in the percentage of inhibited protein denaturation. | The result was statistically significantly different from that of aspirin. It can be used medicinally. | [38] |
| Gelidium pacificum Okamura | In LPS-stimulated human monocytic (THP-1) cells | Significant reduction of NO production in LPS-treated cells | Suppressed the mRNA and protein expression of TLR-4, MyD88, and TRAF-6. | Reduced LPS-induced cell toxicity and presented an anti-inflammatory effect via the TLR4 signaling pathway. | [39] | |
| Gelidium crinale | Lipopolysaccharide (LPS)-induced oxidative stress | Lipopolysaccharide (LPS)-induced oxidative stress and inflammation in macrophages | GNP had fairly strong scavenging activities on ABTS, hydroxyl, and DPPH radicals and had Fe2+-chelating ability in a dose-dependent manner. | GNP may be a latent anti-inflammatory component in pharmaceutical and functional food industries. | [20] | |
| Gracilaria caudate | Male Swiss mice | Reductions in joint oedema, MPO activity cell influx, IL-1β, and NO levels | Improved neutrophil migration to inflamed tissue, inhibited hyper-nociception, oedema. | Treatment for arthritic inflammation. | [40] | |
| Gracilaria lemaneiformis | IEC-6 cells | Inhibition of LPS-induced NO, TNF-α, and IL-6 production in IEC-6 cells | S-PS fractions possess anti-inflammatory activity. | Modulates inflammation and auto-immune diseases. | [41] | |
| Gelidium amansii | Diets-induced obese (DIO) C57BL/6J mice | Increased levels of anti-inflammatory cytokine production and lipolysis protein | Achieved anti-inflammatory and lipolysis-promoting effects. | Improves health conditions related to inflammation. | [42] |
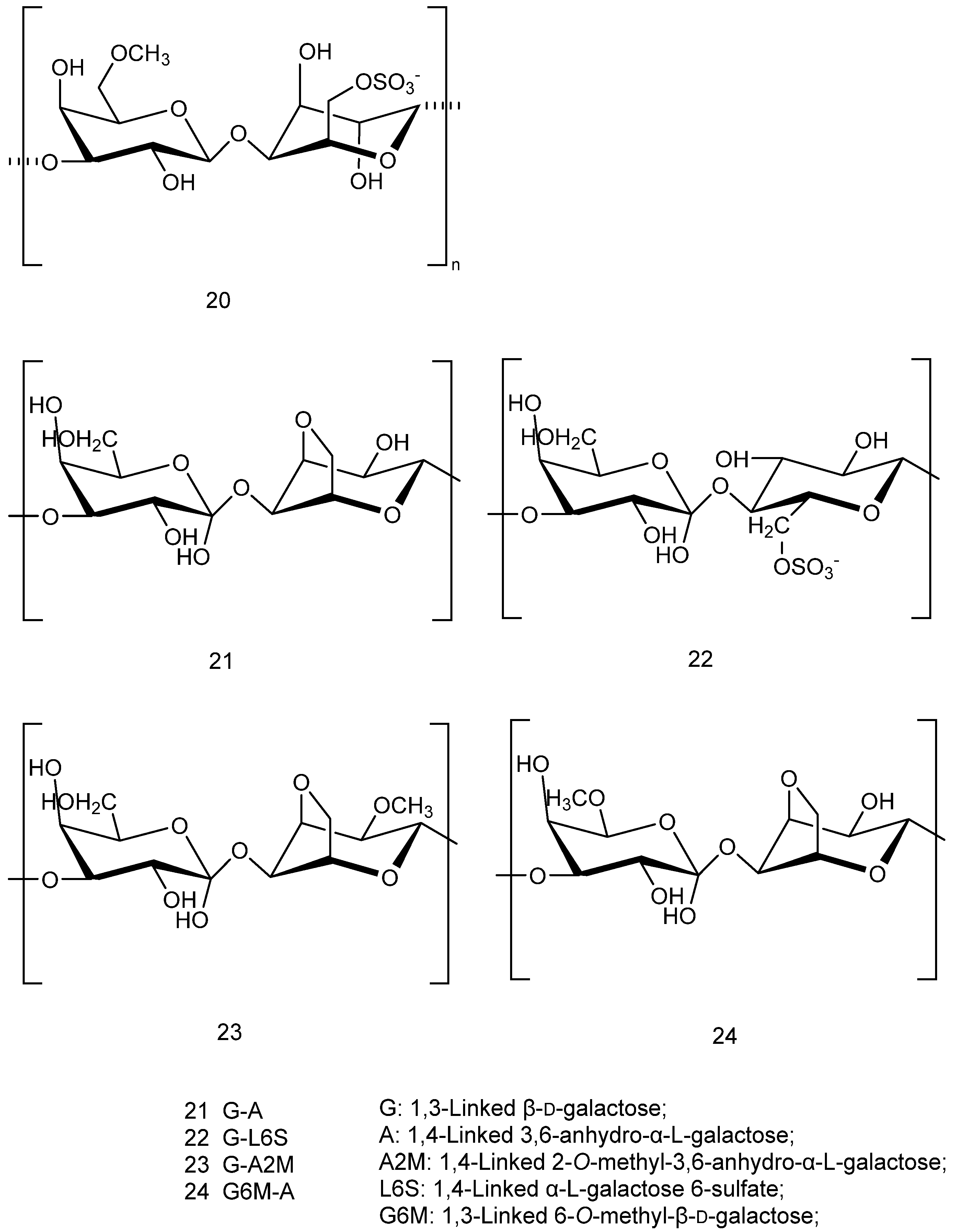
2.1.2. Porphyran
2.2. Pigments
2.2.1. Phycobiliproteins
2.2.2. Carotenoids
2.3. Secondary Metabolites
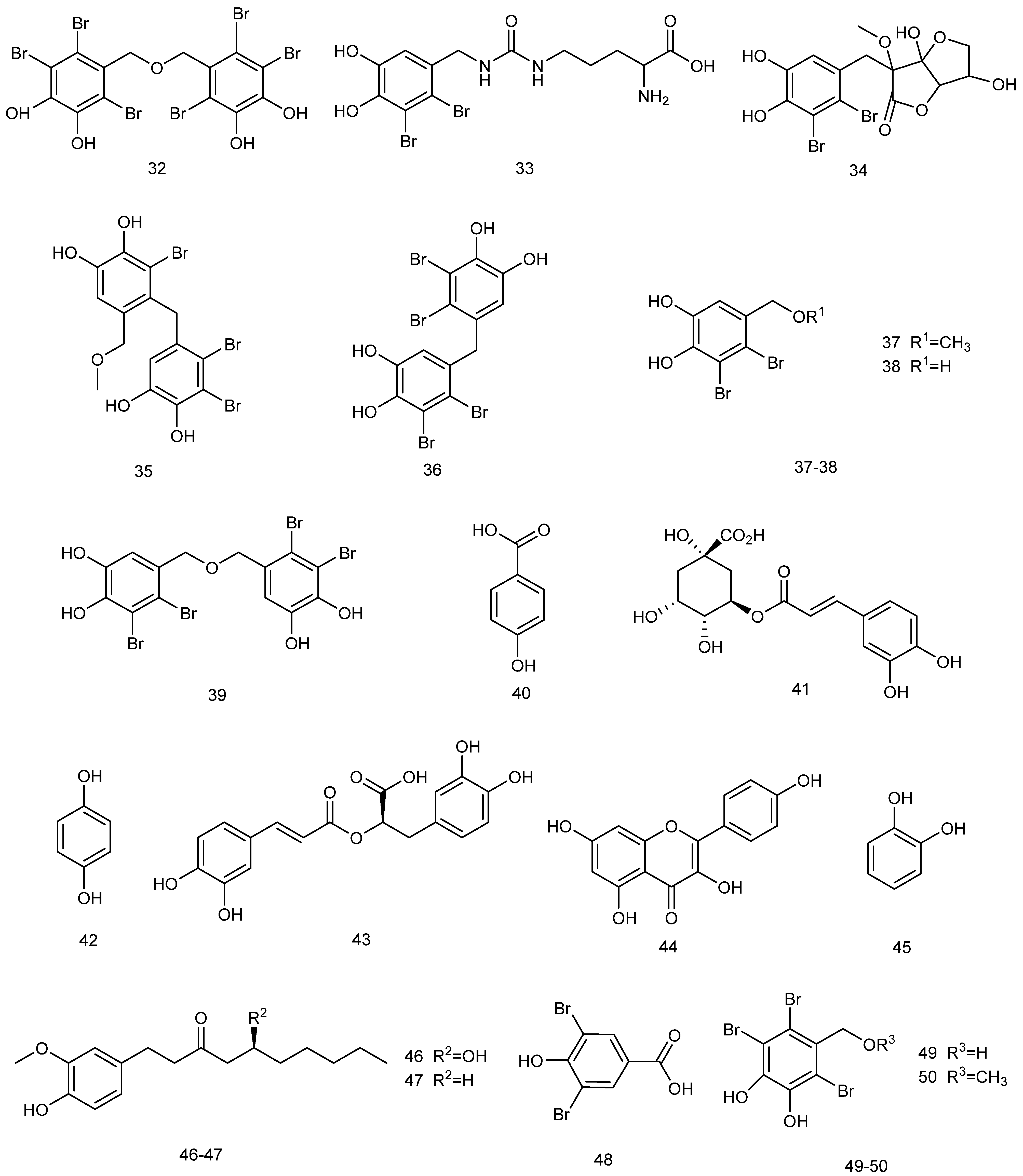
2.3.1. Phenolic Compounds
2.3.2. Mycosporine-like Amino Acids
2.4. Other Neuroprotective Compounds
2.4.1. Lectin
2.4.2. Diketopiperazines
2.4.3. Homotaurine
3. Extraction of Neuroprotective Compounds from Red Seaweed
- Water extraction;
- Acidic and alkaline extraction;
- Soaking-stirring method;
- Innovative techniques;
- Ultrasound-assisted extraction (UAE);
- Microwave-assisted extraction (MAE);
- Ultrasound-microwave assisted extraction (UMAE);
- Enzyme-assisted extraction (EAE).
| Source | Polysaccharide Type | Extraction Method | Yield | Monosaccharides Composition | Time (min) | Temperature (°C) | Water/Material Ratio (mL/g) | References |
|---|---|---|---|---|---|---|---|---|
| Eucheuma gelatinae | β-carrageenan | Maceration-stirred | 87.56 ± 5.61(%) | Rhamnose, mannose, glucose, fucose, and xylose | 115.35 min | 82.35 °C | 36.42 (v/w) | [137] |
| Porphyra haitanensis | Porphyra haitanensis polysaccharides (PHPs) | PHP, UHP-PHP, US-PHP, and M-PHP | 71.33% | Galactose, mannose, glucose and xylose | 120.00 min | 90 °C | 1:40 (v/w) | [138] |
| Porphyridium purpureum | Porphyridium purpureum polysaccharides | A novel three-step extraction strategy | 75.20% | Galactose (39.58%), xylose (38.83%), and glucose (21.59%) | 120.00 min | 80 °C | 1:50 (v/v) | [139] |
| Porphyridium purpureum | Porphyridium purpureum polysaccharides | Response surface methodology, microwave-assisted extraction | 25.48% | Glucuronic acid (150 °C), fucose (90 °C) | 45 min | 87 °C | 1:63 (g/mL) | [140] |
| Porphyra haitanensis | Porphyra haitanensis polysaccharides (PHPs) | Water extraction and alcohol precipitation methods, single-factor and Box-Behnken response surface methodologies | 20.48% | Galactose, glucose, and fucose | 180 min | 80 °C | 0.04 | [141] |
| Rhodymenia intricata | Rhodymenia intricata polysaccharides | Ultrasound-assisted water extraction method | 37.78 ± 0.15% | α-pyranose | 95 min | 80 °C | 1:84 (g/mL) | [142] |
| Gracilaria chouae | Gracilaria chouae sulfated polysaccharides | Citric acid extraction and water extraction | 22.85 ± 0.80% (CGCP), 27.4 ± 0.12% (WGCP) | Galactose, glucose, and xylose | 120 min | 100 °C | 1:25 (w/v) | [143] |
| Sarcopeltis skottsbergii | Carrageenan | Microwave-assisted hydrothermal treatment | 63–64% | Galactose (54.00 ± 0.50%), glucose (3.92 ± 0.41%) | 5-7 min | 110 °C, 160 °C | 1:30 (w/w) | [130] |
| Mastocarpus stellatus | Hybrid carrageenans | Microwave hydrodiffusion and gravity (MHG) | 37% | Galactose + xylose + mannose yielded close to 25%, followed by glucose 3.5% | 120 min | 90 °C | - | [144] |
| Gracilaria lemaneiformis | Agar | Traditional alkaline extraction | 4% | Galactose, anhydro-L-galactose | 180 min | 90 °C | 1:20 (v/w) | [145] |
| Gracilaria lemaneiformis | Agar | Enzyme-assisted extraction technology | 24.70% | Galactose, anhydro-L-galactose | 60 min | 50 °C | 1:20 (v/w) | [145] |
| Gracilaria lemaneiformis | Agar | Enzymatic extraction | 3.40% | Galactose, anhydro-L-galactose | 180 min | 50 °C | 1:20 (v/w) | [145] |
| G. sesquipedale | Agar | Subcritical water extraction, moderate electric fields, and a combination of both methods | - | Galactose, anhydro-L-galactose | 95 °C for 180 min, 110 °C for 60 min, 130 °C for 9 min, and 140 °C for 1 s | 1:30 (w/v) | [146] | |
| G. vermiculophylla | Agar | Subcritical water extraction, moderate electric fields and a combination of both methods | - | Galactose, anhydro-L-galactose | 85 °C for 120 min, 110 °C for 15 min, 120 °C for 5 min, and 125 °C for 1 min. | 1:30 (w/v) | [146] | |
| Porphyra haitanensis | Porphyra haitanensis polysaccharides (PHP) | Ultrasonic/microwave-assisted extraction (UMAE), response surface methodology to optimize | 20.98% | Galactopyranose | 29.64 min | 79.94 °C | 1:41.79 g/mL | [147] |
| Source | Type | Extraction Method | Total Content of Pigments (mg/100 g) | Yield | Solvent | Time (min) | Temperature (°C) | References |
|---|---|---|---|---|---|---|---|---|
| Gelidium sesquipedale | Phycobiliproteins | Traditional serial extraction (5 h) | 147.3 ± 3.2 | 100% | Ethanol, water, and aqueous ethanol solutions with concentrations of 50% and 70% | 300 min | RT or 40 °C | [148] |
| Gelidium sesquipedale | Phycobiliproteins | Ultrasound-assisted extraction (UAE) 10 min | 54.7 ± 1.6 | 37% | - | 10 min | RT or 40 °C | [148] |
| Gelidium sesquipedale | Phycobiliproteins | Ultrasound-assisted extraction (UAE) 15 min | 54.1 ± 2.1 | 37% | - | 15 min | RT or 40 °C | [148] |
| Gelidium sesquipedale | Phycobiliproteins | UAE15min + maceration (Mac) 45 min | 72.4 ± 0.5 | 49% | - | 60 min | RT or 40 °C | [148] |
| Gelidium sesquipedale | Phycobiliproteins | UAE15 min + maceration (Mac) 60 min | 77.9 ± 1.6 | 53% | - | 75 min | RT or 40 °C | [148] |
| Grateloupia turuturu | R-phycoerythrin (R-PE) | Ultrasound-assisted enzymatic hydrolysis (UAEH) | 4.28 ± 0.09 mg/g dry weight (dw) at 180 min | - | - | 180 min | 20 °C | [149] |
| Porphyridium purpureum | Phycoerythrin | Deep eutectic solvent (DES)-based ultrasound-assisted extraction (UAE) | - | - | DES or water | 10 min | 25 ± 1 °C | [150] |
| Solieria filiformis | R-Phycoerythrin (R-PE) | 0.025 M phosphate buffer, pH 6.5 | 0.14 mg/g dry weight (dw) | - | Phosphate buffer | 360 min | 4 °C | [151] |
| Gracilaria gracilis | Phycobiliproteins | Maceration and freeze–thaw | 3.58 mg/g | - | Different phosphate buffer concentrations (0.01 M < C < 1 M, pH 6.8) | 5–30 min | RT | [152] |
| Gracilaria gracilis | Phycobiliproteins | Ultrasound-assisted extraction (ultrasonic water bath and ultrasonic probe) | 1.48–1.99 mg/g | - | Different phosphate buffer concentrations (0.01 M < C < 1 M, pH 6.8) | 10 s–10 min | RT | [152] |
| Gracilaria gracilis | Phycobiliproteins | High pressure-assisted extraction | - | - | Different phosphate buffer concentrations (0.01 M < C < 1 M, pH 6.8) | 5–30 min | RT | [152] |
| Gracilaria gracilis | Phycobiliprotein | Maceration | - | - | Phosphate buffer pH 6.8 (0.1 M) | 10 min | RT | [153] |
| Porphyra sensu lato | Phycobiliproteins | Alkaline hydrolysis | 4.29 ± 0.54 mg/g dry weight (dw) (PE), 1.24 ± 0.24 mg/g DW (PC) | - | 5.25% sodium carbonate (SC) | - | 80 °C | [154] |
| Porphyra sensu lato | Phycoerythrin (PE), phycocyanin (PC) | Enzymatic hydrolysis | 12.20 ± 3.70 mg/g dry weight (dw) (PE), 6.71 ± 1.69 mg/g DW (PC) | - | Miura cocktail | 122 min | 27.66 °C | [154] |
| Gelidium sesquipedale | Phenolic compounds | Ultrasound-assisted extraction (UAE) | 189.3 ± 11.7 mg GAE/100 g dw (40 °C, 15 min) and 205.6 ± 7.7 mg GAE/100 g dw (40 °C, 30 min) | 81% | Ethanol/Water (50:50 v/v) | 15 and 30 min | RT and 40 °C | [148] |
| Porphyra sensu lato | Phenolic compounds | Alkaline hydrolysis | 3.19 ± 0.41 mg/g dry weight (dw) (1%, 3.53 ± 0.64 mmg/g dry weight (dw) (2.5%) | - | 1% and 2.5% sodium carbonate (SC) | - | 80 °C | [154] |
| Porphyra sensu lato | Phenolic compounds | Enzymatic hydrolysis | 3.08 ± 0.22 mg/g dry weight (dw) | - | Miura cocktail | - | - | [154] |
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.; Tan, Y.; Cheng, X.; Zhang, Z.; Huang, J.; Hui, S.; Zhu, L.; Liu, Y.; Zhao, D.; Liu, Z.; et al. Untargeted metabolomics analysis of the hippocampus and cerebral cortex identified the neuroprotective mechanisms of Bushen Tiansui formula in an aβ(25-35)-induced rat model of Alzheimer’s disease. Front. Pharmacol. 2022, 13, 990307. [Google Scholar]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement. J. Alzheimers Assoc. 2024, 20, 3708–3821. [CrossRef] [PubMed]
- Monteiro, A.R.; Barbosa, D.J.; Remião, F.; Silva, R. Alzheimer’s disease: Insights and new prospects in disease pathophysiology, biomarkers and disease-modifying drugs. Biochem. Pharmacol. 2023, 211, 115522. [Google Scholar] [CrossRef] [PubMed]
- Athar, T.; Al Balushi, K.; Khan, S.A. Recent advances on drug development and emerging therapeutic agents for Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 5629–5645. [Google Scholar] [CrossRef]
- Chen, Y.; Lai, M.; Tao, M. Evaluating the efficacy and safety of Alzheimer’s disease drugs: A meta-analysis and systematic review. Medicine 2024, 103, e37799. [Google Scholar] [CrossRef]
- Ismail, M.M.; Alotaibi, B.S.; El-Sheekh, M.M. Therapeutic Uses of Red Macroalgae. Molecules 2020, 25, 4411. [Google Scholar] [CrossRef]
- Van Etten, J.; Cho, C.H.; Yoon, H.S.; Bhattacharya, D. Extremophilic red algae as models for understanding adaptation to hostile environments and the evolution of eukaryotic life on the early earth. Semin. Cell Dev. Biol. 2023, 134, 4–13. [Google Scholar] [CrossRef]
- Park, S.J.; Sharma, A.; Lee, H.J. An Update on the Chemical Constituents and Biological Properties of Selected Species of an Underpinned Genus of Red Algae: Chondrus. Mar. Drugs 2024, 22, 47. [Google Scholar] [CrossRef]
- Venkatraman, K.L.; Mehta, A. Health Benefits and Pharmacological Effects of Porphyra Species. Plant Foods Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef]
- Lee, P.Y.; Costumbrado, J.; Hsu, C.Y.; Kim, Y.H. Agarose gel electrophoresis for the separation of DNA fragments. J. Vis. Exp. JoVE 2012, 62, 3923. [Google Scholar]
- Hannan, M.A.; Haque, M.N.; Mohibbullah, M.; Dash, R.; Hong, Y.K.; Moon, I.S. Gelidium amansii Attenuates Hypoxia/Reoxygenation-Induced Oxidative Injury in Primary Hippocampal Neurons Through Suppressing GluN2B Expression. Antioxidants 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhe, W.; Zhang, R.; Peng, Z.; Wang, Y.; Gao, H.; Guo, Z.; Xiao, J. Ultrasonic-assisted extraction of polyphenolic compounds from Paederia scandens (Lour.) Merr. Using deep eutectic solvent: Optimization, identification, and comparison with traditional methods. Ultrason. Sonochemistry 2022, 86, 106005. [Google Scholar] [CrossRef] [PubMed]
- Dhaouafi, J.; Nedjar, N.; Jridi, M.; Romdhani, M.; Balti, R. Extraction of Protein and Bioactive Compounds from Mediterranean Red Algae (Sphaerococcus coronopifolius and Gelidium spinosum) Using Various Innovative Pretreatment Strategies. Foods 2024, 13, 1362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gu, D.; Lu, C.; Zhang, C.; Chen, J.; Yang, R.; Luo, Q.; Wang, T.; Zhang, P.; Chen, H. Cold stress tolerance of the intertidal red alga Neoporphyra haitanensis. BMC Plant Biol. 2022, 22, 114. [Google Scholar] [CrossRef]
- Nakamura-Gouvea, N.; Alves-Lima, C.; Benites, L.F.; Iha, C.; Maracaja-Coutinho, V.; Aliaga-Tobar, V.; Araujo Amaral Carneiro, M.; Yokoya, N.S.; Marinho-Soriano, E.; Graminha, M.A.S.; et al. Insights into agar and secondary metabolite pathways from the genome of the red alga Gracilaria domingensis (Rhodophyta, Gracilariales). J. Phycol. 2022, 58, 406–423. [Google Scholar] [CrossRef]
- Ismail, M.M.; El Zokm, G.M.; Miranda Lopez, J.M. Nutritional, bioactive compounds content, and antioxidant activity of brown seaweeds from the Red Sea. Front. Nutr. 2023, 10, 1210934. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar]
- Netanel Liberman, G.; Ochbaum, G.; Mejubovsky-Mikhelis, M.; Bitton, R.; Malis Arad, S. Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int. J. Biol. Macromol. 2020, 145, 1171–1179. [Google Scholar] [CrossRef]
- Li, X.; Huang, S.; Chen, X.; Xu, Q.; Ma, Y.; You, L.; Kulikouskaya, V.; Xiao, J.; Piao, J. Structural characteristic of a sulfated polysaccharide from Gracilaria Lemaneiformis and its lipid metabolism regulation effect. Food Funct. 2020, 11, 10876–10885. [Google Scholar] [CrossRef]
- Pei, Y.; Yang, S.; Xiao, Z.; Zhou, C.; Hong, P.; Qian, Z.J. Structural Characterization of Sulfated Polysaccharide Isolated From Red Algae (Gelidium crinale) and Antioxidant and Anti-Inflammatory Effects in Macrophage Cells. Front. Bioeng. Biotechnol. 2021, 9, 794818. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydari, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.H.; et al. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Complement. Integr. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Chang, C.C.; Nagarajan, D.; Chen, C.Y.; Chang, J.S. Algae-derived hydrocolloids in foods: Applications and health-related issues. Bioengineered 2021, 12, 3787–3801. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, L.; Li, X. κ-carrageenan-derived pentasaccharide attenuates Aβ25-35-induced apoptosis in SH-SY5Y cells via suppression of the JNK signaling pathway. Mol. Med. Rep. 2017, 15, 285–290. [Google Scholar] [CrossRef]
- Sun, H.; Xu, L.; Wang, K.; Li, Y.; Bai, T.; Dong, S.; Wu, H.; Yao, Z. κ-Carrageenan Oligosaccharides Protect Nerves by Regulating Microglial Autophagy in Alzheimer’s Disease. ACS Chem. Neurosci. 2023, 14, 3540–3550. [Google Scholar] [CrossRef]
- Yin, M.; Feng, C.; Yu, Z.; Zhang, Y.; Li, Y.; Wang, X.; Song, C.; Guo, M.; Li, C. sc2GWAS: A comprehensive platform linking single cell and GWAS traits of human. Nucleic Acids Res. 2025, 53, D1151–D1161. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Pierre, G.; Barkallah, M.; Ursu, A.V.; Ben Hlima, H.; Desbrières, J.; Le Cerf, D.; Fendri, I.; Michaud, P.; et al. Structural Characterization and Rheological and Antioxidant Properties of Novel Polysaccharide from Calcareous Red Seaweed. Mar. Drugs 2022, 20, 546. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Semyanov, A. The great astroglial metabolic revolution: Mitochondria fuel astrocyte homeostatic support and neuroprotection. Cell Calcium 2022, 104, 102583. [Google Scholar] [CrossRef]
- Li, X., Sr.; Liu, W.; Jiang, G.; Lian, J.; Zhong, Y.; Zhou, J.; Li, H.; Xu, X.; Liu, Y.; Cao, C.; et al. Celastrol Ameliorates Neuronal Mitochondrial Dysfunction Induced by Intracerebral Hemorrhage via Targeting cAMP-Activated Exchange Protein-1. Adv. Sci. 2024, 11, e2307556. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, M.J.; Lee, H.L.; Moon, J.H.; Jeong, H.R.; Kim, H.J.; Chung, M.Y.; Heo, H.J. Porphyra tenera Protects Against PM(2.5)-Induced Cognitive Dysfunction with the Regulation of Gut Function. Mar. Drugs 2022, 20, 439. [Google Scholar] [CrossRef]
- Sun, X.Y.; Zhang, H.; Deng, J.W.; Yu, B.X.; Zhang, Y.H.; Ouyang, J.M. Regulatory Effects of Damaged Renal Epithelial Cells After Repair by Porphyra yezoensis Polysaccharides with Different Sulfation Degree on the Calcium Oxalate Crystal-Cell Interaction. Int. J. Nanomed. 2021, 16, 8087–8102. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.W.; Gu, L.Q.; Zeng, X.Y.; Sun, X.Y.; Ouyang, J.M. Sulfated Pelvetia siliquosa Polysaccharides Inhibit CaOx Stone Formation by Inhibiting Calcium Oxalate Crystallization, Cellular Inflammation, and Crystal Adhesion. J. Agric. Food Chem. 2025, 73, 1542–1562. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 Suppresses Neuroinflammation Against Cognitive Deficit in a Streptozotocin-Induced Rat Model of Alzheimer’s Disease Through Activation of BDNF-TrkB Signaling Pathway. Front. Pharmacol. 2019, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, X.; Yuan, H.; Huang, S.; Park, S. Mitigation of Memory Impairment with Fermented Fucoidan and λ-Carrageenan Supplementation through Modulating the Gut Microbiota and Their Metagenome Function in Hippocampal Amyloid-β Infused Rats. Cells 2022, 11, 2301. [Google Scholar] [CrossRef]
- Barros-Gomes, J.A.C.; Nascimento, D.L.A.; Silveira, A.C.R.; Silva, R.K.; Gomes, D.L.; Melo, K.R.T.; Almeida-Lima, J.; Camara, R.B.G.; Silva, N.B.; Rocha, H.A.O. In Vivo Evaluation of the Antioxidant Activity and Protective Action of the Seaweed Gracilaria birdiae. Oxidative Med. Cell. Longev. 2018, 2018, 9354296. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Sulfated polysaccharides of some seaweeds exhibit neuroprotection via mitigation of oxidative stress, cholinergic dysfunction and inhibition of Zn—Induced neuronal damage in HT-22 cells. BMC Complement. Med. Ther. 2020, 20, 251. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Reddy, S.M.; Suresh, V.; Pitchiah, S.; Subramanian, B., 4th. Anti-inflammatory Activities of Sulfated Polysaccharides from Ethanol Crude Extract of Spyrida Species Red Seaweed. Cureus 2023, 15, e50284. [Google Scholar]
- Cui, M.; Wu, J.; Wang, S.; Shu, H.; Zhang, M.; Liu, K.; Liu, K. Characterization and anti-inflammatory effects of sulfated polysaccharide from the red seaweed Gelidium pacificum Okamura. Int. J. Biol. Macromol. 2019, 129, 377–385. [Google Scholar] [CrossRef]
- Oliveira, F.F.B.; Bingana, R.D.; Morais, P.A.F.; Oliveira, S.; Barbosa, A.; Chaves, L.S.; Alencar, P.O.C.; Soares, P.M.G.; Souza, M.; Freitas, A.L.P.; et al. Sulfated polysaccharide from Gracilaria caudata reduces hypernociception and inflammatory response during arthritis in rodents. Int. J. Biol. Macromol. 2020, 161, 1061–1069. [Google Scholar] [CrossRef]
- Gong, Y.; Ma, Y.; Cheung, P.C.; You, L.; Liao, L.; Pedisić, S.; Kulikouskaya, V. Structural characteristics and anti-inflammatory activity of UV/H(2)O(2)-treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 152, 112157. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Oh, H.; Lee, M. Anti-inflammatory effects of Agar free-Gelidium amansii (GA) extracts in high-fat diet-induced obese mice. Nutr. Res. Pract. 2018, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Humayun, S.; Rjabovs, V.; Justine, E.E.; Darko, C.N.S.; Howlader, M.M.; Reile, I.; Sim, J.H.; Kim, Y.J.; Tuvikene, R. Immunomodulatory activity of red algal galactans and their partially depolymerized derivatives in RAW264.7 macrophages. Carbohydr. Polym. 2025, 347, 122741. [Google Scholar] [CrossRef] [PubMed]
- de Brito, T.V.; da Silva Prudêncio, R.; Sales, A.B.; Vieira, F., Jr.; Candeira, S.J.; Franco, Á.X.; Aragão, K.S.; de Albuquerque Ribeiro, R.; de Souza, M.H.L.P.; de Sousa Chaves, L.; et al. Anti-inflammatory effect of a sulphated polysaccharide fraction extracted from the red algae Hypnea musciformis via the suppression of neutrophil migration by the nitric oxide signalling pathway. J. Pharm. Pharmacol. 2013, 65, 724–733. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Deng, L.; Zhang, H.; He, B.; Cao, W.; Cui, Y. Pexidartinib (PLX3397) through restoring hippocampal synaptic plasticity ameliorates social isolation-induced mood disorders. Int. Immunopharmacol. 2022, 113 Pt B, 109436. [Google Scholar] [CrossRef]
- Lai, N.J.; Ngu, E.L.; Pang, J.R.; Wong, K.H.; Ardianto, C.; Ming, L.C.; Lim, S.H.; Walvekar, S.G.; Anwar, A.; Yow, Y.Y. Carrageenophyte Kappaphycus malesianus Inhibits Microglia-Mediated Neuroinflammation via Suppression of AKT/NF-κB and ERK Signaling Pathways. Mar. Drugs 2022, 20, 534. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Yang, X.; Li, L.; Qi, B.; Hu, X.; Ma, H.; Li, C.; Pan, C. Degradation of sulphated polysaccharides from Grateloupia livida and antioxidant activity of the degraded components. Int. J. Biol. Macromol. 2020, 156, 660–668. [Google Scholar] [CrossRef]
- Khan, B.M.; Qiu, H.M.; Xu, S.Y.; Liu, Y.; Cheong, K.L. Physicochemical characterization and antioxidant activity of sulphated polysaccharides derived from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 145, 1155–1161. [Google Scholar] [CrossRef]
- Casas-Arrojo, V.; Decara, J.; de Los Ángeles Arrojo-Agudo, M.; Pérez-Manríquez, C.; Abdala-Díaz, R.T. Immunomodulatory, Antioxidant Activity and Cytotoxic Effect of Sulfated Polysaccharides from Porphyridium cruentum. (S.F.Gray) Nägeli. Biomolecules 2021, 11, 488. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Mao, G.; Guo, Y.; Wang, G.; Sun, X.; Xu, N.; Zhang, Z. Structural Characterization of Gracilariopsis lemaneiformis Polysaccharide and Its Property in Delaying Cellular Senescence. Front. Nutr. 2022, 9, 876992. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Chen, H.; Sun, P. Extraction, Structural Characterization, and Potential Antioxidant Activity of the Polysaccharides from Four Seaweeds. Int. J. Mol. Sci. 2016, 17, 1988. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, T.; Hu, E.; Yan, Q.; Li, H.; Wang, Y.; Luo, J.; Tang, T. A novel strategy of integrating network pharmacology and transcriptome reveals antiapoptotic mechanisms of Buyang Huanwu Decoction in treating intracerebral hemorrhage. J. Ethnopharmacol. 2024, 319 Pt 1, 117123. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, R.; Cai, W.; Jiang, L.; Chen, K.; Shi, J.; Lou, J.; Yu, L.; Wu, C.; Yang, L.; et al. Davunetide promotes structural and functional recovery of the injured spinal cord by promoting autophagy. Neural Regen. Res. 2025. [Google Scholar] [CrossRef] [PubMed]
- Rudtanatip, T.; Pariwatthanakun, C.; Somintara, S.; Sakaew, W.; Wongprasert, K. Structural characterization, antioxidant activity, and protective effect against hydrogen peroxide-induced oxidative stress of chemically degraded Gracilaria fisheri sulfated galactans. Int. J. Biol. Macromol. 2022, 206, 51–63. [Google Scholar] [CrossRef]
- Liao, X.; Yang, L.; Chen, M.; Yu, J.; Zhang, S.; Ju, Y. The hypoglycemic effect of a polysaccharide (GLP) from Gracilaria lemaneiformis and its degradation products in diabetic mice. Food Funct. 2015, 6, 2542–2549. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Chang, Y.; Shen, J.; Xue, C. Expression and Characterization of a Novel β-Porphyranase from Marine Bacterium Wenyingzhuangia fucanilytica: A Biotechnological Tool for Degrading Porphyran. J. Agric. Food Chem. 2019, 67, 9307–9313. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, T.K.; Ahn, J.H.; Yang, S.R.; Shin, M.C.; Cho, J.H.; Won, M.H.; Kang, I.J.; Park, J.H. Porphyran Attenuates Neuronal Loss in the Hippocampal CA1 Subregion Induced by Ischemia and Reperfusion in Gerbils by Inhibiting NLRP3 Inflammasome-Mediated Neuroinflammation. Mar. Drugs 2024, 22, 170. [Google Scholar] [CrossRef]
- Geng, L.; Wang, J.; Zhang, Z.; Yue, Y.; Zhang, Q. Structure and Bioactivities of Porphyrans and Oligoporphyrans. Curr. Pharm. Des. 2019, 25, 1163–1171. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Pan, Y.; Wang, G.; Mao, G. The degraded polysaccharide from Pyropia haitanensis represses amyloid beta peptide-induced neurotoxicity and memory in vivo. Int. J. Biol. Macromol. 2020, 146, 725–729. [Google Scholar] [CrossRef]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, T.; Cho, K.; Isaka, S.; Ueno, M.; Jin, J.O.; Yamaguchi, K.; Kim, D.; Oda, T. Protective effect of porphyran isolated from discolored nori (Porphyra yezoensis) on lipopolysaccharide-induced endotoxin shock in mice. Int. J. Biol. Macromol. 2016, 93 Pt A, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Kim, S.J.; Yadav, D.; An, E.K.; Zhang, W.; Eom, H.Y.; Kwak, M.; Oda, T.; Lee, P.C.; Jin, J.O. Pyropia yezoensis-derived porphyran attenuates acute and chronic colitis by suppressing dendritic cells. Int. J. Biol. Macromol. 2023, 231, 123148. [Google Scholar] [CrossRef]
- Wang, Y.; Hwang, J.Y.; Park, H.B.; Yadav, D.; Oda, T.; Jin, J.O. Porphyran isolated from Pyropia yezoensis inhibits lipopolysaccharide-induced activation of dendritic cells in mice. Carbohydr. Polym. 2020, 229, 115457. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, Q.M.; Li, G.L.; Sun, L.C.; Gao, Y.Y.; Zhang, Y.F.; Liu, H.; Cao, M.J.; Liu, G.M. The anti-diarrhea activity of red algae-originated sulphated polysaccharides on ETEC-K88 infected mice. RSC Adv. 2019, 9, 2360–2370. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, L.; Zhang, J.; Wang, J.; Zhang, Q.; Duan, D.; Zhang, Q. Oligo-Porphyran Ameliorates Neurobehavioral Deficits in Parkinsonian Mice by Regulating the PI3K/Akt/Bcl-2 Pathway. Mar. Drugs 2018, 16, 82. [Google Scholar] [CrossRef]
- Wang, W.; Song, N.; Jia, F.; Xie, J.; Zhang, Q.; Jiang, H. Neuroprotective effects of porphyran derivatives against 6-hydroxydopamine-induced cytotoxicity is independent on mitochondria restoration. Ann. Transl. Med. 2015, 3, 39. [Google Scholar]
- Oh, J.H.; Kim, E.Y.; Nam, T.J. Phycoerythrin-Derived Tryptic Peptide of a Red Alga Pyropia yezoensis Attenuates Glutamate-Induced ER Stress and Neuronal Senescence in Primary Rat Hippocampal Neurons. Mol. Nutr. Food Res. 2018, 62, e1700469. [Google Scholar] [CrossRef]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse (Palmaria palmata) resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100 Pt 1, 514–521. [Google Scholar] [CrossRef]
- Yi, L.T.; Zhang, M.M.; Cheng, J.; Wan, H.Q.; Li, C.F.; Zhu, J.X.; Zhang, Q.P.; Liu, Q.; Xu, G.H. Antidepressant-like Effects of Degraded Porphyran Isolated from Porphyra haitanensis. Mol. Nutr. Food Res. 2021, 65, e2000869. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, M.; Sun, L.; Fu, X.; Yu, T. Phycocyanin hexamers and their associated linker polypeptides in phycobilisomes from the marine red alga Polysiphonia urceolata. Int. J. Biol. Macromol. 2025, 293, 139398. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Møller, A.H.; Ilmjärv, T.; Dalsgaard, T.K. Stability of R-phycoerythrin from Furcellaria lumbricalis—Dependence on purification strategies and purity. Food Res. Int. 2024, 190, 114595. [Google Scholar] [CrossRef] [PubMed]
- Dagnino-Leone, J.; Figueroa, C.P.; Castañeda, M.L.; Youlton, A.D.; Vallejos-Almirall, A.; Agurto-Muñoz, A.; Pavón Pérez, J.; Agurto-Muñoz, C. Phycobiliproteins: Structural aspects, functional characteristics, and biotechnological perspectives. Comput. Struct. Biotechnol. J. 2022, 20, 1506–1527. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.N.; Lu, L.; Peng, P.P.; Fu, Z.J.; Miao, D.; Zhou, M.; Noy, D.; Zhao, K.H. The phycobilisome core-membrane linkers from Synechocystis sp. PCC 6803 and red-algae assemble in the same topology. Plant J. Cell Mol. Biol. 2021, 107, 1420–1431. [Google Scholar] [CrossRef]
- Kim, E.Y.; Choi, Y.H.; Nam, T.J. Identification and antioxidant activity of synthetic peptides from phycobiliproteins of Pyropia yezoensis. Int. J. Mol. Med. 2018, 42, 789–798. [Google Scholar] [CrossRef]
- Sato, N.; Furuta, T.; Takeda, T.; Miyabe, Y.; Ura, K.; Takagi, Y.; Yasui, H.; Kumagai, Y.; Kishimura, H. Antioxidant activity of proteins extracted from red alga dulse harvested in Japan. J. Food Biochem. 2019, 43, e12709. [Google Scholar] [CrossRef]
- Fang, C.; Song, K.; Yan, Z.; Liu, G. Monitoring phycocyanin in global inland waters by remote sensing: Progress and future developments. Water Res. 2025, 275, 123176. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, H.; Hu, J.; Zheng, B.; Zhang, Y. Anti-Aging Effects of R-Phycocyanin from Porphyra haitanensis on HUVEC Cells and Drosophila melanogaster. Mar. Drugs 2022, 20, 468. [Google Scholar] [CrossRef]
- Xiang, Q.; Xiang, Y.; Liu, Y.; Chen, Y.; He, Q.; Chen, T.; Tang, L.; He, B.; Li, J. Revealing the potential therapeutic mechanism of Lonicerae Japonicae Flos in Alzheimer’s disease: A computational biology approach. Front. Med. 2024, 11, 1468561. [Google Scholar] [CrossRef]
- Remya, C.; Dileep, K.V.; Variyar, E.J.; Zhang, K.Y.J.; Omkumar, R.V.; Sadasivan, C. Chemical similarity assisted search for acetylcholinesterase inhibitors: Molecular modeling and evaluation of their neuroprotective properties. Int. J. Biol. Macromol. 2021, 174, 466–476. [Google Scholar] [CrossRef]
- Liu, Y.; Uras, G.; Onuwaje, I.; Li, W.; Yao, H.; Xu, S.; Li, X.; Li, X.; Phillips, J.; Allen, S.; et al. Novel inhibitors of AChE and Aβ aggregation with neuroprotective properties as lead compounds for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2022, 235, 114305. [Google Scholar] [CrossRef] [PubMed]
- Mune Mune, M.A.; Miyabe, Y.; Shimizu, T.; Matsui, W.; Kumagai, Y.; Kishimura, H. Characterisation of Bioactive Peptides from Red Alga Gracilariopsis chorda. Mar. Drugs 2023, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Windarto, S.; Lee, M.C.; Nursyam, H.; Hsu, J.L. First Report of Screening of Novel Angiotensin-I Converting Enzyme Inhibitory Peptides Derived from the Red Alga Acrochaetium sp. Mar. Biotechnol. 2022, 24, 882–894. [Google Scholar]
- Sumikawa, K.; Takei, K.; Kumagai, Y.; Shimizu, T.; Yasui, H.; Kishimura, H. In Silico Analysis of ACE Inhibitory Peptides from Chloroplast Proteins of Red Alga Grateloupia asiatica. Mar. Biotechnol. 2020, 22, 391–402. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Polyakov, N.E.; Focsan, A.L.; Gao, Y.; Kispert, L.D. The Endless World of Carotenoids-Structural, Chemical and Biological Aspects of Some Rare Carotenoids. Int. J. Mol. Sci. 2023, 24, 9885. [Google Scholar] [CrossRef]
- Kim, D.S.; Kang, S.; Moon, N.R.; Shin, B.K.; Park, S. Zeaxanthin and Lutein Ameliorate Alzheimer’s Disease-like Pathology: Modulation of Insulin Resistance, Neuroinflammation, and Acetylcholinesterase Activity in an Amyloid-β Rat Model. Int. J. Mol. Sci. 2024, 25, 9828. [Google Scholar] [CrossRef]
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids supplementation and inflammation: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 8161–8177. [Google Scholar] [CrossRef]
- Yang, Y.; Li, R.; Hui, J.; Li, L.; Zheng, X. β-Carotene attenuates LPS-induced rat intestinal inflammation via modulating autophagy and regulating the JAK2/STAT3 and JNK/p38 MAPK signaling pathways. J. Food Biochem. 2021, 45, e13544. [Google Scholar] [CrossRef]
- Stringham, N.T.; Green, M.; Roche, W.; Prado-Cabrero, A.; Mulcahy, R.; Nolan, J. Lutein, zeaxanthin, and meso-zeaxanthin supplementation attenuates inflammatory cytokines and markers of oxidative cardiovascular processes in humans. Nutr. Metab. Cardiovasc. Dis. NMCD 2024, 34, 1976–1983. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A promising therapy for diabetic encephalopathy through inhibition of hippocampal ferroptosis. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 126, 154887. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Xu, L.; Zhao, S.; Hu, S.; Ma, A.; Ma, Y. Lutein Can Alleviate Oxidative Stress, Inflammation, and Apoptosis Induced by Excessive Alcohol to Ameliorate Reproductive Damage in Male Rats. Nutrients 2022, 14, 2385. [Google Scholar] [CrossRef]
- Hu, E.; Li, Z.; Li, T.; Yang, X.; Ding, R.; Jiang, H.; Su, H.; Cheng, M.; Yu, Z.; Li, H.; et al. A novel microbial and hepatic biotransformation-integrated network pharmacology strategy explores the therapeutic mechanisms of bioactive herbal products in neurological diseases: The effects of Astragaloside IV on intracerebral hemorrhage as an example. Chin. Med. 2023, 18, 40. [Google Scholar] [CrossRef]
- Liu, D.F.; Bai, M.; Du, N.N.; Shen, S.; Li, Z.Y.; Zhang, X.; Guo, R.; Yao, G.D.; Song, S.J.; Huang, X.X. Insight into Isolation and Characterization of Phenolic Compounds from Hawthorn (Crataegus pinnatifida Bge.) with Antioxidant, Anti-Acetylcholinesterase, and Neuroprotective Activities. Plant Foods Hum. Nutr. 2022, 77, 538–544. [Google Scholar] [CrossRef]
- Paudel, P.; Park, S.E.; Seong, S.H.; Jung, H.A.; Choi, J.S. Bromophenols from Symphyocladia latiuscula Target Human Monoamine Oxidase and Dopaminergic Receptors for the Management of Neurodegenerative Diseases. J. Agric. Food Chem. 2020, 68, 2426–2436. [Google Scholar] [CrossRef]
- Gao, X.H.; Tang, J.J.; Liu, H.R.; Liu, L.B.; Liu, Y.Z. Structure-activity study of fluorine or chlorine-substituted cinnamic acid derivatives with tertiary amine side chain in acetylcholinesterase and butyrylcholinesterase inhibition. Drug Dev. Res. 2019, 80, 438–445. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Chen, Y.M.; Liu, H.R.; Yan, J.Y.; Cui, P.W.; Zhang, Q.F.; Gao, X.H.; Feng, X.; Liu, Y.Z. Nitrogen-containing flavonoid and their analogs with diverse B-ring in acetylcholinesterase and butyrylcholinesterase inhibition. Drug Dev. Res. 2020, 81, 1037–1047. [Google Scholar] [CrossRef]
- Bayrak, C.; Taslimi, P.; Kilinc, N.; Gulcin, I.; Menzek, A. Synthesis and Biological Activity of Some Bromophenols and Their Derivatives Including Natural Products. Chem. Biodivers. 2023, 20, e202300469. [Google Scholar] [CrossRef]
- Oztaskin, N.; Goksu, S.; Demir, Y.; Maras, A.; Gulcin, İ. Synthesis of Novel Bromophenol with Diaryl Methanes-Determination of Their Inhibition Effects on Carbonic Anhydrase and Acetylcholinesterase. Molecules 2022, 27, 7426. [Google Scholar] [CrossRef]
- Dong, H.; Liu, M.; Wang, L.; Liu, Y.; Lu, X.; Stagos, D.; Lin, X.; Liu, M. Bromophenol Bis (2,3,6-Tribromo-4,5-dihydroxybenzyl) Ether Protects HaCaT Skin Cells from Oxidative Damage via Nrf2-Mediated Pathways. Antioxidants 2021, 10, 1436. [Google Scholar] [CrossRef]
- Hofer, S.; Hartmann, A.; Orfanoudaki, M.; Ngoc, H.N.; Nagl, M.; Karsten, U.; Heesch, S.; Ganzera, M. Development and Validation of an HPLC Method for the Quantitative Analysis of Bromophenolic Compounds in the Red Alga Vertebrata lanosa. Mar. Drugs 2019, 17, 675. [Google Scholar] [CrossRef]
- Nabil-Adam, A.; Ashour, M.L.; Shreadah, M.A. Modulation of MAPK/NF-κB Pathway and NLRP3 Inflammasome by Secondary Metabolites from Red Algae: A Mechanistic Study. ACS Omega 2023, 8, 37971–37990. [Google Scholar] [CrossRef]
- Palaniveloo, K.; Ong, K.H.; Satriawan, H.; Abdul Razak, S.; Suciati, S.; Hung, H.Y.; Hirayama, S.; Rizman-Idid, M.; Tan, J.K.; Yong, Y.S.; et al. In vitro and in silico cholinesterase inhibitory potential of metabolites from Laurencia snackeyi (Weber-van Bosse) M. Masuda. 3 Biotech 2023, 13, 337. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Mahendran, S.; Maheswari, P.; Sasikala, V.; Rubika, J.J.; Pandiarajan, J. In vitro antioxidant study of polyphenol from red seaweeds dichotomously branched gracilaria Gracilaria edulis and robust sea moss Hypnea valentiae. Toxicol. Rep. 2021, 8, 1404–1411. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; Farag, M.A.; Kontominas, M.G.; Shakour, Z.T.; Ramadan, A.R. Nanoencapsulated Extract of a Red Seaweed (Rhodophyta) Species as a Promising Source of Natural Antioxidants. ACS Omega 2022, 7, 6539–6548. [Google Scholar] [CrossRef]
- Kazłowska, K.; Hsu, T.; Hou, C.C.; Yang, W.C.; Tsai, G.J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef]
- Vega, J.; Bárcenas-Pérez, D.; Fuentes-Ríos, D.; López-Romero, J.M.; Hrouzek, P.; Figueroa, F.L.; Cheel, J. Isolation of Mycosporine-like Amino Acids from Red Macroalgae and a Marine Lichen by High-Performance Countercurrent Chromatography: A Strategy to Obtain Biological UV-Filters. Mar. Drugs 2023, 21, 357. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Alilou, M.; Hartmann, A.; Mayr, J.; Karsten, U.; Nguyen-Ngoc, H.; Ganzera, M. Isolation and Structure Elucidation of Novel Mycosporine-like Amino Acids from the Two Intertidal Red Macroalgae Bostrychia scorpioides and Catenella caespitosa. Mar. Drugs 2023, 21, 543. [Google Scholar] [CrossRef]
- Sun, Y.; Han, X.; Hu, Z.; Cheng, T.; Tang, Q.; Wang, H.; Deng, X.; Han, X. Extraction, Isolation and Characterization of Mycosporine-like Amino Acids from Four Species of Red Macroalgae. Mar. Drugs 2021, 19, 615. [Google Scholar] [CrossRef]
- Nishida, Y.; Kumagai, Y.; Michiba, S.; Yasui, H.; Kishimura, H. Efficient Extraction and Antioxidant Capacity of Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2020, 18, 502. [Google Scholar] [CrossRef]
- Nishida, Y.; Saburi, W.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2022, 20, 184. [Google Scholar] [CrossRef]
- Dias, L.P.; Santos, A.L.E.; Araújo, N.M.S.; Silva, R.R.S.; Santos, M.H.C.; Roma, R.R.; Rocha, B.A.M.; Oliveira, J.T.A.; Teixeira, C.S. Machaerium acutifolium lectin alters membrane structure and induces ROS production in Candida parapsilosis. Int. J. Biol. Macromol. 2020, 163, 19–25. [Google Scholar] [CrossRef]
- García-Revilla, J.; Boza-Serrano, A.; Espinosa-Oliva, A.M.; Soto, M.S.; Deierborg, T.; Ruiz, R.; de Pablos, R.M.; Burguillos, M.A.; Venero, J.L. Galectin-3, a rising star in modulating microglia activation under conditions of neurodegeneration. Cell Death Dis. 2022, 13, 628. [Google Scholar] [CrossRef]
- Roma, R.R.; Oliveira, F.S.A.; Fernandes, D.G.S.; Garcia, W.; Soares, E.N.; Costa, S.L.; Teixeira, C.S. ConA-glutamate interactions: New insights into its neuroprotective effect. Int. J. Biol. Macromol. 2025, 310, 143463. [Google Scholar] [CrossRef]
- Mesquita, J.X.; de Brito, T.V.; Fontenelle, T.P.C.; Damasceno, R.O.S.; de Souza, M.; de Souza Lopes, J.L.; Beltramini, L.M.; Barbosa, A.; Freitas, A.L.P. Lectin from red algae Amansia multifida Lamouroux: Extraction, characterization and anti-inflammatory activity. Int. J. Biol. Macromol. 2021, 170, 532–539. [Google Scholar] [CrossRef]
- Fontenelle, T.P.C.; Lima, G.C.; Mesquita, J.X.; Lopes, J.L.S.; de Brito, T.V.; Vieira Júnior, F.D.C.; Sales, A.B.; Aragão, K.S.; Souza, M.; Barbosa, A.; et al. Lectin obtained from the red seaweed Bryothamnion triquetrum: Secondary structure and anti-inflammatory activity in mice. Int. J. Biol. Macromol. 2018, 112, 1122–1130. [Google Scholar] [CrossRef]
- Alves, M.F.A.; Barreto, F.K.A.; Vasconcelos, M.A.; Nascimento Neto, L.G.D.; Carneiro, R.F.; Silva, L.T.D.; Nagano, C.S.; Sampaio, A.H.; Teixeira, E.H. Antihyperglycemic and antioxidant activities of a lectin from the marine red algae, Bryothamnion seaforthii, in rats with streptozotocin-induced diabetes. Int. J. Biol. Macromol. 2020, 158, 773–780. [Google Scholar] [CrossRef]
- Cornacchia, C.; Cacciatore, I.; Baldassarre, L.; Mollica, A.; Feliciani, F.; Pinnen, F. 2,5-diketopiperazines as neuroprotective agents. Mini Rev. Med. Chem. 2012, 12, 2–12. [Google Scholar] [CrossRef]
- Li, H.L.; Yang, S.Q.; Li, X.M.; Li, X.; Wang, B.G. Structurally diverse alkaloids produced by Aspergillus creber EN-602, an endophytic fungus obtained from the marine red alga Rhodomela confervoides. Bioorganic Chem. 2021, 110, 104822. [Google Scholar] [CrossRef]
- Turkez, H.; Cacciatore, I.; Arslan, M.E.; Fornasari, E.; Marinelli, L.; Di Stefano, A.; Mardinoglu, A. Histidyl-Proline Diketopiperazine Isomers as Multipotent Anti-Alzheimer Drug Candidates. Biomolecules 2020, 10, 737. [Google Scholar] [CrossRef]
- Kumar, D.; Gupta, S.K.; Ganeshpurkar, A.; Gutti, G.; Krishnamurthy, S.; Modi, G.; Singh, S.K. Development of Piperazinediones as dual inhibitor for treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2018, 150, 87–101. [Google Scholar] [CrossRef]
- Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: A review. Aging Clin. Exp. Res. 2012, 24, 580–587. [Google Scholar] [CrossRef]
- Wu, S.; Yue, Y.; Tian, H.; Tao, L.; Wang, Y.; Xiang, J.; Wang, S.; Ding, H. Tramiprosate protects neurons against ischemic stroke by disrupting the interaction between PSD95 and nNOS. Neuropharmacology 2014, 83, 107–117. [Google Scholar] [CrossRef]
- Minoia, A.; Piritore, F.C.; Bolognin, S.; Pessoa, J.; de Jesus, B.B.; Tiso, N.; Romanelli, M.G.; Schwamborn, J.C.; Dalle Carbonare, L.; Valenti, M.T. Antioxidant, Osteogenic, and Neuroprotective Effects of Homotaurine in Aging and Parkinson’s Disease Models. Antioxidants 2025, 14, 249. [Google Scholar] [CrossRef]
- Spalletta, G.; Cravello, L.; Gianni, W.; Piras, F.; Iorio, M.; Cacciari, C.; Casini, A.R.; Chiapponi, C.; Sancesario, G.; Fratangeli, C.; et al. Homotaurine Effects on Hippocampal Volume Loss and Episodic Memory in Amnestic Mild Cognitive Impairment. J. Alzheimers Dis. JAD 2016, 50, 807–816. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef]
- Yamamoto, R.; Toriumi, S.; Kawagoe, C.; Saburi, W.; Kishimura, H.; Kumagai, Y. Extraction and antioxidant capacity of mycosporine-like amino acids from red algae in Japan. Biosci. Biotechnol. Biochem. 2024, 88, 830–838. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Rivas, S.; Torres, M.D.; Domínguez, H. Microwave-Assisted Extraction of Carrageenan from Sarcopeltis skottsbergii. Mar. Drugs 2023, 21, 83. [Google Scholar] [CrossRef]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum (Rhodophyta). Ultrason. Sonochemistry 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Otero, P.; Carpena, M.; Garcia-Oliveira, P.; Echave, J.; Soria-Lopez, A.; Garcia-Perez, P.; Fraga-Corral, M.; Cao, H.; Nie, S.; Xiao, J.; et al. Seaweed polysaccharides: Emerging extraction technologies, chemical modifications and bioactive properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1901–1929. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Tounsi, L.; Djomdi, D.; Pierre, G.; Delattre, C.; Ursu, A.V.; Fendri, I.; Abdelkafi, S.; Michaud, P. Bioactive Polysaccharides from Seaweeds. Molecules 2020, 25, 3152. [Google Scholar] [CrossRef] [PubMed]
- Wassie, T.; Niu, K.; Xie, C.; Wang, H.; Xin, W. Extraction Techniques, Biological Activities and Health Benefits of Marine Algae Enteromorpha prolifera Polysaccharide. Front. Nutr. 2021, 8, 747928. [Google Scholar] [CrossRef]
- Ma, J.; Hu, J.; Sha, X.; Meng, D.; Yang, R. Phycobiliproteins, the pigment-protein complex form of natural food colorants and bioactive ingredients. Crit. Rev. Food Sci. Nutr. 2024, 64, 2999–3017. [Google Scholar] [CrossRef]
- Goksen, G. Elucidation and quantification health-promoting phenolic compounds, antioxidant properties and sugar levels of ultrasound assisted extraction, aroma compositions and amino acids profiles of macroalgae, Laurencia papillosa. Ultrason. Sonochemistry 2023, 98, 106527. [Google Scholar] [CrossRef]
- Ha, H.T.; Cuong, D.X.; Thuy, L.H.; Thuan, P.T.; Tuyen, D.T.T.; Mo, V.T.; Dong, D.H. Carrageenan of Red Algae Eucheuma gelatinae: Extraction, Antioxidant Activity, Rheology Characteristics, and Physicochemistry Characterization. Molecules 2022, 27, 1268. [Google Scholar] [CrossRef]
- Zheng, M.; Ma, M.; Yang, Y.; Liu, Z.; Liu, S.; Hong, T.; Ni, H.; Jiang, Z. Structural characterization and antioxidant activity of polysaccharides extracted from Porphyra haitanensis by different methods. Int. J. Biol. Macromol. 2023, 242 Pt 2, 125003. [Google Scholar] [CrossRef]
- Li, T.; Xu, J.; Wang, W.; Chen, Z.; Li, C.; Wu, H.; Wu, H.; Xiang, W. A Novel Three-Step Extraction Strategy for High-Value Products from Red Algae Porphyridium purpureum. Foods 2021, 10, 2164. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Xu, B.; Xiang, W.; Li, A.; Li, T. Extraction Optimization of Polysaccharides from Wet Red Microalga Porphyridium purpureum Using Response Surface Methodology. Mar. Drugs 2024, 22, 498. [Google Scholar] [CrossRef]
- Dong, M.; Jiang, Y.; Wang, C.; Yang, Q.; Jiang, X.; Zhu, C. Determination of the Extraction, Physicochemical Characterization, and Digestibility of Sulfated Polysaccharides in Seaweed-Porphyra haitanensis. Mar. Drugs 2020, 18, 539. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wu, Y.; Luo, Y.; Lv, W.; Chen, S.; Wang, N.; Meng, M.; Liao, K.; Yang, Y. Study on the Extraction Technology and Antioxidant Capacity of Rhodymenia intricata Polysaccharides. Foods 2024, 13, 3964. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, Q.; Huang, S.; Shao, P.; You, L.; Pedisić, S. Digestion & fermentation characteristics of sulfated polysaccharides from Gracilaria chouae using two extraction methods in vitro and in vivo. Food Res. Int. 2021, 145, 110406. [Google Scholar] [PubMed]
- Barral-Martínez, M.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Tailoring hybrid carrageenans from Mastocarpus stellatus red seaweed using microwave hydrodiffusion and gravity. Carbohydr. Polym. 2020, 248, 116830. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, X.; Zhang, J.; Zhang, Y.; Chen, J.; Chen, F.; Xiao, A. Pretreatment Techniques and Green Extraction Technologies for Agar from Gracilaria lemaneiformis. Mar. Drugs 2021, 19, 617. [Google Scholar] [CrossRef]
- Pereira, S.G.; Martins, A.A.; Mata, T.M.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Life cycle assessment and cost analysis of innovative agar extraction technologies from red seaweeds. Bioresour. Technol. 2024, 414, 131649. [Google Scholar] [CrossRef]
- Xu, S.Y.; Chen, X.Q.; Liu, Y.; Cheong, K.L. Ultrasonic/microwave-assisted extraction, simulated digestion, and fermentation in vitro by human intestinal flora of polysaccharides from Porphyra haitanensis. Int. J. Biol. Macromol. 2020, 152, 748–756. [Google Scholar] [CrossRef]
- Castejón, N.; Parailloux, M.; Izdebska, A.; Lobinski, R.; Fernandes, S.C.M. Valorization of the Red Algae Gelidium sesquipedale by Extracting a Broad Spectrum of Minor Compounds Using Green Approaches. Mar. Drugs 2021, 19, 574. [Google Scholar] [CrossRef]
- Le Guillard, C.; Bergé, J.P.; Donnay-Moreno, C.; Cornet, J.; Ragon, J.Y.; Fleurence, J.; Dumay, J. Optimization of R-Phycoerythrin Extraction by Ultrasound-Assisted Enzymatic Hydrolysis: A Comprehensive Study on the Wet Seaweed Grateloupia turuturu. Mar. Drugs 2023, 21, 213. [Google Scholar] [CrossRef]
- Li, K.; Jiang, C.; Han, S.I.; Kang, S.; Chen, J.; Won, D.; Kang, Y.; Bae, B.; Choi, Y.E.; Kim, H.S.; et al. Green and efficient method to acquire high-value phycobiliprotein from microalgal biomass involving deep eutectic solvent-based ultrasound-assisted extraction. Food Chem. 2024, 449, 139196. [Google Scholar] [CrossRef]
- Bastos-Filho, A.J.U.; Brito, A.A.M.; Lopes, F.L.S.; Lima, F.E.S.; Araújo-Filho, A.A.L.; Ferreira, V.R.; Martins, J.R.P.; Holanda, T.B.L.; de Oliveira, H.D.; Benevides, N.M.B.; et al. Extraction, purification, physicochemical properties, and spectroscopic stability of the R-Phycoerythrin from the seaweed Solieria filiformis cultivated in Ceara Coast/Brazil. Int. J. Biol. Macromol. 2025, 310 Pt 3, 143035. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Barroso, S.; Mendes, S.; Amaral, R.A.; Dias, J.R.; Baptista, T.; Saraiva, J.A.; Alves, N.M.; Gil, M.M. Optimization of phycobiliprotein pigments extraction from red algae Gracilaria gracilis for substitution of synthetic food colorants. Food Chem. 2020, 321, 126688. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Barroso, S.; Mendes, S.; Gil, M.M. Stability, kinetics, and application study of phycobiliprotein pigments extracted from red algae Gracilaria gracilis. J. Food Sci. 2020, 85, 3400–3405. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.T.; García-García, P.; Korbee, N.; Vega, J.; Señoráns, F.J.; Figueroa, F.L. Optimizing the Extraction of Bioactive Compounds from Porphyra linearis (Rhodophyta): Evaluating Alkaline and Enzymatic Hydrolysis for Nutraceutical Applications. Mar. Drugs 2024, 22, 284. [Google Scholar] [CrossRef]







| Activities | Compounds | In Vitro or In Vivo | Methods | Doses | Results | References |
|---|---|---|---|---|---|---|
| Antioxidant activity | Porphyran | In vitro | Superoxide, hydrogen peroxide, and hydroxyl radical scavenging activity (DC-porphyrin) of porphyrin derived from color-changing seaweed. | 10–1000 µg/mL | Superoxide scavenging activity (IC50: 415.9 μg/mL) and hydroxyl radical scavenging activity (IC50: 32.7 μg/mL). | [61] |
| Anti-inflammatory activity | Porphyran | In vivo | The effects of DC-porphyrin and a DC-porphyrin component F1 on the production of inflammatory mediators induced by LPS in mice. | 100 mg/kg | Significantly reduced the levels of pro-inflammatory mediators NO and TNF-α. | [62] |
| Porphyran | In vivo and in vitro | Oral or intraperitoneal injection of porphyrin to inhibit the progression of DSS-induced colitis in mice. | 50 μg/mL | The levels of interferon-γ and interleukin-17 in T cells of the oral porphyrin group decreased. Inhibited T cell activation by suppressing dendritic cells and macrophages. | [63] | |
| Porphyran | In vivo and in vitro | Inhibit the up-regulation of costimulatory molecules and CCR7 expression in bmdc induced by LPS in vitro and in vivo. | 0, 10, 25, 50, 100 μg/mL | Porphyrin is a very promising drug for the treatment of endotoxin-mediated inflammatory diseases. | [64] | |
| Porphyran | In vivo | The effects of porphyrin on inflammatory factors, IgA antibodies, and non-specific immune factors in mice infected with ETEC-K88. | 70.54 ng/mL, 79.20 ng/mL, 10 mg/d | Reduced the levels of pro-inflammatory factors (MCP-1, TNF-a, IFN-g, IL-6), IgA, and NBT. | [65] | |
| Neuroprotective activity | Oligo-Porphyran | In vivo | PI3K/Akt/Bcl-2 pathway; dopamine (DA) metabolism; behavioral deficits; pole test; traction test. | 25, 50 mg/kg | Regulated the PI3K/Akt/Bcl-2 pathway; ameliorated neurobehavioral deficits. | [66] |
| Porphyran and its derivatives | In vitro | MTT assay; Rhodamine123 using flow cytometry. | <1 mg/mL | Both AP and PP antagonized the weak toxicity of 6-OHDA on MES23.5 dopaminergic cells. | [67] | |
| Porphyran | In vivo | PYP pretreatment can prevent the decline in cell viability and the increase in GRP78 expression caused by glutamate exposure. | 1 μg/mL | Blocking the NMDA receptor reduced the phosphorylation level of JNK. Down-regulation of GRP78 expression, β-galactosidase activity, and neuromutability increased. | [68] | |
| Anti-inflammatory activity | Phycobiliproteins | In vivo | Algae bile protein and chlorophyll a were obtained by water extraction, and d-DWE was obtained by hot lysozyme digestion to evaluate inflammatory factors. | - | Reduced TNF-α, IL-6, and NO in LPS-stimulated RAW 264.7 cells, alleviated the induced acute inflammation. | [69] |
| Type | Color | Absorption Peaks (nm) | Fluorescence Properties | Existence Form | Representative Algae Species |
|---|---|---|---|---|---|
| R-Phycoerythrin,R-PE | Pink-red | 495, 545, 565 | Strong red fluorescence (~575 nm) | Disk-shaped hexamers | Porphyra (Nori) |
| R-Phycocyanin, R-PC | Blue | 615, 650 | Orange fluorescence (~647 nm) | α and β subunits form into trimers | Gracilaria |
| Allophycocyanin, APC | Blue-green | 650, 660 | Deep red fluorescence (~660 nm) | Hexamers or trimers | Chondrus (Irish moss) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Shi, W.; Mao, Z.; Xie, W.; Wan, G. Neuroprotective Mechanisms of Red Algae-Derived Bioactive Compounds in Alzheimer’s Disease: An Overview of Novel Insights. Mar. Drugs 2025, 23, 274. https://doi.org/10.3390/md23070274
Wang T, Shi W, Mao Z, Xie W, Wan G. Neuroprotective Mechanisms of Red Algae-Derived Bioactive Compounds in Alzheimer’s Disease: An Overview of Novel Insights. Marine Drugs. 2025; 23(7):274. https://doi.org/10.3390/md23070274
Chicago/Turabian StyleWang, Tianzi, Wenling Shi, Zijun Mao, Wei Xie, and Guoqing Wan. 2025. "Neuroprotective Mechanisms of Red Algae-Derived Bioactive Compounds in Alzheimer’s Disease: An Overview of Novel Insights" Marine Drugs 23, no. 7: 274. https://doi.org/10.3390/md23070274
APA StyleWang, T., Shi, W., Mao, Z., Xie, W., & Wan, G. (2025). Neuroprotective Mechanisms of Red Algae-Derived Bioactive Compounds in Alzheimer’s Disease: An Overview of Novel Insights. Marine Drugs, 23(7), 274. https://doi.org/10.3390/md23070274