Sequential Extraction of Bioactive Saponins from Cucumaria frondosa Viscera: Supercritical CO2–Ethanol Synergy for Enhanced Yields and Antioxidant Performance
Abstract
1. Introduction
2. Results and Discussion
2.1. Impacts of Interference Compounds on Saponin Quantification and Extraction
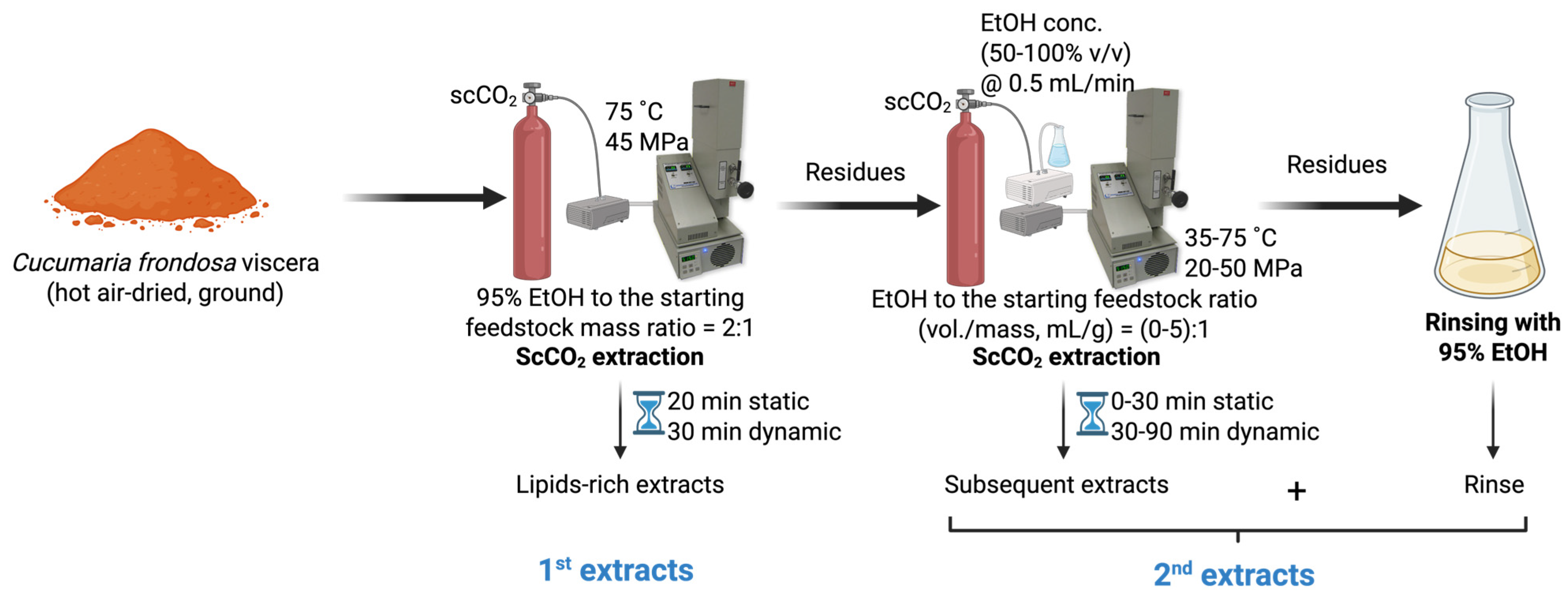
2.2. Sequential ScCO2 Extraction of Saponins
2.3. Sequential Extraction: ScCO2 Extraction Followed by Conventional Extraction
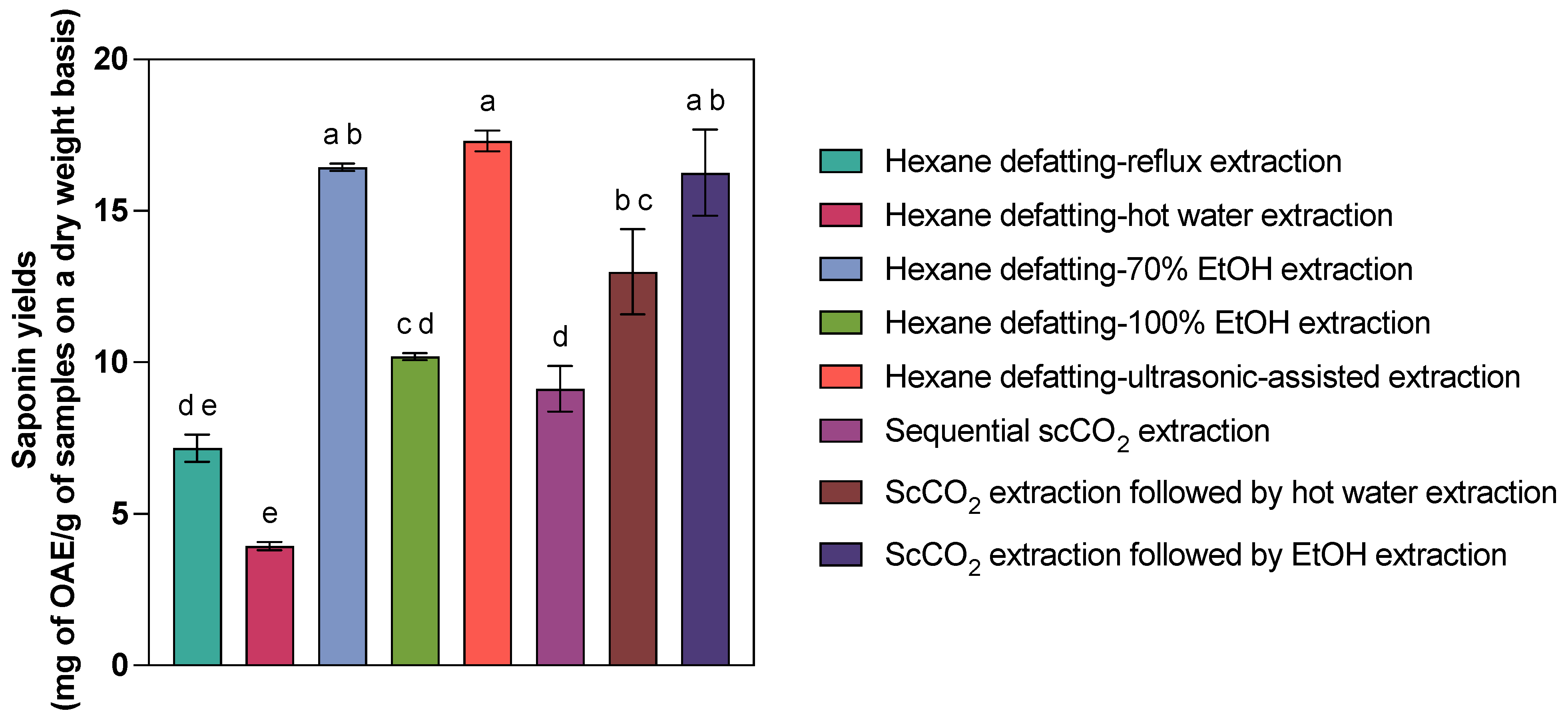
2.4. Comparison of Saponin Yields from Different Methods
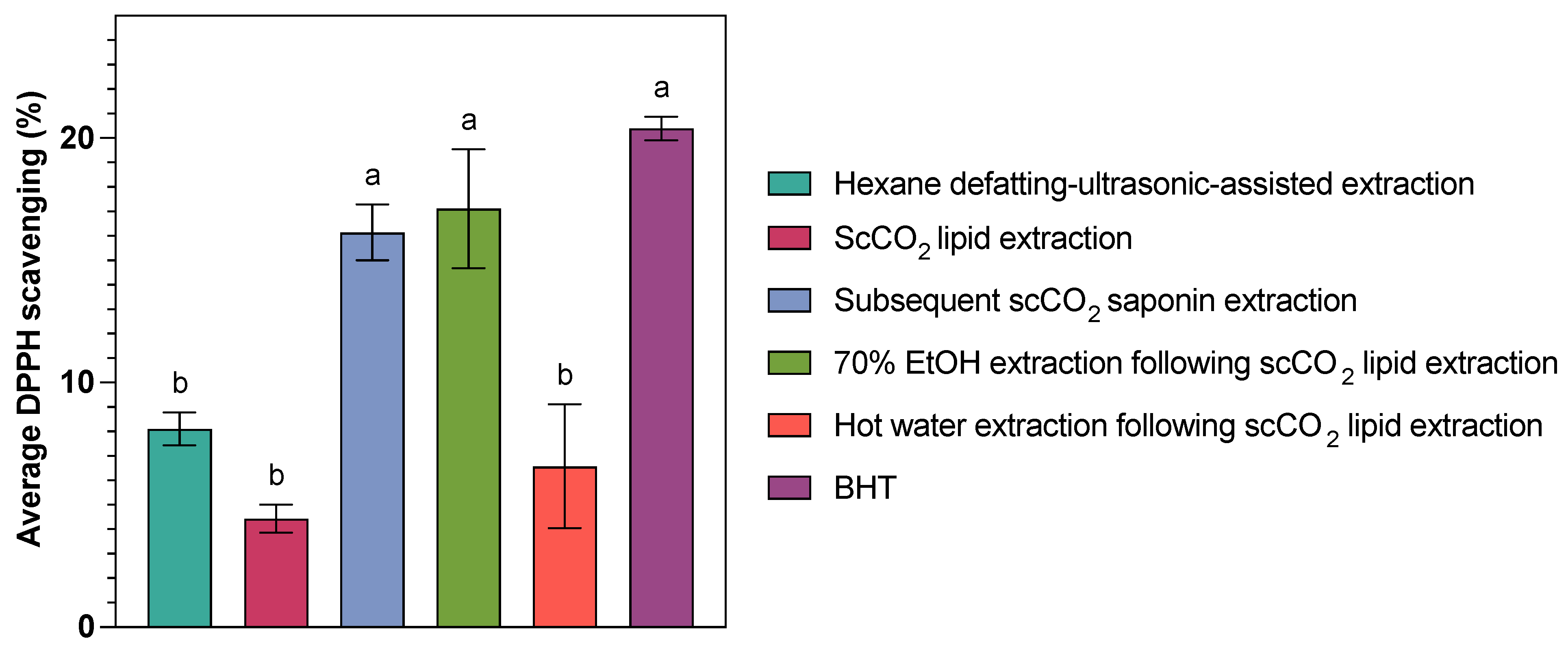
2.5. Antioxidant Activities of Extracts
| Extract 1 | Hexane Defatting–Ultrasonic-Assisted Extraction | ScCO2 Lipid Extraction | Subsequent scCO2 Saponin Extraction | 70% EtOH Extraction Following scCO2 Lipid Extraction | Hot water Extraction Following scCO2 Lipid Extraction | Butylated Hydroxytoluene (BHT) |
|---|---|---|---|---|---|---|
| Average DPPH scavenging (%) 2 | 8.11 ± 1.16 b | 4.43 ± 1.00 b | 16.14 ± 1.98 a | 17.12 ± 4.20 a | 6.57 ± 4.39 b | 20.39 ± 0.68 a (tested)/23.13 (theoretical 3) |
| Antioxidant power (%) 4 | 39.75 | 21.74 | 79.14 | 83.92 | 32.23 | \ |
2.6. Green and Efficient Valorization via Sequential Extraction
3. Materials and Methods
3.1. Chemicals and Materials
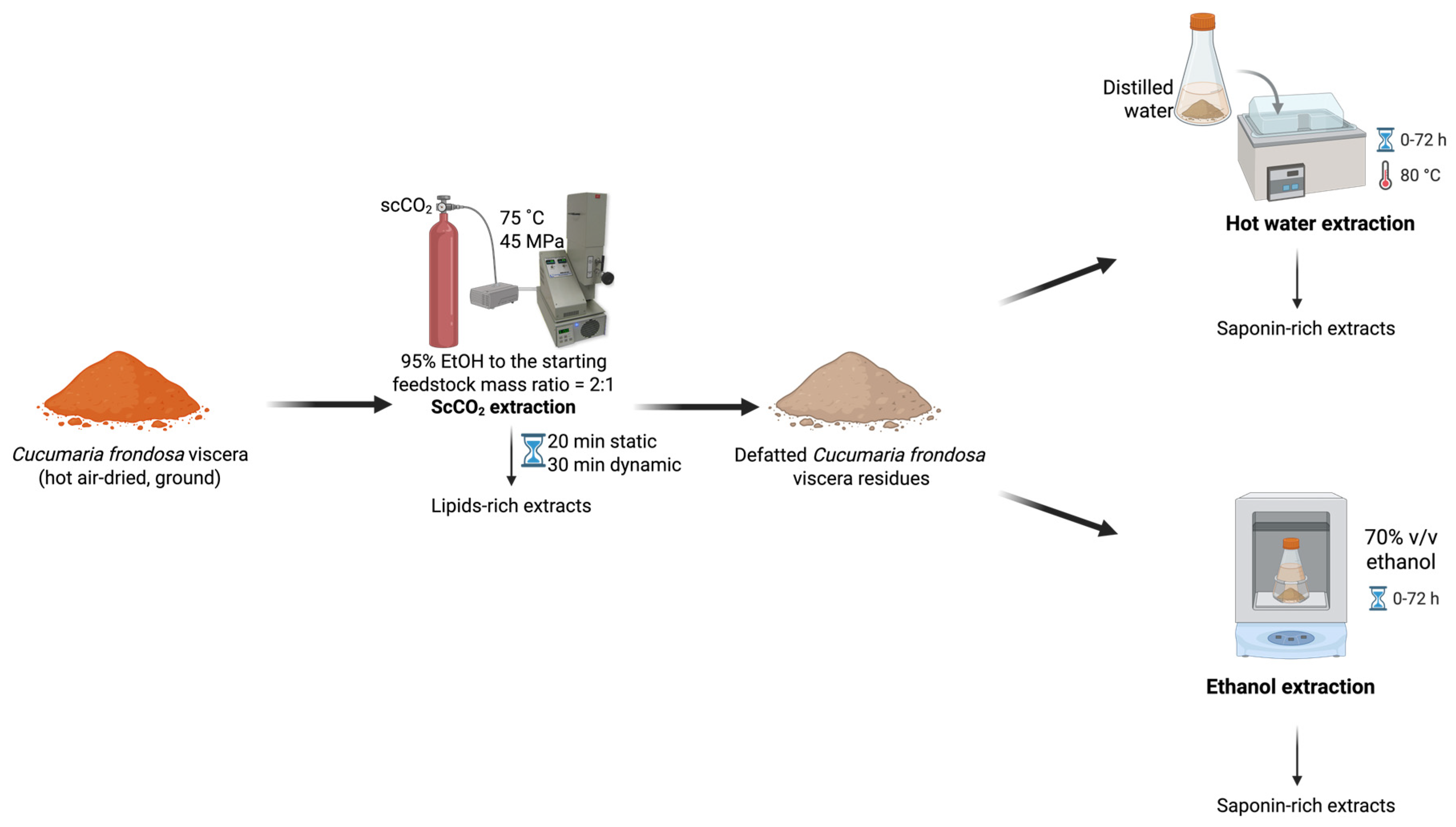
3.2. Sequential ScCO2 Extraction of Lipids and Saponins
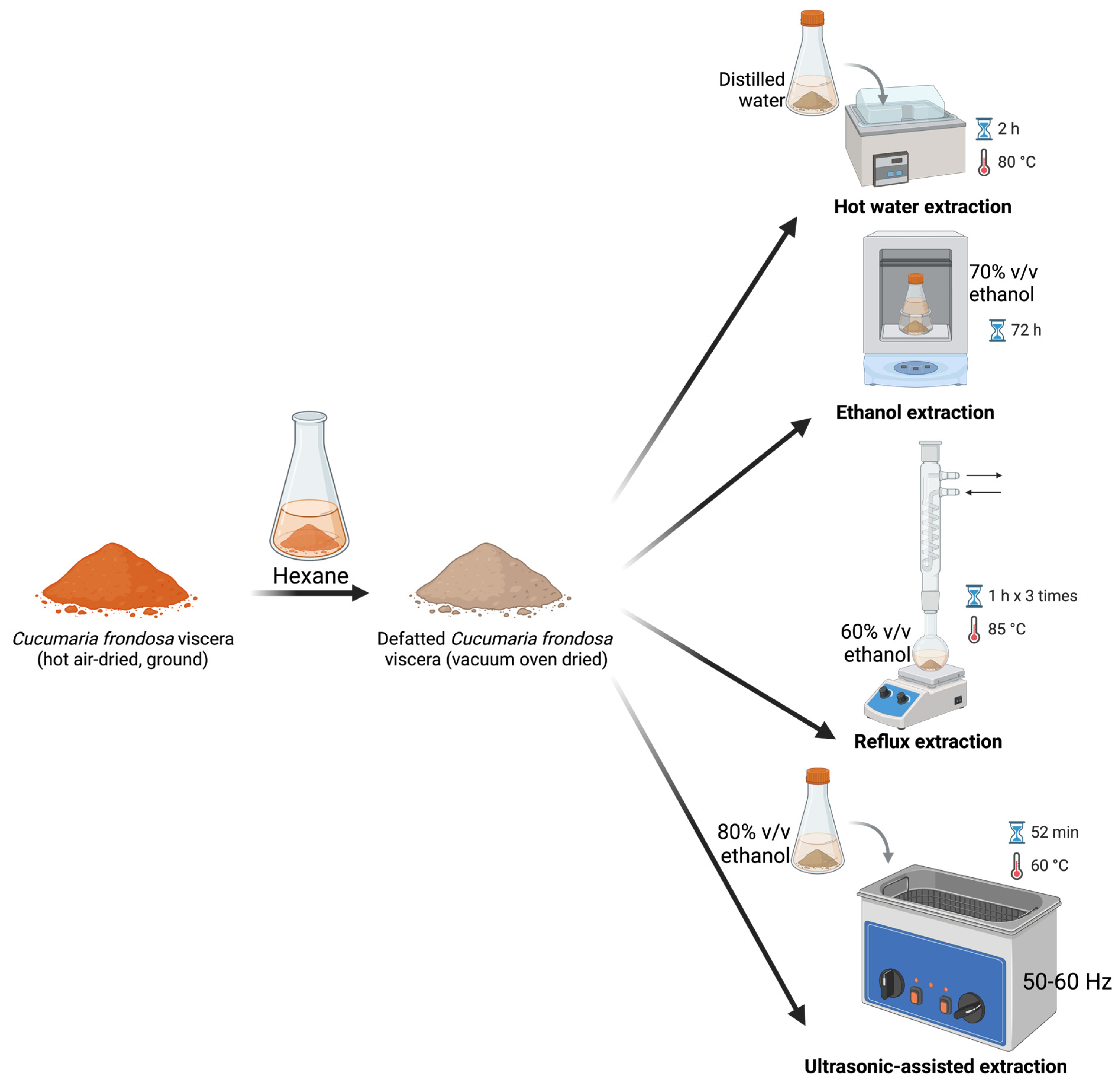
3.3. Conventional Extraction of Saponins
3.3.1. EtOH Extraction
3.3.2. Reflux Extraction
3.3.3. Hot Water Extraction
3.3.4. Ultrasonic-Assisted Extraction
3.4. ScCO2 Extraction Followed by Conventional Extraction
3.5. Purification
3.6. Saponin Yield Determination
3.6.1. Standard Solution and Stock Solution Preparation
3.6.2. Standard Curve Preparation
3.6.3. Total Saponin Content Determination
3.7. Antioxidant Activity Test
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Hao, R.; Chen, J.; Li, S.; Huang, K.; Cao, H.; Farag, M.A.; Battino, M.; Daglia, M.; Capanoglu, E.; et al. Health Benefits of Saponins and Its Mechanisms: Perspectives from Absorption, Metabolism, and Interaction with Gut. Crit. Rev. Food Sci. Nutr. 2024, 64, 9311–9332. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Queiroga, C.L.; Junior, Í.M.; Cabral, F.A. Fractionated Extraction of Saponins from Brazilian Ginseng by Sequential Process Using Supercritical CO2, Ethanol and Water. J. Supercrit. Fluids 2014, 92, 272–281. [Google Scholar] [CrossRef]
- Thimmappa, R.; Wang, S.; Zheng, M.; Misra, R.C.; Huang, A.C.; Saalbach, G.; Chang, Y.; Zhou, Z.; Hinman, V.; Bao, Z.; et al. Biosynthesis of Saponin Defensive Compounds in Sea Cucumbers. Nat. Chem. Biol. 2022, 18, 774–781. [Google Scholar] [CrossRef]
- Fagbohun, O.F.; Joseph, J.S.; Oriyomi, O.V.; Rupasinghe, H.P.V. Saponins of North Atlantic Sea Cucumber: Chemistry, Health Benefits, and Future Prospectives. Mar. Drugs 2023, 21, 262. [Google Scholar] [CrossRef]
- Chen, C.; Han, X.; Dong, P.; Li, Z.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Sea Cucumber Saponin Liposomes Ameliorate Obesity-Induced Inflammation and Insulin Resistance in High-Fat-Diet-Fed Mice. Food Funct. 2018, 9, 861–870. [Google Scholar] [CrossRef]
- Guo, Y.; Han, X.; Che, H.; Li, Z.; Dong, P.; Xue, C.; Zhang, T.; Wang, Y. Synergistic Effect of Eicosapentaenoic Acid-Enriched Phospholipids and Sea Cucumber Saponin on Orotic Acid-Induced Non-Alcoholic Fatty Liver Disease in Rats. R. Soc. Open Sci. 2018, 5, 172182. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, L.; Ding, L.; Shi, H.; Xue, C.; Zhang, T.; Wang, Y. Synergistic Effect of Sea Cucumber Saponins and EPA-Enriched Phospholipids on Insulin Resistance in High-Fat Diet-Induced Obese Mice. Food Funct. 2019, 10, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, Y.; Qiu, L.; Yu, Y.; Wang, C. Saponins of Panax Notoginseng: Chemistry, Cellular Targets and Therapeutic Opportunities in Cardiovascular Diseases. Expert Opin. Investig. Drugs 2014, 23, 523–539. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Zhao, Y.; Xue, C.; Zhang, T.; Wang, Y. Saponins from Sea Cucumber and Their Biological Activities. J. Agric. Food Chem. 2018, 66, 7222–7237. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Khattab, R.A.; Elbandy, M.; Lawrence, A.; Paget, T.; Rae-Rho, J.; Binnaser, Y.S.; Ali, I. Extraction, Identification and Biological Activities of Saponins in Sea Cucumber Pearsonothuria Graeffei. Comb. Chem. High Throughput Screen. 2018, 21, 222–231. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef]
- Findlay, J.A.; Yayli, N.; Radics, L. Novel Sulfated Oligosaccharides from the Sea Cucumber Cucumaria frondosa. J. Nat. Prod. 1992, 55, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea Cucumbers Triterpene Glycosides, the Recent Progress in Structural Elucidation and Chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Yayli, N. Minor Saponins from the Sea Cucumber Cucumaria frondosa. Indian J. Chem. Sect. B 2001, 40B, 399–404. [Google Scholar]
- Fagbohun, O.F.; Hui, J.P.M.; Zhang, J.; Jiao, G.; Rupasinghe, H.P.V. Application of Response Surface Methodology and Artificial Neural Network to Optimize the Extraction of Saponins and Polyphenols from North Atlantic Sea Cucumber. Food Chem. Adv. 2024, 5, 100748. [Google Scholar] [CrossRef]
- Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.M.; Ghandourah, M.; Al-Farawati, R. Sulfated Triterpene Glycosides from the Saudi Red Sea Cucumber Holothuria Atra with Antioxidant and Cytotoxic Activities. Thalass. Int. J. Mar. Sci. 2021, 37, 817–824. [Google Scholar] [CrossRef]
- Nugroho, A.; Harahap, I.A.; Ardiansyah, A.; Bayu, A.; Rasyid, A.; Murniasih, T.; Setyastuti, A.; Putra, M.Y. Antioxidant and Antibacterial Activities in 21 Species of Indonesian Sea Cucumbers. J. Food Sci. Technol. 2022, 59, 239–248. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; MM Franco, C. Distribution of Saponins in the Sea Cucumber Holothuria Lessoni; the Body Wall versus the Viscera, and Their Biological Activities. Mar. Drugs 2018, 16, 423. [Google Scholar] [CrossRef]
- Lin, J.; Jiao, G.; Brooks, M.S.; Budge, S.M.; Kermanshahi-Pour, A. Extraction of Omega-3 Fatty Acids from Atlantic Sea Cucumber (Cucumaria frondosa) Viscera Using Supercritical Carbon Dioxide. Mar. Drugs 2024, 22, 366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ebrahim, Z.M.; Tao, L.; Shi, W.; Li, W.; Lu, W. Optimized Extraction of Saponins from Camelia Oleifera Using Ultrasonic-Assisted Enzymes and Their Surface Performance Evaluation. Processes 2025, 13, 1063. [Google Scholar] [CrossRef]
- Majinda, R. Extraction and Isolation of Saponins. In Natural Products Isolation; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 864, pp. 415–426. ISBN 978-1-61779-624-1. [Google Scholar]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and Quantification of Saponins: A Review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, R.; Guo, F.; Li, Y. Determination Total Saponin Content in Sea Cucumbers. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 89–92. [Google Scholar] [CrossRef]
- Wang, L.; Fang, X.; Du, M.; Long, Q. Effects of Supercritical CO2 Extraction on the Quality of Oil and Physicochemical Properties of Tea Saponin in Oil-Tea Camellia Seed Cake. China Oils Fats 2020, 45, 109–114. [Google Scholar] [CrossRef]
- Yang, D. Optimization of Supercritical CO2 Extraction Process of Quinoa Bran Saponin. Food Res. Dev. 2019, 40, 149–154. [Google Scholar] [CrossRef]
- Liu, X. Optimization of Supercritical CO2 Fluid Extraction Conditions of Saponins from Spina Gleditsiae. IOP Conf. Ser. Mater. Sci. Eng. 2018, 439, 042027. [Google Scholar] [CrossRef]
- Deng, C.; Nie, F. Extraction of Steroidal Saponins from Sisal Hemp by Supercritical CO2. Food Res. Dev. 2008, 2, 41–44. [Google Scholar]
- Zakharenko, A.; Romanchenko, D.; Thinh, P.D.; Pikula, K.; Hang, C.T.; Yuan, W.; Xia, X.; Chaika, V.; Chernyshev, V.; Zakharenko, S.; et al. Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa japonica semper, 1868. Molecules 2020, 25, 4088. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H. Valorization of Bioactive Compounds from Food By-Products Using Supercritical Fluid Extraction: A Technological and Industrial Perspective. Food Chem. 2025, 484, 144277. [Google Scholar] [CrossRef]
- Carvalho, L.M.S.; Oliveira, A.M.B.; Grimaldi, R.; de Souza, P.T.; Batista, E.A.C.; Martínez, J. Supercritical Fluid and Pressurized Liquid Extraction of Spent Tucumã-Do-Amazonas (Astrocaryum aculeatum) Almonds. J. Supercrit. Fluids 2024, 209, 106238. [Google Scholar] [CrossRef]
- Vardanega, R.; Fuentes, F.S.; Palma, J.; Bugueño-Muñoz, W.; Cerezal-Mezquita, P.; Ruiz-Domínguez, M.C. Valorization of Granadilla Waste (Passiflora Ligularis, Juss.) by Sequential Green Extraction Processes Based on Pressurized Fluids to Obtain Bioactive Compounds. J. Supercrit. Fluids 2023, 194, 105833. [Google Scholar] [CrossRef]
- Marcuzzo, N.; Draszewski, C.P.; Wagner, R.; Cordeiro, M.W.S.; Castilhos, F.; Mayer, F.D.; Flores, D.C.B.; Nora, F.M.D.; Abaide, E.R.; Rosa, C.S. Obtaining Oil and Fermentable Sugars from Olive Pomace Using Sequential Supercritical Fluid Extraction and Enzymatic Hydrolysis. J. Supercrit. Fluids 2024, 211, 106288. [Google Scholar] [CrossRef]
- Park, J.; Roy, V.C.; Kim, S.; Lee, S.; Chun, B. Extraction of Edible Oils and Amino Acids from Eel By-Products Using Clean Compressed Solvents: An Approach of Complete Valorization. Food Chem. 2022, 388, 132949. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, X.; Ge, K.; Wu, J.; Wang, Z.; Du, J.; Song, L.; Zhou, Z. Isolation, Identification, and Quantitative Determination of Saponin in Apostichopus Japonicus by HPLC-DAD. J. Ocean Univ. China 2022, 21, 473–478. [Google Scholar] [CrossRef]
- Le Bot, M.; Thibault, J.; Pottier, Q.; Boisard, S.; Guilet, D. An Accurate, Cost-Effective and Simple Colorimetric Method for the Quantification of Total Triterpenoid and Steroidal Saponins from Plant Materials. Food Chem. 2022, 383, 132597. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Huo, Y.; Yang, W.; Chen, J.; Gao, Z.; Yang, Z. Ultrasonic Extraction and Antioxidant Evaluation of Oat Saponins. Ultrason. Sonochem. 2024, 109, 106989. [Google Scholar] [CrossRef]
- Shang, J. Analytical Method Development for Quantification of Total Saponins. Master’s Thesis, National University of Singapore, Singapore, 2016. [Google Scholar]
- Cheng, Y.; Zheng, Y.; VanderGheynst, J.S. Rapid Quantitative Analysis of Lipids Using a Colorimetric Method in a Microplate Format. Lipids 2011, 46, 95–103. [Google Scholar] [CrossRef]
- Ondevilla, J.C.; Hanashima, S.; Mukogawa, A.; Miyazato, D.G.; Umegawa, Y.; Murata, M. Effect of the Number of Sugar Units on the Interaction between Diosgenyl Saponin and Membrane Lipids. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184145. [Google Scholar] [CrossRef]
- Liao, Y.; Li, Z.; Zhou, Q.; Sheng, M.; Qu, Q.; Shi, Y.; Yang, J.; Lv, L.; Dai, X.; Shi, X. Saponin Surfactants Used in Drug Delivery Systems: A New Application for Natural Medicine Components. Int. J. Pharm. 2021, 603, 120709. [Google Scholar] [CrossRef]
- Cai, Y.; Gao, H.; Song, L.; Tao, F.; Ji, X.; Yu, Y.; Cao, Y.; Tang, S.; Xue, P. Optimization of Green Deep Eutectic Solvent (DES) Extraction of Chenopodium Quinoa Willd. Husks Saponins by Response Surface Methodology and Their Antioxidant Activities. RSC Adv. 2023, 13, 29408–29418. [Google Scholar] [CrossRef] [PubMed]
- Ostellari, P.; Tajoli, F.; Fortunati, I.; Carofiglio, T.; Badocco, D.; Pastore, P.; Gross, S. Eu(Iii)-Doped Calcium Molybdate Nano- and Microstructures: Microfluidic Synthesis and Morphology Tuning via Solvent Dielectric Constant and Viscosity Control. CrystEngComm 2024, 26, 6052–6064. [Google Scholar] [CrossRef]
- Dufour, R.; Perry, G.; Harnois, M.; Coffinier, Y.; Thomy, V.; Senez, V.; Boukherroub, R. From Micro to Nano Reentrant Structures: Hysteresis on Superomniphobic Surfaces. Colloid Polym. Sci. 2013, 291, 409–415. [Google Scholar] [CrossRef]
- Wood, J.A.; Bernards, M.A.; Wan, W.; Charpentier, P.A. Extraction of Ginsenosides from North American Ginseng Using Modified Supercritical Carbon Dioxide. J. Supercrit. Fluids 2006, 39, 40–47. [Google Scholar] [CrossRef]
- Yeo, S.; Park, S.; Kim, J.; Kim, J. Critical Properties of Carbon Dioxide + Methanol, + Ethanol, + 1-Propanol, and + 1-Butanol. J. Chem. Eng. Data 2000, 45, 932–935. [Google Scholar] [CrossRef]
- Ansharullah; Tamrin; Patadjai, A.B.; Asranuddin. Powder Production of Sea Cucumber (Holothuria Scabra): Effect of Processing Methods on the Antioxidant Activities and Physico-Chemical Characteristics. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012024. [Google Scholar] [CrossRef]
- Shanei, A.; Sazgarnia, A. An Overview of Therapeutic Applications of Ultrasound Based on Synergetic Effects with Gold Nanoparticles and Laser Excitation. Iran. J. Basic Med. Sci. 2019, 22, 848–855. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, H.; Zheng, T.; Jiang, J.; Xu, Y.; Jia, F.; He, K.; Yang, Y. Quantitative Changes and Transformation Mechanisms of Saponin Components in Chinese Herbal Medicines during Storage and Processing: A Review. Molecules 2024, 29, 4486. [Google Scholar] [CrossRef]
- Kang, Q.; Chen, S.; Li, S.; Wang, B.; Liu, X.; Hao, L.; Lu, J. Comparison on Characterization and Antioxidant Activity of Polysaccharides from Ganoderma Lucidum by Ultrasound and Conventional Extraction. Int. J. Biol. Macromol. 2019, 124, 1137–1144. [Google Scholar] [CrossRef]
- Higgins, C.L.; Filip, S.V.; Afsar, A.; Colquhoun, H.M.; Hayes, W. From Food to Mobility: Investigating a Screening Assay for New Automotive Antioxidants Using the Stable Radical DPPH. ChemistrySelect 2021, 6, 9179–9184. [Google Scholar] [CrossRef]
- Kodali, S.T.; Kauffman, P.; Kotha, S.R.; Yenigalla, A.; Veeraraghavan, R.; Pannu, S.R.; Hund, T.J.; Satoskar, A.R.; McDaniel, J.C.; Maddipati, R.K.; et al. Oxidative Lipidomics: Analysis of Oxidized Lipids and Lipid Peroxidation in Biological Systems with Relevance to Health and Disease. In Measuring Oxidants and Oxidative Stress in Biological Systems; Berliner, L.J., Parinandi, N.L., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 61–92. ISBN 978-3-030-47318-1. [Google Scholar]
- Khan, F.I.; Ghoshal, A.K. Removal of Volatile Organic Compounds from Polluted Air. J. Loss Prev. Process Ind. 2000, 13, 527–545. [Google Scholar] [CrossRef]
- Barjoveanu, G.; Pătrăuțanu, O.-A.; Teodosiu, C.; Volf, I. Life Cycle Assessment of Polyphenols Extraction Processes from Waste Biomass. Sci. Rep. 2020, 10, 13632. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Kermanshahi Pour, A. Extraction of Anthocyanins from Haskap Berry Pulp Using Supercritical Carbon Dioxide: Influence of Co-Solvent Composition and Pretreatment. LWT 2018, 98, 237–244. [Google Scholar] [CrossRef]
| Treatment * | Reflux-Native | Reflux-Defatted | 70% EtOH-Native | 70% EtOH-Defatted | 100% EtOH-Native | 100% EtOH-Defatted |
|---|---|---|---|---|---|---|
| Yields (mg OAE/g) | 4.18 ± 0.82 | 6.71 ± 0.73 | 36.34 ± 0.64 | 16.44 ± 0.21 | 38.22 ± 0.83 | 10.19 ± 0.20 |
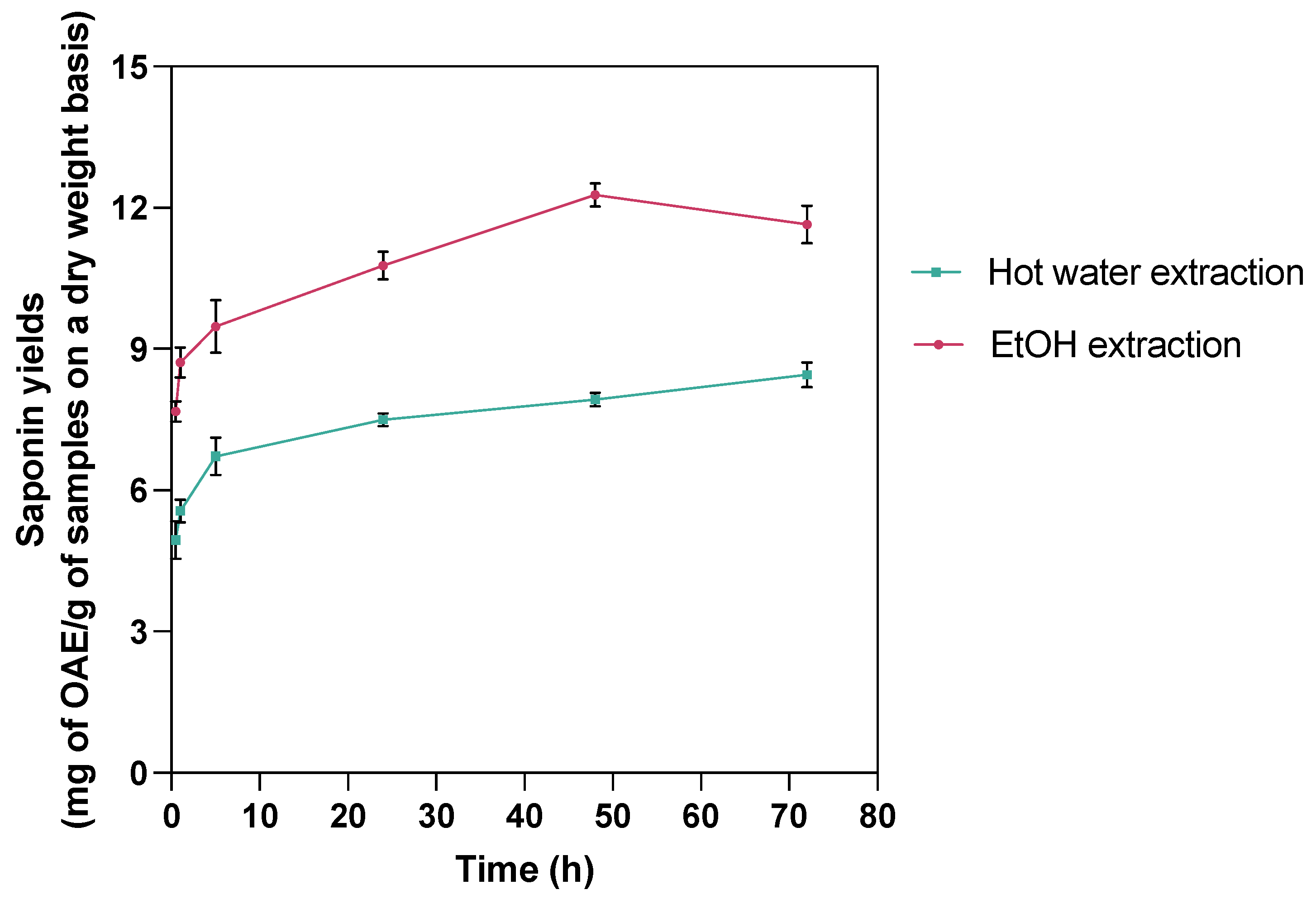
| Time (h) | 0.5 | 1 | 5 | 24 | 48 | 72 | |
|---|---|---|---|---|---|---|---|
| Treatment | |||||||
| Hot water extraction | 4.94 ± 0.69 a | 5.57 ± 0.41 a,b | 6.72 ± 0.69 b,c | 7.50 ± 0.25 c,d | 7.93 ± 0.24 c,d | 8.45 ± 0.46 d | |
| EtOH extraction | 7.68 ± 0.38 a | 8.72 ± 0.55 a,b | 9.47 ± 0.97 b,c | 10.77 ± 0.51 c,d | 12.27 ± 0.43 d | 11.65 ± 0.69 d | |
| Method 1 | Yields (mg OAE/g) 2 | Recovery Efficiency 3 |
|---|---|---|
| Hexane defatting–70% EtOH extraction | 16.44 ± 0.21 a,b | 96.13% |
| Hexane defatting–100% EtOH extraction | 10.19 ± 0.20 c,d | 58.87% |
| Hexane defatting–hot water extraction | 3.94 ± 0.23 e | 22.76% |
| Hexane defatting–reflux extraction | 7.17 ± 0.78 d,e | 41.42% |
| Hexane defatting–ultrasonic-assisted extraction | 17.31 ± 0.60 a | / |
| Sequential scCO2 extraction | 9.13 ± 1.30 d | 52.74% |
| ScCO2 extraction followed by hot water extraction | 12.99 ± 2.43 b,c | 75.04% |
| ScCO2 extraction followed by EtOH extraction | 16.26 ± 2.47 a,b | 93.93% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; Jiao, G.; Kermanshahi-pour, A. Sequential Extraction of Bioactive Saponins from Cucumaria frondosa Viscera: Supercritical CO2–Ethanol Synergy for Enhanced Yields and Antioxidant Performance. Mar. Drugs 2025, 23, 272. https://doi.org/10.3390/md23070272
Lin J, Jiao G, Kermanshahi-pour A. Sequential Extraction of Bioactive Saponins from Cucumaria frondosa Viscera: Supercritical CO2–Ethanol Synergy for Enhanced Yields and Antioxidant Performance. Marine Drugs. 2025; 23(7):272. https://doi.org/10.3390/md23070272
Chicago/Turabian StyleLin, Jianan, Guangling Jiao, and Azadeh Kermanshahi-pour. 2025. "Sequential Extraction of Bioactive Saponins from Cucumaria frondosa Viscera: Supercritical CO2–Ethanol Synergy for Enhanced Yields and Antioxidant Performance" Marine Drugs 23, no. 7: 272. https://doi.org/10.3390/md23070272
APA StyleLin, J., Jiao, G., & Kermanshahi-pour, A. (2025). Sequential Extraction of Bioactive Saponins from Cucumaria frondosa Viscera: Supercritical CO2–Ethanol Synergy for Enhanced Yields and Antioxidant Performance. Marine Drugs, 23(7), 272. https://doi.org/10.3390/md23070272








