Cladolosides of Groups S and T: Triterpene Glycosides from the Sea Cucumber Cladolabes schmeltzii with Unique Sulfation; Human Breast Cancer Cytotoxicity and QSAR
Abstract
1. Introduction
2. Results and Discussion
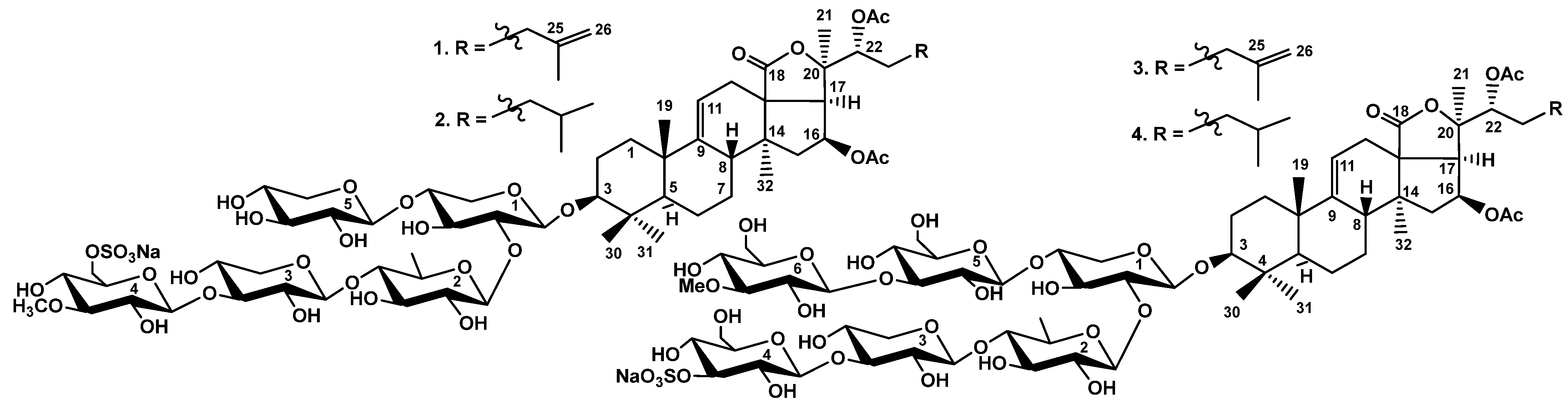
2.1. Structure Elucidation of Glycosides
2.2. Biological Activity of the Glycosides
2.3. Correlational Analysis and QSAR Model
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animals and Cells
3.3. Extraction and Isolation
3.3.1. Cladoloside S (1)
3.3.2. Cladoloside S1 (2)
3.3.3. Cladoloside T (3)
3.3.4. Cladoloside T1 (4)
3.4. Cytotoxic Activity (MTT Assay)
3.5. Hemolytic Activity
3.6. Building a QSAR Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A. Progress in the studies of triterpene glycosides from sea cucumbers (Holothuroidea, Echinodermata) between 2017 and 2021. Nat. Prod. Commun. 2021, 16, 10. [Google Scholar] [CrossRef]
- Kamyab, E.; Kellermann, M.Y.; Kunzmann, A.; Schupp, P.J. Chemical biodiversity and bioactivities of saponins in Echinodermata with an emphasis on sea cucumbers (Holothuroidea). In YOUMARES 9–The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 121–157. [Google Scholar] [CrossRef]
- Popov, R.S.; Ivanchina, N.V.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I.; Dolmatov, I.Y.; Stonik, V.A.; Dmitrenok, P.S. Metabolite profiling of triterpene glycosides of the Far Eastern sea cucumber Eupentacta fraudatrix and their distribution in various body components using LC-ESI QTOF-MS. Mar. Drugs 2017, 15, 302. [Google Scholar] [CrossRef] [PubMed]
- Khodja, I.; Mezali, K.; Savarino, P.; Gerbaux, P.; Flammang, P.; Caulier, G. Structural characterization and profiles of saponins from Two Algerian Sea Cucumbers. Molecules 2024, 29, 5346. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, Y.E.; Tuenter, E.; Foubert, K.; Herawati, H.; Hariati, A.M.; Aulanni’am, A.; Pieters, L.; De Bruyne, T.; Hermans, N. Saponin and fatty acid profiling of the sea cucumber Holothuria atra, α-glucosidase inhibitory activity and the identification of a novel triterpene glycoside. Nutrients 2023, 15, 1033. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Separation procedures for complicated mixtures of sea cucumber triterpene glycosides with isolation of individual glycosides, their comparison with HPLC/MS metabolomic approach, and biosynthetic interpretation of the obtained structural data. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2022; Volume 72, pp. 103–146. [Google Scholar] [CrossRef]
- Van Dyck, S.; Flammang, P.; Meriaux, C.; Bonnel, D.; Salzet, M.; Fourmier, I.; Wisztorski, M. Localization of secondary metabolites in marine invertebrates: Contribution of MALDI MSI for the study of saponins in Cuvierian tubules of H. forskali. PLoS ONE 2010, 5, 11. [Google Scholar] [CrossRef]
- Van Dyck, S.; Caulier, G.; Todeso, M.; Gebraux, P.; Fournier, I.; Wisztorsky, M.; Flammang, P. The triterpene glycosides of Holohuria forskali: Usefulness and efficiency as a chemical defense mechanism against predatory fish. J. Exp. Biol. 2011, 214, 1347–1356. [Google Scholar] [CrossRef]
- Van Dyck, S.; Gerbaux, P.; Flammang, P. Quialitative and quantitative saponin contents in five sea cucumbers from Indian Ocean. Mar. Drugs 2010, 8, 173–189. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; Franco, C.M.M. Distribution of saponins in the sea cucumber Holothuria lessoni; the body wall versus the viscera, and their biological activities. Mar. Drugs 2018, 16, 423. [Google Scholar] [CrossRef]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Sea cucumber triterpene glycosides as anticancer agents. In Studies in Natural Product Chemistry; Rahman, A., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 49, pp. 55–105. [Google Scholar]
- Careaga, V.P.; Maier, M.S. Cytotoxic triterpene glycosides from sea cucumbers. In Handbook of Anticancer Drugs from Marine Origin; Kim, S.-K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 515–528. [Google Scholar]
- Pislyagin, E.A.; Manzhulo, I.V.; Gorpenchenko, T.Y.; Dmitrenok, P.S.; Avilov, S.A.; Silchenko, A.S.; Wang, Y.-M.; Aminin, D.L. Cucumarioside A2-2 causes macrophage activation in mouse spleen. Mar. Drugs 2017, 15, 341. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Saponins from sea cucumber and their biological activities. J. Agric. Food Chem. 2018, 66, 7222–7237. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Popov, R.S.; Dmitrenok, P.S.; Chingizova, E.A.; Menchinskaya, E.S.; Panina, E.G.; Stepanov, V.G.; Kalinin, V.I.; et al. Djakonoviosides A, A1, A2, B1–B4—Triterpene monosulfated tetra- and pentaosides from the sea cucumber Cucumaria djakonovi: The first finding of a hemiketal fragment in the aglycones; Activity against human breast Cancer Cell Lines. Int. J. Mol. Sci. 2023, 24, 11128. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Popov, R.S.; Chingizova, E.A.; Menchinskaya, E.S.; Zelepuga, E.A.; Panina, E.G.; Stepanov, V.G.; Kalinin, V.I.; et al. Sulfated triterpene glycosides from the Far Eastern sea cucumber Cucumaria djakonovi: Djakonoviosides C1, D1, E1, and F1; cytotoxicity against human breast cancer cell lines; quantitative structure–activity relationships. Mar. Drugs 2023, 21, 602. [Google Scholar] [CrossRef] [PubMed]
- Menchinskaya, E.S.; Chingizova, E.A.; Pislyagin, E.A.; Yurchenko, E.A.; Klimovich, A.A.; Zelepuga, E.A.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S. Mechanisms of Action of Sea Cucumber Triterpene Glycosides Cucumarioside A0-1 and Djakonovioside A Against Human Triple-Negative Breast Cancer. Mar. Drugs 2024, 22, 474. [Google Scholar] [CrossRef]
- Kalinin, V.I. System-theoretical (holistic) approach to the modelling of structural-functional relationships of Biomolecules and their evolution: An example of triterpene glycosides from sea cucumbers (Echinodermata, Holothurioidea). J. Theor. Biol. 2000, 206, 151–168. [Google Scholar] [CrossRef]
- Park, J.-I.; Bae, H.-R.; Kim, C.G.; Stonik, V.A.; Kwak, J.Y. Relationships between chemical structures and functions of triterpene glycosides isolated from sea cucumbers. Front. Chem. 2014, 2, 77. [Google Scholar] [CrossRef]
- Zelepuga, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Structure-activity relationships of holothuroid’s triterpene glycosides and some in silico insights obtained by molecular dynamics study on the mechanisms of their membranolytic action. Mar. Drugs 2021, 19, 604. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjascchenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Kalinin, V.I.; Stonik, V.A. Structure and biological action of cladolosides B1, B2, C, C1, C2 and D, six new triterpene glycosides from the sea cucumber Cladolabes schmeltzii. Nat. Prod. Commun. 2013, 8, 1527–1534. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjascchenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Savchenko, A.M.; Kalinin, V.I. Triterpene glycosides from the sea cucumber Cladolabes schmeltzii II. Structure and Biological Action of Cladolosides A1–A6. Nat. Prod. Commun. 2014, 9, 1421–1428. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjascchenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Dolmatov, I.Y.; Kalinin, V.I. Structures and biological activities of cladolosides C3, E1, E2, F1, F2, G, H1 and H2, eight triterpene glycosides from the sea cucumber Cladolabes schmeltzii with one known and four new carbohydrate chains. Carb. Res. 2015, 414, 22–31. [Google Scholar] [CrossRef]
- Shashkov, A.S.; Chizhov, O.S. C-13-NMR spectroscopy in chemistry of carbohydrates and related compounds. Bioorg. Khim. 1976, 2, 437–497. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjascchenko, P.V.; Dmitrenok, P.S.; Chingizova, E.A.; Dolmatov, I.Y.; Kalinin, V.I. Cladolosides I1, I2, J1, K1 and L1, monosulfated triterpene glycosides with new carbohydrate chains from the sea cucumber Cladolabes schmeltzii. Carb. Res. 2017, 445, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjascchenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dolmatov, I.Y.; Kalinin, V.I. Cladolosides C4, D1, D2, M, M1, M2, N and Q, new triterpene glycosides with diverse carbohydrate chains from sea cucumber Cladolabes schmeltzii. An uncommon 20,21,22,23,24,25,26,27-Okta-nor-lanostane aglycone. The synergism of inhibitory action of non-toxic dose of the glycosides and radioactive irradiation on colony formation of HT-29 cancer cells. Carb. Res. 2018, 468, 36–44. [Google Scholar]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjascchenko, P.V.; Dmitrenok, P.S.; Yurchenko, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dolmatov, I.Y.; Kalinin, V.I. Cladolosides O, P, P1–P3 and R, triterpene glycosides with two novel types of carbohydrate chains from the sea cucumber Cladolabes schmeltzii. Inhibition of cancer cells colony formation and its synergy with radioactive irradiation. Carb. Res. 2018, 468, 73–79. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andrijaschenko, P.V.; Popov, R.S.; Dmitrenok, P.S.; Chingizova, E.A.; Kalinin, V.I. Kurilosides A1, A2, C1, D, E and F—Triterpene glycosides from the Far Eastern sea sucumber Thyonidium (=Duasmodactyla) kurilensis (Levin): Structures with unusual non-holostane aglycones and cytotoxicities. Mar. Drugs 2020, 18, 551. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S.; Popov, R.S.; Chingizova, E.A. Structures and bioactivities of psolusosides B1, B2, J, K, L, M, N, O, P, and Q from the sea cucumber Psolus fabricii. The first finding of tetrasulfated marine low molecular weight metabolites. Mar. Drugs 2019, 17, 631. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Avilov, S.A.; Andrijaschenko, P.V.; Popov, R.S.; Chingizova, E.A.; Grebnev, B.B.; Rasin, A.B.; Kalinin, V.I. The isolation, structure elucidation and bioactivity study of chilensosides A, A1, B, C, and D, holostane triterpene di-, tri- and tetrasulfated pentaosides from the sea cucumber Paracaudina chilensis (Caudinidae, Molpadida). Molecules 2022, 27, 7655. [Google Scholar] [CrossRef]
- Hall, L.H.; Kier, L.B. The molecular connectivity chi indices and kappa shape indices in structure-property modeling. In Reviwes in Computational Chemistry; Lipkowitz, K.B., Boyd, D.B., Eds.; Wiley-VCH, Inc.: Hoboken, NJ, USA, 1991; Volume 2, Chapter 9; pp. 367–422. [Google Scholar] [CrossRef]
- Labute, P. LowModeMD—Implicit Low Mode Velocity Filtering Applied to Conformational Search of Macrocycles and Protein Loops. J. Chem. Inf. Model. 2010, 50, 792–800. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 1987; pp. 231–232. [Google Scholar]

| Position | δC, mult. a | δH, mult. (J in Hz) b | HMBC | ROESY |
|---|---|---|---|---|
| 1 | 36.1, CH2 | 1.81, m | H-11 | |
| 1.44, m | H-3, H-5, H-11 | |||
| 2 | 26.9, CH2 | 2.22, m | ||
| 1.97, m | H-19, H-30 | |||
| 3 | 88.6, CH | 3.25, dd (12.0; 3.9) | C: 4, 30, 31, C:1 Xyl1 | H-1, H-5, H-31, H1-Xyl1 |
| 4 | 39.8, C | |||
| 5 | 52.7, CH | 0.92, m | C: 6, 19, 30, 31 | H-1, H-3, H-31 |
| 6 | 20.7, CH2 | 1.71, m | ||
| 1.53, m | H-8, H-19, H-30 | |||
| 7 | 27.8, CH2 | 1.64, m | H-15, H-32 | |
| 1.24, m | ||||
| 8 | 39.5, CH | 3.25, brd (13.0) | H-6, H-15, H-19 | |
| 9 | 150.9, C | |||
| 10 | 39.3, C | |||
| 11 | 110.4, CH | 5.23, m | C: 10, 13 | |
| 12 | 33.9, CH2 | 2.53, brdd (17.2; 6.1) | C: 9, 11, 14, 18 | H-17, H-32 |
| 2.50, brd (17.2) | C: 9, 11, 14, 18 | H-21 | ||
| 13 | 59.0, C | |||
| 14 | 43.6, C | |||
| 15 | 44.4, CH2 | 2.40, dd (12.1; 6.1) | C: 13, 14, 16, 17, 32 | H-7, H-32 |
| 1.40, brdd (12.1; 9.1) | C: 14, 16, 32 | H-8 | ||
| 16 | 75.2, CH | 5.90, brq (9.1) | C: 13, 20, OAc-16 | |
| 17 | 53.6, CH | 2.91, d (9.1) | C: 12, 13, 15, 16, 18, 20, 21 | H-12, H-21, H-32 |
| 18 | 176.0, C | |||
| 19 | 22.0, CH3 | 1.35, s | C: 1, 5, 9, 10 | H-1, H-2, H-6, H-8, H-30 |
| 20 | 85.8, C | |||
| 21 | 22.4, CH3 | 1.64, s | C: 17, 20, 22 | H-12, H-17 |
| 22 | 74.7, CH | 6.66, d (11.1) | C: 20, 21, OAc-22 | |
| 23 | 29.3, CH2 | 1.78, m | ||
| 24 | 34.3, CH2 | 2.26, m | C: 25 | |
| 2.16, m | H-22 | |||
| 25 | 144.9, C | |||
| 26 | 110.6, CH2 | 4.84, brs | C: 24, 25, 27 | |
| 4.83, brs | C: 24, 25, 27 | |||
| 27 | 22.2, CH3 | 1.74, s | C: 24, 25, 26 | H-26 |
| 30 | 16.5, CH3 | 1.11, s | C: 3, 4, 5, 31 | H-2, H-6, H-19, H-31 |
| 31 | 27.9, CH3 | 1.29, s | C: 3, 4, 5, 30 | H-3, H-5, H-6, H-30, H-1Xyl1 |
| 32 | 21.0, CH3 | 0.91, s | C: 8, 13, 14, 15 | H-7, H-12, H-15, H-16, H-17 |
| OAc-16 | 169.4, C | |||
| 21.4, CH3 | 2.28, s | C: 16 | H-22 | |
| OAc-22 | 170.3, C | |||
| 20.7, CH3 | 2.08, s | C: 22 |
| Atom | δC, mult. a,b,c | δH, mult. (J in Hz) d | HMBC | ROESY |
|---|---|---|---|---|
| Xyl1 (1→C-3) | ||||
| 1 | 105.1, CH | 4.76, d (7.5) | C-3 | H-3; H-3, 5 Xyl1 |
| 2 | 83.4, CH | 4.09, t (8.8) | C: 1 Xyl1, 1 Qui2 | H-1 Qui2 |
| 3 | 75.3, CH | 4.22, t (8.8) | C: 2 Xyl 1 | |
| 4 | 76.2, CH | 4.29, m | C: 1 Xyl5 | |
| 5 | 64.0, CH2 | 4.40, dd (11.9; 4.4) | C: 1 Xyl1 | |
| 3.63, brdd (11.9; 9.4) | H-1, 3 Xyl1 | |||
| Qui2 (1→2Xyl1) | ||||
| 1 | 105.4, CH | 5.17, d (7.5) | C: 2 Xyl1 | H-2 Xyl1, H-3, 5 Qui2 |
| 2 | 76.4, CH | 4.05, t (9.2) | C: 1, 3 Qui2 | |
| 3 | 75.1, CH | 4.12, t (9.2) | C: 2 Qui2 | H-1 Qui2 |
| 4 | 85.6, CH | 3.67, t (9.2) | C: 3, 5 Qui2, 1 Xyl3 | H-1 Xyl3, H-2 Qui2 |
| 5 | 71.6, CH | 3.82, dd (9.2; 5.9) | H-1 Qui2 | |
| 6 | 18.1, CH3 | 1.76, d (5.9) | C: 4, 5 Qui2 | |
| Xyl3 (1→4Qui2) | ||||
| 1 | 104.6, CH | 4.87, d (7.9) | C: 4 Qui2 | H-4 Qui2, H-3, 5 Xyl3 |
| 2 | 73.5, CH | 3.97, t (8.9) | C: 1, 3 Xyl3 | |
| 3 | 86.6, CH | 4.14, t (8.9) | C: 2, 4 Xyl3; 1 MeGlc4 | H-1 MeGlc4, H-1, 5 Xyl3 |
| 4 | 68.7, CH | 4.05, m | ||
| 5 | 66.3, CH2 | 4.20, dd (11.6; 4.8) | C: 3, 4 Xyl3 | |
| 3.64, brt (11.6) | C: 1 Xyl3 | H-1 Xyl3 | ||
| MeGlc4 (1→3Xyl3) | ||||
| 1 | 105.0, CH | 5.28, d (8.5) | C: 3 Xyl3 | H-3 Xyl3, H-3, 5 MeGlc4 |
| 2 | 74.4, CH | 3.85, t (8.5) | C: 1 MeGlc4 | |
| 3 | 87.1, CH | 3.64, t (8.5) | C: 2, 4 MeGlc4, OMe | |
| 4 | 70.0, CH | 4.19, t (8.5) | ||
| 5 | 76.3, CH | 4.01, m | H-1 MeGlc4 | |
| 6 | 66.6, CH2 | 5.01, m | C: 4, 5 MeGlc4 | |
| OMe | 60.5, CH3 | 3.81, s | C: 3 MeGlc4 | H-3 MeGlc4 |
| Xyl5 (1→4Xyl1) | ||||
| 1 | 103.5, CH | 4.91, d (8.0) | C: 4 Xyl1 | H-4 Xyl1, H-3, 5 Xyl5 |
| 2 | 73.7, CH | 4.00, t (8.6) | C: 1, 3 Xyl5 | |
| 3 | 77.8, CH | 4.12, t (8.6) | C: 2, 4 Xyl5 | H-1 Xyl5 |
| 4 | 70.7, CH | 4.18, m | ||
| 5 | 67.2, CH2 | 4.33, dd (11.7; 4.9) | C: 1, 3, 4 Xyl5 | |
| 3.68, t (11.7) | C: 1, 3, 4 Xyl5 |
| Position | δC, mult. a | δH, mult. (J in Hz) b | HMBC | ROESY |
|---|---|---|---|---|
| 1 | 36.1, CH2 | 1.70, m | H-19 | |
| 1.31, m | H-3, H-5 | |||
| 2 | 26.7, CH2 | 2.07, m | ||
| 1.85, m | H-19, H-30 | |||
| 3 | 88.8, CH | 3.14, brd (11.7) | C:1 Xyl1 | H-1, H-5, H-31, H1-Xyl1 |
| 4 | 39.7, C | |||
| 5 | 52.7, CH | 0.78, brd (11.7) | H-3, H-31 | |
| 6 | 21.0, CH2 | 1.57, m | ||
| 1.42, m | ||||
| 7 | 27.7, CH2 | 1.60, m | ||
| 1.15, m | H-5, H-32 | |||
| 8 | 39.5, CH | 3.12, brd (16.0) | H-15 | |
| 9 | 150.6, C | |||
| 10 | 39.2, C | |||
| 11 | 110.7, CH | 5.18, m | H-1 | |
| 12 | 33.9, CH2 | 2.62, brd (16.8) | ||
| 2.52, dd (16.8; 6.0) | C: 17 | H-21 | ||
| 13 | 59.2, C | |||
| 14 | 43.6, C | |||
| 15 | 44.4, CH2 | 2.35, dd (12.0; 7.2) | C: 13, 17, 32 | H-32 |
| 1.28, brd (12.0) | H-8 | |||
| 16 | 75.5, CH | 5.83, brq (9.6) | H-32 | |
| 17 | 53.4, CH | 2.97, d (9.6) | C: 12, 13, 18, 21 | H-12, H-21, H-32 |
| 18 | 176.9, C | |||
| 19 | 22.0, CH3 | 1.23, s | C: 1, 5, 9, 10 | H-1, H-30 |
| 20 | 86.5, C | |||
| 21 | 22.5, CH3 | 1.65, s | C: 17, 20, 22 | |
| 22 | 75.4, CH | 6.48, d (10.8) | ||
| 23 | 28.5, CH2 | 1.52, m | ||
| 24 | 35.1, CH2 | 1.29, m | ||
| 1.15, m | ||||
| 25 | 28.1, CH | 1.52, m | ||
| 26 | 22.5, CH3 | 0.83, s | C: 24, 25, 27 | |
| 27 | 22.8, CH3 | 0.82, s | C: 24, 25, 26 | H-31 |
| 30 | 16.5, CH3 | 0.98, s | C: 3, 4, 5, 31 | H-2, H-6, H-19, H-30, |
| 31 | 27.9, CH3 | 1.16, s | C: 3, 4, 5, 30 | H-1 Xyl1 |
| 32 | 21.0, CH3 | 0.91, s | C: 8, 13, 14, 15 | H-12, H-15, H-17, H-24 |
| OAc-16 | 171.4, C | |||
| 20.9, CH3 | 2.07, s | |||
| OAc-22 | 170.5, C | |||
| 21.6, CH3 | 2.25, s | H-22, H-24, H-26, H-27 |
| Atom | δC, mult. a,b,c | δH, mult. (J in Hz) d | HMBC | ROESY |
|---|---|---|---|---|
| Xyl1 (1→C-3) | ||||
| 1 | 104.8, CH | 4.66, d (6.9) | C-3 | H-3; H-3, 5 Xyl1 |
| 2 | 82.5, CH | 3.96, t (7.6) | C: 1,3 Xyl1, C: 1 Qui2 | H-1 Qui2; H-4 Xyl1 |
| 3 | 75.1, CH | 4.16, t (7.6) | C: 2,4 Xyl 1 | H-1 Xyl1 |
| 4 | 77.1, CH | 4.22, m | C: 3 Xyl1, C: 1 Glc5 | H-1 Glc5 |
| 5 | 63.5, CH2 | 4.39, m | C: 1, 3 Xyl1 | |
| 3.63, m | H-1 Xyl1 | |||
| Qui2 (1→2Xyl1) | ||||
| 1 | 104.7, CH | 5.02, d (7.7) | C: 2 Xyl1 | H-2 Xyl1, H-3, 5 Qui2 |
| 2 | 75.7, CH | 3.87, t (8.7) | C: 1, 3 Qui2 | H-4 Qui2 |
| 3 | 74.8, CH | 3.94, t (8.7) | C: 2, 4 Qui2 | H-1 Qui2 |
| 4 | 85.7, CH | 3.49, t (8.7) | C: 3, 5 Qui2, 1 Xyl3 | H-1 Xyl3, H-2 Qui2 |
| 5 | 71.5, CH | 3.67, dd (8.7; 6.8) | H-1 Qui2 | |
| 6 | 17.8, CH3 | 1.63, d (6.8) | C: 4, 5 Qui2 | |
| Xyl3 (1→4Qui2) | ||||
| 1 | 104.4, CH | 4.76, d (8.3) | C: 4 Qui2 | H-4 Qui2, H-3, 5 Xyl3 |
| 2 | 73.6, CH | 3.88, t (8.3) | C: 1, 3 Xyl3 | |
| 3 | 86.5, CH | 4.12, t (8.3) | C: 2, 4 Xyl3; 1 Glc4 | H-1 Glc4, H-1 Xyl3 |
| 4 | 68.6, CH | 3.94, m | ||
| 5 | 65.9, CH2 | 4.09, dd (10.8; 5.0) | C: 1, 3, 4 Xyl3 | |
| 3.60, t (10.8) | C: 1, 3, 4 Xyl3 | H-1, 3 Xyl3 | ||
| Glc4 (1→3Xyl3) | ||||
| 1 | 104.0, CH | 5.27, d (8.3) | C: 3 Xyl3 | H-3 Xyl3, H-3, 5 Glc4 |
| 2 | 73.6, CH | 3.98, t (8.3) | C: 1, 3 Glc4 | |
| 3 | 84.2, CH | 5.03, t (8.3) | C: 2, 4 Glc4 | H-1, 5 Glc4 |
| 4 | 70.0, CH | 3.95, t (8.3) | C: 3, 5 Glc4 | H-6 Glc4 |
| 5 | 77.1, CH | 3.85, t (8.3) | C: 4 Glc4 | H-1, 3 Glc4 |
| 6 | 61.7, CH2 | 4.33, dd (11.6; 2.1) | C: 4 Glc4 | |
| 3.99, m | C: 5 Glc4 | |||
| Glc5 (1→4Xyl1) | ||||
| 1 | 102.2, CH | 4.92, d (6.8) | C: 4 Xyl1 | H-4 Xyl1, H-3, 5 Glc5 |
| 2 | 73.3, CH | 3.92, t (7.9) | C: 1 Glc5 | |
| 3 | 86.9, CH | 4.19, t (7.9) | C: 2, 4 Glc5, C: 1 MeGlc6 | H-1 MeGlc6; H-1, 5 Glc5 |
| 4 | 69.4, CH | 3.87, t (7.9) | C: 3, 5 Glc5 | |
| 5 | 77.5, CH | 3.87, t (7.9) | C: 6 Glc5 | H-3 Glcl5 |
| 6 | 61.7, CH2 | 4.29, brd (11.7) | ||
| 4.02, brd (11.7) | C: 5 Glc5 | |||
| MeGlc6 (1→3Glc5) | ||||
| 1 | 104.5, CH | 5.19, d (7.5) | C: 3 Glc5 | H-3 Glc5; H-3, 5 MeGlc6 |
| 2 | 74.5, CH | 3.85, t (9.1) | C: 1, 3 MeGlc6 | |
| 3 | 86.9, CH | 3.67, t (9.1) | C: 2, 4 MeGlc6; OMe | H-1 MeGlc6 |
| 4 | 70.3, CH | 3.87, t (9.1) | C: 3, 5 MeGlc6 | |
| 5 | 77.5, CH | 3.93, t (9.1) | C: 6 MeGlc6 | H-1 MeGlc6 |
| 6 | 61.7, CH2 | 4.37, brd (11.9) | C: 4 MeGlc6 | H-4 MeGlc6 |
| 4.04, dd (11.9; 6.0) | C: 5 MeGlc6 | |||
| OMe | 60.7, CH3 | 3.80, s | C: 3 MeGlc6 |
| Glycosides (Cladolosides) | ED50, µM, Erythrocytes | Cytotoxicity, IC50 µM | |||
|---|---|---|---|---|---|
| MCF-10A | MCF-7 | T-47D | MDA-MB-231 | ||
| S (1) | 3.40 ± 0.25 | >20.00 | >20.00 | 14.85 ± 0.71 | 13.24 ± 1.08 |
| S1 (2) | 4.78 ± 0.30 | >20.00 | >20.00 | >20.00 | >20.00 |
| T (3) | 0.39 ± 0.03 | 8.23 ± 0.52 | 8.99 ± 0.61 | 8.21 ± 0.72 | 3.41 ± 0.33 |
| T1 (4) | 0.12 ± 0.02 | 8.41 ± 0.40 | 7.78 ± 0.62 | 7.41 ± 0.69 | 2.94 ± 0.21 |
| A1 (5) | 6.17 ± 0.51 | >20.00 | >20.00 | >20.00 | >20.00 |
| A2 (6) | 2.78 ± 0.32 | >20.00 | >20.00 | >20.00 | >20.00 |
| A3 (7) | 7.58 ± 0.69 | >20.00 | >20.00 | >20.00 | >20.00 |
| A4 (8) | 13.60 ± 0.70 | >20.00 | >20.00 | >20.00 | >20.00 |
| A5 (9) | 7.19 ± 0.53 | >20.00 | >20.00 | >20.00 | >20.00 |
| A6 (10) | 3.43 ± 0.22 | >20.00 | >20.00 | >20.00 | >20.00 |
| B1 (11) | 0.55 ± 0.04 | >20.00 | >20.00 | 12.99 ± 1.07 | 10.12 ± 1.00 |
| B2 (12) | 0.31 ± 0.02 | 12.23 ± 1.20 | 14.52 ± 0.48 | 12.50 ±0.96 | 3.92 ± 0.35 |
| C (13) | 0.44 ± 0.05 | >20.00 | >20.00 | 12.39 ± 0.66 | 13.01 ± 0.80 |
| C1 (14) | 0.15 ± 0.02 | >20.00 | >20.00 | >20.00 | 15.27 ± 0.71 |
| C3 (16) | 12.10 ± 0.58 | >20.00 | >20.00 | >20.00 | >20.00 |
| D (18) | 0.29 ± 0.03 | >20.00 | 15.31 ± 0.94 | 14.93 ± 0.85 | 2.98 ± 0.24 |
| G (25) | 0.35 ± 0.04 | >20.00 | > 20.00 | >20.00 | 3.95 ± 0.38 |
| H1 (26) | 0.21 ± 0.02 | >20.00 | 15.93 ± 1.14 | 13.20 ± 0.72 | 2.47 ± 0.32 |
| I1 (28) | 1.72 ± 0.10 | 13.44 ± 0.92 | 11.23 ± 1.02 | 9.91 ± 0.68 | 3.02 ± 0.28 |
| I2 (29) | 4.24 ± 0.31 | >20.00 | >20.00 | >20.00 | >20.00 |
| J1 (30) | 1.10 ± 0.10 | >20.00 | >20.00 | >20.00 | 6.18 ± 0.54 |
| K1 (31) | 0.31 ± 0.02 | >20.00 | >20.00 | 12.13 ± 1.00 | 5.83 ± 0.41 |
| K2 (32) | 4.63 ± 0.41 | 14.45 ± 1.07 | >20.00 | 14.08 ± 0.91 | 6.81 ± 0.73 |
| L1 (33) | 0.20 ± 0.02 | 17.88 ± 1.12 | 12.70 ± 1.01 | 9.03 ± 0.81 | 13.36 ± 1.08 |
| M1 (35) | 0.08 ± 0.01 | 9.81 ± 0.86 | >20.00 | 17.14 ± 1.52 | 5.05 ± 0.38 |
| P2 (41) | 0.17 ± 0.02 | 11.10 ± 0.74 | 16.32 ± 1.03 | 10.59 ± 0.61 | 6.92 ± 0.44 |
| Cuc A0-1 | 1.63 ± 0.21 | 7.51 ± 0.63 | 11.33 ± 1.00 | 8.46 ± 0.53 | 3.53 ± 0.54 |
| cysplatin | - | 82.33 ± 5.21 | 152.00 ± 8.02 | ≥160.00 | >160.00 |
| Glycoside | Selectivity Index (SI) | ||
|---|---|---|---|
| MCF-7 | T-47D | MDA-MB-231 | |
| Cladoloside S (1) | - | >1.35 | >1.51 |
| Cladoloside T (3) | 0.92 | 1.00 | 2.41 |
| Cladoloside T1 (4) | 1.08 | 1.13 | 2.86 |
| Cladoloside B1 (11) | - | >1.54 | >1.98 |
| Cladoloside B2 (12) | 0.84 | 0.98 | 3.12 |
| Cladoloside C (13) | - | >1.61 | >1.54 |
| Cladoloside C1 (14) | - | - | >1.13 |
| Cladoloside D (18) | >1.31 | >1.34 | >6.7 |
| Cladoloside G (25) | - | - | >5.06 |
| Cladoloside H1 (26) | 1.26 | 1.51 | 8.10 |
| Cladoloside I1 (28) | 1.20 | 1.36 | 4.45 |
| Cladoloside J1 (30) | - | - | >3.24 |
| Cladoloside K1 (31) | - | >1.65 | >3.83 |
| Cladoloside K2 (32) | - | 1.03 | 2.17 |
| Cladoloside L1 (33) | 1.41 | 1.98 | 1.34 |
| Cladoloside M1 (35) | - | 0.57 | 1.94 |
| Cladoloside P2 (41) | 0.68 | 1.05 | 1.60 |
| Cucumarioside A0-1 | 0.66 | 0.89 | 2.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silchenko, A.S.; Zelepuga, E.A.; Chingizova, E.A.; Menchinskaya, E.S.; Tabakmakher, K.M.; Kalinovsky, A.I.; Avilov, S.A.; Popov, R.S.; Dmitrenok, P.S.; Kalinin, V.I. Cladolosides of Groups S and T: Triterpene Glycosides from the Sea Cucumber Cladolabes schmeltzii with Unique Sulfation; Human Breast Cancer Cytotoxicity and QSAR. Mar. Drugs 2025, 23, 265. https://doi.org/10.3390/md23070265
Silchenko AS, Zelepuga EA, Chingizova EA, Menchinskaya ES, Tabakmakher KM, Kalinovsky AI, Avilov SA, Popov RS, Dmitrenok PS, Kalinin VI. Cladolosides of Groups S and T: Triterpene Glycosides from the Sea Cucumber Cladolabes schmeltzii with Unique Sulfation; Human Breast Cancer Cytotoxicity and QSAR. Marine Drugs. 2025; 23(7):265. https://doi.org/10.3390/md23070265
Chicago/Turabian StyleSilchenko, Alexandra S., Elena A. Zelepuga, Ekaterina A. Chingizova, Ekaterina S. Menchinskaya, Kseniya M. Tabakmakher, Anatoly I. Kalinovsky, Sergey A. Avilov, Roman S. Popov, Pavel S. Dmitrenok, and Vladimir I. Kalinin. 2025. "Cladolosides of Groups S and T: Triterpene Glycosides from the Sea Cucumber Cladolabes schmeltzii with Unique Sulfation; Human Breast Cancer Cytotoxicity and QSAR" Marine Drugs 23, no. 7: 265. https://doi.org/10.3390/md23070265
APA StyleSilchenko, A. S., Zelepuga, E. A., Chingizova, E. A., Menchinskaya, E. S., Tabakmakher, K. M., Kalinovsky, A. I., Avilov, S. A., Popov, R. S., Dmitrenok, P. S., & Kalinin, V. I. (2025). Cladolosides of Groups S and T: Triterpene Glycosides from the Sea Cucumber Cladolabes schmeltzii with Unique Sulfation; Human Breast Cancer Cytotoxicity and QSAR. Marine Drugs, 23(7), 265. https://doi.org/10.3390/md23070265











