Prickly Defenders: A Review of Venomous Sea Urchins (Echinoidea)
Abstract
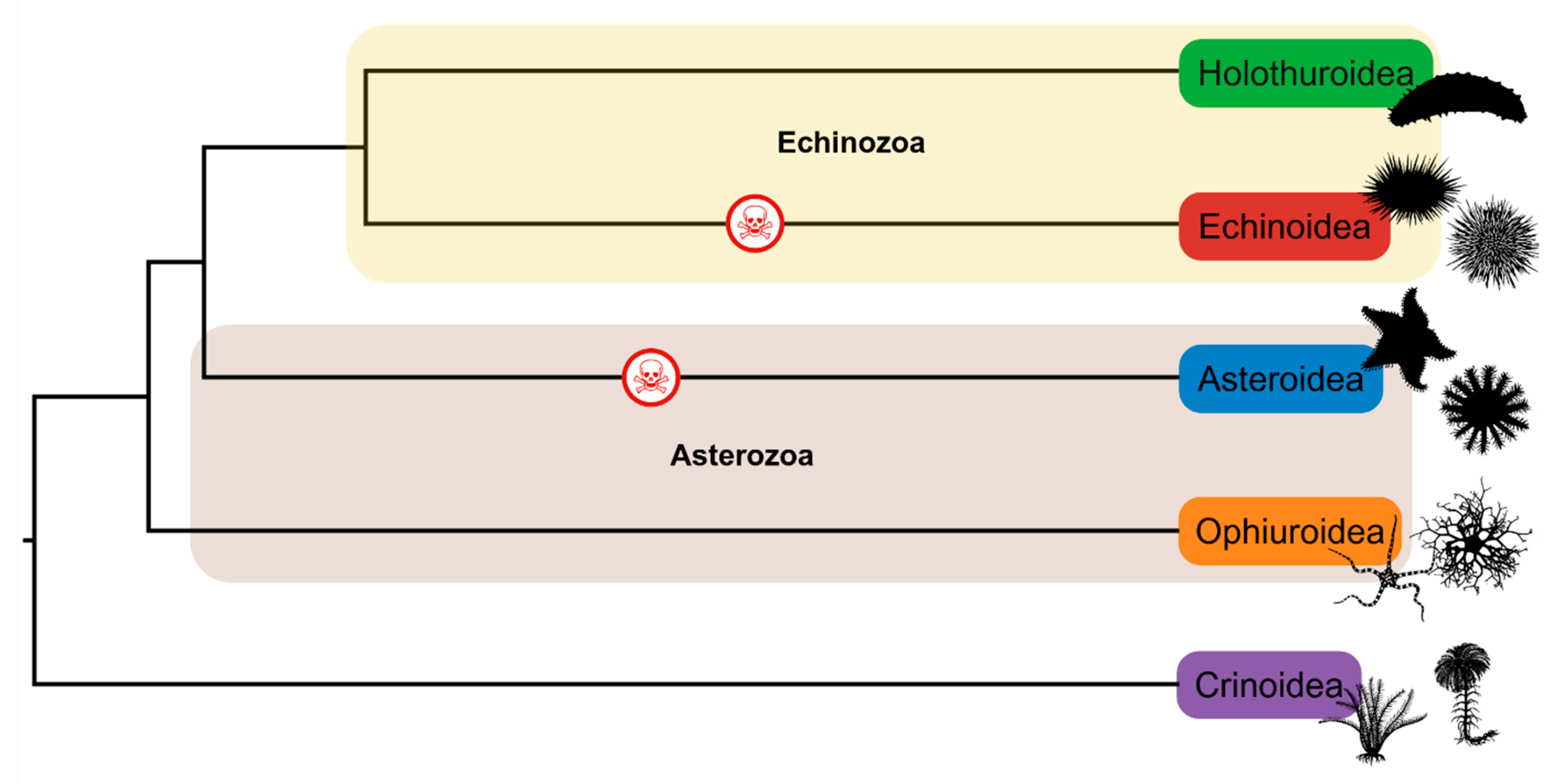
1. Introduction
2. Venom System
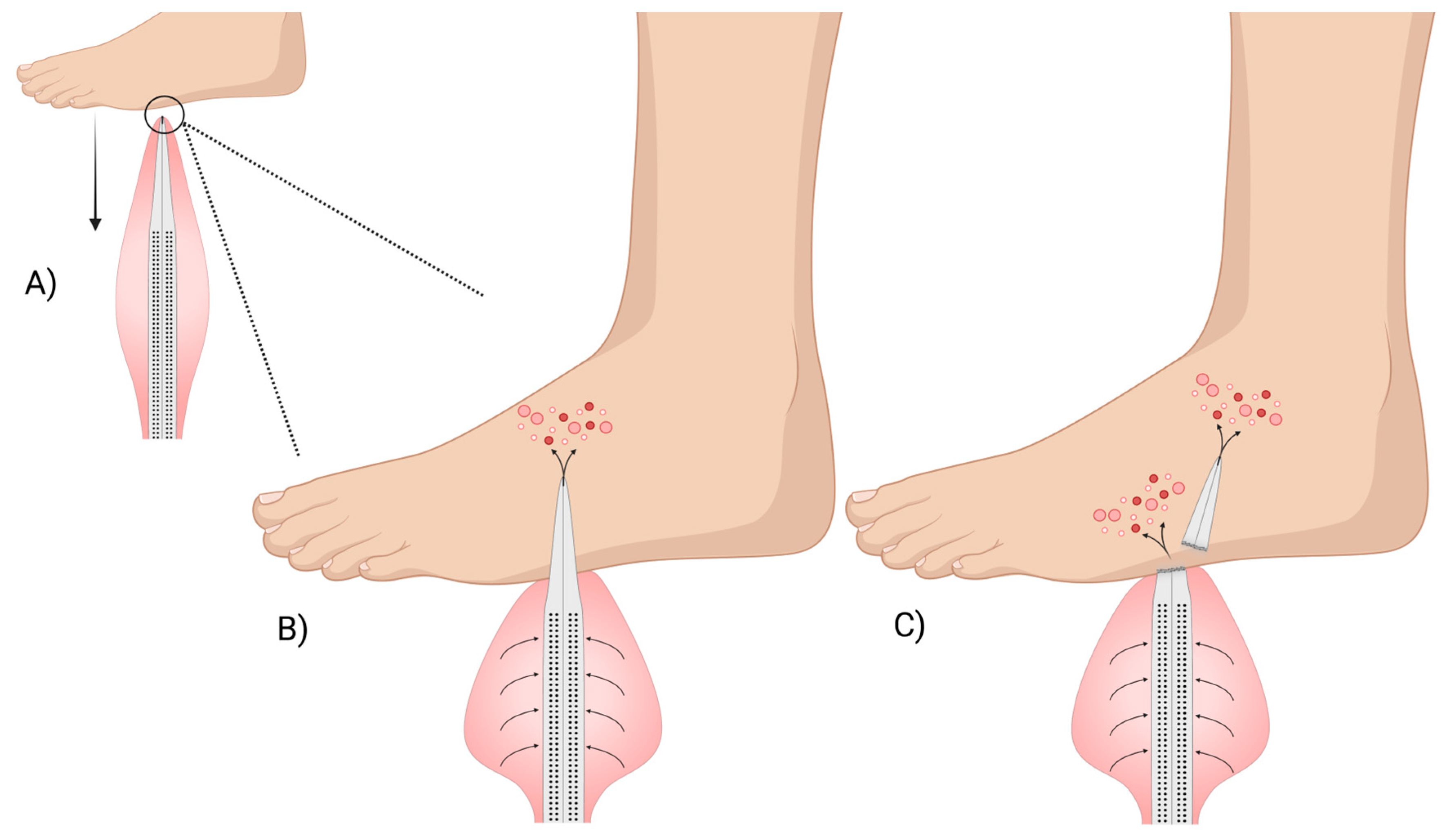
2.1. Spines
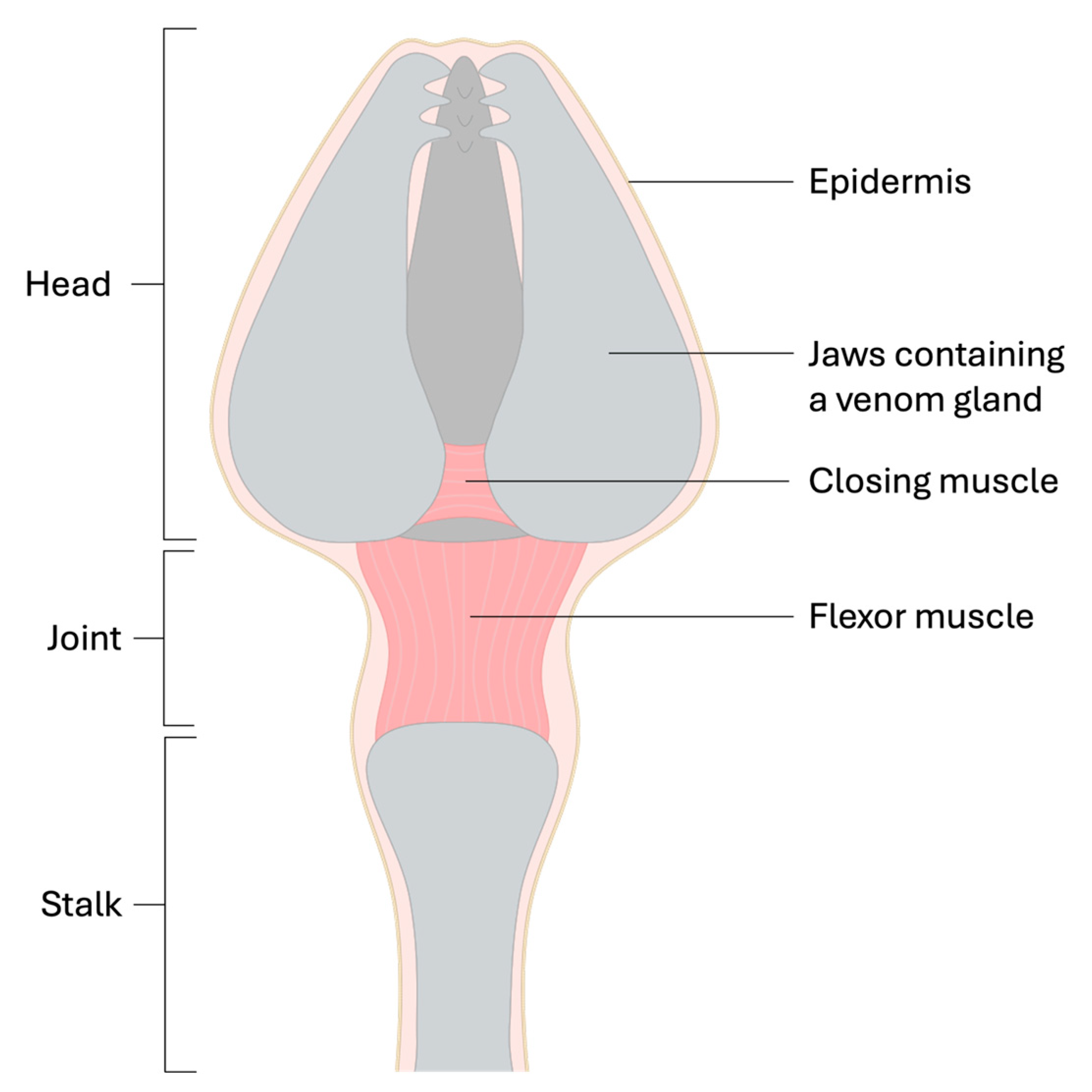
2.2. Pedicellariae
3. Venom Toxins
3.1. Spine Venom
3.1.1. Echinothrix calamaris and E. diadema
3.1.2. Echinometra mathaei
3.1.3. Echinometra lucunter
3.1.4. Lytechinus variegatus and Arbacia lixula
3.2. Pedicellariae Toxins
3.2.1. Toxopneustes pileolus
3.2.2. Toxopneustes roseus
3.2.3. Tripneustes gratilla
3.2.4. Lytechinus variegatus
3.2.5. Lytechinus pictus
3.2.6. Briefly Noted Toxins from Other Urchin Species
4. Envenomation and Human Interactions
5. Conclusions
6. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, J.R.; Petsios, E.; Davidson, E.H.; Erkenbrack, E.M.; Gao, F.; Bottjer, D.J. Reorganization of sea urchin gene regulatory networks at least 268 million years ago as revealed by oldest fossil cidaroid echinoid. Sci. Rep. 2015, 5, 15541. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.R.; Erkenbrack, E.M.; Hinman, V.F.; McCauley, B.S.; Petsios, E.; Bottjer, D.J. Paleogenomics of echinoids reveals an ancient origin for the double-negative specification of micromeres in sea urchins. Proc. Natl. Acad. Sci. USA 2017, 114, 5870–5877. [Google Scholar] [CrossRef] [PubMed]
- Kroh, A.; Mooi, R. The World Echinoidea Database. Available online: https://www.marinespecies.org/echinoidea/index.php (accessed on 21 January 2025).
- Lawrence, J.M. Sea Urchins: Biology and Ecology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 38, p. 509. [Google Scholar]
- Telford, M.J.; Lowe, C.J.; Cameron, C.B.; Ortega-Martinez, O.; Aronowicz, J.; Oliveri, P.; Copley, R.R. Phylogenomic analysis of echinoderm class relationships supports Asterozoa. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140479. [Google Scholar] [CrossRef] [PubMed]
- Reich, A.; Dunn, C.; Akasaka, K.; Wessel, G. Phylogenomic analyses of Echinodermata support the sister groups of Asterozoa and Echinozoa. PLoS ONE 2015, 10, e0119627. [Google Scholar] [CrossRef]
- Kikuchi, D.W.; Allen, W.L.; Arbuckle, K.; Aubier, T.G.; Briolat, E.S.; Burdfield-Steel, E.R.; Cheney, K.L.; Daňková, K.; Elias, M.; Hämäläinen, L. The evolution and ecology of multiple antipredator defences. J. Evol. Biol. 2023, 36, 975–991. [Google Scholar] [CrossRef]
- Arbuckle, K. Evolutionary context of venom in animals. Evol. Venom. Anim. Their Toxins 2017, 24, 3–31. [Google Scholar]
- Shiomi, K.; Yamamoto, S.; Yamanaka, H.; Kikuchi, T. Purification and characterization of a lethal factor in venom from the crown-of-thorns starfish (Acanthaster planci). Toxicon 1988, 26, 1077–1083. [Google Scholar] [CrossRef]
- Shiomi, K.; Midorikawa, S.; Ishida, M.; Nagashima, Y.; Nagai, H. Plancitoxins, lethal factors from the crown-of-thorns starfish Acanthaster planci, are deoxyribonucleases II. Toxicon 2004, 44, 499–506. [Google Scholar] [CrossRef]
- Hillberg, A.K.; Smith, M.K.; Lausen, B.S.; Suwansa-Ard, S.; Johnston, R.; Mitu, S.A.; MacDonald, L.E.; Zhao, M.; Motti, C.A.; Wang, T. Crown-of-thorns starfish spines secrete defence proteins. PeerJ 2023, 11, e15689. [Google Scholar] [CrossRef]
- Chanley, J.; Mezzetti, T.; Sobotka, H. The holothurinogenins. Tetrahedron 1966, 22, 1857–1884. [Google Scholar] [CrossRef]
- Smith, S.J.; Cummins, S.F.; Motti, C.A.; Wang, T. A mass spectrometry database for the identification of marine animal saponin-related metabolites. Anal. Bioanal. Chem. 2024, 416, 6893–6907. [Google Scholar] [CrossRef]
- Smith, S.J.; Wang, T.; Cummins, S.F. Asteroid Saponins: A Review of Their Bioactivity and Selective Cytotoxicity. Mar. Drugs 2024, 22, 552. [Google Scholar] [CrossRef]
- Honey-Escandón, M.; Arreguín-Espinosa, R.; Solís-Marín, F.A.; Samyn, Y. Biological and taxonomic perspective of triterpenoid glycosides of sea cucumbers of the family Holothuriidae (Echinodermata, Holothuroidea). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 180, 16–39. [Google Scholar] [CrossRef] [PubMed]
- Thimmappa, R.; Wang, S.; Zheng, M.; Misra, R.C.; Huang, A.C.; Saalbach, G.; Chang, Y.; Zhou, Z.; Hinman, V.; Bao, Z. Biosynthesis of saponin defensive compounds in sea cucumbers. Nat. Chem. Biol. 2022, 18, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Motti, C.A.; Bose, U.; Roberts, R.E.; McDougall, C.; Smith, M.K.; Hall, M.R.; Cummins, S.F. Chemical ecology of chemosensation in Asteroidea: Insights towards management strategies of pest species. J. Chem. Ecol. 2018, 44, 147–177. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J. The piscine arsenal: An updated review of venomous fishes. Rev. Fish Biol. Fish. 2024, 34, 539–574. [Google Scholar] [CrossRef]
- Xie, B.; Huang, Y.; Baumann, K.; Fry, B.G.; Shi, Q. From marine venoms to drugs: Efficiently supported by a combination of transcriptomics and proteomics. Mar. Drugs 2017, 15, 103. [Google Scholar] [CrossRef]
- von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L. Modern venomics—Current insights, novel methods, and future perspectives in biological and applied animal venom research. GigaScience 2022, 11, giac048. [Google Scholar] [CrossRef]
- Von Reumont, B.M.; Campbell, L.I.; Jenner, R.A. Quo vadis venomics? A roadmap to neglected venomous invertebrates. Toxins 2014, 6, 3488–3551. [Google Scholar] [CrossRef]
- Sunagar, K.; Morgenstern, D.; Reitzel, A.M.; Moran, Y. Ecological venomics: How genomics, transcriptomics and proteomics can shed new light on the ecology and evolution of venom. J. Proteom. 2016, 135, 62–72. [Google Scholar] [CrossRef]
- Wilson, D.; Daly, N.L. Venomics: A Mini-Review. High-Throughput 2018, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Oldrati, V.; Arrell, M.; Violette, A.; Perret, F.; Sprüngli, X.; Wolfender, J.-L.; Stöcklin, R. Advances in Venomics. Mol. Biosyst. 2016, 12, 3530–3543. [Google Scholar] [CrossRef] [PubMed]
- Vetter, I.; Davis, J.L.; Rash, L.D.; Anangi, R.; Mobli, M.; Alewood, P.F.; Lewis, R.J.; King, G.F. Venomics: A new paradigm for natural products-based drug discovery. Amino Acids 2011, 40, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, Z.; Cohen, M.; Lipner, S.R. Sea urchin injuries: A review and clinical approach algorithm. J. Dermatol. Treat. 2021, 32, 150–156. [Google Scholar] [CrossRef]
- Haddad Junior, V. Observation of initial clinical manifestations and repercussions from the treatment of 314 human injuries caused by black sea urchins (Echinometra lucunter) on the southeastern Brazilian coast. Rev. Da Soc. Bras. De Med. Trop. 2012, 45, 390–392. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Caballes, C.F.; Wilmes, J.C.; Matthews, S.; Mellin, C.; Sweatman, H.P.; Nadler, L.E.; Brodie, J.; Thompson, C.A.; Hoey, J. Thirty years of research on crown-of-thorns starfish (1986–2016): Scientific advances and emerging opportunities. Diversity 2017, 9, 41. [Google Scholar] [CrossRef]
- Coppard, S.E.; Kroh, A.; Smith, A.B. The evolution of pedicellariae in echinoids: An arms race against pests and parasites. Acta Zool. 2012, 93, 125–148. [Google Scholar] [CrossRef]
- Pechenik, J.A. The Echinoderms. In Biology of the Invertebrates, 6th ed.; McGraw-Hill: New York, NY, USA, 2010; pp. 497–527. [Google Scholar]
- Voulgaris, K.; Varkoulis, A.; Zaoutsos, S.; Stratakis, A.; Vafidis, D. Mechanical defensive adaptations of three Mediterranean sea urchin species. Ecol. Evol. 2021, 11, 17734–17743. [Google Scholar] [CrossRef]
- Mongiardino Koch, N.; Coppard, S.E.; Lessios, H.A.; Briggs, D.E.; Mooi, R.; Rouse, G.W. A phylogenomic resolution of the sea urchin tree of life. BMC Evol. Biol. 2018, 18, 189. [Google Scholar] [CrossRef]
- Coppard, S.E.; Campbell, A.C. Taxonomic significance of spine morphology in the echinoid genera Diadema and Echinothrix. Invertebr. Biol. 2004, 123, 357–371. [Google Scholar] [CrossRef]
- Regis, M.; Thomassin, B. Macro- and microstructure of the primary spines in Asthenosoma varium Grube (Echinothuridae: Echinoidea): Affinities with the Diadematidae and Toxopneustidae. In Proceedings of the Fifth International Echinoderm Conference, Galway, Ireland, 24–29 September 1984; pp. 321–332. [Google Scholar]
- Alender, C.B. A biologically active substance from spines of two diadematid sea urchins. In Proceedings of the Animal Toxins: A Collection of Papers Presented at the First International Symposium on Animal Toxins, Atlantic City, NJ, USA, 9–11 April 1966; p. 145. [Google Scholar]
- Emson, R.H.; Young, C. Form and function of the primary spines of two bathyal echinothuriid sea urchins. Acta Zool. 1998, 79, 101–111. [Google Scholar] [CrossRef]
- Hebert, E.; Silvia, M.; Wessel, G.M. Structural and molecular distinctions of primary and secondary spines in the sea urchin Lytechinus variegatus. Sci. Rep. 2024, 14, 28525. [Google Scholar] [CrossRef]
- Moureaux, C.; Perez-Huerta, A.; Compère, P.; Zhu, W.; Leloup, T.; Cusack, M.; Dubois, P. Structure, composition and mechanical relations to function in sea urchin spine. J. Struct. Biol. 2010, 170, 41–49. [Google Scholar] [CrossRef]
- Tsafnat, N.; Fitz Gerald, J.D.; Le, H.N.; Stachurski, Z.H. Micromechanics of sea urchin spines. PLoS ONE 2012, 7, e44140. [Google Scholar] [CrossRef]
- Drozdov, A.; Sharmankina, V.; Zemnukhova, L.; Polyakova, N. Chemical composition of spines and tests of sea urchins. Biol. Bull. 2016, 43, 521–531. [Google Scholar] [CrossRef]
- Cavey, M.; Märkel, K. Echinoidea. In Microscopic Anatomy of Invertebrates; Harrison, F., Ed.; Wiley-Liss: New York, NY, USA, 1994; Volume 14, pp. 345–400. [Google Scholar]
- Stevenson, A.; Kroh, A. Deep-sea sea urchins. In Developments in Aquaculture and Fisheries Science; Elsevier: Amsterdam, The Netherlands, 2020; Volume 43, pp. 237–254. [Google Scholar]
- Campbell, A.C. Form and function of pedicellariae. In Echinoderm Studies 1; CRC Press: Boca Raton, FL, USA, 1983; pp. 139–167. [Google Scholar]
- Sladen, W.P. XII.—On a remarkable form of Pedicellaria, and the functions performed thereby; together with general observations on the allied forms of this organ in the Echinidæ. J. Nat. Hist. 1880, 6, 101–114. [Google Scholar] [CrossRef]
- von Uexküll, J. Die physiologie der Pedicellarien. In Zeitschrift für Biologie; Kühne, W., Voit, C., Eds.; Urban & Schwarzenberg München Lehmann: München, Germany, 1899; pp. 334–403. [Google Scholar]
- Lambert, A.; De Vos, L.; Jangoux, M. Functional morphology of the pedicellariae of the asteroid Marthasterias glacialis (Echinodermata). Zoomorphology 1984, 104, 122–130. [Google Scholar] [CrossRef]
- Gale, A.S. The phylogeny of post-Palaeozoic Asteroidea (Echinodermata: Neoasteroidea). Spec. Pap. Palaeontol. 2011, 85, 1–112. [Google Scholar]
- Cannone, A. The anatomy and venom-emitting mechanism of the globiferous pedicellariae of the urchin Parechinus Angulosus (Leske) with notes of their behaviour. Afr. Zool. 1970, 5, 179–190. [Google Scholar]
- Lewis, J.B.; Saluja, G. The claviform pedicellariae and their stalk glands in the tropical sea urchin Diadema antillarum Philippi. Can. J. Zool. 1967, 45, 1211–1214. [Google Scholar] [CrossRef]
- Ghyoot, M.; Dubois, P.; Jangoux, M. The venom apparatus of the globiferous pedicellariae of the toxopneustid Sphaerechinus granularis (Echinodermata, Echinoida): Fine structure and mechanism of venom discharge. Zoomorphology 1994, 114, 73–82. [Google Scholar] [CrossRef]
- Oldfield, S.C. Surface fine structure of the globiferous pedicellariae of the regular echinoid, Psammechinus miliaris Gmelin. Cell Tissue Res. 1975, 162, 377–385. [Google Scholar] [CrossRef]
- Campbell, A.; Laverack, M. The responses of pedicellariae from Echinus esculentus (L.). J. Exp. Mar. Biol. Ecol. 1968, 2, 191–214. [Google Scholar] [CrossRef]
- Campbell, A.C. Observations on the activity of echinoid pedicellariae: III. Jaw responses of globiferous pedicellariae and their significance. Mar. Freshw. Behav. Phy 1976, 4, 25–39. [Google Scholar] [CrossRef]
- Sheppard-Brennand, H.; Poore, A.G.; Dworjanyn, S.A. A waterborne pursuit-deterrent signal deployed by a sea urchin. Am. Nat. 2017, 189, 700–708. [Google Scholar] [CrossRef]
- Bretschneider, A.; Pomory, C. Autotomy of Globiferous Pedicellariae in the Sea Urchin: Lytechinus variegatus (Echinodermata: Echinoidea). Gulf Caribb. Res. 2024, 35, 16–22. [Google Scholar] [CrossRef]
- Chia, F.S. Histology of the globiferous pedicellariae of Psammechinus miliaris (Echinodermata: Echinoidea). J. Zool. 1970, 160, 9–16. [Google Scholar] [CrossRef]
- Vermeij, G.J. The Mesozoic marine revolution: Evidence from snails, predators and grazers. Paleobiology 1977, 3, 245–258. [Google Scholar] [CrossRef]
- Chia, F.-S.; Amerongen, H. On the prey-catching pedicellariae of a starfish, Stylasterias forreri (de Loriol). Can. J. Zool. 1975, 53, 748–755. [Google Scholar] [CrossRef]
- Emson, R.; Young, C. Feeding mechanism of the brisingid starfish Novodinia antillensis. Mar. Biol. 1994, 118, 433–442. [Google Scholar] [CrossRef]
- Blowes, L.M.; Egertová, M.; Liu, Y.; Davis, G.R.; Terrill, N.J.; Gupta, H.S.; Elphick, M.R. Body wall structure in the starfish Asterias rubens. J. Anat. 2017, 231, 325–341. [Google Scholar] [CrossRef]
- Ha, D.T.; Kicha, A.A.; Kalinovsky, A.I.; Malyarenko, T.V.; Popov, R.S.; Malyarenko, O.S.; Ermakova, S.P.; Thuy, T.T.; Long, P.Q.; Ivanchina, N.V. Asterosaponins from the tropical starfish Acanthaster planci and their cytotoxic and anticancer activities in vitro. Nat. Prod. Res. 2021, 35, 548–555. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kicha, A.A.; Malyarenko, T.V.; Ivanchina, N.V. Asterosaponins: Structures, taxonomic distribution, biogenesis and biological activities. Mar. Drugs 2020, 18, 584. [Google Scholar] [CrossRef]
- Mebs, D. Toxicity in animals. Trends in evolution? Toxicon 2001, 39, 87–96. [Google Scholar] [CrossRef]
- Dehghani, H.; Rashedinia, M.; Mohebbi, G.; Vazirizadeh, A.; Baghban, N. Antioxidant and anticholinesterase properties of Echinometra mathaei and Ophiocoma erinaceus venoms from the Persian Gulf. Front. Chem. 2024, 11, 1332921. [Google Scholar] [CrossRef]
- Moreno-Garcıa, D.; Salas-Rojas, M.; Ferná ndez-Martınez, E.; del Rocıo López-Cuellar, M.; Sosa-Gutierrez, C.; Pelá ez-Acero, A. Sea urchins: An update on their pharmacological properties. PeerJ 2022, 10, e13606. [Google Scholar] [CrossRef]
- He, D.; Wu, H.; Wei, Y.; Liu, W.; Huang, F.; Shi, H.; Zhang, B.; Wu, X.; Wang, C. Effects of harmine, an acetylcholinesterase inhibitor, on spatial learning and memory of APP/PS1 transgenic mice and scopolamine-induced memory impairment mice. Eur. J. Pharmacol. 2015, 768, 96–107. [Google Scholar] [CrossRef]
- Rossetto, A.L.; Mora, J.d.M.; Haddad Junior, V. Sea urchin granuloma. Rev. Do Inst. De Med. Trop. De São Paulo 2006, 48, 303–306. [Google Scholar] [CrossRef]
- Cotrin, S.S.; Puzer, L.; de Souza Judice, W.A.; Juliano, L.; Carmona, A.K.; Juliano, M.A. Positional-scanning combinatorial libraries of fluorescence resonance energy transfer peptides to define substrate specificity of carboxydipeptidases: Assays with human cathepsin B. Anal. Biochem. 2004, 335, 244–252. [Google Scholar] [CrossRef]
- Sciani, J.M.; Antoniazzi, M.M.; Neves, A.d.C.; Pimenta, D.C. Cathepsin B/X is secreted by Echinometra lucunter sea urchin spines, a structure rich in granular cells and toxins. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 1–8. [Google Scholar] [CrossRef]
- Sciani, J.M.; Emerenciano, A.K.; Silva, J.R.M.C.d.; Pimenta, D.C. Initial peptidomic profiling of Brazilian sea urchins: Arbacia lixula, Lytechinus variegatus and Echinometra lucunter. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 17. [Google Scholar] [CrossRef]
- Sciani, J.M.; Zychar, B.; Gonçalves, L.R.; Giorgi, R.; Nogueira, T.; Pimenta, D.C. Preliminary molecular characterization of a proinflammatory and nociceptive molecule from the Echinometra lucunter spines extracts. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 43. [Google Scholar] [CrossRef]
- Halstead, B.W. Poisonous and Venomous Marine Animals of the World, 2nd ed.; Darwin Press Inc.: Princeton, NJ, USA, 1988. [Google Scholar]
- Mebs, D. Clinical toxicology of sea urchin and starfish injuries. In Handbook of Clinical Toxicology of Animal Venoms and Poisons; CRC Press: Boca Raton, FL, USA, 2017; pp. 129–133. [Google Scholar]
- Endean, R. The venomous sea-urchin Toxopneustes pileolus. Med. J. Aust. 1961, 1, 320. [Google Scholar] [CrossRef]
- Nakagawa, H.; Kimura, A. Partial purification and characterization of a toxic substance from pedicellariae of the sea urchin Toxopneustes pileolus. Jpn. J. Pharmacol. 1982, 32, 966–968. [Google Scholar] [CrossRef]
- Kimura, A.; Nakagawa, H. Action of an extract from the sea urchin Toxopneustes pileolus on isolated smooth muscle. Toxicon 1980, 18, 689–693. [Google Scholar] [CrossRef]
- Kimura, A.; Hayashi, H.; Kuramoto, M. Studies of urchi-toxins: Separation, purification and pharmacological actions of toxinic substances. Jpn. J. Pharmacol. 1975, 25, 109–120. [Google Scholar] [CrossRef]
- Takei, M.; Nakagawa, H.; Kimura, A.; Endo, K. A toxic substance from the sea urchin Toxopneustes pileolus induces histamine release from rat peritoneal mast cells. Agents Actions 1991, 32, 224–228. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yanagihara, N.; Izumi, F.; Wada, A.; Kimura, A. Inhibition of nicotinic acetylcholine receptor-mediated secretion and synthesis of catecholamines by sea urchin toxin in cultured bovine adrenal medullary cells. Biochem. Pharmacol. 1992, 44, 1779–1785. [Google Scholar]
- Nakagawa, H.; Hashimoto, H.; Hayashi, H.; Shinohara, M.; Ohura, K.; Tachikawa, E.; Kashimoto, T. Isolation of a novel lectin from the globiferous pedicellariae of the sea urchin Toxopneustes pileolus. In Natural Toxins 2: Structure, Mechanism of Action, and Detection; Springer: Boston, MA, USA, 1996; pp. 213–223. [Google Scholar]
- Sakai, H.; Edo, K.; Nakagawa, H.; Shinohara, M.; Nishiitsutsuji, R.; Ohura, K. Isolation and partial characterization of a L-rhamnose-binding lectin from the globiferous pedicellariae of the toxopneustid sea urchin, Toxopneustes pileolus. Int. Aquat. Res. 2013, 5, 12. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yamaguchi, C.; Tomiyoshi, F.; Hayashi, H. A Novel Mitogenic Lectin from the Globiferous Pedicellariae of Sea Urchin, Toxopneustes pileolus. Chem. Soc. Pak. 1999, 21, 305–310. [Google Scholar]
- Edo, K.; Sakai, H.; Nakagawa, H.; Hashimoto, T.; Shinohara, M.; Ohura, K. Immunomodulatory activity of a pedicellarial venom lectin from the toxopneustid sea urchin, Toxopneustes pileolus. Toxin Rev. 2012, 31, 54–60. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Higashi, E.; Nakagawa, H. cDNA cloning and expression of Contractin A, a phospholipase A2-like protein from the globiferous pedicellariae of the venomous sea urchin Toxopneustes pileolus. Toxicon 2015, 108, 46–52. [Google Scholar] [CrossRef]
- Nakagawa, H.; Tu, A.T.; Kimura, A. Purification and characterization of Contractin A from the pedicellarial venom of sea urchin, Toxopneustes pileolus. Arch. Biochem. Biophys. 1991, 284, 279–284. [Google Scholar] [CrossRef]
- Zhang, Y.-a.; Abe, J.; Siddiq, A.; Nakagawa, H.; Honda, S.; Wada, T.; Ichida, S. UT841 purified from sea urchin (Toxopneustes pileolus) venom inhibits time-dependent 45Ca2+ uptake in crude synaptosome fraction from chick brain. Toxicon 2001, 39, 1223–1229. [Google Scholar] [CrossRef]
- Kuwabara, S. Purification and properties of peditoxin and the structure of its prosthetic group, pedoxin, from the sea urchin Toxopneustes pileolus (Lamarck). J. Biol. Chem. 1994, 269, 26734–26738. [Google Scholar] [CrossRef]
- Drickamer, K.; Taylor, M.E. Biology of animal lectins. Annu. Rev. Cell Biol. 1993, 9, 237–264. [Google Scholar] [CrossRef]
- Drickamer, K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J. Biol. Chem. 1988, 263, 9557–9560. [Google Scholar] [CrossRef]
- Arason, G.J. Lectins as defence molecules in vertebrates and invertebrates. Fish Shellfish Immunol. 1996, 6, 277–289. [Google Scholar] [CrossRef]
- Arlinghaus, F.; Fry, B.; Sunagar, K.; Jackson, T.; Eble, J.; Reeks, T.; Clemetson, K. Lectin proteins. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B., Ed.; Ocford University Press: Oxford, UK, 2015; pp. 299–311. [Google Scholar]
- Hatakeyama, T.; Ichise, A.; Unno, H.; Goda, S.; Oda, T.; Tateno, H.; Hirabayashi, J.; Sakai, H.; Nakagawa, H. Carbohydrate recognition by the rhamnose-binding lectin SUL-I with a novel three-domain structure isolated from the venom of globiferous pedicellariae of the flower sea urchin Toxopneustes pileolus. Protein Sci. 2017, 26, 1574–1583. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Ichise, A.; Yonekura, T.; Unno, H.; Goda, S.; Nakagawa, H. cDNA cloning and characterization of a rhamnose-binding lectin SUL-I from the toxopneustid sea urchin Toxopneustes pileolus venom. Toxicon 2015, 94, 8–15. [Google Scholar] [CrossRef]
- Takei, M.; Nakagawa, H. A sea urchin lectin, SUL-1, from the Toxopneustid sea urchin induces DC maturation from human monocyte and drives Th1 polarization in vitro. Toxicol. Appl. Pharmacol. 2006, 213, 27–36. [Google Scholar] [CrossRef]
- Nakagawa, H.; Tanigawa, T.; Tomita, K.; Tomihara, Y.; Araki, Y.; Tachikawa, E. Recent studies on the pathological effects of purified sea urchin toxins. J. Toxicol. Toxin Rev. 2003, 22, 633–649. [Google Scholar] [CrossRef]
- Edo, K. Study on a Pedicellarial Venom Lectin from the Sea Urchin Toxopneustes pileolus, in the Coast of Tokushima Prefecture, Japan; The University of Tokushima: Tokushima, Japan, 2014. [Google Scholar]
- Suzuki-Nishimura, T.; Nakagawa, H.; Uchida, M.K. D-galactose-specific sea urchin lectin sugar-specifically inhibited histamine release induced by Datura stramonium agglutinin: Differences between sugar-specific effects of sea urchin lectin and those of D-galactose-or L-fucose-specific plant lectins. Jpn. J. Pharmacol. 2001, 85, 443–452. [Google Scholar] [CrossRef]
- Kimura, A.; Nakagawa, H.; Hayashi, H.; Endo, K. Seasonal changes in contractile activity of a toxic substance from the pedicellaria of the sea urchin Toxopneustes pileolus. Toxicon 1984, 22, 353–358. [Google Scholar] [CrossRef]
- Herzig, V.; Ward, R.J.; dos Santos, W.F. Intersexual variations in the venom of the Brazilian ‘armed’spider Phoneutria nigriventer (Keyserling, 1891). Toxicon 2002, 40, 1399–1406. [Google Scholar] [CrossRef]
- Harris, R.J.; Jenner, R.A. Evolutionary ecology of fish venom: Adaptations and consequences of evolving a venom system. Toxins 2019, 11, 60. [Google Scholar] [CrossRef]
- Robles-Gómez, E.; Benítez-Villalobos, F.; Soriano-García, M.; Antúnez-Argüelles, E. Non-peptide molecules in the pedicellariae of Toxopneustes roseus. Toxicon 2020, 184, 143–151. [Google Scholar] [CrossRef]
- Klupczynska, A.; Pawlak, M.; Kokot, Z.J.; Matysiak, J. Application of metabolomic tools for studying low molecular-weight fraction of animal venoms and poisons. Toxins 2018, 10, 306. [Google Scholar] [CrossRef]
- Alender, C.B. The Venom from the Heads of the Globiferous Pedicellariae of the Sea Urchin, Tripneustes gratilla (Linnaeus); University of Hawai’i at Manoa: Honolulu, HI, USA, 1964. [Google Scholar]
- Alender, C.B.; Feigen, G.A.; Tomita, J.T. Isolation and characterization of sea urchin toxin. Toxicon 1965, 3, 9–17. [Google Scholar] [CrossRef]
- Feigen, G.A.; Sanz, E.; Alender, C.B. Studies on the mode of action of sea urchin toxin—I. Conditions affecting release of histamine and other agents from isolated tissues. Toxicon 1966, 4, 161–175. [Google Scholar] [CrossRef]
- Feigen, G.A.; Sanz, E.; Tomita, J.T.; Alender, C.B. Studies on the mode of action of sea urchin toxin—II Enzymatic and immunological behavior. Toxicon 1968, 6, 17–43. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D. A toxin from the sea urchin Tripneustes gratilla. Toxicon 1984, 22, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Lennox-Bulow, D.; Courtney, R.; Seymour, J. Geographic variation in stonefish (Synanceia spp.) venom. Toxicon 2025, 254, 108222. [Google Scholar] [CrossRef]
- Winter, K.L.; Isbister, G.K.; McGowan, S.; Konstantakopoulos, N.; Seymour, J.E.; Hodgson, W.C. A pharmacological and biochemical examination of the geographical variation of Chironex fleckeri venom. Toxicol. Lett. 2010, 192, 419–424. [Google Scholar] [CrossRef]
- Nakagawa, H.; Yamaguchi, C.; Sakai, H.; Kanemaru, K.; Hayashi, H.; Araki, Y.; Tomihara, Y.; Shinohara, M.; Ohura, K.; Kitagawa, H. Biochemical and physiological properties of pedicellarial lectins from the toxopneustid sea urchins. J. Nat. Toxins 1999, 8, 297–308. [Google Scholar]
- Mendes, E.G.; Abbud, L.; Umiji, S. Cholinergic action of homogenates of sea urchin pedicellariae. Science 1963, 139, 408–409. [Google Scholar] [CrossRef]
- Biedebach, M.C.; Jacobs, G.P.; Langjahr, S.W. Muscle membrane potential effects of a toxin extract from the sea urchin Lytechinus pictus (verrill). Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1978, 59, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Kihara, H.; Hashimura, S. A new sea urchin toxin and its effect on spontaneous transmitter release at frog neuromuscular junctions. Jpn. J. Physiol. 1984, 34, 839–847. [Google Scholar] [CrossRef]
- Weisler, M.I.; Mihaljević, M.; Rogers, A.J. Sea urchins: Improving understanding of prehistoric subsistence, diet, foraging behavior, tool use, and ritual practices in Polynesia. J. Isl. Coast. Archaeol. 2020, 15, 547–575. [Google Scholar] [CrossRef]
- Sun, J.; Chiang, F.S. Use and exploitation of sea urchins. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 25–45. [Google Scholar]
- Strauss, M.B.; MacDonald, R.I. Hand injuries from sea urchin spines. Clin. Orthop. Relat. Res. 1976, 114, 216–218. [Google Scholar] [CrossRef]
- De la Torre, C.; Vega, A.; Carracedo, A.; Toribio, J. Identification of Mycobacterium marinum in sea-urchin granulomas. Br. J. Dermatol. 2001, 145, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Vargas, C.R.; Kanwar, A.; Dousa, K.M.; Skalweit, M.J.; Rowe, D.; Gatherwright, J. Mycobacterial tenosynovitis after sea urchin spine injury in an immunocompromised patient. Open Forum Infect. Dis. 2018, 5, ofy285. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Soma, T.; Gaman, K.; Usui, M.; Yamashita, T. Sea urchin spine arthritis of the hand. J. Hand Surg. 2008, 33, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Schefflein, J.; Umans, H.; Ellenbogen, D.; Abadi, M. Sea urchin spine arthritis in the foot. Skelet. Radiol. 2012, 41, 1327–1331. [Google Scholar] [CrossRef]
- Nassab, R.; Rayatt, S.; Peart, F. The management of hand injuries caused by sea urchin spines. J. Hand Surg. 2005, 30, 432–433. [Google Scholar] [CrossRef]
- Dahl, W.J.; Jebson, P.; Louis, D.S. Sea urchin injuries to the hand: A case report and review of the literature. Iowa Orthop. J. 2010, 30, 153. [Google Scholar]
- Pattanaprichakul, P.; Bunyaratavej, S.; Leeyaphan, C.; Sitthinamsuwan, P.; Sudhadham, M.; Muanprasart, C.; Feng, P.; Badali, H.; De Hoog, G.S. An unusual case of eumycetoma caused by Exophiala jeanselmei after a sea urchin injury. Mycoses 2013, 56, 491–494. [Google Scholar] [CrossRef]
- López Zabala, I.; Poggio Cano, D.; García-Elvira, R.; Asunción Márquez, J. Mycobacterium marinum osteomyelitis of the first metatarsal. Eur. J. Orthop. Surg. Traumatol. 2012, 22, 225–228. [Google Scholar] [CrossRef]
- Dreyfus, P.; Daupleix, D. Synovitis caused by a sea-urchin sting associated with an inoculation pasteurella infection. La Nouv. Presse Medicale 1979, 8, 2199–2200. [Google Scholar]
- Mouchbahani-Constance, S.; Choinière, M.; Sharif-Naeini, R. Understanding the pain experience of lionfish envenomation. Pain Rep. 2023, 8, e1090. [Google Scholar] [CrossRef]
- Harris, R.J.; Saggiomo, S.L.; Paxton, G.; Motti, C.A. Sting Stories: Firsthand Experiences of Fish Envenomation Through a Small-Scale Questionnaire. Toxins 2025, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Ward-Smith, H.; Arbuckle, K.; Naude, A.; Wüster, W. Fangs for the memories? A survey of pain in snakebite patients does not support a strong role for defense in the evolution of snake venom composition. Toxins 2020, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, T.M.; Soares, A.G.; Stockand, J.D. Advances in venomics: Modern separation techniques and mass spectrometry. J. Chromatogr. B 2020, 1160, 122352. [Google Scholar] [CrossRef] [PubMed]


| Species | Toxin | Structural Family | Mass | Tested Effects | Apparatus | References |
|---|---|---|---|---|---|---|
| Echinometra lucunter | Cathepsin B/X | Peptidase | 60–80 kDa | Inflammation in mice | Sp | [69] |
| p3E | Small molecule | 598 Da | Nociceptive response in rats; inflammatory responses in cremaster muscle of mice | Sp | [71] | |
| Echinometra mathaei | † | Proteins and small molecules | † | Cholinesterase inhibition; antioxidant activity | Sp | [64] |
| Echinothrix calamaris and Echinothrix diadema | - | - | - | Stimulation of smooth muscle; pain; hypotension | Sp | [35] |
| Noradrenaline * | Catecholamine neurotransmitter | 169.18 g/mol | - | |||
| Lytenchinus pictus | - | - | - | Reduced excitatory synaptic potential. | Ped | [112] |
| Lytenchinus variegatus | - | Acetylcholine-like substance | - | Effects on cholinergic system | Ped | [111] |
| Tripneustes gratilla | TGL-I | Lectin | 23 kDa | Ca2+-independent heparin-binding | Ped | [95,110] |
| - | - | 25 kDa | - | Ped | [107] | |
| - | - | - | Respiratory distress; hypotension; death in rabbits | Ped | [103] | |
| Toxopneustes pileolus | Peditoxin (Pedin + Pedoxin) | Holoprotein (Apoprotein + prosthetic group) | 10.2 kDa (10 kDa + 200 Da) | Anaphylaxis-like shock and death in mice | Ped | [87] |
| SUL-I | Lectin | 32 kDa | chemotactic properties for guinea-pig neutrophils; mitogenic activity on murine T-lymphocytes; D-galactose- and L-rhamnose-specific binding | Ped | [80,81,82,83,92,93,94] | |
| SUL-IA # | Lectin | 32 kDa | “ | Ped | [96] | |
| SUL-II | First identified as a lectin but likely a PLA2 | 23 kDa | D-galactose-specific binding | Ped | [95,97] | |
| SUL-III | Homohexameric lectin | 170 kDa (28 kDa subunits) | L-rhamnose-binding lectin; agglutination of rabbit erythrocytes; mitogenic stimulation on murine splenocytes | Ped | [81] | |
| Contractin-A | PLA2 | 14.9 kDa | Mitogenic stimulation on murine splenocytes | Ped | [84,85] | |
| UT841 | PLA2 | 18 kDa | - | Ped | [86] | |
| Toxopneustes roseus | CLX (2-[1-(4-chlorphenyl)-1-phenylethoxy]-N,N-dimethylethanamine) | Small molecule | 376.7 g/mol | Likely analgesic effects through NaV channel blocking | Ped | [101] |
| EMB (2-[1-(4-bromophenyl)-1-phenylethoxy]-N,N-dimethylethanamine) | Small molecule | 348.3 g/mol | “ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehlert-Flaskämper, S.; Motti, C.A.; Harris, R.J. Prickly Defenders: A Review of Venomous Sea Urchins (Echinoidea). Mar. Drugs 2025, 23, 253. https://doi.org/10.3390/md23060253
Ehlert-Flaskämper S, Motti CA, Harris RJ. Prickly Defenders: A Review of Venomous Sea Urchins (Echinoidea). Marine Drugs. 2025; 23(6):253. https://doi.org/10.3390/md23060253
Chicago/Turabian StyleEhlert-Flaskämper, Sina, Cherie A. Motti, and Richard J. Harris. 2025. "Prickly Defenders: A Review of Venomous Sea Urchins (Echinoidea)" Marine Drugs 23, no. 6: 253. https://doi.org/10.3390/md23060253
APA StyleEhlert-Flaskämper, S., Motti, C. A., & Harris, R. J. (2025). Prickly Defenders: A Review of Venomous Sea Urchins (Echinoidea). Marine Drugs, 23(6), 253. https://doi.org/10.3390/md23060253








