Preparation and Characterization of Polyphenol-Functionalized Chitooligosaccharide Pyridinium Salts with Antioxidant Activity
Abstract
1. Introduction
2. Results
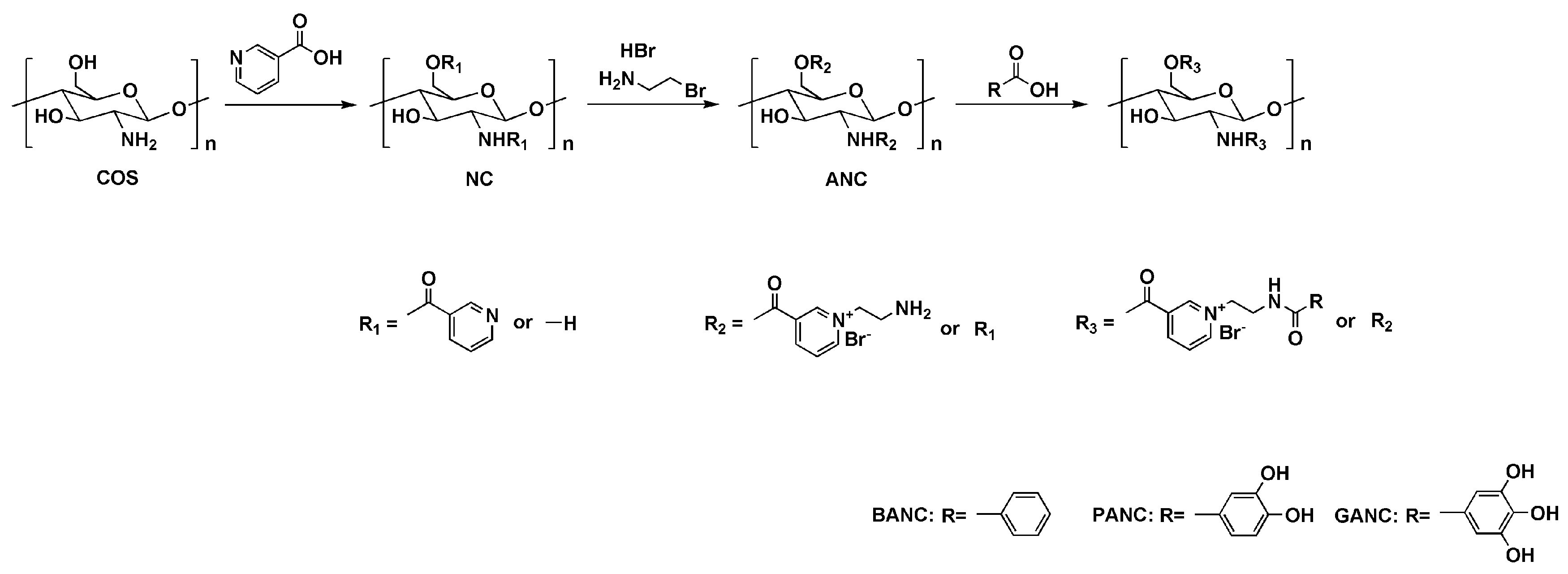
2.1. Chemical Synthesis
2.2. Characterization
2.2.1. FT–IR Spectra
2.2.2. 1H NMR Spectra
2.3. Degrees of Substitution (DS) Analysis
2.4. Solubility Assay
2.5. Antioxidant Activity
2.5.1. The Scavenging Ability of the DPPH Radical

2.5.2. The Scavenging Ability of the Superoxide Anion Radical

2.5.3. Reducing Power

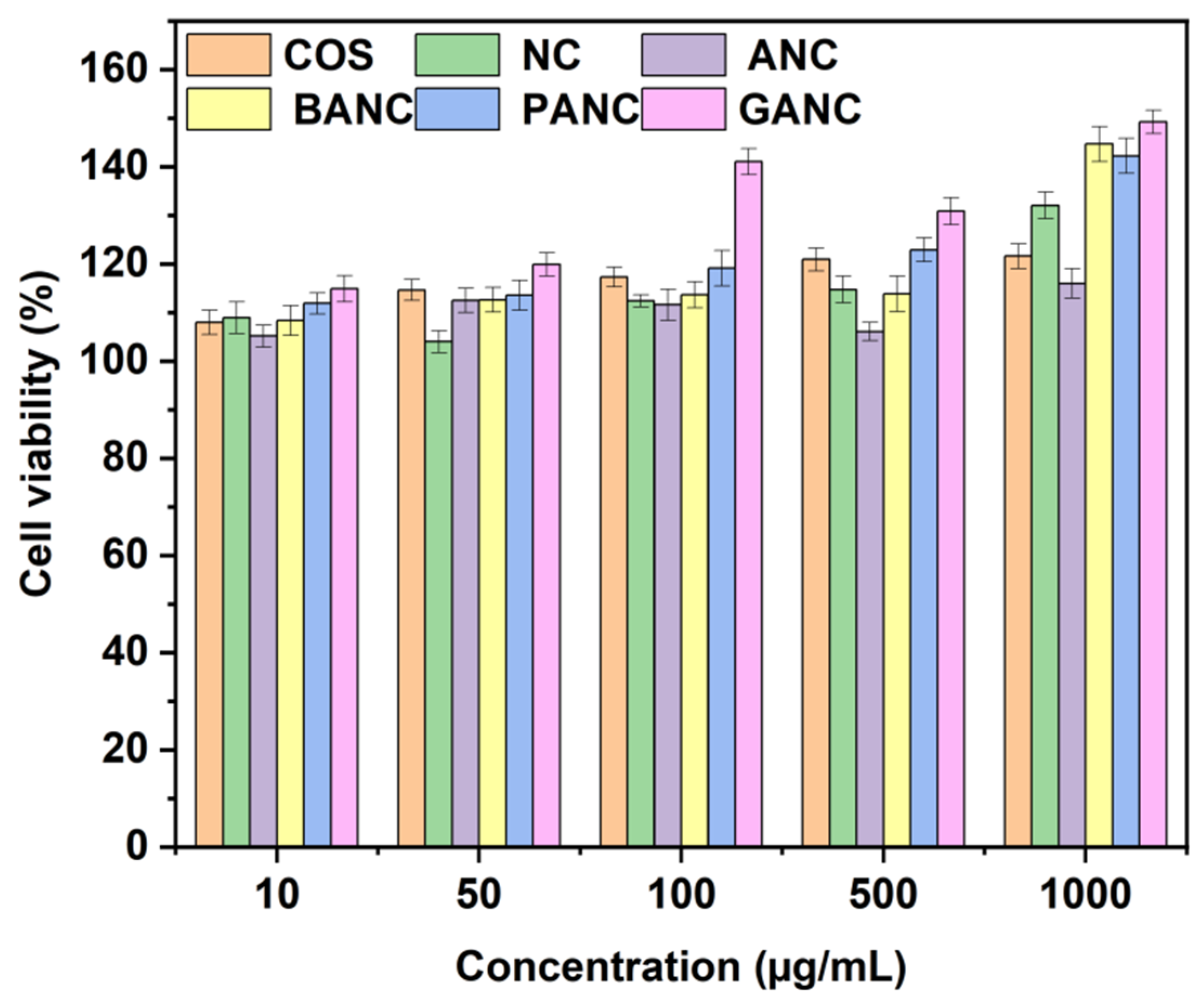
2.6. Cytotoxicity Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Chemical Synthesis
4.2.1. Synthesis of Nicotinated Chitooligosaccharide (NC)
4.2.2. Synthesis of Aminoethyl Nicotinated Chitooligosaccharide (ANC)
4.2.3. Synthesis of Benzoic Acid, Protocatechuic Acid, and Gallic Acid-Functionalized Chitooligosaccharide Pyridinium Salts (BANC, PANC, and GANC)
4.3. Analytical Methods
4.3.1. Fourier Transform Infrared (FT-IR) Spectroscopy
4.3.2. Proton Nuclear Magnetic Resonance (1H NMR) Spectroscopy
4.3.3. Degrees of Substitution (DS)
4.4. Solubility Assay
4.5. Antioxidant Assays In Vitro
4.5.1. DPPH Radical Scavenging Ability Assay
4.5.2. Superoxide Anion Radical Scavenging Activity Assay
4.5.3. Reducing Power Assay
4.6. Cytotoxicity Assay
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Duanghathaipornsuk, S.; Farrell, E.J.; Alba-Rubio, A.C.; Zelenay, P.; Kim, D.S. Detection Technologies for Reactive Oxygen Species: Fluorescence and Electrochemical Methods and Their Applications. Biosensors 2021, 11, 30. [Google Scholar] [CrossRef] [PubMed]
- Dayem, A.A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ji, X.; Cui, J.; Mi, Y.; Zhang, J.; Guo, Z. Synthesis, Characterization, and the Antioxidant Activity of Phenolic Acid Chitooligosaccharide Derivatives. Mar. Drugs. 2022, 20, 489. [Google Scholar] [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Razis, A.F.A.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative stress, free radicals and antioxidants: Potential crosstalk in the pathophysiology of human diseases. Front. Chem. 2023, 11, 1158198. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Kumari, R.; Dkhar, D.S.; Mahapatra, S.; Kumar, R.; Chandra, P. Nano-bioengineered sensing technologies for real-time monitoring of reactive oxygen species in in vitro and in vivo models. Microchem. J. 2022, 180, 107615. [Google Scholar] [CrossRef]
- Tan, W.; Lin, C.; Zhang, J.; Li, Q.; Guo, Z. Synthesis of Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Thioctate and the Potential for Antioxidant Application. Molecules 2022, 27, 2682. [Google Scholar] [CrossRef]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants-A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef]
- Mi, Y.; Tan, W.; Zhang, J.; Guo, Z. Modification of Hydroxypropyltrimethyl Ammonium Chitosan with Organic Acid: Synthesis, Characterization, and Antioxidant Activity. Polymers 2020, 12, 2460. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiong, P.; Zhang, H.; Zhu, Q.; Liao, C.; Jiang, G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021, 201, 111531. [Google Scholar] [CrossRef] [PubMed]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of antioxidants: Mechanisms and pharmaceutical applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Sang, S.; McClements, D.J.; Chen, L.; Long, J.; Jiao, A.; Jin, Z.; Qiu, C. Polyphenols as plant-based nutraceuticals: Health effects, encapsulation, nano-delivery, and application. Foods 2022, 11, 2189. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary Polyphenols and Their Biological Significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T. Possible side effects of polyphenols and their interactions with medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Stojković, D.S.; Živković, J.; Soković, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Janković, T.; Maksimović, Z. Antibacterial activity of Veronica montana L. extract and of protocatechuic acid incorporated in a food system. Food Chem. Toxicol. 2013, 55, 209–213. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Wang, Q. Antibacterial activity and mechanism of Protocatechuic acid against bacterial canker of tomato fruits caused by Clavibacter michiganensis subsp. michiganensis. Food Biosci. 2024, 60, 104437. [Google Scholar] [CrossRef]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lan, W.; Sun, X. Antibacterial and antioxidant properties of phenolic acid grafted chitosan and its application in food preservation: A review. Food Chem. 2023, 428, 136788. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Huang, H.; Wen, Z.; Fan, Y. Fabrication of chitosan-gallic acid conjugate for improvement of physicochemical stability of β-carotene nanoemulsion: Impact of Mw of chitosan. Food Chem. 2021, 362, 130218. [Google Scholar] [CrossRef]
- Pakizeh, M.; Moradi, A.; Ghassemi, T. Chemical extraction and modification of chitin and chitosan from shrimp shells. Eur. Polym. J. 2021, 159, 110709. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Nie, Y.; Sun, D.; Wu, D.; Ban, L.; Zhang, H.; Yang, S.; Chen, J.; Du, H. Recent advances and perspectives in functional chitosan-based composites for environmental remediation, energy, and biomedical applications. Prog. Mater. Sci. 2025, 152, 101460. [Google Scholar] [CrossRef]
- Ngo, D.H.; Qian, Z.; Vo, T.S.; Ryu, B.; Ngo, D.N.; Kim, S.K. Antioxidant activity of gallate-chitooligosaccharides in mouse macrophage RAW264.7 cells. Carbohydr. Polym. 2011, 84, 1282–1288. [Google Scholar] [CrossRef]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Lund, M.N. Reactions of plant polyphenols in foods: Impact of molecular structure. Trends Food Sci. Technol. 2021, 112, 241–251. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef]
- Xie, M.; Hu, B.; Wang, Y.; Zeng, X. Grafting of Gallic Acid onto Chitosan Enhances Antioxidant Activities and Alters Rheological Properties of the Copolymer. J. Agric. Food Chem. 2014, 62, 9128–9136. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Singh, A.; Zhang, B.; Visessanguan, W.; Benjakul, S. Chitooligosaccharide Conjugates Prepared Using Several Phenolic Compounds via Ascorbic Acid/H2O2 Free Radical Grafting: Characteristics, Antioxidant, Antidiabetic, and Antimicrobial Activities. Foods 2022, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Benjakul, S.; Huda, N.; Xu, C.A.; Wu, P. Preparation and characterization of squid pen chitooligosaccharide–epigallocatechin gallate conjugates and their antioxidant and antimicrobial activities. RSC Adv. 2020, 10, 33196–33204. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Wang, M.; Khan, I.M.; Niazi, S.; Wang, B.; Ma, X.; Wang, Z.; Xia, W. Preparation and characterization of chitosan oligosaccharide derivatives containing cinnamyl moieties with enhanced antibacterial activities. LWT 2021, 147, 111663. [Google Scholar] [CrossRef]
- Lin, C.; Luan, F.; Su, S.; Jiang, A.; Tan, W.; Guo, Z. Water-soluble fluorine-functionalized chitooligosaccharide derivatives: Synthesis, characterization and antimicrobial activity. Carbohydr. Res. 2023, 533, 108935. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, Z.; Su, S.; Lin, C.; Mi, Y.; Tan, W.; Guo, Z. Self-assembled low molecular weight chitosan-based cationic micelle for improved water solubility, stability and sustained release of α-tocopherol. Food Chem. 2023, 429, 136886. [Google Scholar] [CrossRef]
- Li, Q.; Mi, Y.; Tan, W.; Guo, Z. Highly efficient free radical-scavenging property of phenolic-functionalized chitosan derivatives: Chemical modification and activity assessment. Int. J. Biol. Macromol. 2020, 164, 4279–4288. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Chen, Y.; Mi, Y.; Tan, W.; Miao, Q.; Li, Q.; Dong, F.; Guo, Z. Preparation of 2,6-diurea-chitosan oligosaccharide derivatives for efficient antifungal and antioxidant activities. Carbohydr. Polym. 2020, 234, 115903. [Google Scholar] [CrossRef]
- Ali, I.O.; Nassar, H.S.; El-Nasser, K.S.; Bougarech, A.; Abid, M.; Elhenawy, A.A. Synthesis and characterization of MnII and CoII complexes with poly (vinyl alcohol-nicotinic acid) for photocatalytic degradation of Indigo carmine dye. Inorg. Chem. Commun. 2021, 124, 108360. [Google Scholar] [CrossRef]
- Lin, C.; Yuan, Y.; Tan, W.; Guo, Z.; Jiang, A. Preparation of cationic chitooligosaccharide derivatives bearing N-halogenated benzyl pyridinium and assessment of their antimicrobial activities. Polymers 2023, 80, 12193–12210. [Google Scholar] [CrossRef]
- Wang, L.; Liu, X.; Tan, W.; Li, Q.; Guo, Z.; Zhang, J. Preparation and antioxidant activity of novel chitosan oligosaccharide quinolinyl urea derivatives. Carbohydr. Res. 2022, 521, 108678. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Gebicki, J.M.; Iohara, D.; Tomida, H.; Uekama, K.; Maruyama, T.; Hirayama, F.; Otagiri, M. Antioxidant activities of chitosans and its derivatives in in vitro and in vivo studies. Carbohydr. Polym. 2018, 199, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Asouri, M.; Ataee, R.; Ahmadi, A.; Amini, A.; Moshaei, M.R. Antioxidant and Free Radical Scavenging Activities of Curcumin. Asian J. Chem. 2013, 25, 7593–7595. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Barroso, M.F.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Delerue-Matos, C.; Oliveira, M.B.P.P.; Tuñón-Blanco, P. Electrocatalytic evaluation of DNA damage by superoxide radical for antioxidant capacity assessment. J. Electroanal. Chem. 2011, 659, 43–49. [Google Scholar] [CrossRef]
- Ma, B.; Zhang, J.; Mi, Y.; Miao, Q.; Tan, W.; Guo, Z. Preparation of imidazole acids grafted chitosan with enhanced antioxidant, antibacterial and antitumor activities. Carbohydr. Polym. 2023, 315, 120978. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Teng, A.; Li, Y.; Jiao, Y.; Zhao, K.; Wang, Y.; Li, R.; Yang, N.; Wang, W. Polyphenols and polyphenols-based biopolymer materials: Regulating iron absorption and availability from spontaneous to controllable. Crit. Rev. Food Sci. Nutr. 2022, 63, 12341–12359. [Google Scholar] [CrossRef]
- Rosales, T.K.O.; Alves da Silva, F.F.; Bernardes, E.S.; Fabi, J.P. Plant-derived polyphenolic compounds: Nanodelivery through polysaccharide-based systems to improve the biological properties. Crit. Rev. Food Sci. Nutr. 2023, 64, 11894–11918. [Google Scholar] [CrossRef]
- Gonta, A.; Lupascu, T.; Povar, I.; Timbaliuc, N.; Sukhanov, T.E.; Petrova, V.; Skorik, I.A. Enhancement of Antioxidant and Antibacterial Activities by Immobilization of Natural Bactericide into Hybrid Supra-molecular Chitosan Bio-composite Gel. IFMBE Proc. 2016, 55, 301–304. [Google Scholar] [CrossRef]
- Wang, L.; Guo, R.; Liang, X.; Ji, Y.; Zhang, J.; Gai, G.; Guo, Z. Preparation and Antioxidant Activity of New Carboxymethyl Chitosan Derivatives Bearing Quinoline Groups. Mar. Drugs 2023, 21, 606. [Google Scholar] [CrossRef]
- Han, X.; Mi, Y.; Ji, Y.; Sun, M.; Tang, H.; Dong, F.; Guo, Z. A novel chitosan antioxidant bearing sulfhydryl group: Synthesis, characterization and activity assessment. Int. J. Biol. Macromol. 2024, 261, 129816. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ji, X.; Mi, Y.; Miao, Q.; Dong, F.; Tan, W.; Guo, Z. Antimicrobial and Antioxidant Activities of N-2-Hydroxypropyltrimethyl Ammonium Chitosan Derivatives Bearing Amino Acid Schiff Bases. Mar. Drugs 2022, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Synthesis, characterization, and evaluation of antifungal and antioxidant properties of cationic chitosan derivative via azide-alkyne click reaction. Int. J. Biol. Macromol. 2018, 120, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Li, Q.; Tan, W.; Wei, L.; Zhang, J.; Dong, F.; Gu, G.; Guo, Z. The evaluation of antioxidant and antifungal properties of 6-amino-6-deoxychitosan in vitro. Int. J. Biol. Macromol. 2018, 107, 595–603. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, J.; Tian, L.; Mi, Y.; Guo, Z. Phenolic Acid Functional Quaternized Chitooligosaccharide Derivatives: Preparation, Characterization, Antioxidant, Antibacterial, and Antifungal Activity. Mar. Drugs 2023, 21, 535. [Google Scholar] [CrossRef]



| Compounds | NC | ANC | BANC | PANC | GANC |
| DS | 1.80 | 0.97 | 0.27 | 0.30 | 0.30 |
| Solvents | Samples | |||||
|---|---|---|---|---|---|---|
| COS | NC | ANC | BANC | PANC | GANC | |
| deionized water | √ | × | √ | √ | √ | √ |
| dimethyl sulfoxide | √ | √ | √ | √ | √ | √ |
| N,N’-dimethylformamide | × | × | × | × | × | × |
| N-methyl-2-pyrrolidone | × | √ | × | × | × | × |
| anhydrous ethanol | × | × | × | × | × | × |
| acetone | × | × | × | × | × | × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Tan, W.; Guo, Z.; Jiang, A. Preparation and Characterization of Polyphenol-Functionalized Chitooligosaccharide Pyridinium Salts with Antioxidant Activity. Mar. Drugs 2025, 23, 150. https://doi.org/10.3390/md23040150
Qi Z, Tan W, Guo Z, Jiang A. Preparation and Characterization of Polyphenol-Functionalized Chitooligosaccharide Pyridinium Salts with Antioxidant Activity. Marine Drugs. 2025; 23(4):150. https://doi.org/10.3390/md23040150
Chicago/Turabian StyleQi, Zhen, Wenqiang Tan, Zhanyong Guo, and Aili Jiang. 2025. "Preparation and Characterization of Polyphenol-Functionalized Chitooligosaccharide Pyridinium Salts with Antioxidant Activity" Marine Drugs 23, no. 4: 150. https://doi.org/10.3390/md23040150
APA StyleQi, Z., Tan, W., Guo, Z., & Jiang, A. (2025). Preparation and Characterization of Polyphenol-Functionalized Chitooligosaccharide Pyridinium Salts with Antioxidant Activity. Marine Drugs, 23(4), 150. https://doi.org/10.3390/md23040150







