1. Introduction
Vascular inflammation serves as a common early pathological event in the development of atherosclerosis, diabetes-associated vascular complications, and sepsis, acting as a precursor to accelerated atherogenesis and multi-organ dysfunction [
1]. The vascular endothelium, a critical interface between circulating blood and peripheral tissues, plays a crucial role in vascular inflammation. Endothelial activation, characterized by the upregulation of cell adhesion molecules (such as VCAM-1 and ICAM-1) and pro-inflammatory cytokines and chemokines (such as IL-6, TNF-α, and MCP-1), represents a central feature of vascular inflammatory pathologies [
2,
3]. The dysregulated activation of endothelial cells leads to enhanced leukocyte adhesion and impaired endothelial barrier integrity. These perturbations disrupt endothelial homeostasis, accelerate atherosclerotic plaque formation, and contribute to end-organ damage in diverse inflammatory disorders [
4]. Consequently, therapeutic strategies targeting endothelial inflammatory resolution emerge as essential paradigms for mitigating vascular dysfunction and tissue injury in inflammation-driven disease states.
Over the past decade, marine ecosystems have emerged as a prolific source of novel bioactive compounds, with natural products from marine organisms yielding a plethora of therapeutically promising agents. Isaridin E, a cyclodepsipeptide isolated from marine-derived fungus
Beauveria felina (SYSU-MS7908) from the South China Sea, exhibits diverse biological activities. A prior study has established its insecticidal efficacy [
5]. In addition, it has been demonstrated that isaridin E remarkably attenuates neutrophil activity via the suppression of formyl-methionyl-leucyl-phenylalanine (FMLP)-induced superoxide anion release [
6]. Furthermore, our previous findings revealed that isaridin E exerts excellent antiplatelet and antithrombotic effects, suggesting its multi-targeted therapeutic potential [
7]. Endothelial activation is a key initiating factor for platelet adhesion and thrombus formation. More recently, in a murine model of sepsis induced by caecal ligation and puncture (CLP), a study from our group demonstrated the protective effects of isaridin E against sepsis through the suppression of von Willebrand factor (vWF)-mediated platelet–endothelial interactions [
8]. While pretreatment with isaridin E substantially attenuated sepsis-associated pulmonary vascular hyperpermeability in this paradigm, its direct modulatory effects on vascular endothelial inflammation and the precise molecular mechanisms underlying these actions remain to be elucidated.
In this study, we aimed to investigate the anti-inflammatory efficacy of isaridin E in both in vitro and in vivo models of lipopolysaccharide (LPS)-induced vascular inflammation, with a focus on its impact on pro-inflammatory cytokine expression, endothelial barrier function, and underlying molecular mechanisms.
3. Discussion
In the present study, we demonstrated the robust anti-inflammatory efficacy of isaridin E in both in vitro and in vivo models of LPS-induced endothelial inflammation. Notably, our findings establish isaridin E as a novel natural marine metabolite with significant therapeutic potential for mitigating endothelial inflammation during LPS-induced endotoxemia. Specifically, we show that isaridin E attenuates LPS-driven inflammatory responses in vascular and pulmonary tissues by disrupting the TLR4/NF-κB signaling cascade, a pivotal pathway in the pathogenesis of sepsis and other inflammatory diseases.
Endothelial cells, strategically positioned at the interface of blood and vessel walls, serve as dynamic sensors and effectors in inflammatory responses [
9,
10]. While not viewed as classical immune cells, upon infection or other injury, they orchestrate immune activation through the secretion of various pro-inflammatory cytokines (e.g., TNF-α and IL-6) and chemokines (e.g., MCP-1). Furthermore, endothelial cells are critical for mediating leukocyte trafficking into tissues via the upregulation of adhesion molecule expression (such as ICAM-1 and VCAM-1) on their surface [
11,
12,
13]. Endothelial dysfunction has been regarded as a central pathophysiological feature in sepsis, atherosclerosis, and other inflammation-driven diseases [
1,
2,
3]. Consequently, targeting endothelial activation represents a promising therapeutic strategy to mitigate systemic inflammation and organ injury. The search for novel therapeutic agents to reduce endothelial-mediated inflammation is an area of intense research.
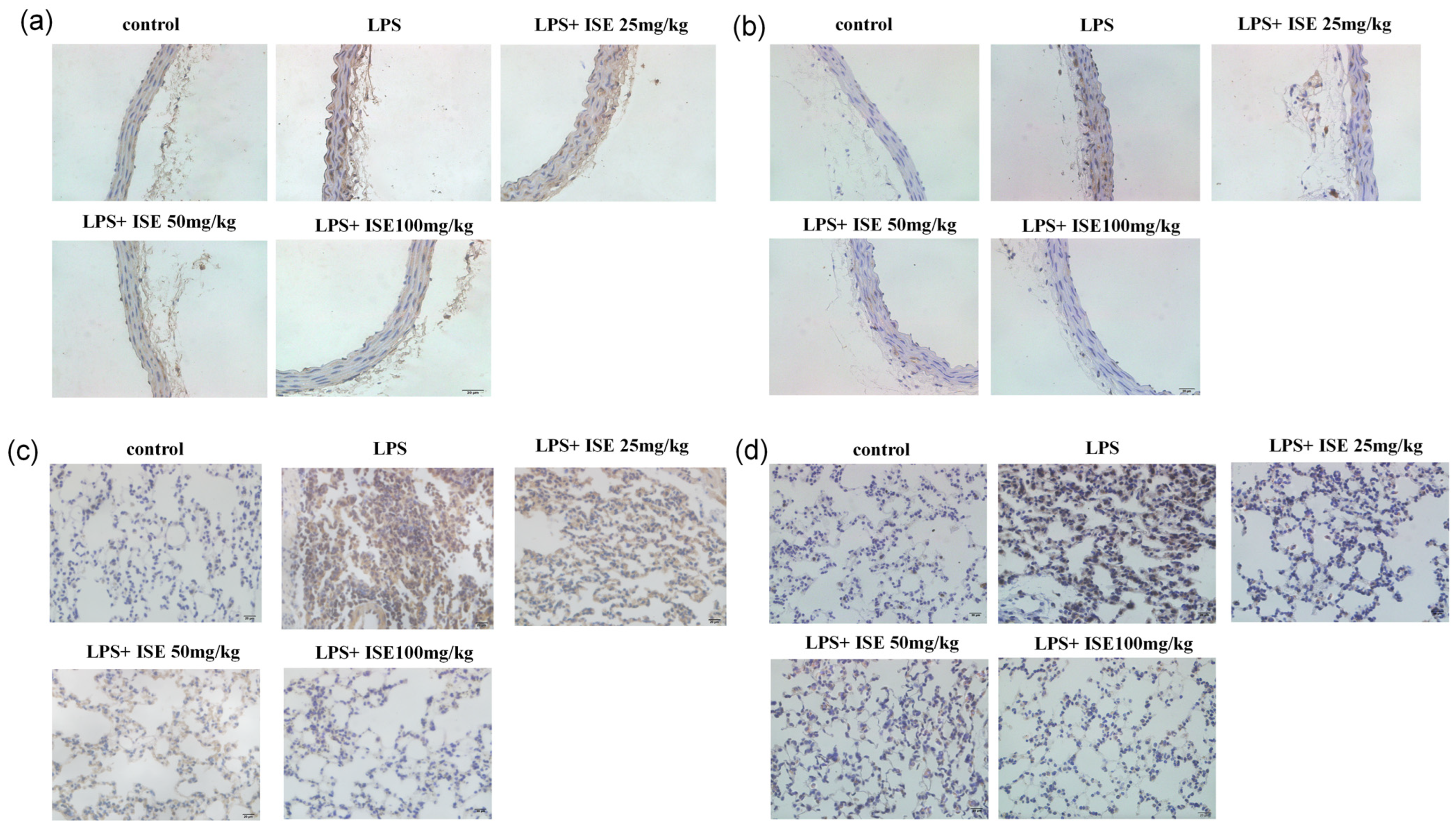
In the present study, utilizing a widely used LPS-induced inflammation model, we provide compelling evidence that isaridin E, a cyclic hexapeptide compound derived from the marine fungus
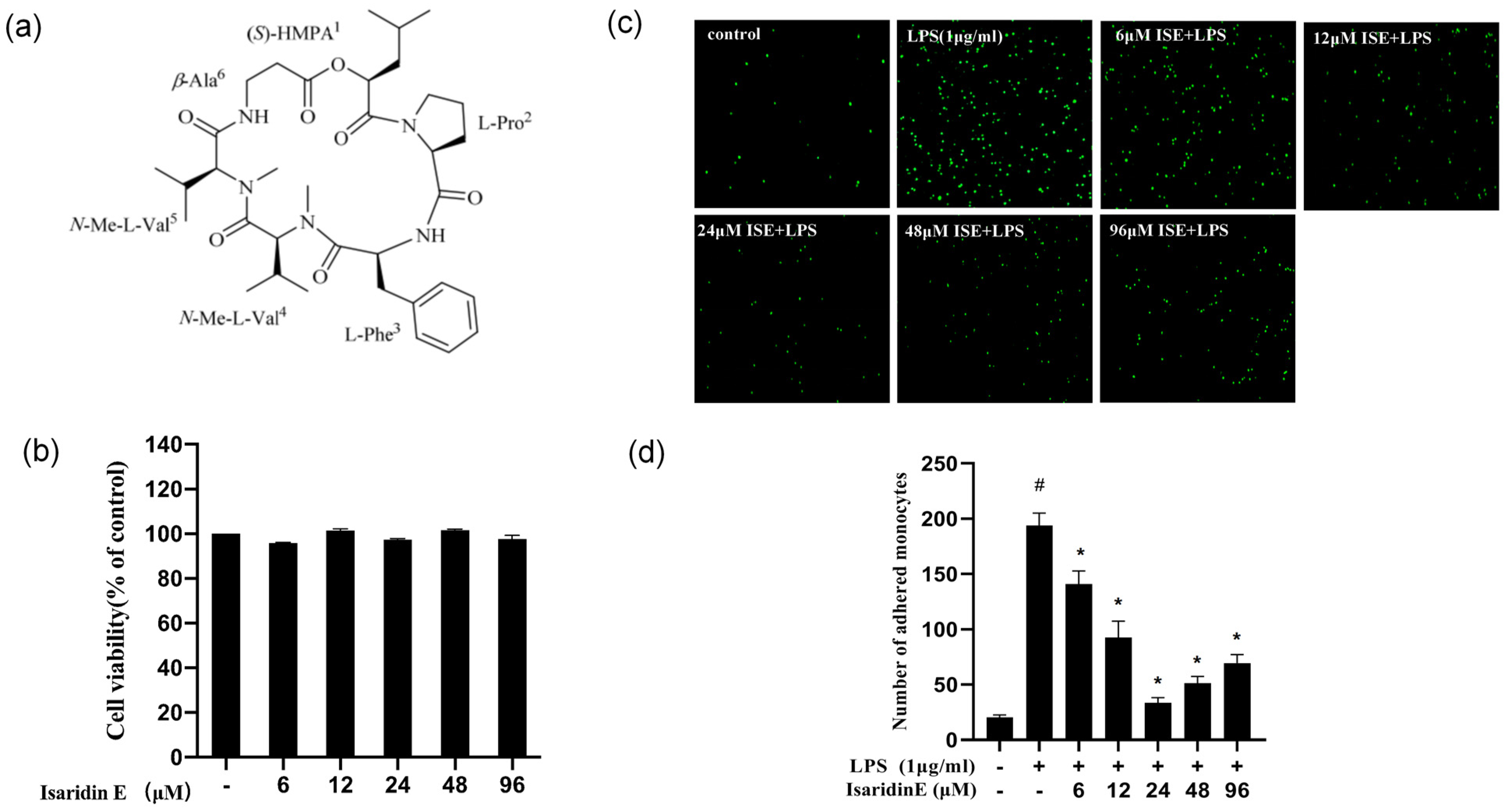
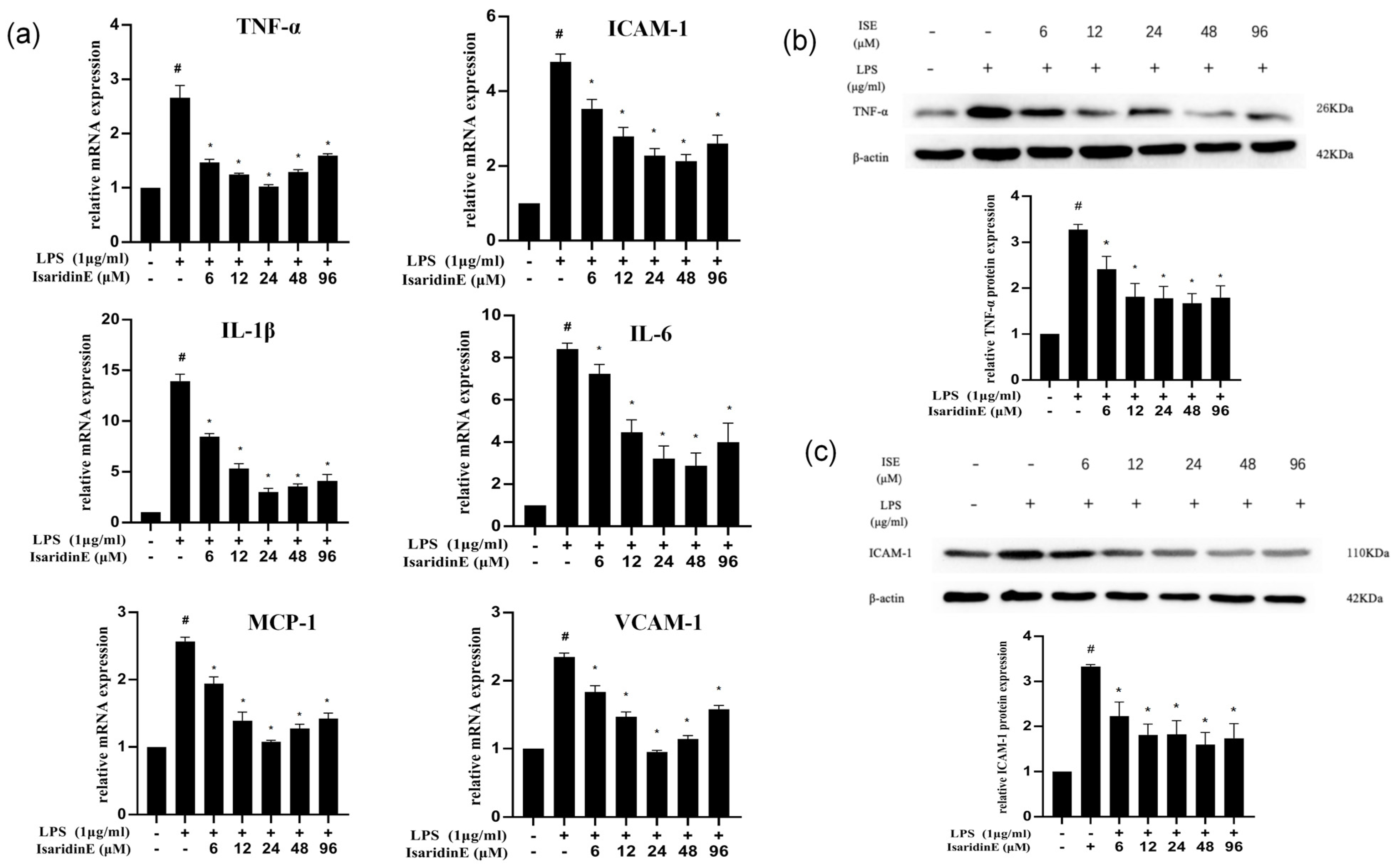
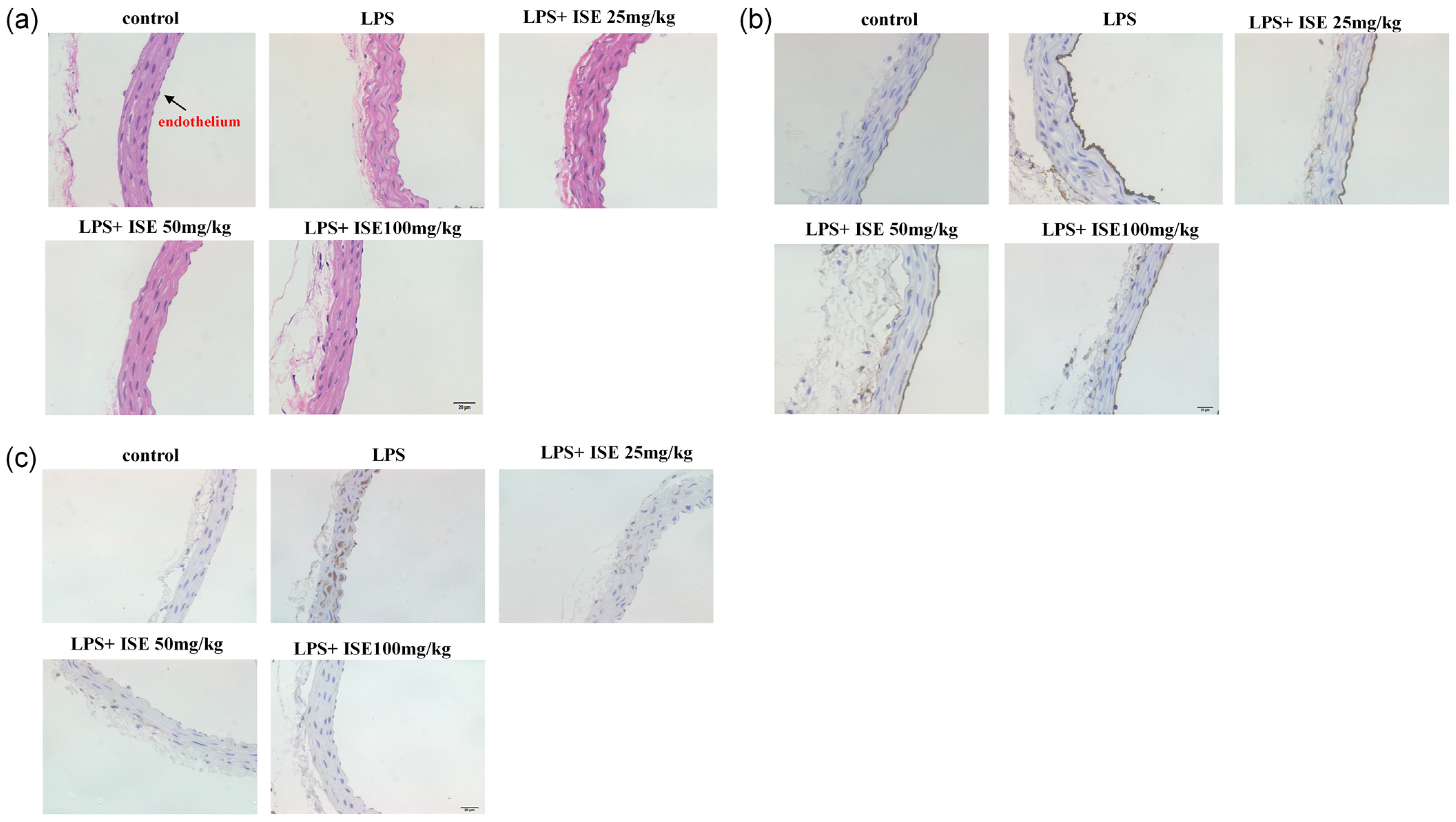
Beauveria felina, potently suppresses LPS-triggered inflammatory responses in HUVECs and in a murine endotoxemia model. The administration of isaridin E markedly decreased the LPS-induced secretion of pro-inflammatory cytokines and adhesion molecule expression, accompanied with a marked reduction in monocyte adhesion to activated endothelial monolayers, which is a critical step in leukocyte extravasation during inflammation. In line with the in vitro findings, the administration of isaridin E to LPS-challenged mice profoundly suppressed the aortic expression of TNF-α and ICAM-1, with a remarkable improvement in vascular architecture. Importantly, the anti-inflammatory effects of isaridin E were achieved without overt cytotoxicity (
Figure 1). Together with the previous finding that isaridin E inhibits neutrophil activation [
6], the beneficial effects of this compound on endothelial cells collectively position isaridin E as a promising candidate for therapeutic intervention in inflammatory vascular diseases.
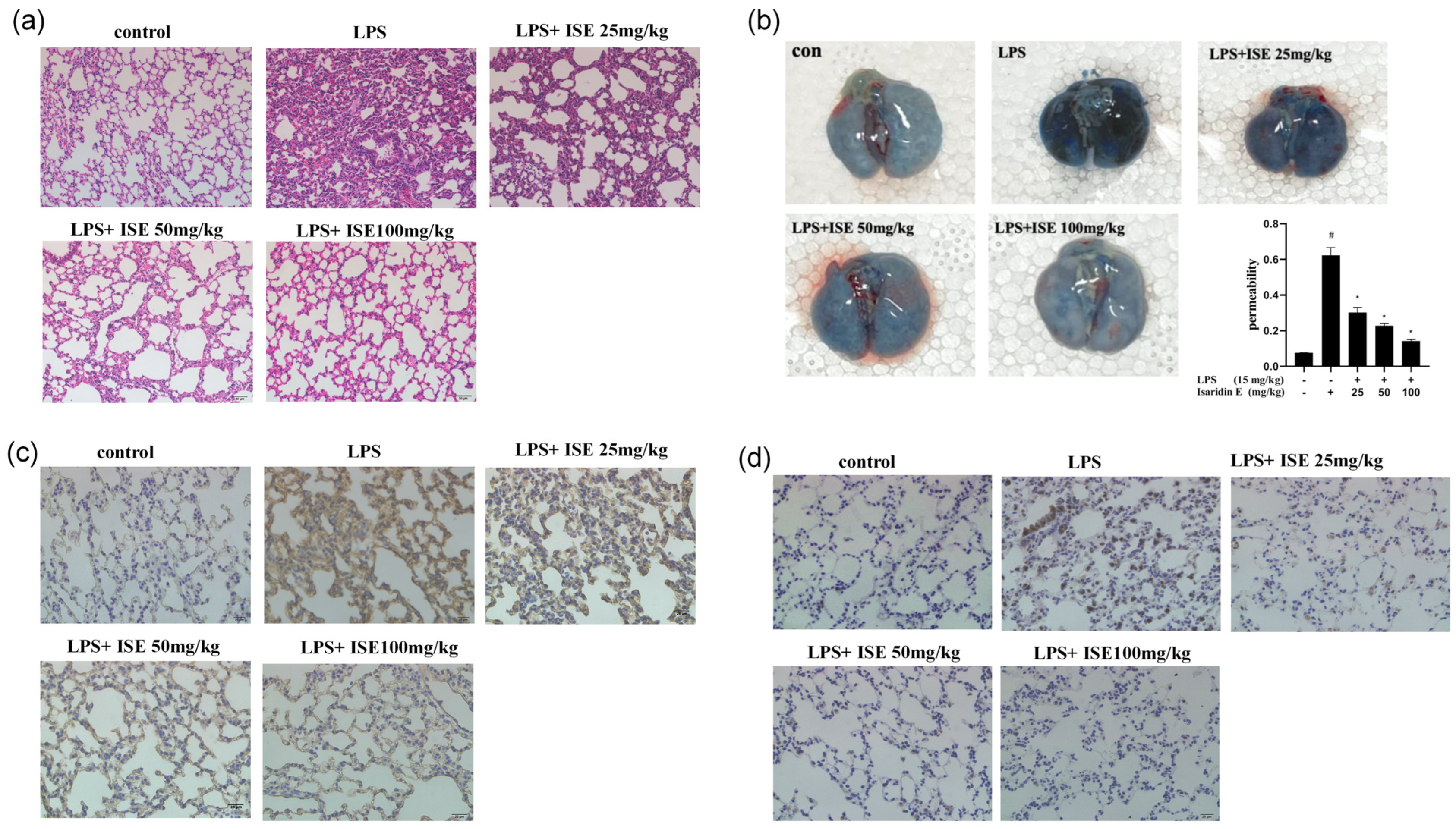
Accumulating evidence shows that endothelial hyperpermeability is a key driver of inflammation-driven pathologies. During inflammatory states, endothelial cells respond to stimuli like LPS by secreting cytokines and upregulating adhesion molecules, which increase vascular permeability. This disruption of endothelial barrier function is particularly evident in the lung, where increased permeability contributes to pulmonary edema and inflammatory cell infiltration, which are hallmarks of LPS-induced lung injury [
14,
15]. Experimental approaches targeting endothelial integrity have proven to be promising therapies during sepsis [
16,
17]. In a recent study from our group, we observed that isaridin E pretreatment significantly ameliorated pulmonary vascular permeability in a CLP-induced sepsis model. This protective effect was mechanistically linked to a significant reduction in vWF levels and a suppression of platelet–endothelial interactions [
8]. To further characterize the therapeutic potential of isaridin E in inflammatory diseases, we evaluated its ability to protect lung tissue from LPS-induced damage. In line with previous findings [
8], our results demonstrate that isaridin E significantly attenuates LPS-induced endothelial hyperpermeability in the lung, as quantified by reduced Evans Blue dye extravasation into the lung interstitium. This preservation of endothelial barrier integrity was accompanied by a marked reduction in LPS-stimulated cytokine (TNF-α) and adhesion molecule (ICAM-1) expression within the lung tissue. Moreover, isaridin E treatment effectively suppressed inflammatory cell transmigration and blood cell extravasation into the lung parenchyma, as evidenced by histological analysis. These findings collectively highlight isaridin E’s capacity to safeguard the vascular endothelium against LPS-induced injury, thereby mitigating downstream organ dysfunction.
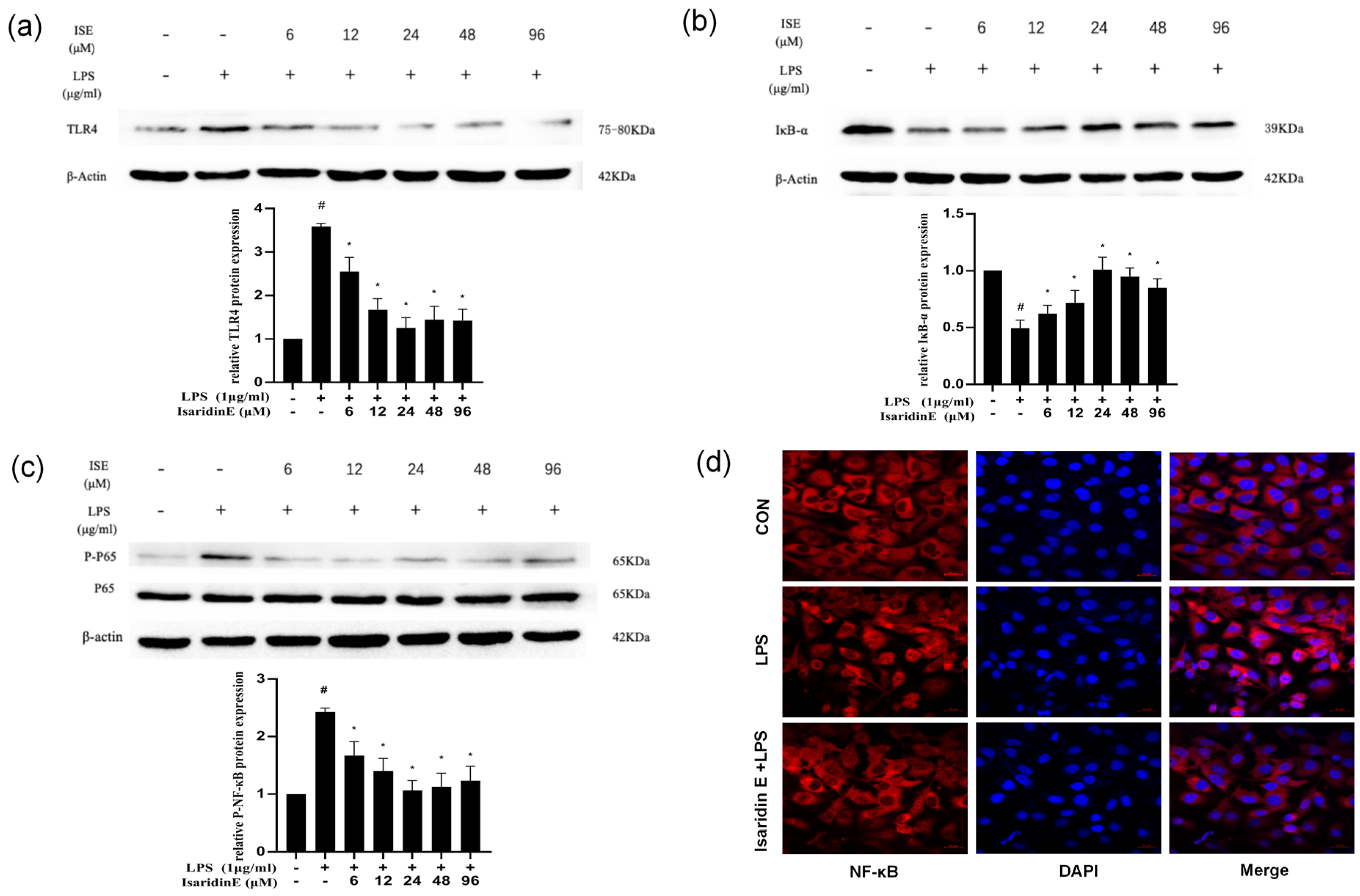
Emerging evidence underscores the TLR4/NF-κB signaling axis as a central regulator of inflammatory pathologies, including chronic inflammation and sepsis [
18]. The stimulation of endothelial cells by LPS activates TLR4, which triggers a downstream cascade that culminates in NF-κB activation [
19,
20]. NF-κB, which is a crucial transcription factor acting as a switch to regulate early gene expression, is critically implicated in the pathogenesis of inflammation and immune responses [
21,
22,
23]. Numerous studies have shown that the inhibition of the TLR4/NF-κB pathway alleviates the inflammatory response of endothelial cells [
24,
25,
26]. Given the pivotal role of TLR4/NF-κB signaling in endothelial inflammation, we investigated whether isaridin E modulates this pathway. Our findings demonstrated that isaridin E significantly suppressed TLR4 expression and inhibited NF-κB p65 phosphorylation and nuclear translocation in LPS-stimulated HUVECs, as demonstrated using immunoblotting and confocal microscopy. These in vitro findings were recapitulated in vivo, where immunohistochemical analysis revealed that isaridin E treatment reduced TLR4 expression and NF-κB p65 phosphorylation in the aortic and lung tissues from LPS-challenged mice. Collectively, these results suggest that isaridin E attenuates endothelial inflammation, at least in part, by suppressing the TLR4/NF-κB signaling cascade.
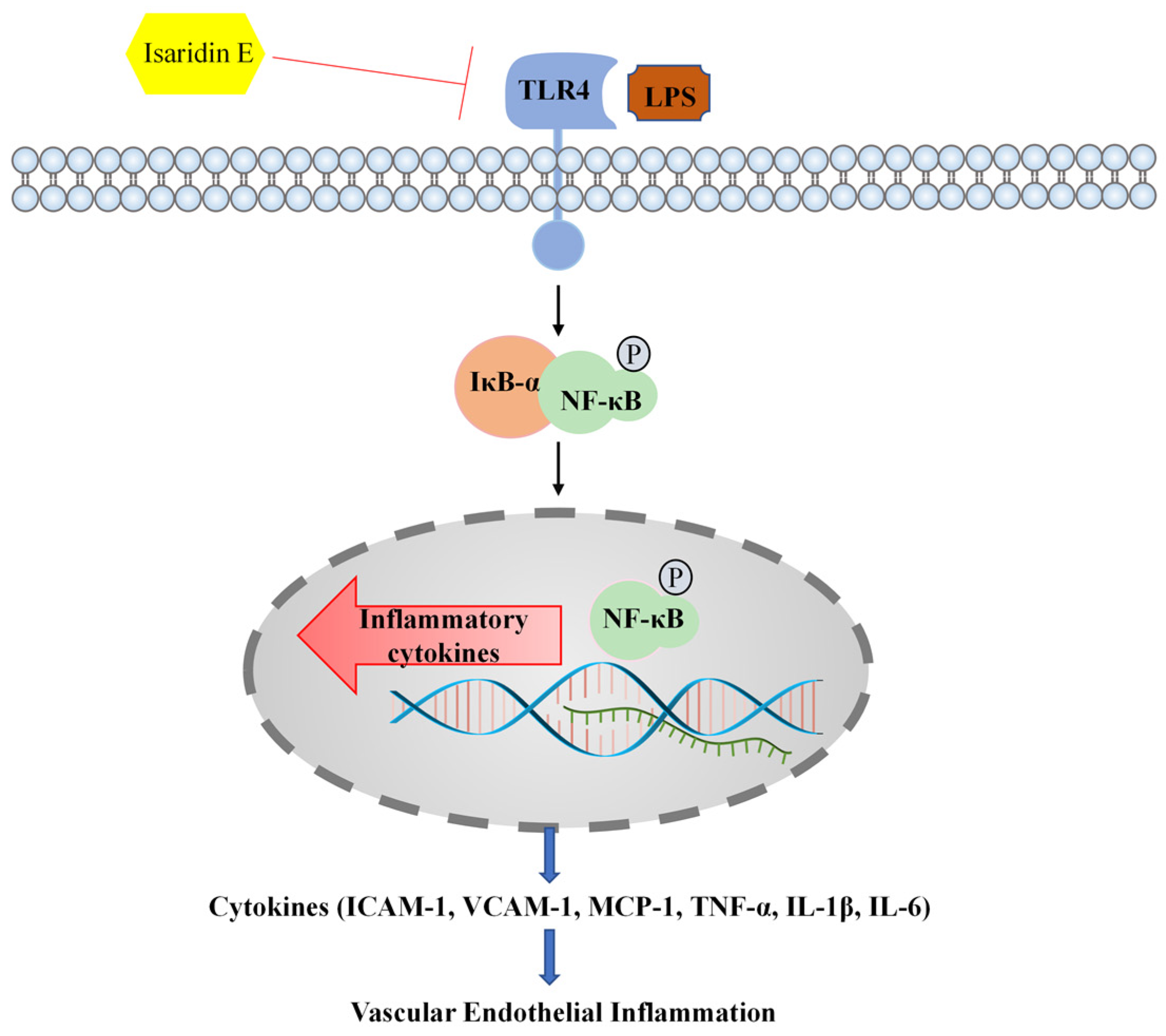
In summary, our research has provided evidence demonstrating that isaridin E exerts potent anti-inflammatory effects against LPS-induced endothelial inflammation in both cellular and preclinical models. Mechanistically, these effects are attributed to the suppression of TLR4/NF-κB signaling, which is a critical pathway driving inflammatory responses in sepsis and other vascular pathologies. Consequently, isaridin E may emerge as a potential therapeutic agent to mitigate inflammatory tissue injury for conditions characterized by endothelial inflammation or TLR4/NF-κB dysregulation. A schematic overview of this study is shown in
Figure 7.
4. Materials and Methods
4.1. Materials
Isaridin E was isolated from the ascidian-derived fungal strain
Amphichorda felina (syn.
B. felina) sp. SYSU-MS7908, as described previously by the School of Marine Sciences, Sun Yat-Sen University [
7]. Isaridin E was dissolved in dimethyl sulfoxide (DMSO, at a final concentration of less than 0.1%) and stored at 4 °C, before being diluted to different concentrations as needed for in vitro experiments. For intragastric administrations, isaridin E was dissolved in a saline solution containing 10% Tween 80 and 15% propylene glycol to 2.5, 5, and 10 mg/mL, respectively. LPS was purchased from Sigma-Aldrich (Saint Louis, MO, USA).
4.2. Animals
Male SPF-grade C57BL/6 mice, aged 8–10 weeks and weighing approximately 20–25 g, were supplied by the Experimental Animal Center of Zhongshan Medical School, Sun Yat-Sen University in Guangzhou, China. Animal experiments were approved by the Animal Care and Use Committee of Sun Yat-Sen University (Approval No: SYSU-IACUC-2021-B0064), in accordance with the relevant regulations on animal welfare and ethics for experimental animals in China.
Mice were fed a standard diet and randomly divided into 5 groups. Vascular inflammation was induced by LPS injection (intraperitoneally; 15 mg/kg), and mice were sacrificed after 12 h. To examine the effects of isaridin E, mice were intragastrically administrated multiple doses of vehicle or isaridin E (25, 50, or 100 mg/kg) for 3 days before LPS injection. Mice injected intraperitoneally with 15 mg/kg saline were used as controls.
4.3. Cell Culture and Treatment
The experiments were approved by the medical research ethics committee of Sun Yat-Sen University and were conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was obtained from all subjects. In brief, HUVECs were harvested from the umbilical vein and digested by 0.125% trypsin (Sigma-Aldrich, USA) with 0.01% ethylene glycol-bis (β-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA). After that, the cells were cultured in M199 culture medium (Sigma-Aldrich, USA) containing 20% fetal calf serum, 100 U/mL penicillin, 100 U/mL streptomycin, 25 U/mL heparin, 2 mmol/L L-glutamine, and 5 ng/mL p-ECGF at 37 °C under a 5% CO2-humidified atmosphere. Cells between passages 4 and 8 were used in this study.
4.4. Monocyte Adhesion Assay
THP-1 cells (Procell, Wuhan, China) were labeled with 3 μm Calcein-AM (Beyotime Biotechnology, Haimen, China) for 30 min at 37 °C in 5% CO2. HUVECs pretreated with isaridin E (6 μm, 12 μm, 24 μm, 48 μm, and 96 μm) for 2 h were plated in 35 mm culture dishes at a density of 2 × 105 cells/mL. After incubation with LPS (1 μg/mL) for 18 h, the cells were washed twice; then, the Calcein-AM-labeled monocytes were added into each culture dish for 1 h at 37 °C, under 5% CO2. To remove non-adherent cells, the dishes were gently washed with pre-warmed RPMI-1640. This was repeated twice and then 1 mL RPMI-1640 medium was added into each dish and the images were captured using Olympus Fluoview 500 laser confocal scanning microscopy with an excitation wavelength of 485 nm and emission at 530 nm. At least 6 fields randomly selected in each dish were observed.
4.5. Quantitative Real-Time PCR
Quantitative RT-PCR was performed following the manufacturer’s instructions. Briefly, total RNA was extracted using Trizol reagent (Invitrogen, Waltham, MA, USA); then, the RNA (500 ng) was used for reverse transcription using the PrimeScript RT reagent Kit Perfect Real-Time kit (Takara Bio Inc., Shiga, Japan). The cDNA was used for quantitative real-time PCR analysis using SYBR Green reagents (Takara Bio Inc., Japan). The 2ΔΔCt method was applied for the relative quantification of the target mRNA. The primers used for human IL-6 are forward AGACAGCCACTCACCTCTTCAG and reverse TTCTGCCAGTGCCTCTTTGCTG. The primers used for human TNF are forward CTCTTCTGCCTGCTGCACTTTG and reverse ATGGGCTACAGGCTTGTCACTC. The primers used for human VCAM-1 are forward GATTCTGTGCCCACAGTAAGGC and reverse TGGTCACAGAGCCACCTTCTTG. The primers used for human ICAM-1 are forward GTATGAACTGAGCAATGTGCAAG and reverse GTTCCACCCGTTCTGGAGTC. The primers used for human MCP-1 are forward ACGGGATCGTGGTTTCCAAC and reverse TGGCTTCTTACGCAGGAAGTT. The primers used for human IL-1β are forward ATGATGGCTTATTACAGTGGCAA and reverse GTCGGAGATTCGTAGCTGGA. All primers were synthesized by Guangzhou Huiyuan Biotechnology Co., Ltd. (Guangzhou, China).
4.6. Western Blot
Western blot was performed according to the protocol described previously [
7,
8]. HUVECs were rinsed with ice-cold PBS and lysed with lysis buffer containing a protease inhibitor cocktail (Merck, Darmstadt, Germany). The protein concentration was determined with a BCA kit. Protein was separated using SDS-PAGE and transformed into PVDF membranes (Millipore, Burlington, MA, USA). After blocking in 5% skimmed milk for 1 h at room temperature, the membranes were incubated with primary antibodies against TNF-α (Servicebio, Wuhan, China), ICAM-1 (Servicebio, China), TLR-4 (Proteintech Group, Rosemont, IL, USA), IκBα (Cell Signaling Technology, USA), and p-p65 (Cell Signaling Technology, USA) at 4 °C overnight. Incubation with monoclonal mouse β-actin (Proteintech Group, USA) antibody was performed as the loading sample control. After incubation with the secondary antibody conjugated to horseradish peroxidase (Cell Signaling Technology, Danvers, MA, USA) for 1 h at room temperature, bands were detected using the Pierce ECL Western blotting substrate (Thermo Scientific, Waltham, MA, USA) and were quantified using a computer-aided one-dimensional gel analysis system.
4.7. Evans Blue Staining of the Lungs
A 5% working solution of Evans blue was prepared by dissolving it in sterile physiological saline. Upon injection into mice, their bodies exhibited immediate blue discoloration. After a 2 h circulation period, the mice were anesthetized and placed in a supine position on a mouse board. The abdominal and thoracic cavities were surgically opened to expose the heart and lungs. A small incision was made in the left atrium, followed by the injection of chilled PBS solution containing 0.1% sodium heparin into the right ventricle for perfusion. Perfusion was discontinued upon the absence of red blood spots in the lungs. The lungs, stained with Evans blue, were harvested, photographed, and homogenized in centrifuge tubes containing 1 mL of acetamide per 30 mg of lung tissue. The tubes were subsequently incubated in a dark 37 °C chamber for 24 h. Following incubation, the tubes were centrifuged at 14,000 rpm and room temperature for 30 min, and the resulting supernatant was transferred to fresh 1.5 mL EP tubes for future use. The absorbance of the supernatant was quantified at 620 nm using a UV spectrophotometer.
4.8. Immunohistochemistry
Mice were anesthetized with 2% pentobarbital sodium and were perfused intracardiacly with Kreb’s solution containing 137 mmol/L NaCl, 5.4 mmol/L KCl, 2.0 mmol/L CaCl2, 1.1 mmol/L MgCl2, 0.4 mmol/L NaH2PO4, 5.6 mmol/L Glucose, 11.9 mmol/L NaHCO3, and 10 U/mL heparin, followed by 4 °C fixative solution containing 4% freshly depolymerized paraformaldehyde in 0.1 mol/L phosphate buffer for 10–15 min. After the fat and connective tissue was cleaned, the thoracic aorta was embedded and frozen using an optimal cutting temperature compound (OCT, Sakura, Japan). Immunohistochemistry was performed using the streptavidin–biotin–peroxidase complex system, according to the manufacturer’s instructions (SABC Peroxidase Kit, Absin Bioscience, Shanghai, China). Cryostat sections (8 μm) were pretreated with the solution of 3% hydrogenperoxide and methanol at a ratio of 1:50 for 30 min at room temperature, and were then blocked with 5% bovine serum albumin in PBS for 30 min. Then, the sections were incubated with ICAM-1 (1:200 dilution; Santa Cruz Biotechnology, USA) or TNF-α (1:200 dilution; Santa Cruz Biotechnology, USA) polyclonal antibody at 4 °C overnight, and were then treated with a biotinylated secondary anti-rabbit antibody for 30 min at room temperature. After that, the sections were incubated with the streptavidin–biotin–peroxidase complex for 30 min at room temperature. Staining was performed with DAB chromogen. Slides were counterstained with hematoxylin.
4.9. Nuclear Translocation of NF-κB
The cells underwent 2–3 washes with PBS before being fixed with 1 mL of 4% paraformaldehyde for 1 h. Following three additional washes with PBS, 1 mL of 1% Triton X-100 was added to permeabilize the cells for 1 h. Subsequently, the cells were incubated with 100 μL of immunostaining blocking solution at 37 °C for 1 h. After removing the excess blocking solution, the cells were incubated with NF-κB p65 antibody (diluted 1:100, Santa Cruz Biotechnology, Dallas, TX, USA) overnight in a humid chamber at 4 °C. After another round of PBS washes, an immunofluorescence secondary antibody solution was added, and the cells were incubated in a dark, humid chamber at 37 °C for 1 h. The cells were then treated with a fluorescence quenching solution containing DAPI and stored in the dark. Images were visualized and captured using a laser scanning confocal microscope.
4.10. Statistical Analyses
Blinded data analysis was performed using GraphPad Prism 8.0 (GraphPad software, 20 La Jolla, CA, USA, RRID:SCR_002798). All data were presented as mean ± SEM, and the n value represents the number of independent experiments on different batches of cells or different mice. Statistical analysis was determined using an unpaired two-tailed Student t test or ANOVA followed by a Bonferroni multiple comparison test with a 95% confidence interval. Values of p < 0.05 were considered significant.