Encapsulation Techniques to Enhance Astaxanthin Utilization as Functional Feed Ingredient
Abstract
1. Introduction
2. Results and Discussion
2.1. Encapsulation Assessment
2.1.1. Encapsulation Efficiency
2.1.2. Particle Characterization
2.1.3. Colour Measurement
2.1.4. Hygroscopicity
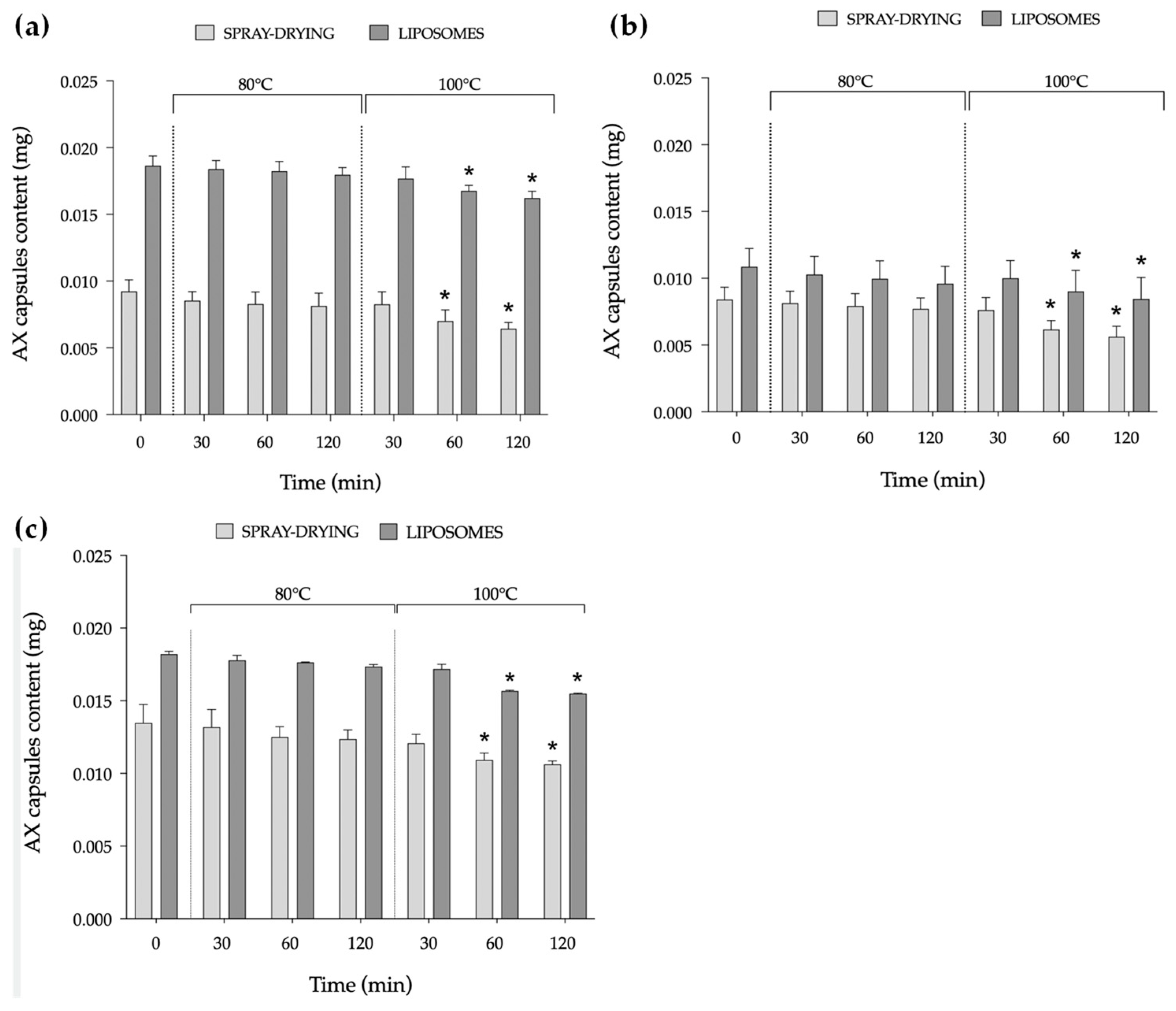
2.1.5. Temperature Stability
2.2. In Vitro Study
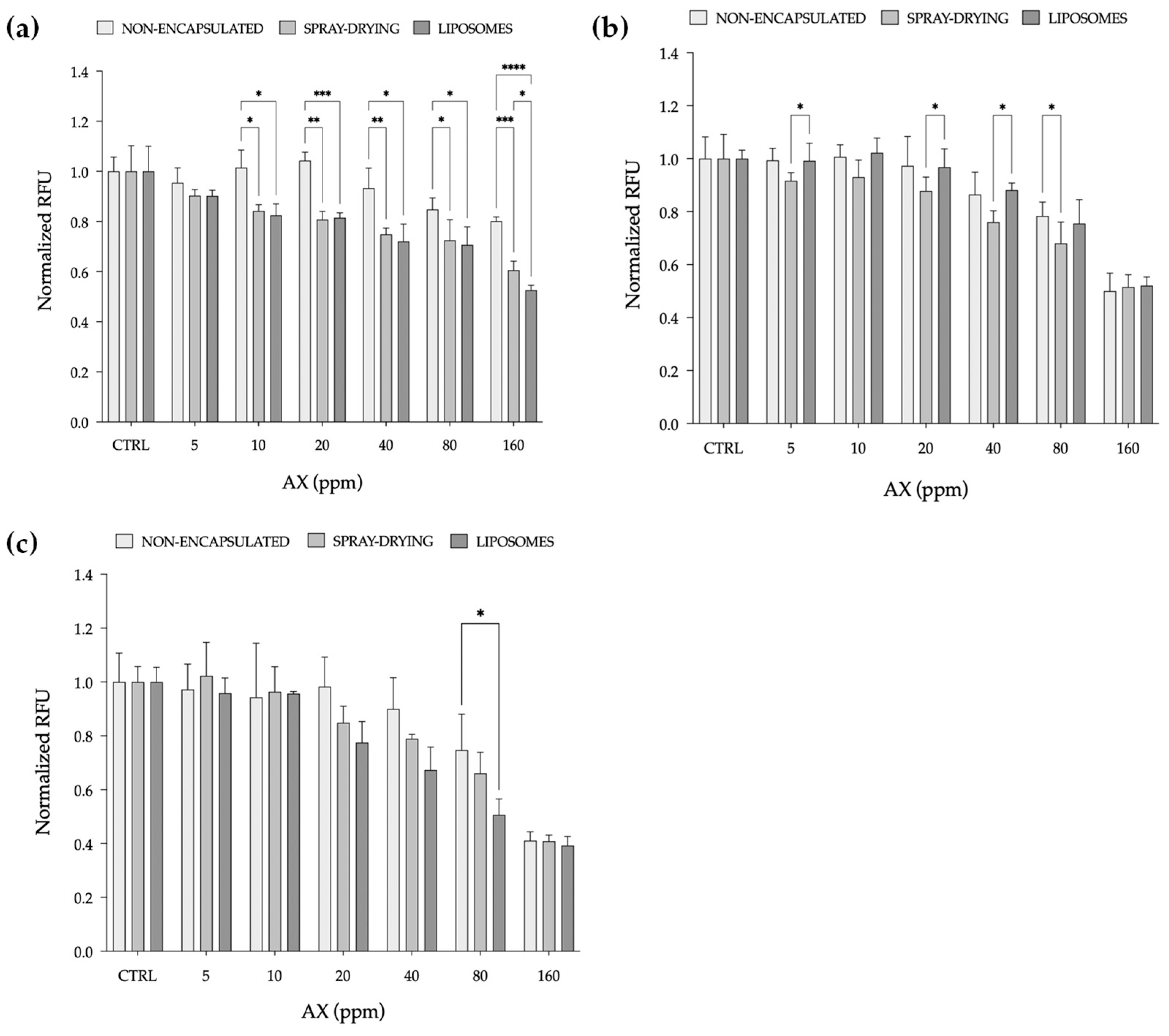
2.2.1. Cell Viability Assay
2.2.2. ROS Production Assay
3. Materials and Methods
3.1. Astaxanthin Sources
3.2. Shrimp Lipid Extraction
3.3. Encapsulation Processes
3.3.1. Spray-Drying
3.3.2. Liposome Entrapment
3.4. Encapsulation Properties
3.4.1. Encapsulation Efficiency
3.4.2. Microstructure and Ultrastructure Analysis
3.4.3. Hydrodynamic Properties and Particle Size Measurement
3.4.4. Colour Measurement
3.4.5. Hygroscopicity
3.4.6. Temperature Stability
3.5. In Vitro Study
3.5.1. RTL-W1 Cell Culture
3.5.2. Cell Viability Assay
3.5.3. ROS Production Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AX | Astaxanthin |
| EE | Encapsulation efficiency |
| H2DCF-DA | 2′,7′-dichlorodihydrofluorescin diacetate |
| M-GA | Maltodextrin–gum Arabic |
| ML | Modified lecithin |
| PBS | Phosphate-buffered saline |
| PDI | Polydispersity index |
| PUFAs | Polyunsaturated fatty acids |
| RFU | Relative fluorescence units |
| ROS | Reactive oxygen species |
| SC | Sodium caseinate |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
References
- Meor Mohd Affandi, M.M.R.; Julianto, T.; Majeed, A.B.A. Enhanced Oral Bioavailability of Astaxanthin with Droplet Size Reduction. FSTR 2012, 18, 549–554. [Google Scholar] [CrossRef]
- Elbahnaswy, S.; Elshopakey, G.E. Recent Progress in Practical Applications of a Potential Carotenoid Astaxanthin in Aquaculture Industry: A Review. Fish Physiol. Biochem. 2024, 50, 97–126. [Google Scholar] [CrossRef]
- Rahman, M.M.; Khosravi, S.; Chang, K.H.; Lee, S.-M. Effects of Dietary Inclusion of Astaxanthin on Growth, Muscle Pigmentation and Antioxidant Capacity of Juvenile Rainbow Trout (Oncorhynchus mykiss). JFN 2016, 21, 281–288. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.M.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Miki, W. Biological Functions and Activities of Animal Carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Lin, S.-F.; Chen, Y.-C.; Chen, R.-N.; Chen, L.-C.; Ho, H.-O.; Tsung, Y.-H.; Sheu, M.-T.; Liu, D.-Z. Improving the Stability of Astaxanthin by Microencapsulation in Calcium Alginate Beads. PLoS ONE 2016, 11, e0153685. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of Its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Anarjan, N.; Tan, C.P.; Nehdi, I.A.; Ling, T.C. Colloidal Astaxanthin: Preparation, Characterization and Bioavailability Evaluation. Food Chem. 2012, 135, 1303–1309. [Google Scholar] [CrossRef]
- Dang, Y.; Li, Z.; Yu, F. Recent Advances in Astaxanthin as an Antioxidant in Food Applications. Antioxidants 2024, 13, 879. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as Feed Supplement in Aquatic Animals. Rev. Aquac. 2018, 10, 738–773. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An Economic Assessment of Astaxanthin Production by Large Scale Cultivation of Haematococcus Pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Fei, Z.; Fan, F.; Liao, J.; Wan, M.; Bai, W.; Wang, W.; He, M.; Li, Y. Improving Astaxanthin Production of Haematococcus Pluvialis on the Outdoor Large Scale Cultivation by Optimizing the Disinfection Strategy of Photobioreactor. Algal Res. 2022, 64, 102708. [Google Scholar] [CrossRef]
- Tran, T.N.; Tran, N.-T.; Tran, T.-A.; Pham, D.-C.; Su, C.-H.; Nguyen, H.C.; Barrow, C.J.; Ngo, D.-N. Highly Active Astaxanthin Production from Waste Molasses by Mutated Rhodosporidium Toruloides G17. Fermentation 2023, 9, 148. [Google Scholar] [CrossRef]
- Li, Z.; You, L.; Du, X.; Yang, H.; Yang, L.; Zhu, Y.; Li, L.; Jiang, Z.; Li, Q.; He, N.; et al. New Strategies to Study in Depth the Metabolic Mechanism of Astaxanthin Biosynthesis in Phaffia Rhodozyma. Crit. Rev. Biotechnol. 2025, 45, 454–472. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Dufossé, L. A Review of Natural Astaxanthin Production in a Circular Bioeconomy Context Using Paracoccus carotinifaciens. Bioresour. Technol. 2023, 369, 128499. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Calvo, M.M.; Álvarez-Acero, I.; Montero, P.; Gómez-Guillén, M.C. Characterization and Storage Stability of Astaxanthin Esters, Fatty Acid Profile and α-Tocopherol of Lipid Extract from Shrimp (L. Vannamei) Waste with Potential Applications as Food Ingredient. Food Chem. 2017, 216, 37–44. [Google Scholar] [CrossRef]
- Montero, P.; Calvo, M.M.; Gómez-Guillén, M.C.; Gómez-Estaca, J. Microcapsules Containing Astaxanthin from Shrimp Waste as Potential Food Coloring and Functional Ingredient: Characterization, Stability, and Bioaccesibility. LWT 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Takeungwongtrakul, S.; Benjakul, S.; Santoso, J.; Trilaksani, W.; Nurilmala, M. Extraction and Stability of Carotenoid-Containing Lipids from Hepatopancreas of Pacific White Shrimp (Litopenaeus vannamei): Carotenoid and Lipids from Shrimp Hepatopancreas. J. Food Process. Preserv. 2015, 39, 10–18. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Calvo, M.M.; Sánchez-Faure, A.; Montero, P.; Gómez-Guillén, M.C. Development, Properties, and Stability of Antioxidant Shrimp Muscle Protein Films Incorporating Carotenoid-Containing Extracts from Food by-products. LWT—Food Sci. Technol. 2015, 64, 189–196. [Google Scholar] [CrossRef]
- Shahidi, F.; Han, X. Encapsulation of Food Ingredients. Crit. Rev. Food Sci. Nutr. 1993, 33, 501–547. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.H.; Bhanvase, B.A.; Sivakumar, M.; Potdar, S.B. Current Overview of Encapsulation. In Encapsulation of Active Molecules and Their Delivery System; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–8. [Google Scholar] [CrossRef]
- Panagiotakopoulos, I.; Nasopoulou, C. Extraction Methods, Encapsulation Techniques, and Health Benefits of Astaxanthin. Sustainability 2024, 16, 10859. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of Spray-Drying in Microencapsulation of Food Ingredients: An Overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, S.; Gu, K.; Zhang, N. Preparation of Astaxanthin-Loaded Liposomes: Characterization, Storage Stability and Antioxidant Activity. CyTA—J. Food 2018, 16, 607–618. [Google Scholar] [CrossRef]
- Marín, D.; Alemán, A.; Sánchez-Faure, A.; Montero, P.; Gómez-Guillén, M.C. Freeze-Dried Phosphatidylcholine Liposomes Encapsulating Various Antioxidant Extracts from Natural Waste as Functional Ingredients in Surimi Gels. Food Chem. 2018, 245, 525–535. [Google Scholar] [CrossRef]
- Anarjan, N.; Tan, C. Effects of Selected Polysorbate and Sucrose Ester Emulsifiers on the Physicochemical Properties of Astaxanthin Nanodispersions. Molecules 2013, 18, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-H.; Chang, C.-H.; Peng, R.Y.; Chyau, C.-C. Improved Membrane Transport of Astaxanthin by Liposomal Encapsulation. Eur. J. Pharm. Biopharm. 2010, 75, 154–161. [Google Scholar] [CrossRef]
- Khalid, N.; Shu, G.; Holland, B.J.; Kobayashi, I.; Nakajima, M.; Barrow, C.J. Formulation and Characterization of O/W Nanoemulsions Encapsulating High Concentration of Astaxanthin. Food Res. Int. 2017, 102, 364–371. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Comunian, T.A.; Montero, P.; Ferro-Furtado, R.; Favaro-Trindade, C.S. Encapsulation of an Astaxanthin-Containing Lipid Extract from Shrimp Waste by Complex Coacervation Using a Novel Gelatin–Cashew Gum Complex. Food Hydrocoll. 2016, 61, 155–162. [Google Scholar] [CrossRef]
- Nguyen, K.D. Astaxanthin: A Comparative Case of Synthetic VS. Natural Production. Chem. Biomol. Eng. Publ. Other Work. 2013, 1, 1–11. Available online: http://trace.tennessee.edu/utk_chembiopubs/94 (accessed on 24 March 2025).
- Lakshmi, P.; Kumar, G.A. Nanosuspension Technology: A Review. Int. J. Pharm. Pharm. Sci. 2010, 2, 35–40. [Google Scholar]
- Gómez-Estaca, J.; Pérez-García, A.; Alemán, A.; Gómez-Guillén, M.C.; Montero, P. Drying Soy Phosphatidylcholine Liposomal Suspensions in Alginate Matrix: Effect of Drying Methods on Physico-Chemical Properties and Stability. Food Hydrocoll. 2021, 111, 106357. [Google Scholar] [CrossRef]
- Müller, R.H.; Jacobs, C.; Kayser, O. Nanosuspensions as Particulate Drug Formulations in Therapy. Adv. Drug Deliv. Rev. 2001, 47, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A. Preparation and In Vitro Evaluation of A Pegylated Nano-Liposomal Formulation Containing Docetaxel. Sci. Pharm. 2009, 77, 453–464. [Google Scholar] [CrossRef]
- Reza Mozafari, M.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and Their Applications in Food Nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef]
- Liang, C.; Du, J.; Hou, T.; Sui, L.; Li, J.; Zhao, Y.; Wu, D. Improvement of Membrane Stabilizer on the Rehydrated Reconstruction of Spray-Dried Mannitol-Based Liposome Powder. Colloid Polym. Sci. 2024, 302, 711–720. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, Z.E.; Singh, H. Recent Trends in the Lipid-based Nanoencapsulation of Antioxidants and Their Role in Foods. J. Sci. Food Agric. 2006, 86, 2038–2045. [Google Scholar] [CrossRef]
- Mahdavi, S.A.; Jafari, S.M.; Ghorbani, M.; Assadpoor, E. Spray-Drying Microencapsulation of Anthocyanins by Natural Biopolymers: A Review. Dry. Technol. 2014, 32, 509–518. [Google Scholar] [CrossRef]
- Rocha-Selmi, G.A.; Bozza, F.T.; Thomazini, M.; Bolini, H.M.A.; Fávaro-Trindade, C.S. Microencapsulation of Aspartame by Double Emulsion Followed by Complex Coacervation to Provide Protection and Prolong Sweetness. Food Chem. 2013, 139, 72–78. [Google Scholar] [CrossRef]
- Comunian, T.A.; Thomazini, M.; Alves, A.J.G.; De Matos, F.E., Jr.; De Carvalho Balieiro, J.C.; Favaro-Trindade, C.S. Microencapsulation of Ascorbic Acid by Complex Coacervation: Protection and Controlled Release. Food Res. Int. 2013, 52, 373–379. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Sørensen, M. Nutritional and Physical Quality of Fish Feeds Extruded at Various Temperatures. Ph.D. Thesis, Department of Animal and Aquacultural Sciences, Agricultural University of Norway, Ås, Norway, 2003; p. 3. [Google Scholar]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M.; Argüelles-Monal, W. Microencapsulation of Astaxanthin in a Chitosan Matrix. Carbohydr. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Bustos-Garza, C.; Yáñez-Fernández, J.; Barragán-Huerta, B.E. Thermal and pH Stability of Spray-Dried Encapsulated Astaxanthin Oleoresin from Haematococcus Pluvialis Using Several Encapsulation Wall Materials. Food Res. Int. 2013, 54, 641–649. [Google Scholar] [CrossRef]
- Mayor, J.; Cuesta, A.; Espinosa-Ruíz, C.; Esteban, M.Á. In Vitro Effects of Astaxanthin on Bacterial and Cell Viability, Cell Migration and Mitochondrial Activities in Four Fish Cell Lines. Aquac. Rep. 2023, 31, 101636. [Google Scholar] [CrossRef]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for Cell Viability Assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Rengel, D.; Dıez-Navajas, A.; Serna-Rico, A.; Veiga, P.; Muga, A.; Milicua, J.C.G. Exogenously Incorporated Ketocarotenoids in Large Unilamellar Vesicles. Protective Activity against Peroxidation. Biochim. Biophys. Acta (BBA)—Biomembr. 2000, 1463, 179–187. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Scita, G.; Freisleben, H.-J.; Kagan, V.E.; Packer, L. Antioxidant Radical-Scavenging Activity of Carotenoids and Retinoids Compared to α-Tocopherol. In Methods in Enzymology; Carotenoids Part A: Chemistry, Separation, Quantitation, and Antioxidation; Academic Press: Cambridge, MA, USA, 1992; Volume 213, pp. 460–472. [Google Scholar] [CrossRef]
- Zhu, X.; Hao, R.; Zhang, J.; Tian, C.; Hong, Y.; Zhu, C.; Li, G. Dietary Astaxanthin Improves the Antioxidant Capacity, Immunity and Disease Resistance of Coral Trout (Plectropomus leopardus). Fish Shellfish. Immunol. 2022, 122, 38–47. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Guo, Z.-X.; Ye, C.-X.; Wang, A.-L. Effect of Dietary Astaxanthin on the Growth Performance, Non-Specific Immunity, and Antioxidant Capacity of Pufferfish (Takifugu obscurus) under High Temperature Stress. Fish Physiol. Biochem. 2018, 44, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Abdol Wahab, N.R.; Meor Mohd Affandi, M.M.R.; Fakurazi, S.; Alias, E.; Hassan, H. Nanocarrier System: State-of-the-Art in Oral Delivery of Astaxanthin. Antioxidants 2022, 11, 1676. [Google Scholar] [CrossRef]
- Smith, C.T.; Gomez, L.A.; Chile, C.; Cortes, R.A. Astaxanthin effect on reactive oxygen species and leukocytes counts in rainbow trout (Oncorhynchus mykiss). Glob. Virtual Conf. 2013, 8, 451–454. [Google Scholar]
- Shabanzadeh, S.; Vatandoust, S.; Hosseinifard, S.M.; Sheikhzadeh, N.; Shahbazfar, A.-A. Dietary Astaxanthin (Lucantin® Pink) Mitigated Oxidative Stress Induced by Diazinon in Rainbow Trout (Oncorhynchus mykiss). Vet. Res. Forum 2023, 14, 97–104. [Google Scholar] [CrossRef]
- Kalinowski, C.T.; Larroquet, L.; Véron, V.; Robaina, L.; Izquierdo, M.S.; Panserat, S.; Kaushik, S.; Fontagné-Dicharry, S. Influence of Dietary Astaxanthin on the Hepatic Oxidative Stress Response Caused by Episodic Hyperoxia in Rainbow Trout. Antioxidants 2019, 8, 626. [Google Scholar] [CrossRef]
- Hassanzadeh, P.; Ahmadvand, M.; Aslani, S.; Sheikhzadeh, N.; Mousavi, S.; Khatibi, S.A.; Ahmadifar, E. Dietary Astaxanthin Mitigated Paraquat-Induced Oxidative Stress in Rainbow Trout (Oncorhynchus mykiss) Fillet. Aquac. Res. 2022, 53, 5300–5309. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Storebakken, T.; Liaaen-Jensen, S. Response to Carotenoids by Rainbow Trout in the Sea: Resorption and Metabolism of Dietary Astaxanthin and Canthaxanthin. Aquaculture 1990, 91, 153–162. [Google Scholar] [CrossRef]
- Saleh, N.; Wassef, E.A.; Shalaby, S.M. The Role of Dietary Astaxanthin in European Sea Bass (Dicentrarchus labrax) Growth, Immunity, Antioxidant Competence and Stress Tolerance. Egypt. J. Aquat. Biol. Fish. 2018, 22, 189–200. [Google Scholar] [CrossRef]
- Shastak, Y.; Pelletier, W. Captivating Colors, Crucial Roles: Astaxanthin’s Antioxidant Impact on Fish Oxidative Stress and Reproductive Performance. Animals 2023, 13, 3357. [Google Scholar] [CrossRef]
- Britton, B. UV/visible spectroscopy. In Carotenoids Volume 1B: Spectroscopy; Britton, S., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser-Verlag: Berlin, Germany, 1995. [Google Scholar]
- Mosquera, M.; Giménez, B.; Montero, P.; Gómez-Guillén, M.C. Incorporation of Liposomes Containing Squid Tunic ACE -inhibitory Peptides into Fish Gelatin. J. Sci. Food Agric. 2016, 96, 769–776. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Gómez-Estaca, J.; Gavara, R.; Hernandez-Munoz, P. Functional Properties of Bioplastics Made from Wheat Gliadins Modified with Cinnamaldehyde. J. Agric. Food Chem. 2011, 59, 6689–6695. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the Probe 2‘,7‘-Dichiorofluorescin as an Indicator of Reactive Oxygen Species Formation and Oxidative Stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitale, M.; Gomez-Estaca, J.; Chung, J.; Chua, S.-C.; Pampanin, D.M. Encapsulation Techniques to Enhance Astaxanthin Utilization as Functional Feed Ingredient. Mar. Drugs 2025, 23, 143. https://doi.org/10.3390/md23040143
Vitale M, Gomez-Estaca J, Chung J, Chua S-C, Pampanin DM. Encapsulation Techniques to Enhance Astaxanthin Utilization as Functional Feed Ingredient. Marine Drugs. 2025; 23(4):143. https://doi.org/10.3390/md23040143
Chicago/Turabian StyleVitale, Matteo, Joaquin Gomez-Estaca, Janete Chung, Seong-Chea Chua, and Daniela Maria Pampanin. 2025. "Encapsulation Techniques to Enhance Astaxanthin Utilization as Functional Feed Ingredient" Marine Drugs 23, no. 4: 143. https://doi.org/10.3390/md23040143
APA StyleVitale, M., Gomez-Estaca, J., Chung, J., Chua, S.-C., & Pampanin, D. M. (2025). Encapsulation Techniques to Enhance Astaxanthin Utilization as Functional Feed Ingredient. Marine Drugs, 23(4), 143. https://doi.org/10.3390/md23040143






