Marine Sponge-Derived Gukulenin A Sensitizes Ovarian Cancer Cells to PARP Inhibition via Ferroptosis Induction
Abstract
1. Introduction
2. Results
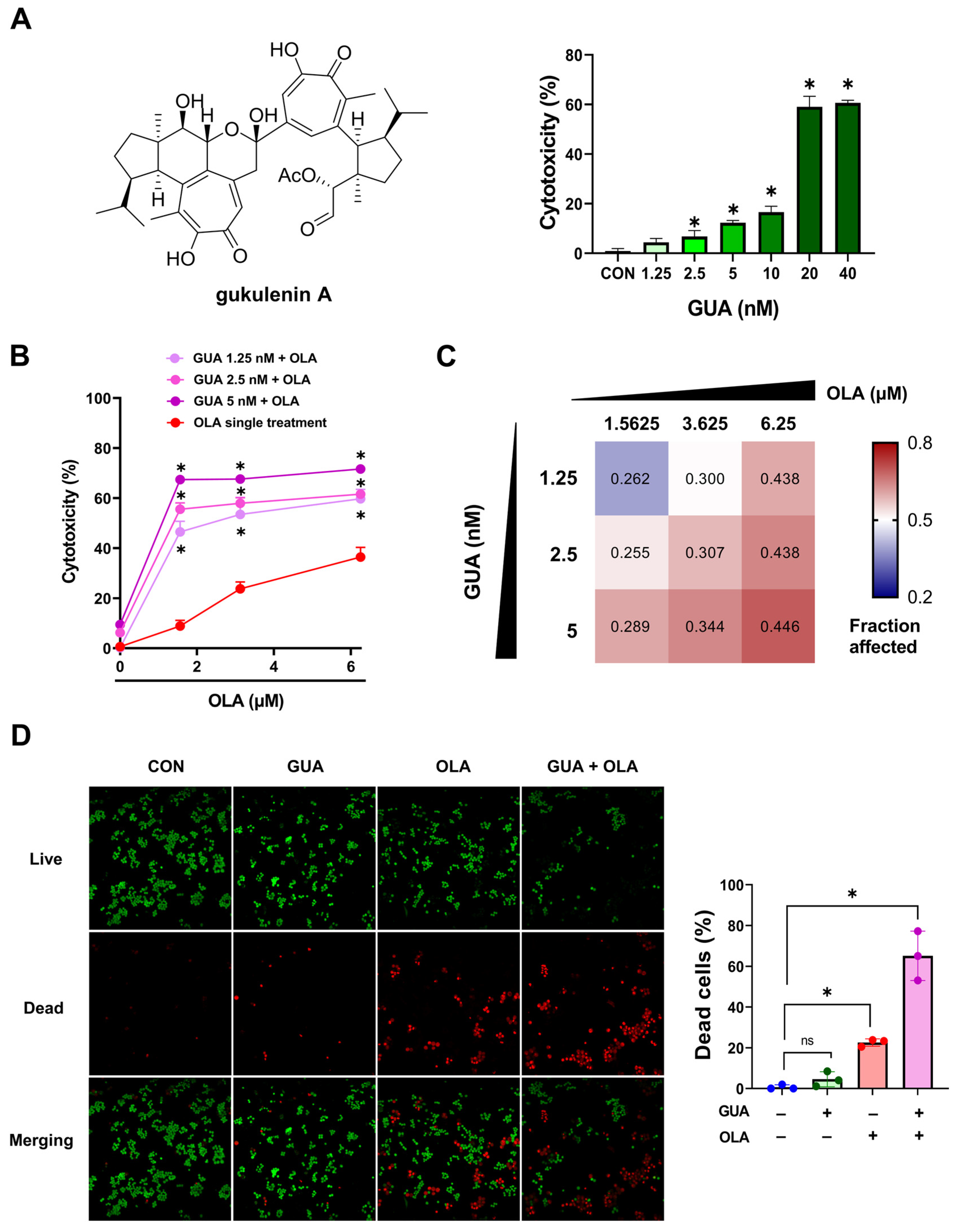
2.1. GUA-Mediated Enhancement of OLA Sensitivity in Human Ovarian Cancer Cells
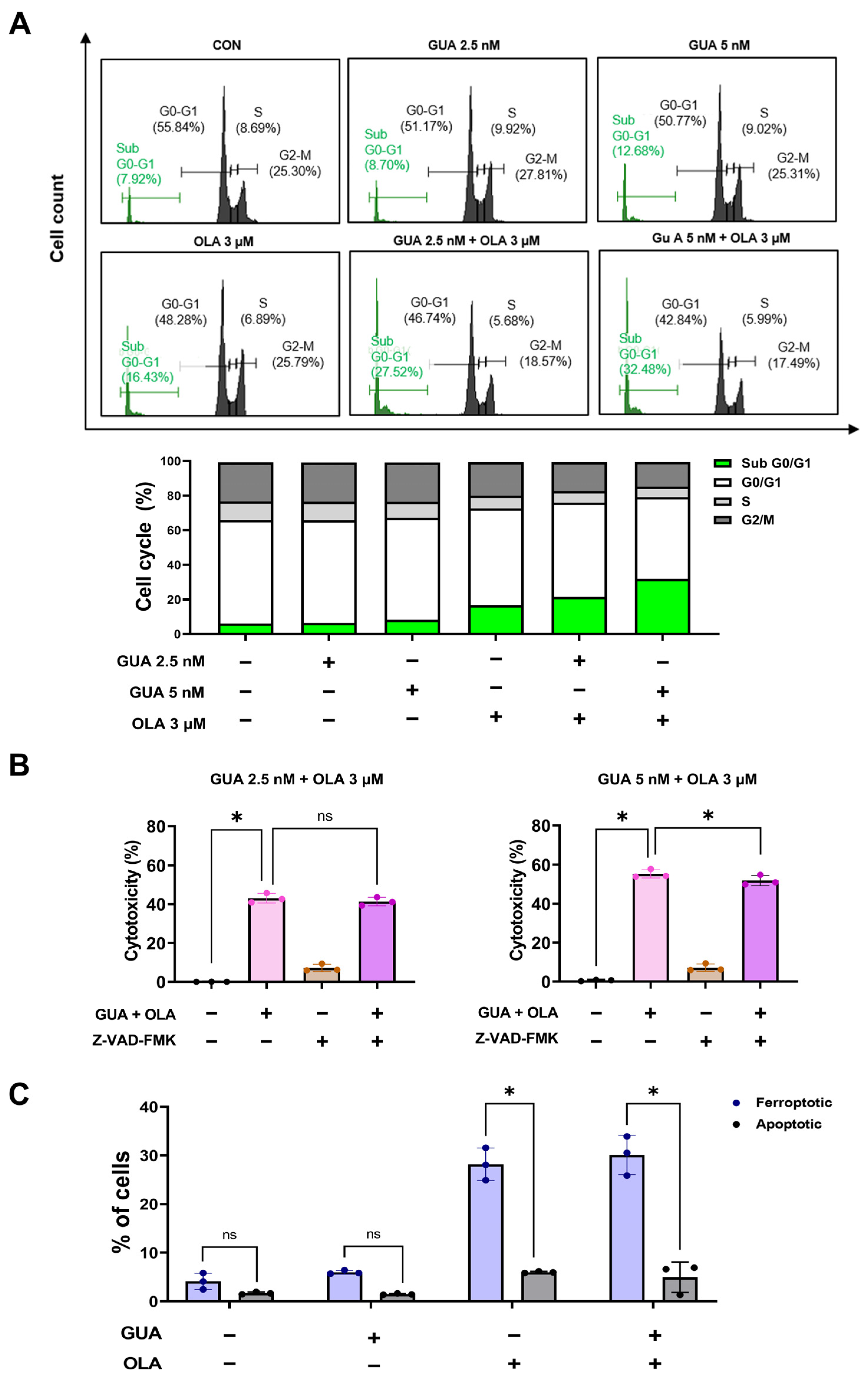
2.2. Effects of GUA and OLA Combination Treatment on Cell Cycle Distribution and Apoptosis
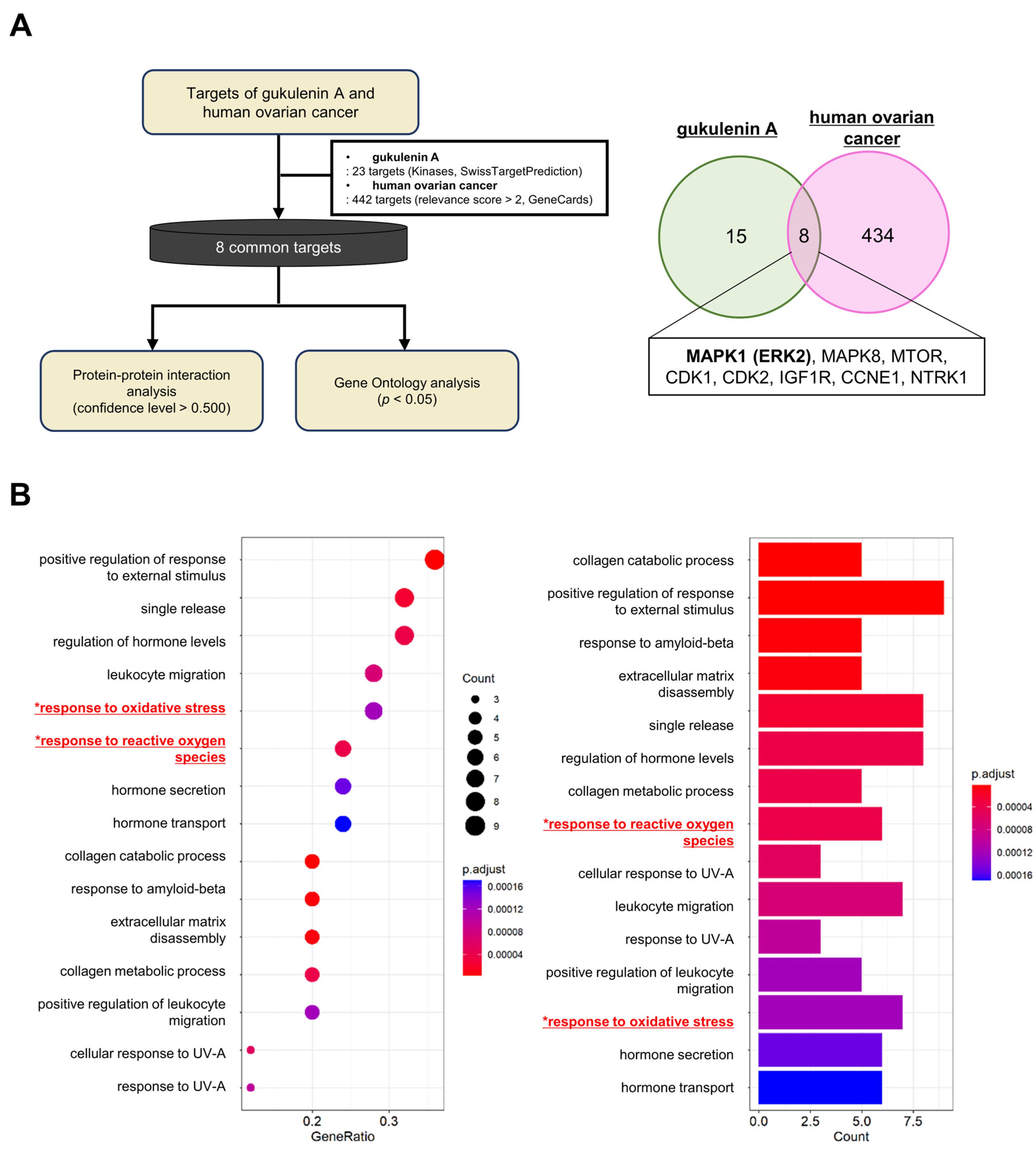
2.3. Network Pharmacology and GO Enrichment Analysis of GUA Targets in Ovarian Cancer
2.4. Effects of GUA and OLA Combination Treatment on ROS Production and NOX
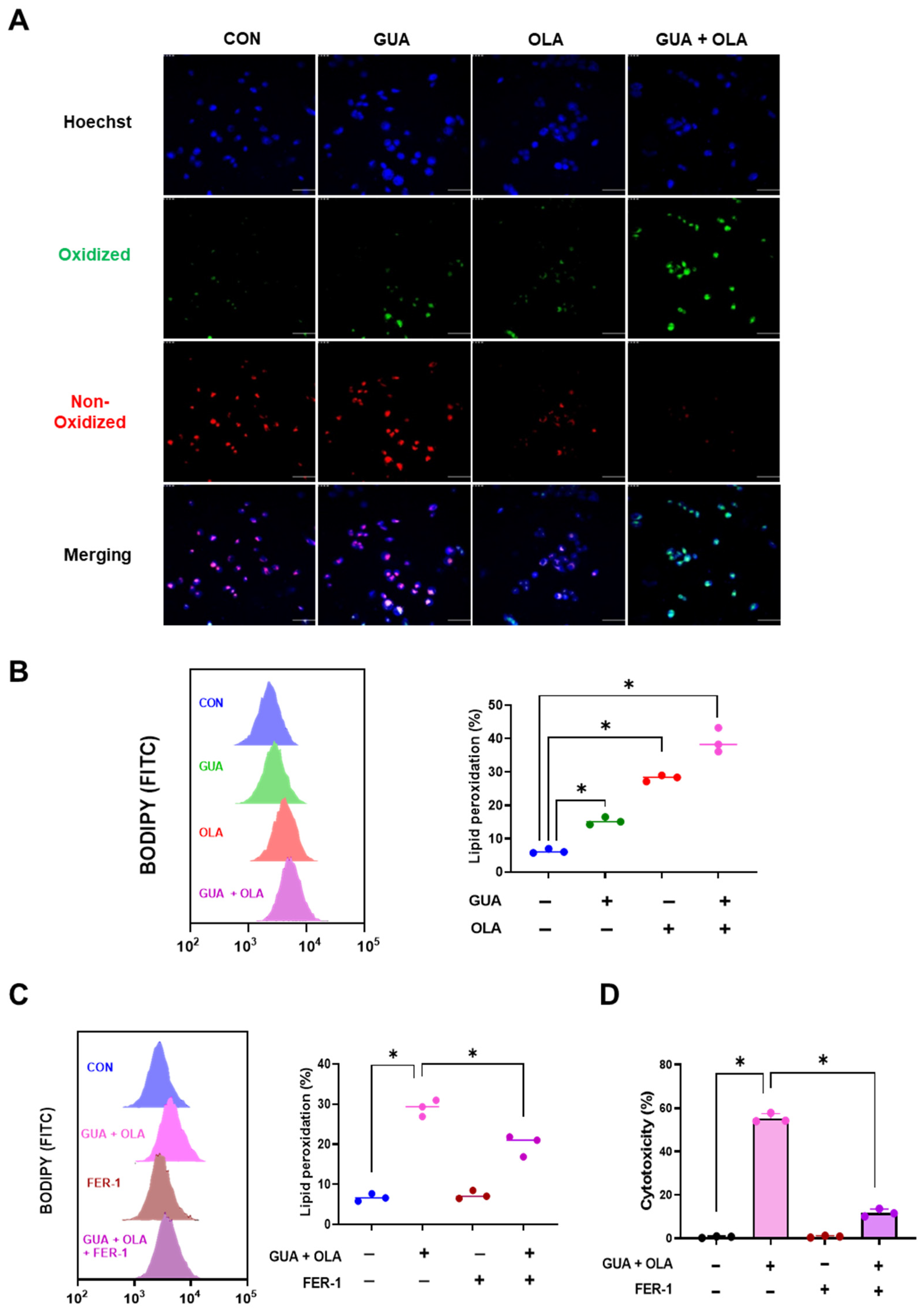
2.5. Involvement of Lipid Peroxidation in GUA and OLA Combination Treatment-Induced Ferroptosis
2.6. Involvement of ERK Signaling in GUA and OLA Combination Treatment-Induced Ferroptosis
3. Discussion
4. Materials and Methods
4.1. Extraction and Purification of GUA
4.2. Materials
4.3. Cell Culture and Cell Viability/Cytoxicity Assay
4.4. Annexin-V-FITC and PI Staining Assay
4.5. Transfection
4.6. Network Pharmacology and GO Enrichment Analysis
4.7. Detection of Intracellular ROS
4.8. Lipid Peroxidation Assay
4.9. Western Blot Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tavares, V.; Marques, I.S.; Melo, I.G.; Assis, J.; Pereira, D.; Medeiros, R. Paradigm Shift: A Comprehensive Review of Ovarian Cancer Management in an Era of Advancements. Int. J. Mol. Sci. 2024, 25, 1845. [Google Scholar] [CrossRef] [PubMed]
- Moufarrij, S.; O’Cearbhaill, R.E. Novel Therapeutics in Ovarian Cancer: Expanding the Toolbox. Curr. Oncol. 2023, 31, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidi, A.; Fountzilas, E.; Fostira, F.; Psyrri, A.; Gogas, H.; Papadimitriou, C. Neoadjuvant treatment in ovarian cancer: New perspectives, new challenges. Front. Oncol. 2022, 12, 820128. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, J.; Mishail, D.; Wang, C.Y. Recent advancements in PARP inhibitors-based targeted cancer therapy. Precis. Clin. Med. 2020, 3, 187–201. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Haro-Silerio, J.I.; Yap, T.A.; Ngoi, N. PARP inhibitors: Enhancing efficacy through rational combinations. Br. J. Cancer 2023, 129, 904–916. [Google Scholar] [CrossRef]
- Esposito, R.; Federico, S.; Glaviano, F.; Somma, E.; Zupo, V.; Costantini, M. Bioactive Compounds from Marine Sponges and Algae: Effects on Cancer Cell Metabolome and Chemical Structures. Int. J. Mol. Sci. 2022, 23, 10680. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J. Genet. Eng. Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Kudryashova, E.K.; Kaune, M.; Makarieva, T.N.; Shubina, L.K.; Busenbender, T.; Denisenko, V.A.; Popov, R.S.; Hauschild, J.; Fedorov, S.N.; et al. Urupocidin C: A new marine guanidine alkaloid which selectively kills prostate cancer cells via mitochondria targeting. Sci. Rep. 2020, 10, 9764. [Google Scholar] [CrossRef]
- Dyshlovoy, S.A.; Kaune, M.; Kriegs, M.; Hauschild, J.; Busenbender, T.; Shubina, L.K.; Makarieva, T.N.; Hoffer, K.; Bokemeyer, C.; Graefen, M.; et al. Marine alkaloid monanchoxymycalin C: A new specific activator of JNK1/2 kinase with anticancer properties. Sci. Rep. 2020, 10, 13178. [Google Scholar] [CrossRef]
- Roel, M.; Rubiolo, J.A.; Guerra-Varela, J.; Silva, S.B.; Thomas, O.P.; Cabezas-Sainz, P.; Sanchez, L.; Lopez, R.; Botana, L.M. Marine guanidine alkaloids crambescidins inhibit tumor growth and activate intrinsic apoptotic signaling inducing tumor regression in a colorectal carcinoma zebrafish xenograft model. Oncotarget 2016, 7, 83071–83087. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Parrino, B.; Barraja, P.; Spano, V.; Cirrincione, G.; Diana, P.; Maier, A.; Kelter, G.; Fiebig, H.H. Synthesis and antiproliferative activity of 2,5-bis(3′-indolyl)pyrroles, analogues of the marine alkaloid nortopsentin. Mar. Drugs 2013, 11, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Avila-Arroyo, S.; Nunez, G.S.; Garcia-Fernandez, L.F.; Galmarini, C.M. Synergistic Effect of Trabectedin and Olaparib Combination Regimen in Breast Cancer Cell Lines. J. Breast Cancer 2015, 18, 329–338. [Google Scholar] [CrossRef]
- Jeon, J.E.; Liao, L.; Kim, H.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Cytotoxic diterpenoid pseudodimers from the Korean sponge Phorbas gukhulensis. J. Nat. Prod. 2013, 76, 1679–1685. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, H.; Hwang, H.; Kang, H.; Rho, J.R. Gukulenins A and B, cytotoxic tetraterpenoids from the marine sponge Phorbas gukulensis. J. Nat. Prod. 2010, 73, 734–737. [Google Scholar] [CrossRef]
- Ahn, J.H.; Woo, J.H.; Rho, J.R.; Choi, J.H. Anticancer Activity of Gukulenin A Isolated from the Marine Sponge Phorbas gukhulensis In Vitro and In Vivo. Mar. Drugs 2019, 17, 126. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef]
- Ye, L.F.; Chaudhary, K.R.; Zandkarimi, F.; Harken, A.D.; Kinslow, C.J.; Upadhyayula, P.S.; Dovas, A.; Higgins, D.M.; Tan, H.; Zhang, Y.; et al. Radiation-Induced Lipid Peroxidation Triggers Ferroptosis and Synergizes with Ferroptosis Inducers. ACS Chem. Biol. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Liu, Z.; He, X.; Tang, W.; He, L.; Feng, Y.; Liu, D.; Yin, Y.; Li, T. Ferroptosis and Its Multifaceted Role in Cancer: Mechanisms and Therapeutic Approach. Antioxidants 2022, 11, 1504. [Google Scholar] [CrossRef]
- Yan, H.F.; Zou, T.; Tuo, Q.Z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and links with diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, Y.; Mao, C.; Liu, S.; Xiao, D.; Huang, J.; Tao, Y. Emerging mechanisms and targeted therapy of ferroptosis in cancer. Mol. Ther. 2021, 29, 2185–2208. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Hu, F.; Feng, H.; Linkermann, A.; Min, W.; Stockwell, B.R. Determination of the Subcellular Localization and Mechanism of Action of Ferrostatins in Suppressing Ferroptosis. ACS Chem. Biol. 2018, 13, 1013–1020. [Google Scholar] [CrossRef]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef]
- Cipriano, A.; Viviano, M.; Feoli, A.; Milite, C.; Sarno, G.; Castellano, S.; Sbardella, G. NADPH Oxidases: From Molecular Mechanisms to Current Inhibitors. J. Med. Chem. 2023, 66, 11632–11655. [Google Scholar] [CrossRef]
- Eun, H.S.; Cho, S.Y.; Joo, J.S.; Kang, S.H.; Moon, H.S.; Lee, E.S.; Kim, S.H.; Lee, B.S. Gene expression of NOX family members and their clinical significance in hepatocellular carcinoma. Sci. Rep. 2017, 7, 11060. [Google Scholar] [CrossRef]
- Grauers Wiktorin, H.; Aydin, E.; Hellstrand, K.; Martner, A. NOX2-Derived Reactive Oxygen Species in Cancer. Oxid. Med. Cell. Longev. 2020, 2020, 7095902. [Google Scholar] [CrossRef]
- Zhang, Z.; Luan, Q.; Hao, W.; Cui, Y.; Li, Y.; Li, X. NOX4-derived ROS Regulates Aerobic Glycolysis of Breast Cancer through YAP Pathway. J. Cancer 2023, 14, 2562–2573. [Google Scholar] [CrossRef]
- Zipper, L.M.; Mulcahy, R.T. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol. Sci. 2003, 73, 124–134. [Google Scholar] [CrossRef]
- Cheung, K.L.; Lee, J.H.; Shu, L.; Kim, J.H.; Sacks, D.B.; Kong, A.N. The Ras GTPase-activating-like protein IQGAP1 mediates Nrf2 protein activation via the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)-ERK pathway. J. Biol. Chem. 2013, 288, 22378–22386. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L.; et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef]
- Xia, Q.; Gao, S.; Rapael Gnanamuthu, S.R.; Zhuang, K.; Song, Z.; Zhang, Y.; Wang, X.; Tu, P.; Li, J.; Liu, K. Involvement of Nrf2-HO-1/JNK-Erk Signaling Pathways in Aconitine-Induced Developmental Toxicity, Oxidative Stress, and ROS-Mitochondrial Apoptosis in Zebrafish Embryos. Front. Pharmacol. 2021, 12, 642480. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.Y.; Park, S.G.; Yu, S.N.; Kim, Y.W.; Nam, H.W.; An, H.H.; Kim, Y.W.; Ahn, S.C. Mitochondrial ROS activates ERK/autophagy pathway as a protected mechanism against deoxypodophyllotoxin-induced apoptosis. Oncotarget 2017, 8, 111581–111596. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, J.; Zeng, L.; Yang, Y.; Liu, L.; Yao, M.; Chai, L.; Zhang, L.; Li, Y.; Zhang, L.; et al. Natural product manoalide promotes EGFR-TKI sensitivity of lung cancer cells by KRAS-ERK pathway and mitochondrial Ca2+ overload-induced ferroptosis. Front. Pharmacol. 2022, 13, 1109822. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-H.; Rho, J.-R.; Choi, J.-H. Marine Sponge-Derived Gukulenin A Sensitizes Ovarian Cancer Cells to PARP Inhibition via Ferroptosis Induction. Mar. Drugs 2025, 23, 138. https://doi.org/10.3390/md23040138
Kim J-H, Rho J-R, Choi J-H. Marine Sponge-Derived Gukulenin A Sensitizes Ovarian Cancer Cells to PARP Inhibition via Ferroptosis Induction. Marine Drugs. 2025; 23(4):138. https://doi.org/10.3390/md23040138
Chicago/Turabian StyleKim, Jin-Hyung, Jung-Rae Rho, and Jung-Hye Choi. 2025. "Marine Sponge-Derived Gukulenin A Sensitizes Ovarian Cancer Cells to PARP Inhibition via Ferroptosis Induction" Marine Drugs 23, no. 4: 138. https://doi.org/10.3390/md23040138
APA StyleKim, J.-H., Rho, J.-R., & Choi, J.-H. (2025). Marine Sponge-Derived Gukulenin A Sensitizes Ovarian Cancer Cells to PARP Inhibition via Ferroptosis Induction. Marine Drugs, 23(4), 138. https://doi.org/10.3390/md23040138





