Synthesis of 5-Hydroxy-5-vinyl-2-cyclopentenones, a Family of Rare-Type Natural Products Mostly Recovered from Marine Sources
Abstract
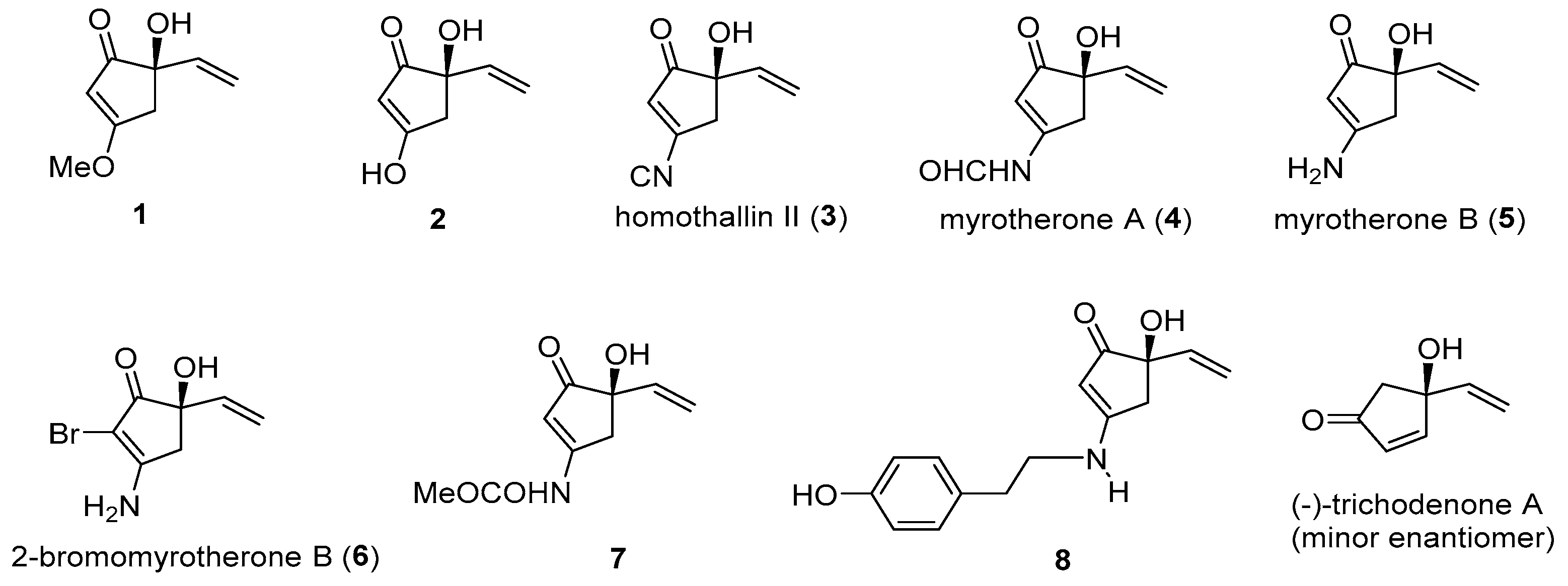
1. Introduction
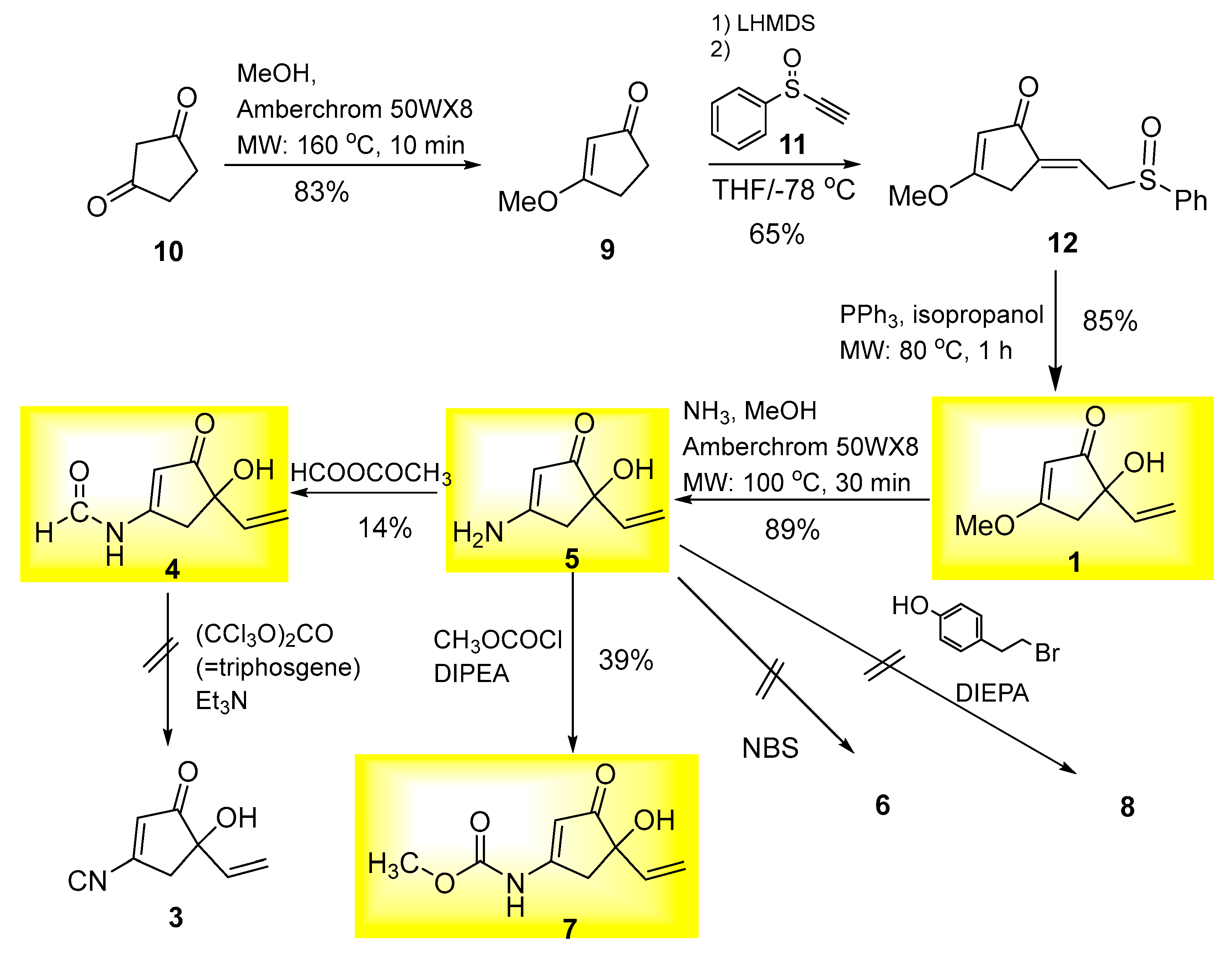
2. Results and Discussions
3. Materials and Methods
3.1. General Methods
3.2. Synthesis of 5-Hydroxy-5-vinyl-2-cyclopentenone-Type Natural Products
3.2.1. (E)-3-Methoxy-5-(2-(phenylthio)ethylidene)cyclopent-2-enone (12)
3.2.2. 5-Hydroxy-3-methoxy-5-vinylcyclopent-2-en-1-one (1)
3.2.3. 3-Amino-5-hydroxy-5-vinylcyclopent-2-en-1-one, Myrothenone B (5)
3.2.4. N-(4-Hydroxy-3-oxo-4-vinylcyclopent-1-en-1-yl)formamide, Myrothenone A (4)
3.2.5. Methyl (4-hydroxy-3-oxo-4-vinylcyclopent-1-en-1-yl)carbamate (7)
3.2.6. 5-Hydroxy-3-((4-hydroxyphenethyl)amino)-5-vinylcyclopent-2-en-1-one (8)
3.2.7. 2-Bromo-5-hydroxy-3-methoxy-5-vinylcyclopent-2-en-1-one (13)
3.2.8. 3-Amino-2-bromo-5-hydroxy-5-vinylcyclopent-2-en-1-one (6) (Bromomyrothenone B)
3.2.9. Reaction of Myrothenone B (5) with Difluorocarbene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations in novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2007, 7, 89–123. [Google Scholar] [CrossRef]
- Strunz, G.M.; Ren, W.-Y.; Stillwell, M.A.; Valenta, Z. Structure and synthesis of a new cyclopentenone derivative from Trichoderma album. Can. J. Chem. 1977, 55, 2610–2612. [Google Scholar] [CrossRef]
- Mukhopadhyay, T.; Roy, K.; Sawant, S.N.; Deshmukh, S.K.; Ganguli, B.N.; Fehlhaber, H.W. On an unstable antifungal metabolite from trichoderma koningiiIsolation and structure elucidation of a new cyclopentenone derivative(3-Dimethylamino-S-hydroxy-5-vinyl-2-cyclopenten-1-one). J. Antibiot. 1996, 49, 210–211. [Google Scholar] [CrossRef]
- Faull, J.L.; Graeme-Cook, K.A.; Pilkington, B.L. Production of an isonitrile antibiotic by an UV-induced mutant of Trichoderma harzianum. Phytochemistry 1994, 36, 1273–1276. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Yamada, K.; Minoura, K.; Miyamoto, K.; Usami, Y.; Kobayashi, T.; Hamada-Sato, N.; Imada, C.; Tsujibo, H. Purification and determination of the chemical structure of the tyrosinase inhibitor produced by Trichoderma viride strain H1-7 from a marine environment. Biol. Pharm. Bull. 2008, 31, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, M.K.; Lee, U.; Kim, S.-K.; Kang, J.S.; Choi, H.D.; Son, B.W. Myrothenones A and B, Cyclopentenone derivatives with tyrosinase inhibitory activity from the marine-derived fungus Myrothecium sp. Chem. Pharm. Bull. 2005, 53, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, D.; Lee, U.; Li, X.; Cheng, J.; Zhu, W.; Jung, J.H.; Choi, H.D.; Son, B.W.; Bromomyrothenone, B.; et al. Bromomyrothenone B and botrytinone, cyclopentenone Derivatives from a marine isolate of the fungus Botrytis. J. Nat. Prod. 2007, 70, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Leutou, A.S.; Yang, G.; Nenkep, V.N.; Siwe, X.N.; Choi, H.-D.; Kang, J.-S.; Son, B.-W. Bioactive cyclopentenone derivatives from marine isolates of fungi. Bull. Korean Chem. Soc. 2009, 30, 2345–2350. [Google Scholar] [CrossRef]
- Lin, W.; Li, L.; Fu, H.; Sattler, I.; Huang, X.; Grabley, S. New Cyclopentenone Derivatives from an Endophytic Streptomyces sp. Isolated from the Mangrove Plant Aegiceras comiculatum. J. Antibiot. 2005, 58, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.B.; Liebeskind, L.S. Acid-catalyzed ring expansion of 1-(1-methoxy-1,2-propadienyl)-2-cyclobuten-1-ols. Synthesis of 5-hydroxy-5-vinyl-2-cyclopenten-1-ones and their stereoselective transformation to 5-(2-acetoxyethylidene)-2-cyclopenten-1-ones. J. Org. Chem. 1990, 55, 4614–4622. [Google Scholar] [CrossRef]
- Usami, Y. Synthesis of marine-derived carbasugar pericosines. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Pergamon Science Publishers: Amsterdam, The Netherlands, 2014; Volume 41, pp. 287–319. [Google Scholar]
- Ichikawa, H.; Watanabe, R.; Fujino, Y.; Usami, Y. Divergent synthesis of withasomnines via synthesis of 4-hydroxy-1H-pyrazoles and Claisen rearrangement of their 4-O-allylethers. Tetrahedron Lett. 2011, 52, 4448–4451. [Google Scholar] [CrossRef]
- Debien, L.; Zard, S.Z. A two-step synthesis of α-keto vinyl carbinols from ketones. Org. Lett. 2013, 15, 6066–6069. [Google Scholar] [CrossRef]
- Ruangsiyanand, C.; Rimek, H.-J.; Zymalkowski, F. Enamine cyclischer 1.3-diketone als Ausgangsstoffe für Pyridinringsynthesen. Chem. Ber. 1970, 103, 2403–2410. [Google Scholar] [CrossRef]
- Nagashima, E.; Suzuki, K.; Sekiya, M. Syntheses and reactions of phenylthio- and propylthioacetylenic compounds. Chem. Pharm. Bull. 1981, 29, 1274–1279. [Google Scholar] [CrossRef]
- Lopes, E.F.; Dalberto, B.T.; Perin, G.; Alves, D.; Barcellos, T.; Lenardão, E.J. Synthesis of terminal ethynyl aryl selenides and sulfides based on the retro-Favorskii reaction of hydroxypropargyl precursors. Chem. Eur. J. 2017, 23, 13760–13765. [Google Scholar] [CrossRef]
- Kikani, B.B.; Mckee, J.R.; Zanger, M. An efficient synthesis of 3-aminocyclo-pent-2-en-1-one. Synthesis 1991, 176. [Google Scholar] [CrossRef]
- Song, Y.S.; Kim, B.T.; Heo, J.-N. An efficient synthesis of 2-aryl-3-methoxy-2-cycloalkenones via Suzuki–Miyaura reaction under microwave irradiation. Tetrahedron Lett. 2005, 46, 5987–5990. [Google Scholar] [CrossRef]
- Si, Y.-X.; Zhu, P.-F.; Zhang, S.-L. Synthesis of isocyanides by reacting primary amines with difluorocarbene. Org. Lett. 2020, 22, 9086–9090. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usami, Y.; Asada, N.; Shizuma, C.; Negoro, K.; Kawai, R.; Kaneda, S.; Hayama, N. Synthesis of 5-Hydroxy-5-vinyl-2-cyclopentenones, a Family of Rare-Type Natural Products Mostly Recovered from Marine Sources. Mar. Drugs 2025, 23, 449. https://doi.org/10.3390/md23120449
Usami Y, Asada N, Shizuma C, Negoro K, Kawai R, Kaneda S, Hayama N. Synthesis of 5-Hydroxy-5-vinyl-2-cyclopentenones, a Family of Rare-Type Natural Products Mostly Recovered from Marine Sources. Marine Drugs. 2025; 23(12):449. https://doi.org/10.3390/md23120449
Chicago/Turabian StyleUsami, Yoshihide, Natsuki Asada, Chihiro Shizuma, Karin Negoro, Ryosuke Kawai, Sayaka Kaneda, and Noboru Hayama. 2025. "Synthesis of 5-Hydroxy-5-vinyl-2-cyclopentenones, a Family of Rare-Type Natural Products Mostly Recovered from Marine Sources" Marine Drugs 23, no. 12: 449. https://doi.org/10.3390/md23120449
APA StyleUsami, Y., Asada, N., Shizuma, C., Negoro, K., Kawai, R., Kaneda, S., & Hayama, N. (2025). Synthesis of 5-Hydroxy-5-vinyl-2-cyclopentenones, a Family of Rare-Type Natural Products Mostly Recovered from Marine Sources. Marine Drugs, 23(12), 449. https://doi.org/10.3390/md23120449






