Enzymatic Spirulina Extract Enhances the Vasodilation in Aorta and Mesenteric Arteries of Aged Rats
Abstract
1. Introduction
2. Results
2.1. Composition and Antioxidant Activity of the Enz-Spir-E
2.2. Animal Weight and Systolic Blood Pressure
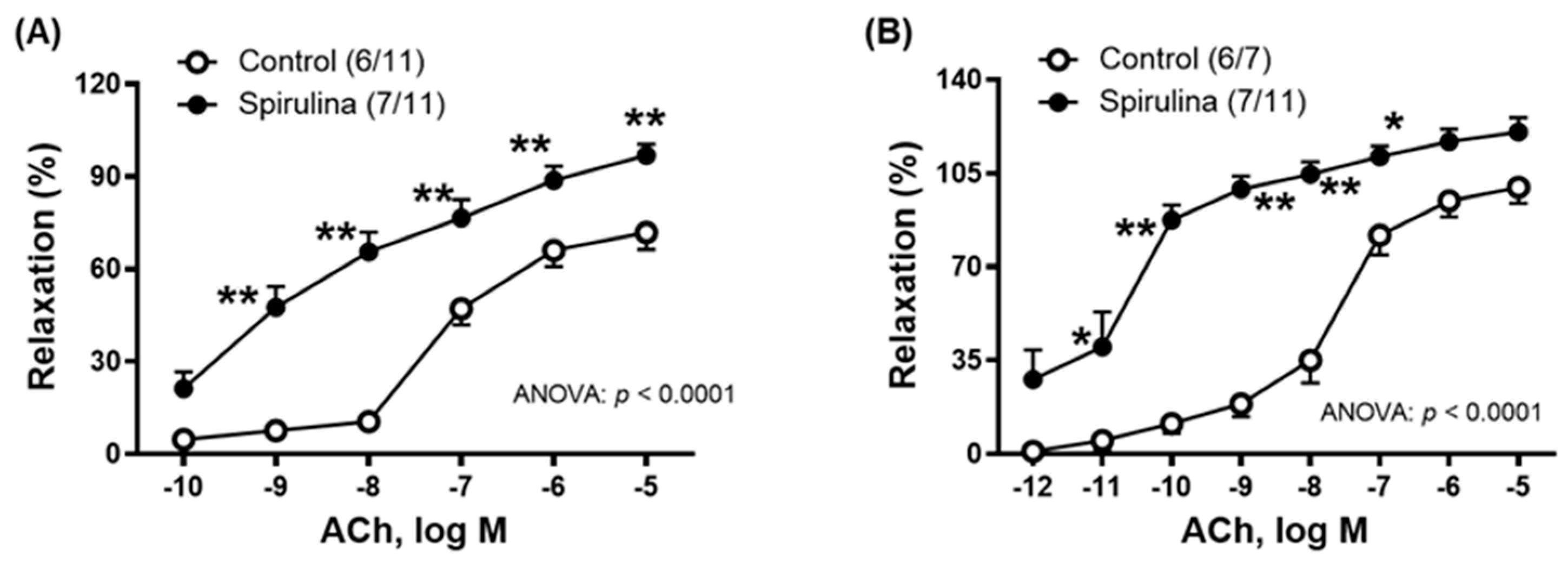
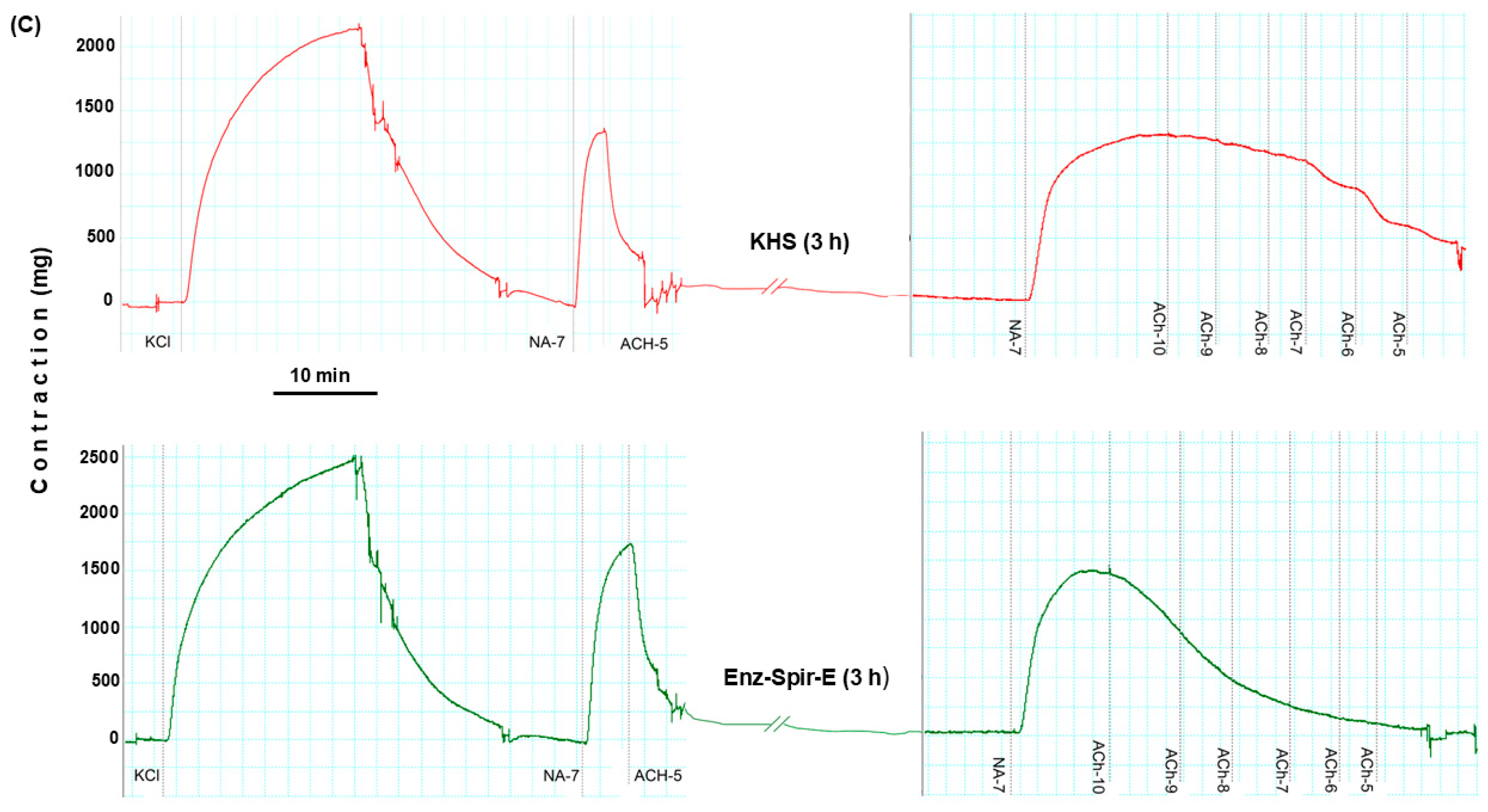
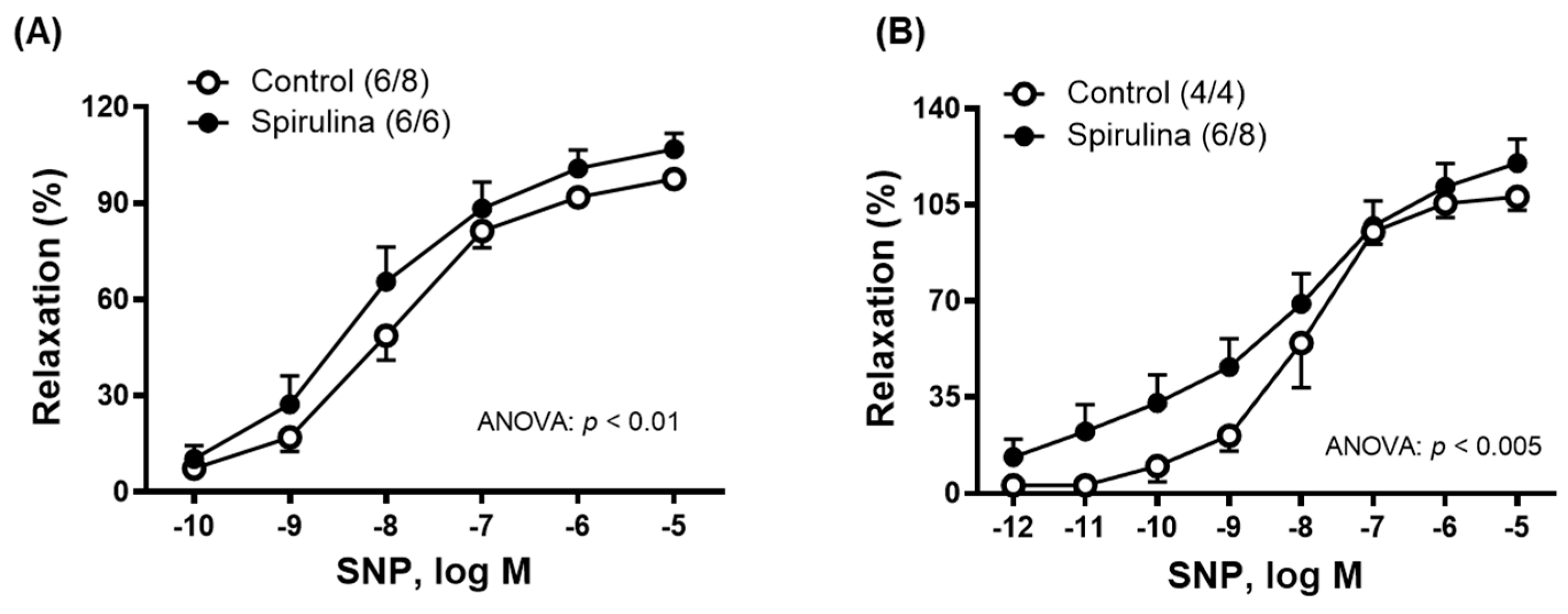
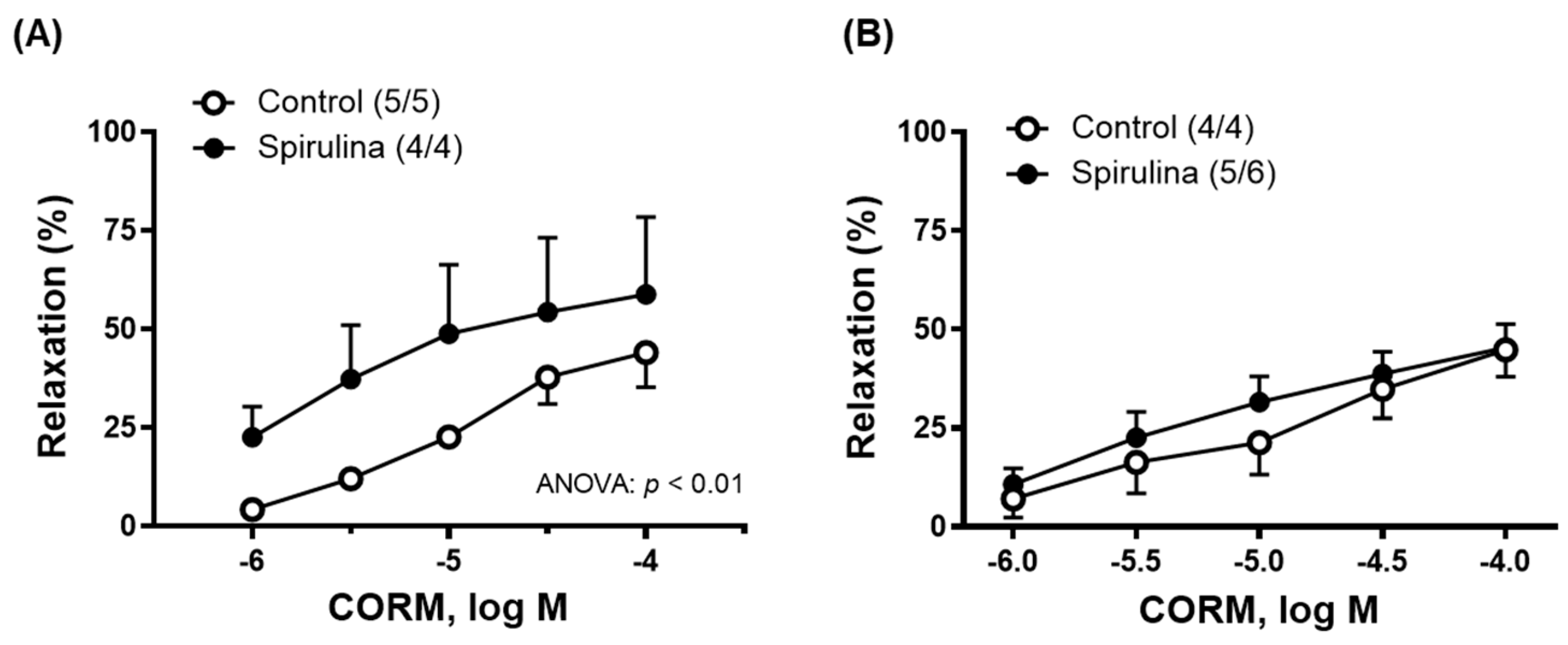
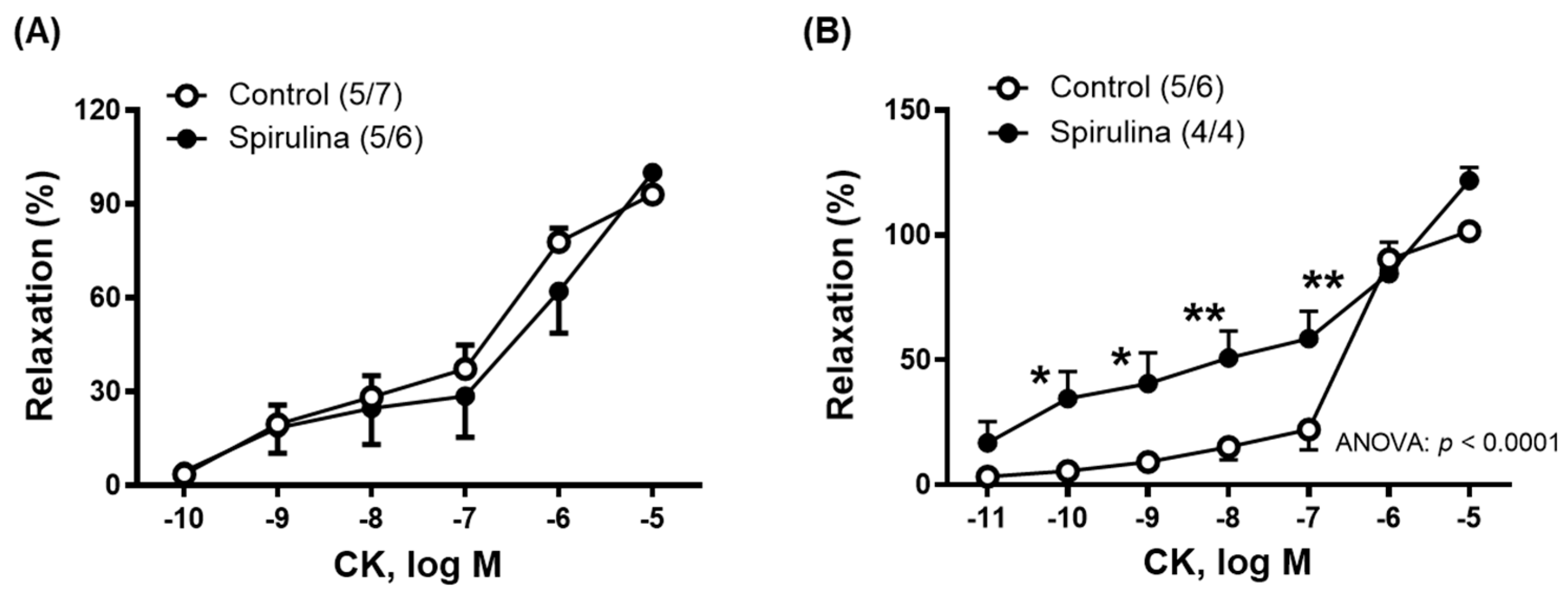
2.3. Effect of Enz-Spir-E on Vascular Functioning
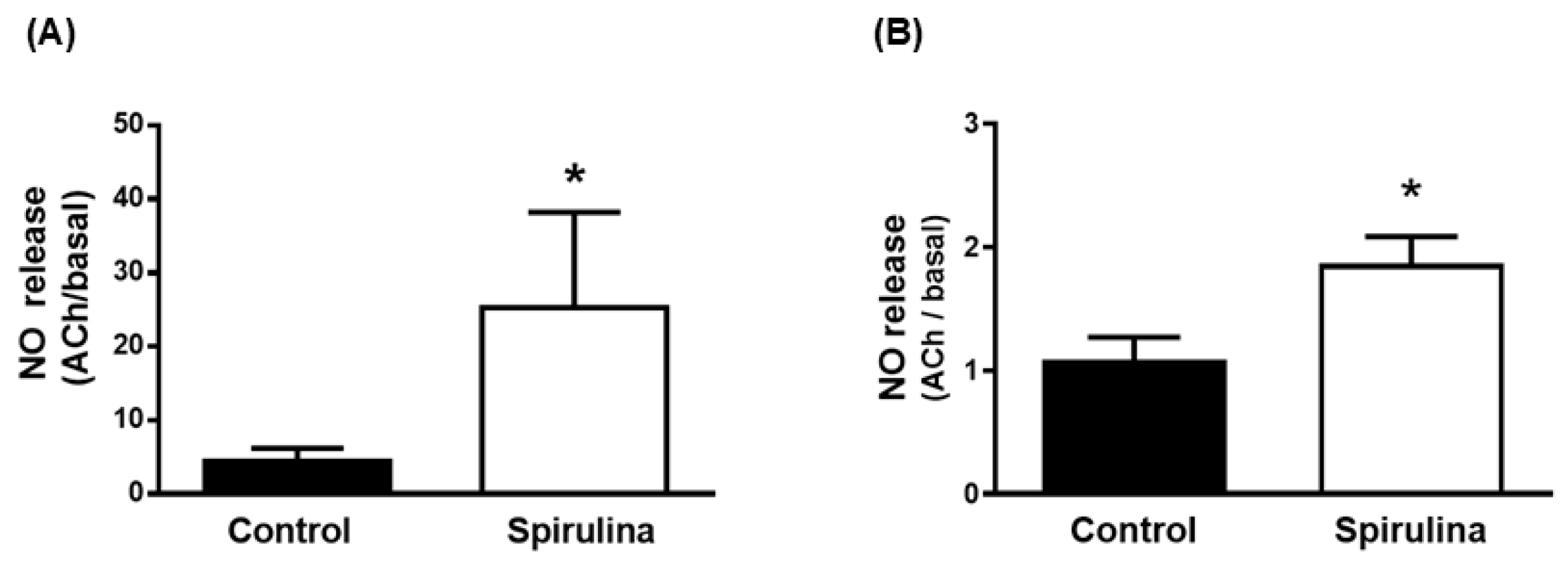
2.4. Effect of Enz-Spir-E on Nitric Oxide Release
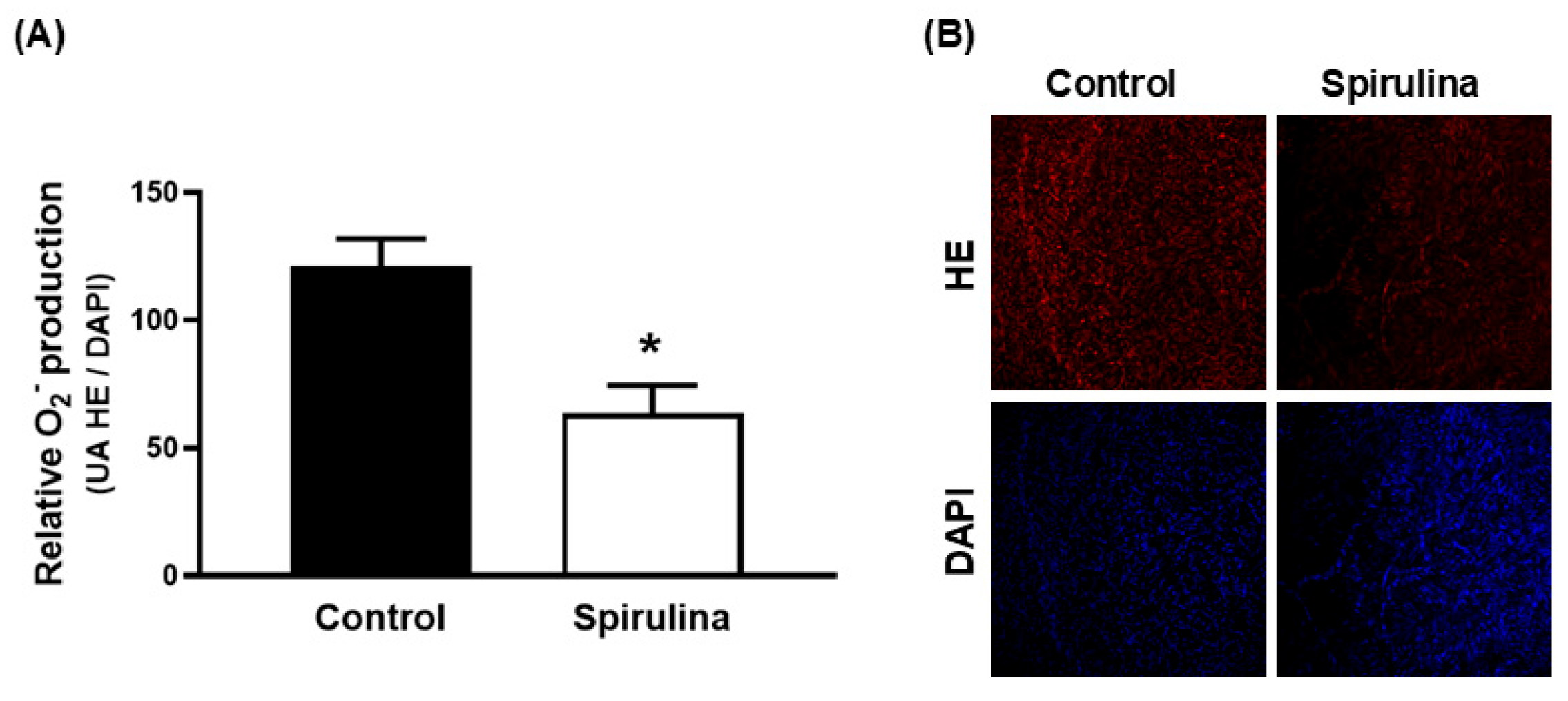
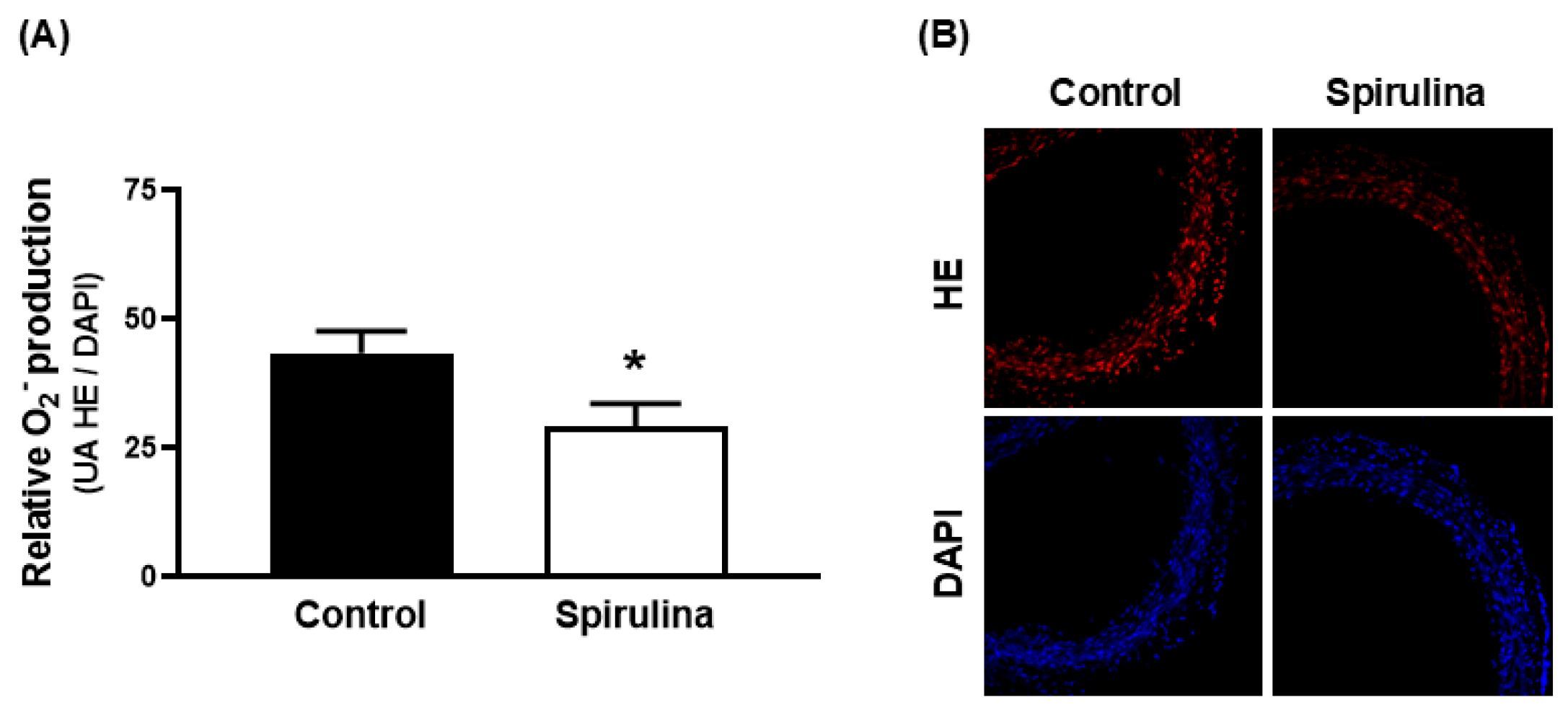
2.5. Effect of Enz-Spir-E on Superoxide Anion Production
3. Discussion
4. Materials and Methods
4.1. Animal Ethical Procedures
4.2. Enzymatic Spirulina Extract
4.2.1. Enzymatic-Assisted Extraction
4.2.2. Elemental Analysis
4.2.3. Total Carbohydrate Content
4.2.4. Amino Acid Composition
4.2.5. Proteomic Analysis
4.2.6. Total Polyphenol Content
4.2.7. Antioxidant Activity
4.3. Vascular Function
4.4. Release of Nitric Oxide
4.5. Detection of Superoxide Anion
4.6. Reagents
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| CO | Carbon monoxide |
| CORM | Carbon monoxide releasing molecules |
| CVD | Cardiovascular diseases |
| DAF-2 | 4,5-diaminofluorescein |
| Enz-Spir-E | Enzymatic Spirulina Extract |
| HE | Hydroethidine |
| KATP | ATP dependent potassium channel |
| KHS | Krebs–Henseleit solution |
| NO | Nitric oxide |
| ROS | Reactive oxygen species |
| SNP | Sodium nitroprusside |
References
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Ageing and Health—World Health Organization (WHO). Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 15 December 2023).
- Brandes, R.P.; Fleming, I.; Busse, R. Endothelial aging. Cardiovasc. Res. 2005, 66, 286–294. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Carter, S.E.; Green, D.J. Arterial structure and function in vascular ageing: Are you as old as your arteries? J. Physiol. 2016, 594, 2275–2284. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Dey, N.; Sellak, H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: From the regulation of tone to gene expression. J. Appl. Physiol. 2001, 91, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.F.; Lemmey, H.A.L.; Borysova, L.; Hiley, C.R.; Dora, K.A.; Garland, C.J. Endothelial Nitric Oxide Suppresses Action-Potential-Like Transient Spikes and Vasospasm in Small Resistance Arteries. Hypertension 2020, 76, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Hossain, E.; Sarkar, O.; Li, Y.; Anand-Srivastava, M.B. Sodium nitroprusside attenuates hyperproliferation of vascular smooth muscle cells from spontaneously hypertensive rats through the inhibition of overexpression of AT1 receptor, cell cycle proteins, and c-Src/growth factor receptor signaling pathways. Can. J. Physiol. Pharmacol. 2020, 98, 35–43. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, P.; Xu, S.F.; Li, Y.Q.; Deng, J.; Yang, D.L. Ginsenoside Re inhibits PDGF-BB-induced VSMC proliferation via the eNOS/NO/cGMP pathway. Biomed. Pharmacother. 2019, 115, 108934. [Google Scholar] [CrossRef]
- Wu, J.; Xia, S.; Kalionis, B.; Wan, W.; Sun, T. The role of oxidative stress and inflammation in cardiovascular aging. Biomed Res. Int. 2014, 2014, 615312. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, K.; Altmann, H.M.; Straub, A.C.; Isenberg, J.S. Nitric oxide: What’s new to NO? Am. J. Physiol. Cell Physiol. 2017, 312, 254–262. [Google Scholar] [CrossRef]
- Vatner, S.F.; Zhang, J.; Oydanich, M.; Berkman, T.; Naftalovich, R.; Vatner, D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020, 64, 101194. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef]
- El Assar, M.; Álvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodríguez-Mañas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Daiber, A.; Di Lisa, F.; Oelze, M.; Kröller-Schön, S.; Steven, S.; Schulz, E.; Münzel, T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2017, 174, 1670–1689. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular smooth muscle cell death, autophagy and senescence in atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Zinovkin, R.A.; Kondratenko, N.D.; Zinovkina, L.A. Does Nrf2 Play a Role of a Master Regulator of Mammalian Aging? Biochemistry 2022, 87, 1465–1476. [Google Scholar] [CrossRef]
- Piacenza, L.; Zeida, A.; Trujillo, M.; Radi, R. The superoxide radical switch in the biology of nitric oxide and peroxynitrite. Physiol. Rev. 2022, 102, 1881–1906. [Google Scholar] [CrossRef]
- Murray, K.O.; Berryman-Maciel, M.; Darvish, S.; Coppock, M.E.; You, Z.; Chonchol, M.; Seals, D.R.; Rossman, M.J. Mitochondrial-targeted antioxidant supplementation for improving age-related vascular dysfunction in humans: A study protocol. Front. Physiol. 2022, 13, 980783. [Google Scholar] [CrossRef]
- Chen, Q.M. Nrf2 for cardiac protection: Pharmacological options against oxidative stress. Trends Pharmacol. Sci. 2021, 42, 729–744. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Hillestad, M.L.; Grande, J.P.; Croatt, A.J.; Barry, M.A.; Farrugia, G.; Katusic, Z.S.; Nath, K.A. Induction and functional significance of the heme oxygenase system in pathological shear stress in vivo. Am. J. Physiol. Heart Circ. Physiol. 2015, 308, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 108073. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Leffler, C.W.; Parfenova, H.; Jaggar, J.H.; Wang, R. Carbon monoxide and hydrogen sulfide: Gaseous messengers in cerebrovascular circulation. J. Appl. Physiol. 2006, 100, 1065–1076. [Google Scholar] [CrossRef]
- Lee, C.W.; Chi, M.C.; Hsu, L.F.; Yang, C.M.; Hsu, T.H.; Chuang, C.C.; Lin, W.N.; Chu, P.M.; Lee, I.T. Carbon monoxide releasing molecule-2 protects against particulate matter-induced lung inflammation by inhibiting TLR2 and 4/ROS/NLRP3 inflammasome activation. Mol. Immunol. 2019, 112, 163–174. [Google Scholar] [CrossRef]
- Lin, C.C.; Hsiao, L.D.; Cho, R.L.; Yang, C.M. Carbon Monoxide Releasing Molecule-2-Upregulated ROS-Dependent Heme Oxygenase-1 Axis Suppresses Lipopolysaccharide-Induced Airway Inflammation. Int. J. Mol. Sci. 2019, 20, 3157. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Villalpando, D.M.; Navarro, R.; Del Campo, L.; Largo, C.; Muñoz, D.; Tabernero, M.; Baeza, R.; Otero, C.; García, H.S.; Ferrer, M. Effect of Dietary Docosahexaenoic Acid Supplementation on the Participation of Vasodilator Factors in Aorta from Orchidectomized Rats. PLoS ONE 2015, 10, e0142039. [Google Scholar] [CrossRef]
- Villalpando, D.M.; Rojas, M.M.; García, H.S.; Ferrer, M. Dietary docosahexaenoic acid supplementation prevents the formation of cholesterol oxidation products in arteries from orchidectomized rats. PLoS ONE 2017, 12, e0185805. [Google Scholar] [CrossRef]
- Rojas, M.M.; Villalpando, D.M.; Alexander-Aguilera, A.; Ferrer, M.; García, H.S. Effect of CLA supplementation on factors related to vascular dysfunction in arteries of orchidectomized rats. Prostaglandins Other Lipid Mediat. 2021, 157, 106586. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.A.A.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef]
- Ghaem Far, Z.; Babajafari, S.; Kojuri, J.; Mohammadi, S.; Nouri, M.; Rostamizadeh, P.; Rahmani, M.H.; Azadian, M.; Ashrafi-Dehkordi, E.; Zareifard, A.; et al. Antihypertensive and antihyperlipemic of spirulina (Arthrospira platensis) sauce on patients with hypertension: A randomized triple-blind placebo-controlled clinical trial. Phytother. Res. 2021, 35, 6181–6190. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Villalpando, D.M.; Verdasco-Martín, C.M.; Plaza, I.; Gómez-Rivas, J.; R de Bethencourt, F.; Villarroel, M.; García, J.L.; Otero, C.; Ferrer, M. Beneficial Effects of Spirulina Aqueous Extract on Vasodilator Function of Arteries from Hypertensive Rats. Int. J. Vasc. Med. 2020, 2020, 6657077. [Google Scholar] [CrossRef] [PubMed]
- Majewski, M.; Klett-Mingo, M.; Verdasco-Martín, C.M.; Otero, C.; Ferrer, M. Spirulina extract improves age-induced vascular dysfunction. Pharm. Biol. 2022, 60, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Verdasco-Martín, C.M.; Echevarrieta, L.; Otero, C. Advantageous Preparation of Digested Proteic Extracts from Spirulina platensis Biomass. Catalysts 2019, 9, 145. [Google Scholar] [CrossRef]
- Verdasco-Martín, C.M.; Díaz-Lozano, A.; Otero, C. Advantageous enzyme selective extraction process of essential spirulina oil. Catal. Today 2020, 346, 121–131. [Google Scholar] [CrossRef]
- Otero, C.; Verdasco-Martín, C.M. Preparation and Characterization of a Multicomponent Arthrospira platensis Biomass Hydrolysate with Superior Anti-Hypertensive, Anti-Hyperlipidemic and Antioxidant Activities via Selective Proteolysis. Mar. Drugs 2023, 21, 255. [Google Scholar] [CrossRef]
- Ferrer, M.; Balfagón, G. Aging alters neuronal nitric oxide release from rat mesenteric arteries: Role of presynaptic beta-adrenoceptors. Clin. Sci. 2001, 101, 321–328. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- van der Rijt, S.; Molenaars, M.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Integrating the Hallmarks of Aging Throughout the Tree of Life: A Focus on Mitochondrial Dysfunction. Front. Cell Dev. Biol. 2020, 8, 594416. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Hallmarks of cancer and hallmarks of aging. Aging 2022, 14, 4176–4187. [Google Scholar] [CrossRef]
- Gkaliagkousi, E.; Lazaridis, A.; Dogan, S.; Fraenkel, E.; Tuna, B.G.; Mozos, I.; Vukicevic, M.; Yalcin, O.; Gopcevic, K. Theories and Molecular Basis of Vascular Aging: A Review of the Literature from VascAgeNet Group on Pathophysiological Mechanisms of Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8672. [Google Scholar] [CrossRef]
- Sharma, R.; Diwan, B.; Sharma, A.; Witkowski, J.M. Emerging cellular senescence-centric understanding of immunological aging and its potential modulation through dietary bioactive components. Biogerontology 2022, 23, 699–729. [Google Scholar] [CrossRef]
- de Freitas Brito, A.; Silva, A.S.; de Souza, A.A.; Ferreira, P.B.; de Souza, I.L.L.; da Cunha Araujo, L.C.; da Silva, F.G.; de Souza Sampaio, R.; da Conceição Correia, S.M.; Tavares, R.L.; et al. Supplementation with Spirulina platensis Modulates Aortic Vascular Reactivity through Nitric Oxide and Antioxidant Activity. Oxidative Med. Cell. Longev. 2019, 2019, 7838149. [Google Scholar] [CrossRef]
- Vanhoutte, P.M. Endothelial control of vasomotor function: From health to coronary disease. Circ. J. 2003, 67, 572–575. [Google Scholar] [CrossRef]
- Long, C.; Liu, H.; Zhan, W.; Chen, L.; Yu, Z.; Tian, S.; Xiang, Y.; Chen, S.; Tian, X.L. Chronological attenuation of NPRA/PKG/AMPK signaling promotes vascular aging and elevates blood pressure. Aging Cell 2022, 21, e13699. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, M.; Li, Z.; Liu, J.; Li, Y.; Yang, Z.; Wang, X.; Huang, X.; Yu, B.; Hou, J.; et al. A landscape of Long non-coding RNAs reveals the leading transcriptome alterations in murine aorta during aging. Genomics 2023, 115, 110573. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Tejera, N.; Marín, J.; Balfagón, G. Effect of age on the vasorelaxation elicited by cromakalim. Role of K+ channels and cyclic GMP. Life Sci. 1998, 63, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ahmet, I.; Griess, B.; Tweedie, D.; Greig, N.H.; Mattson, M.P. Age-related impairment of cerebral blood flow response to KATP channel opener in Alzheimer’s disease mice with presenilin-1 mutation. J. Cereb. Blood Flow Metab. 2021, 41, 1579–1591. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, J.; Jiang, C. Differential expression of Kir6.1 and SUR2B mRNAs in the vasculature of various tissues in rats. J. Membr. Biol. 2003, 196, 61–69. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Stressler, T.; Fischer, L. Flavourzyme, an Enzyme Preparation with Industrial Relevance: Automated Nine-Step Purification and Partial Characterization of Eight Enzymes. J. Agric. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef]
- Zuluaga, J.; Rodríguez, N.; Rivas-Ramirez, I.; de la Fuente, V.; Rufo, L.; Amils, R. An improved semiquantitative method for elemental analysis of plants using inductive coupled plasma mass spectrometry. Biol. Trace Elem. Res. 2011, 144, 1302–1317. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Moore, S.; Spackman, D.H.; Stein, W.H. Automatic recording apparatus for use in the chromatography of amino acids. Fed. Proc. 1958, 17, 1107–1115. [Google Scholar] [PubMed]
- Perkins, D.N.; Pappin, D.J.; Creasy, D.M.; Cottrell, J.S. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis 1999, 20, 3551–3567. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Nielsen, K.C.; Owman, C. Contractile response and amine receptor mechanisms in isolated middle cerebral artery of the cat. Brain Res. 1971, 27, 33–42. [Google Scholar] [CrossRef]
- Isidoro-García, L.; Villalpando, D.M.; Ferrer, M. Vasomotor action of androgens in the mesenteric artery of hypertensive rats. Role of perivascular innervation. PLoS ONE 2021, 16, e0246254. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.C.; Balfagón, G.; Minoves, N.; Blanco-Rivero, J.; Ferrer, M. Androgen deprivation increases neuronal nitric oxide metabolism and its vasodilator effect in rat mesenteric arteries. Nitric Oxide 2005, 12, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Sagredo, A.; del Campo, L.; Martorell, A.; Navarro, R.; Martín, M.C.; Blanco-Rivero, J.; Ferrer, M. Ovariectomy increases the participation of hyperpolarizing mechanisms in the relaxation of rat aorta. PLoS ONE 2013, 8, e73474. [Google Scholar] [CrossRef] [PubMed][Green Version]
| % w/w | µmol/g Extract | |
|---|---|---|
| Asp | 4.65 ± 1.05 | 404 ± 9 |
| Thr | 2.56 ± 0.62 | 222 ± 5 |
| Ser | 2.41 ± 0.79 | 209 ± 7 |
| Glu | 6.81 ± 1.23 | 591 ± 11 |
| Pro | 2.07 ± 0.12 | 180 ± 1 |
| Gly | 1.99 ± 0.31 | 173 ± 3 |
| Ala | 3.70 ± 0.43 | 322 ± 4 |
| Cys | 0.34 ± 0.04 | 30 ± 4 |
| Val | 2.80 ± 0.33 | 243 ± 3 |
| Met | 1.23 ± 0.15 | 107 ± 1 |
| Ile | 2.52 ± 0.49 | 219 ± 4 |
| Leu | 4.54 ± 0.74 | 394 ± 6 |
| Tyr | 1.71 ± 0.05 | 178 ± 0 |
| Phe | 2.40 ± 0.49 | 209 ± 4 |
| His | 0.66 ± 0.10 | 57 ± 1 |
| Lys | 2.28 ± 0.11 | 198 ± 1 |
| Arg | 2.31 ± 0.51 | 201 ± 4 |
| Total | 45.0 ± 7.1 | 3907 ± 69 |
| RT (min) | Peptide Sequence (Seq. ID:) | Mr (Da, Calc.) | M*exp (Da) | MASCOT IONS SCORE ** | Data Base |
|---|---|---|---|---|---|
| 42.83 | MKKIEAIIRPF (SEQ ID NO: 1) | 1344.8 | 1344.7 | 46 | gi|291569590, nitrogen regulatory protein P-II [Arthrospira platensis NIES-39] |
| 51.60 | LPPL (SEQ ID NO: 2) | 438.3 | 438.3 | N.P. | Not assignable to a concrete protein |
| 52.48 | ALAVGIGSIGPGLGQGQ (SEQ ID NO: 3) | 1493.8 | 1493.7 | 90 | gi|146186464, AtpH [Arthrospira platensis HN01]; gi|355333248, Chain B Microscopic Rotary Mechanism Of Ion Translocation In The Fo Complex Of Atp Synthases; gi|310689674, Chain E Microscopic Rotary Mechanism Of Ion Translocation In The Fo Complex Of Atp Synthases; gi|375325268, ATP synthase subunit C membrane-bound F0 sector; DCCD-binding [Arthrospira sp. PCC 8005] |
| 53.79 | TTAASVIAAAL (SEQ ID NO: 4) | 987.6 | 987.4 | 40 | gi|291566395, ATP synthase c chain [Arthrospira platensis NIES-39] |
| 54.56 | DFPGDDIPIVS (SEQ ID NO: 5) | 1173.6 | 1173.5 | 40 | gi|119213EFTU_ARTPT, RecName: full = elongation factor Tu; short = EF-Tu |
| 54.90 | LELL (SEQ ID NO: 6) | 486.3 | 486.3 | N.P. | Not assignable to a concrete protein |
| 48.10 | WKLLP (SEQ ID NO: 7) | 655.4 | de novo *** | ||
| 48.97 | CHLLLSM (+15.99) (SEQ ID NO: 8) | 831.4 | de novo *** |
| Emax | pEC50 | AUC | ||||
|---|---|---|---|---|---|---|
| Control | Enz-Spir-E | Control | Enz-Spir-E | Control | Enz-Spir-E | |
| Aorta | ||||||
| ACh | 71.8 ± 5.5 | 96.8 ± 3.6 * | –7.2 ± 0.1 | –8.5 ± 0.2 * (7/11) | (6/11) 69.7 ± 21.7 | 337.4 ± 30.0 * |
| SNP | 97.5 ± 5.5 | 106.8 ± 4.9 | –7.9 ± 0.1 | –8.2 ± 0.2 (6/6) | (6/8) 291.4 ± 20.9 | 340.6 ± 20.9 * |
| CORM | 44.0 ± 8.8 | 58.7 ± 19.7 | –5.0 ± 0.3 | –5.6 ± 1.2 (4/4) | (5/5) 48.2 ± 7.8 | 90.4 ± 23.2 * |
| CK | 93.0 ± 3.1 | 100.0 ± 5.9 | –6.6 ± 0.4 | –6.0 ± 0.3 * (5/6) | (5/7) 211.3 ± 24.1 | 185.8 ± 41.3 |
| Mesenteric Artery | ||||||
| ACh | 99.7 ± 6 | 120.3 ± 5.3 * | –7.7 ± 0.1 | –10.4 ± 0.2 * (7/11) | (6/7) 297.1 ± 28.4 | 633.4 ± 44.3 * |
| SNP | 108.4 ± 4.9 | 120.2 ± 8.7 | –8.0 ± 0.2 | –8.0 ± 0.5 (6/8) | (4/4) 345.0 ± 28.1 | 446.4 ± 50.5 * |
| CORM | 45.3 ± 5.9 | 44.7 ± 6.8 | –4.7 ± 0.5 | –5.3 ± 0.4 * (5/6) | (4/4) 49.1 ± 10.3 | 60.3 ± 10.3 * |
| CK | 101.5 ± 3.2 | 121.7 ± 5.3 | –6.5 ± 0.1 | –6.1 ± 0.3 (4/4) | (5,6) 194.8 ± 19.8 | 338.0 ± 37.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewski, M.S.; Klett-Mingo, M.; Verdasco-Martín, C.M.; Otero, C.; Ferrer, M. Enzymatic Spirulina Extract Enhances the Vasodilation in Aorta and Mesenteric Arteries of Aged Rats. Mar. Drugs 2025, 23, 395. https://doi.org/10.3390/md23100395
Majewski MS, Klett-Mingo M, Verdasco-Martín CM, Otero C, Ferrer M. Enzymatic Spirulina Extract Enhances the Vasodilation in Aorta and Mesenteric Arteries of Aged Rats. Marine Drugs. 2025; 23(10):395. https://doi.org/10.3390/md23100395
Chicago/Turabian StyleMajewski, Michal S., Mercedes Klett-Mingo, Carlos M. Verdasco-Martín, Cristina Otero, and Mercedes Ferrer. 2025. "Enzymatic Spirulina Extract Enhances the Vasodilation in Aorta and Mesenteric Arteries of Aged Rats" Marine Drugs 23, no. 10: 395. https://doi.org/10.3390/md23100395
APA StyleMajewski, M. S., Klett-Mingo, M., Verdasco-Martín, C. M., Otero, C., & Ferrer, M. (2025). Enzymatic Spirulina Extract Enhances the Vasodilation in Aorta and Mesenteric Arteries of Aged Rats. Marine Drugs, 23(10), 395. https://doi.org/10.3390/md23100395







