Exploring the Pharmacological Potential of Carrageenan Disaccharides as Antitumor Agents: An In Silico Approach
Abstract
1. Introduction
2. Results
2.1. ADMET Profile
2.1.1. Absorption
2.1.2. Distribution
2.1.3. Metabolism and Excretion
2.1.4. Toxicity
2.2. Drug-Likeness Physicochemical Property Analysis
2.3. Identification of Cancer-Related Molecular Targets
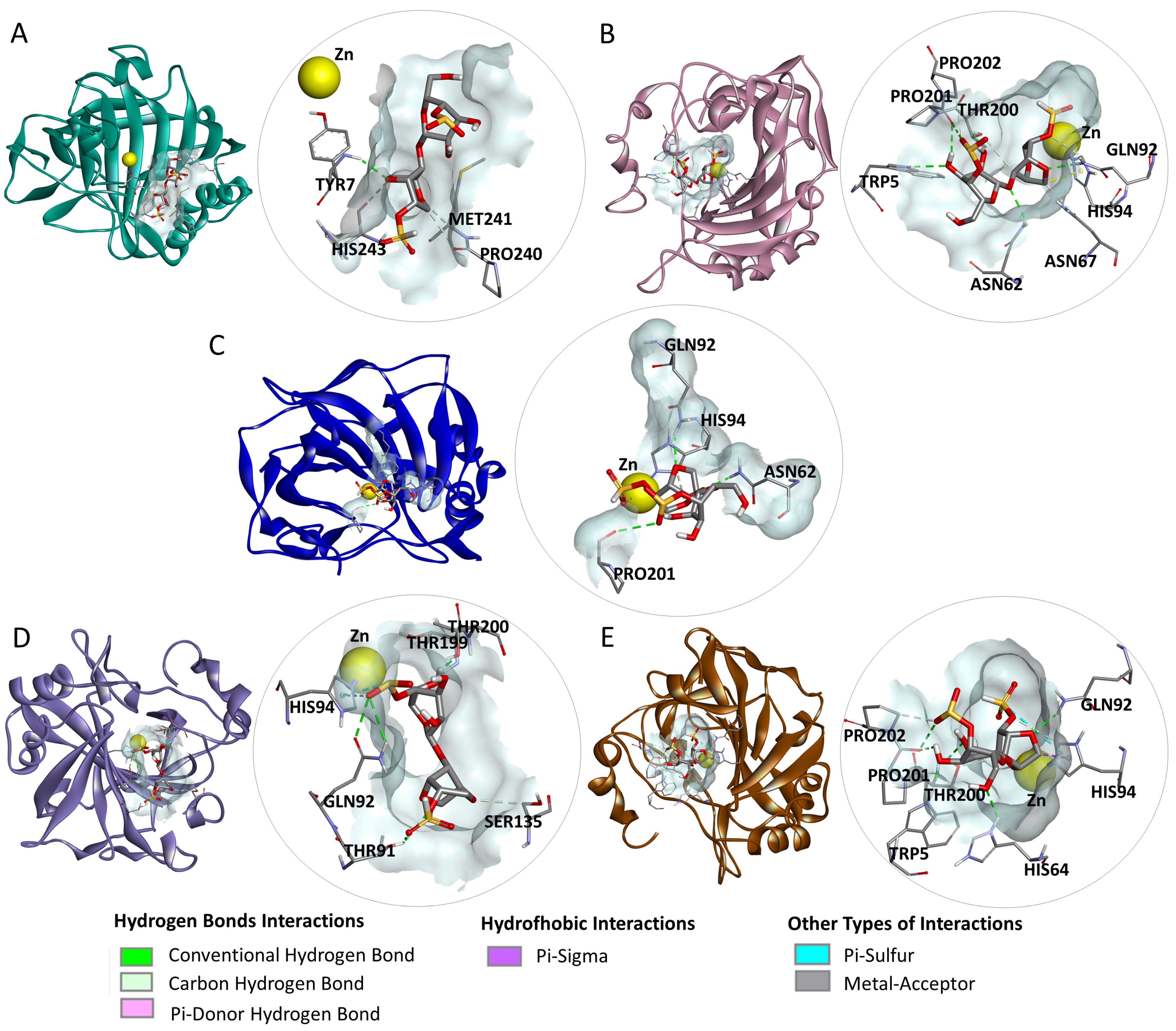
2.4. Molecular Docking
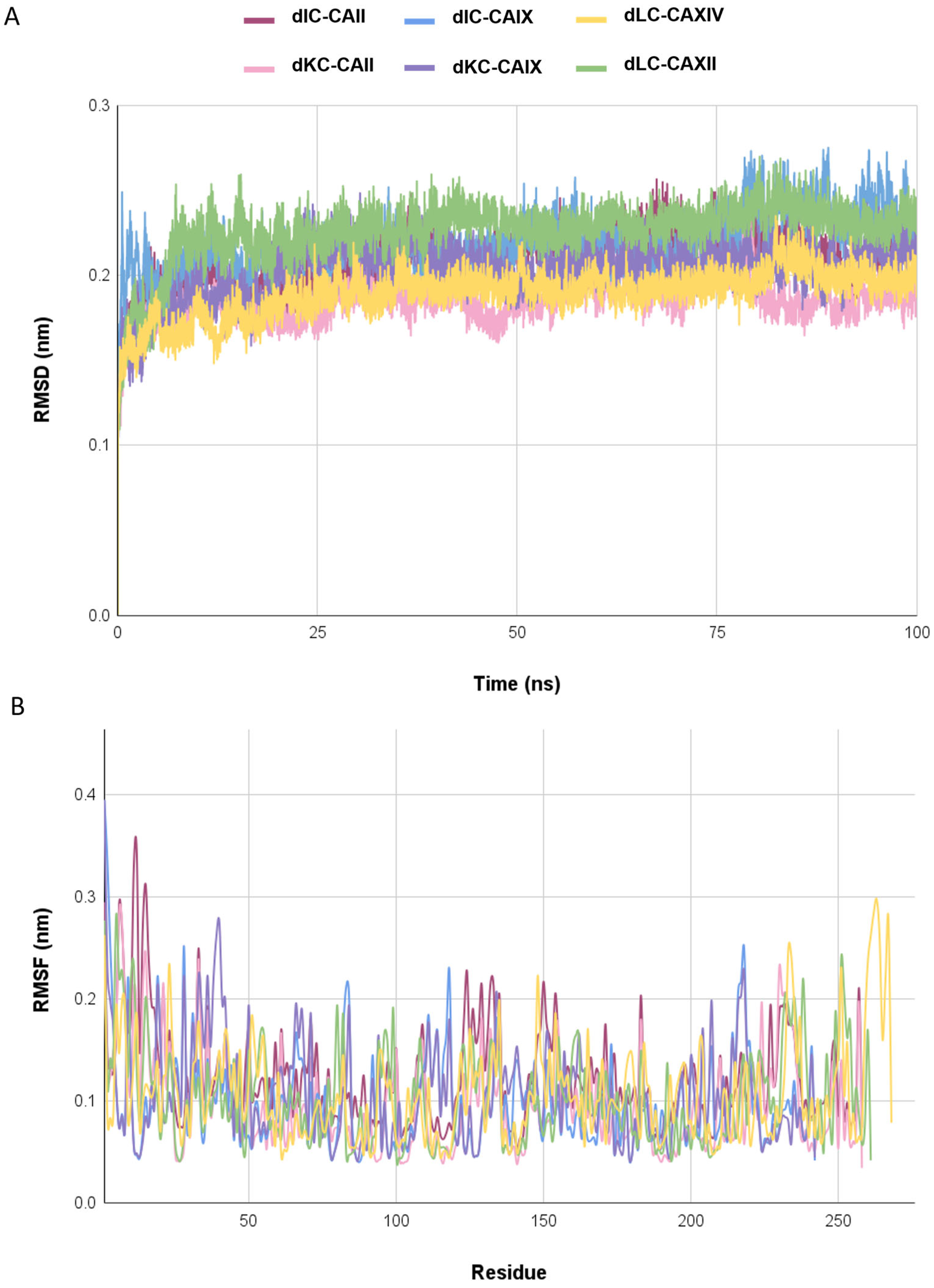
2.5. Molecular Dynamics Simulations
3. Discussion
4. Materials and Methods
4.1. Acquisition of the Disaccharide Structures of Iota, Kappa, and Lambda Carrageenans
4.2. ADMET Assessment
4.3. Drug-Likeness Physicochemical Property Analysis
4.4. Identification of Cancer-Related Molecular Targets
4.5. Molecular Docking
4.6. Molecular Dynamics Simulations
4.7. Language Editing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer#:~:text=The%20problem-,Cancer%20is%20a%20leading%20cause%20of%20death%20worldwide%2C%20accounting%20for,lung%20(2.21%20million%20cases)%3B (accessed on 5 July 2023).
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Salem, H.; Attiya, G.; El-Fishawy, N. Classification of human cancer diseases by gene expression profiles. Appl. Soft Comput. 2017, 50, 124–134. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Dupont, C.A.; Riegel, K.; Pompaiah, M.; Juhl, H.; Rajalingam, K. Druggable genome and precision medicine in cancer: Current challenges. FEBS J. 2021, 288, 6142–6158. [Google Scholar] [CrossRef] [PubMed]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar] [CrossRef]
- Charlton, P.; Spicer, J. Targeted therapy in cancer. Medicine 2016, 44, 34–38. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-cancer activity of porphyran and carrageenan from red seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Alsahli, M.A.; Khan, M.A.; Khan, A.A.; Rahmani, A.H. Natural products: Implication in cancer prevention and treatment through modulating various biological activities. Anti-Cancer Agents Med. Chem. 2020, 20, 2025–2040. [Google Scholar] [CrossRef] [PubMed]
- Assef, A.N.B.; da Costa, B.B.; Moreira, T.A.; do Carmo, L.D.; de Souza, T.F.G.; Alencar, N.M.N.; Alves, A.P.N.N.; Cinelli, L.P.; Wilke, D.V. Antitumor and immunostimulating sulfated polysaccharides from brown algae Dictyota caribaea. Carbohydr. Polym. Technol. Appl. 2021, 2, 100142. [Google Scholar] [CrossRef]
- da Silva Chagas, F.D.; Lima, G.C.; Dos Santos, V.I.N.; Costa, L.E.; de Sousa, W.M.; Sombra, V.G.C.; de Araújo, D.F.; Barros, F.C.N.; Marinho-Soriano, E.; de Andrade Feitosa, J.P.; et al. Sulfated polysaccharide from the red algae Gelidiella acerosa: Anticoagulant, antiplatelet and antithrombotic effects. Int. J. Biol. Macromol. 2020, 159, 415–421. [Google Scholar] [CrossRef]
- Figueroa, F.A.; Abdala-Díaz, R.T.; Pérez, C.; Casas-Arrojo, V.; Nesic, A.; Tapia, C.; Durán, C.; Valdes, O.; Parra, C.; Bravo-Arrepol, G.; et al. Sulfated polysaccharide extracted from the green algae Codium bernabei: Physicochemical characterization and antioxidant, anticoagulant and antitumor activity. Mar. Drugs 2022, 20, 458. [Google Scholar] [CrossRef]
- Panggabean, J.A.; Adiguna, S.P.; Rahmawati, S.I.; Ahmadi, P.; Zainuddin, E.N.; Bayu, A.; Putra, M.Y. Antiviral activities of algal-based sulfated polysaccharides. Molecules 2022, 27, 1178. [Google Scholar] [CrossRef]
- Qin, L.; Xu, H.; He, Y.; Liang, C.; Wang, K.; Cao, J.; Qu, C.; Miao, J. Purification, chemical characterization and immunomodulatory activity of a sulfated polysaccharide from marine brown algae Durvillaea antarctica. Mar. Drugs 2022, 20, 223. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.W.S.; Cerqueira, M.A.; Bourbon, A.I.; Pinheiro, A.C.; Martins, J.T.; Teixeira, J.A.; Coimbra, M.A.; Vicente, A.A. Chemical characterization and antioxidant activity of sulfated polysaccharide from the red seaweed Gracilaria birdiae. Food Hydrocoll. 2012, 27, 287–292. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; da Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Khotimchenko, M.; Tiasto, V.; Kalitnik, A.; Begun, M.; Khotimchenko, R.; Leonteva, E.; Bryukhovetskiy, I.; Khotimchenko, Y. Antitumor potential of carrageenans from marine red algae. Carbohydr. Polym. 2020, 246, 116568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sun, Y.P.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Groult, H.; Cousin, R.; Chot-Plassot, C.; Maura, M.; Bridiau, N.; Piot, J.-M.; Maugard, T.; Fruitier-Arnaudin, I. λ-Carrageenan oligosaccharides of distinct anti-heparanase and anticoagulant activities inhibit MDA-MB-231 breast cancer cell migration. Mar. Drugs 2019, 17, 140. [Google Scholar] [CrossRef]
- Calvo, G.H.; Cosenza, V.A.; Sáenz, D.A.; Navarro, D.A.; Stortz, C.A.; Céspedes, M.A.; Mamone, L.A.; Casas, A.G.; Di Venosa, G.M. Disaccharides obtained from carrageenans as potential antitumor agents. Sci. Rep. 2019, 9, 6654. [Google Scholar] [CrossRef]
- Jin, Z.; Han, Y.-X.; Han, X.-R. Degraded iota-carrageenan can induce apoptosis in human osteosarcoma cells via the Wnt/β-catenin signaling pathway. Nutr. Cancer 2013, 65, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Baghel, R.S.; Choudhary, B.; Pandey, S.; Pathak, P.K.; Patel, M.K.; Mishra, A. Rehashing Our Insight of Seaweeds as a Potential Source of Foods, Nutraceuticals, and Pharmaceuticals. Foods. 2023, 12, 3642. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Andricopulo, A.D. ADMET modeling approaches in drug discovery. Drug Discov. Today 2019, 24, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Agoni, C.; Olotu, F.A.; Ramharack, P.; Soliman, M.E. Druggability and drug-likeness concepts in drug design: Are biomodelling and predictive tools having their say? J. Mol. Model. 2020, 26, 120. [Google Scholar] [CrossRef]
- Agamah, F.E.; Mazandu, G.K.; Hassan, R.; Bope, C.D.; Thomford, N.E.; Ghansah, A.; Chimusa, E.R. Computational/in silico methods in drug target and lead prediction. Brief. Bioinform. 2020, 21, 1663–1675. [Google Scholar] [CrossRef]
- Xia, X. Bioinformatics and drug discovery. Curr. Top. Med. Chem. 2017, 17, 1709–1726. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H. Solution stability-plasma, gastrointestinal, bioassay. Curr. Drug Metab. 2008, 9, 860–868. [Google Scholar] [CrossRef]
- Ntie-Kang, F.; Lifongo, L.L.; Mbah, J.A.; Owono Owono, L.C.; Megnassan, E.; Mbaze, L.M.; Judson, P.N.; Sippl, W.; Efange, S.M.N. In silico drug metabolism and pharmacokinetic profiles of natural products from medicinal plants in the Congo basin. Silico Pharmacol. 2013, 1, 12. [Google Scholar] [CrossRef]
- Pantaleão, S.Q.; Fernandes, P.O.; Gonçalves, J.E.; Maltarollo, V.G.; Honorio, K.M. Recent advances in the prediction of pharmacokinetics properties in drug design studies: A review. ChemMedChem 2022, 17, e202100542. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, S.; Sahoo, S.K. In silico ADMET and molecular docking study on searching potential inhibitors from limonoids and triterpenoids for COVID-19. Comput. Biol. Med. 2020, 124, 103936. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. Int. Sch. Res. Not. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Elmeliegy, M.; Vourvahis, M.; Guo, C.; Wang, D.D. Effect of P-glycoprotein (P-gp) inducers on exposure of P-gp substrates: Review of clinical drug-drug interaction studies. Clin. Pharmacokinet. 2020, 59, 699–714. [Google Scholar] [CrossRef]
- Ayrton, A.; Morgan, P. Role of transport proteins in drug absorption, distribution and excretion. Xenobiotica 2001, 31, 469–497. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S.; Vivès, R.R.; Schaefer, L.; Götte, M.; Merline, R.; Passi, A.; Heldin, P.; Magalhães, A.; Reis, C.A.; Skandalis, S.S.; et al. A biological guide to glycosaminoglycans: Current perspectives and pending questions. FEBS J. 2024, 291, 3331–3366. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Duan, Z.; Wang, X. An update on circumventing multidrug resistance in cancer by targeting P-glycoprotein. Curr. Cancer Drug Targets 2018, 18, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Gombar, V.K.; Hall, S.D. Quantitative structure–activity relationship models of clinical pharmacokinetics: Clearance and volume of distribution. J. Chem. Inf. Model. 2013, 53, 948–957. [Google Scholar] [CrossRef]
- Song, Y.; Li, C.; Liu, G.; Liu, R.; Chen, Y.; Li, W.; Cao, Z.; Zhao, B.; Lu, C.; Liu, Y. Drug-metabolizing cytochrome P450 enzymes have multifarious influences on treatment outcomes. Clin. Pharmacokinet. 2021, 60, 585–601. [Google Scholar] [CrossRef]
- Kaur, G.; Gupta, S.K.; Singh, P.; Ali, V.; Kumar, V.; Verma, M. Drug-metabolizing enzymes: Role in drug resistance in cancer. Clin. Transl. Oncol. 2020, 22, 1667–1680. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Price, A.M.Y. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar] [PubMed]
- Price, G.; Patel, D.A. Drug Bioavailab.; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- McCann, J.; Ames, B.N. Detection of carcinogens as mutagens in the Salmonella/microsome test: Assay of 300 chemicals: Discussion. Proc. Natl. Acad. Sci. USA 1976, 73, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Matthews, E.J.; Kruhlak, N.L.; Benz, R.D.; Contrera, J.F. Assessment of the health effects of chemicals in humans: I. QSAR estimation of the maximum recommended therapeutic dose (MRTD) and no effect level (NOEL) of organic chemicals based on clinical trial data. Curr. Drug Discov. Technol. 2004, 1, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Nemati, M.; Hosseinzadeh, Z.; Nemati, F.; Ebrahimi, B. Impact of antipsychotics and antidepressants drugs on long QT syndrome induction related to hERG channel dysfunction: A systematic review. Biochem. Biophys. Res. Commun. 2023, 681, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Belal, A. Drug likeness, targets, molecular docking and ADMET studies for some indolizine derivatives. Pharmazie 2018, 73, 635–642. [Google Scholar] [CrossRef]
- Ariffin, S.H.Z.; Yeen, W.W.; Abidin, I.Z.Z.; Wahab, R.M.A.; Ariffin, Z.Z.; Senafi, S. Cytotoxicity effect of degraded and undegraded kappa and iota carrageenan in human intestine and liver cell lines. BMC Complement. Altern. Med. 2014, 14, 508. [Google Scholar] [CrossRef]
- McKim, J.M., Jr.; Baas, H.; Rice, G.P.; Willoughby Sr, J.A.; Weiner, M.L.; Blakemore, W. Effects of carrageenan on cell permeability, cytotoxicity, and cytokine gene expression in human intestinal and hepatic cell lines. Food Chem. Toxicol. 2016, 96, 1–10. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Aungst, B.J. Optimizing oral bioavailability in drug discovery: An overview of design and testing strategies and formulation options. J. Pharm. Sci. 2017, 106, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.V.; Raub, T.J.; Blanco, M.J. How hydrogen bonds impact P-glycoprotein transport and permeability. Bioorg. Med. Chem. Lett. 2012, 22, 6540–6548. [Google Scholar] [CrossRef]
- Waring, M.J. Defining optimum lipophilicity and molecular weight ranges for drug candidates—Molecular weight dependent lower log D limits based on permeability. Bioorg. Med. Chem. Lett. 2009, 19, 2844–2851. [Google Scholar] [CrossRef] [PubMed]
- Keilin, D.; Mann, T. Carbonic anhydrase. Nature 1939, 144, 442–443. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Hompland, T.; Fjeldbo, C.S.; Lyng, H. Tumor hypoxia as a barrier in cancer therapy: Why levels matter. Cancers 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.; Meehan, J.; Gray, M.E.; Murray, A.F.; Argyle, D.; Kunkler, I.; Langdon, S.P. The impact of tumour pH on cancer progression; strategies for clinical intervention. Explor. Target. Anti-Tumour Ther. 2020, 1, 71–100. [Google Scholar] [CrossRef] [PubMed]
- Mboge, M.Y.; Mahon, B.P.; McKenna, R.; Frost, S.C. Carbonic anhydrases: Role in pH control and cancer. Metabolites 2018, 8, 19. [Google Scholar] [CrossRef]
- Gerni, S.; Ozturk, C.; Almaz, Z.; Bayrak, C.; Tan, A. Celecoxib derivatives containing pyrazole linked-sulfonamide moiety: Carbonic anhydrase I–II and acetylcholinesterase inhibition profiles, molecular docking studies. ChemistrySelect 2023, 8, e202302088. [Google Scholar] [CrossRef]
- Giovannuzzi, S.; Nikitjuka, A.; Resende, B.R.P.; Smietana, M.; Nocentini, A.; Supuran, C.T.; Winum, J.Y. Boron-containing carbonic anhydrases inhibitors. Bioorg. Chem. 2023, 143, 106976. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Mojzych, M.; Marciniak, B.; Drozda, R.; Kontek, R. Targeting carbonic anhydrase IX and XII isoforms with small molecule inhibitors and monoclonal antibodies. J. Enzym. Inhib. Med. Chem. 2022, 37, 1278–1298. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Marques, S.; Vullo, D.; Innocenti, A.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Inhibition of cytosolic/tumor-associated isoforms I, II, and IX with iminodiacetic carboxylates/hydroxamates also incorporating benzenesulfonamide moieties. Bioorg. Med. Chem. Lett. 2007, 17, 1538–1543. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Hydroxyurea is a carbonic anhydrase inhibitor. Bioorg. Med. Chem. 2003, 11, 2241–2246. [Google Scholar] [CrossRef]
- Uslu, A.G.; Maz, T.G.; Nocentini, A.; Banoglu, E.; Supuran, C.T.; Çalışkan, B. Benzimidazole derivatives as potent and isoform selective tumor-associated carbonic anhydrase IX/XII inhibitors. Bioorg. Chem. 2020, 95, 103544. [Google Scholar] [CrossRef] [PubMed]
- Winum, J.Y.; Maresca, A.; Carta, F.; Scozzafava, A.; Supuran, C.T. Polypharmacology of sulfonamides: Pazopanib, a multitargeted receptor tyrosine kinase inhibitor in clinical use, potently inhibits several mammalian carbonic anhydrases. Chem. Commun. 2012, 48, 8177–8179. [Google Scholar] [CrossRef]
- Mu, Y.; Meng, Q.; Fan, X.; Xi, S.; Xiong, Z.; Wang, Y.; Huang, Y.; Liu, Z. Identification of the inhibition mechanism of carbonic anhydrase II by fructooligosaccharides. Front. Mol. Biosci. 2024, 11, 1398603. [Google Scholar] [CrossRef] [PubMed]
- Mollica, A.; Costante, R.; Akdemir, A.; Carradori, S.; Stefanucci, A.; Macedonio, G.; Ceruso, M.; Supuran, C.T. Exploring new Probenecid-based carbonic anhydrase inhibitors: Synthesis, biological evaluation and docking studies. Bioorg. Med. Chem. 2015, 23, 5311–5318. [Google Scholar] [CrossRef]
- Krebs, J.F.; Fierke, C.A. Determinants of catalytic activity and stability of carbonic anhydrase II as revealed by random mutagenesis. J. Biol. Chem. 1993, 268, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, S.; Maresca, A.; Alper, M.; Kockar, F.; Işik, S.; Sinan, S.; Ozensoy, O.; Arslan, O.; Supuran, C.T. Mutation of active site residues Asn67 to Ile, Gln92 to Val and Leu204 to Ser in human carbonic anhydrase II: Influences on the catalytic activity and affinity for inhibitors. Bioorg. Med. Chem. 2012, 20, 2208–2213. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Patra, S.; Saha, P.; Chaudhuri, S.; Ghosh, A. Cancer drug development of carbonic anhydrase inhibitors beyond the active site. Molecules 2018, 23, 1045. [Google Scholar] [CrossRef]
- John, A.; Sivashanmugam, M.; Umashankar, V.; Natarajan, S.K. Virtual screening, molecular dynamics, and binding free energy calculations on human carbonic anhydrase IX catalytic domain for deciphering potential leads. J. Biomol. Struct. Dyn. 2017, 35, 2155–2168. [Google Scholar] [CrossRef] [PubMed]
- Khedraoui, M.; Abchir, O.; Nour, H.; Yamari, I.; Errougui, A.; Samadi, A.; Chtita, S. An in silico study based on QSAR and molecular docking and molecular dynamics simulation for the discovery of novel potent inhibitor against AChE. Pharmaceuticals 2024, 17, 830. [Google Scholar] [CrossRef]
- Moulishankar, A.; Sundarrajan, T. QSAR modeling, molecular docking, dynamic simulation and ADMET study of novel tetrahydronaphthalene derivatives as potent antitubercular agents. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 111. [Google Scholar] [CrossRef]
- Shoaib, T.H.; Abdelmoniem, N.; Mukhtar, R.M.; Alqhtani, A.T.; Alalawi, A.L.; Alawaji, R.; Althubyani, M.S.; Mohamed, S.G.A.; Mohamed, G.A.; Ibrahim, S.R.M.; et al. Molecular docking and molecular dynamics studies reveal the anticancer potential of medicinal-plant-derived lignans as MDM2-P53 interaction inhibitors. Molecules 2023, 28, 6665. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ali, A.; Warsi, M.H.; Rahman, M.A.; Ahsan, M.J.; Azam, F. Toward the discovery of a novel class of leads for high altitude disorders by virtual screening and molecular dynamics approaches targeting carbonic anhydrase. Int. J. Mol. Sci. 2022, 23, 5054. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; A Shoemaker, B.; A Thiessen, P.; Yu, B.; et al. PubChem 2023 update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.; Molina, M.; Arnold, S.; Woodward, A.; Na, J.Y.; Nuckels, E.; Wang, Y. Metabolite Fragmentation Visualization. J. Syst. Cybern. Inform. 2022, 20, 138–147. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Awale, M.; Reymond, J.L. Polypharmacology browser PPB2: Target prediction combining nearest neighbors with machine learning. J. Chem. Inf. Model. 2018, 59, 10–17. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Battistuz, T.; Bhat, T.N.; Bluhm, W.F.; Bourne, P.E.; Burkhardt, K.; Feng, Z.; Gilliland, G.L.; Iype, L.; Jain, S.; et al. The protein data bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002, 58, 899–907. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Schmid, N.; Eichenberger, A.P.; Choutko, A.; Riniker, S.; Winger, M.; Mark, A.E.; van Gunsteren, W.F. Definition and testing of the GROMOS force-field versions 54A7 and 54B7. Eur. Biophys. J. 2011, 40, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A fast force field generation tool for small organic molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.; Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Pele, R.; Marc, G.; Mogoșan, C.; Apan, A.; Ionuț, I.; Tiperciuc, B.; Moldovan, C.; Araniciu, C.; Oniga, I.; Pîrnău, A.; et al. Synthesis, in vivo anticonvulsant activity evaluation and in silico studies of some quinazolin-4 (3H)-one derivatives. Molecules 2024, 29, 1951. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Nasir, H.H.; Zaib, S.; Ali, S.; Mahmood, T.; Ayub, K.; Tahir, M.N.; Iqbal, J. Carbonic anhydrase inhibition of Schiff base derivative of imino-methyl-naphthalen-2-ol: Synthesis, structure elucidation, molecular docking, dynamic simulation and density functional theory calculations. J. Mol. Struct. 2018, 1156, 193–200. [Google Scholar] [CrossRef]


| Property | Test | Predicted Value | Parameter | Unit | ||
|---|---|---|---|---|---|---|
| dIC | dKC | dLC | ||||
| Absorption | Water solubility | −2.832 | −2.663 | −2.892 | n.a | Numeric (log mol/L) |
| Caco2 permeability | −0.709 | −0.611 | −1.113 | High if >0.90 | Numeric (log Papp in 10−6 cm/s) | |
| Intestinal absorption (human) | 0 | 6.094 | 0 | Low if <30% | Numeric (% Absorbed) | |
| Skin Permeability | −2.735 | −2.735 | −2.735 | Low if >−2.5 | Numeric (log Kp) | |
| P-glycoprotein substrate | Yes | Yes | Yes | n.a | Categorical (Yes/No) | |
| P-glycoprotein I inhibitor | No | No | No | n.a | Categorical (Yes/No) | |
| P-glycoprotein II inhibitor | No | No | No | n.a | Categorical (Yes/No) | |
| Distribution | VDss (human) | −0.647 | −1.167 | −0.413 | Low if <−0.15 and high if >0.45 | Numeric (log L/kg) |
| BBB permeability | −1.873 | −1.387 | −2.38 | Low if <−1 and high if >0.3 | Numeric (log BB) | |
| CNS permeability | −3.69 | −3.924 | −3.961 | Low if <−3 and high if >−2 | Numeric (log PS) | |
| Metabolism | CYP2D6 substrate | No | No | No | n.a | Categorical (Yes/No) |
| CYP3A4 substrate | No | No | No | n.a | Categorical (Yes/No) | |
| CYP1A2 inhibitor | No | No | No | n.a | Categorical (Yes/No) | |
| CYP2C19 inhibitor | No | No | No | n.a. | Categorical (Yes/No) | |
| CYP2C9 inhibitor | No | No | No | n.a. | Categorical (Yes/No) | |
| CYP2D6 inhibitor | No | No | No | n.a. | Categorical (Yes/No) | |
| CYP3A4 inhibitor | No | No | No | n.a. | Categorical (Yes/No) | |
| Excretion | Total Clearance | 1.6 | 1.504 | 1.851 | n.a. | Numeric (log mL/min/kg) |
| Renal OCT2 substrate | No | No | No | n.a. | Categorical (Yes/No) | |
| Property | Test | Predicted Value | Parameter | Unit | ||
|---|---|---|---|---|---|---|
| dIC | dKC | dLC | ||||
| Toxicity | AMES toxicity | No | No | No | n.a. | Categorical (Yes/No) |
| Max. tolerated dose (human) | 0.855 | 1.031 | 0.477 | Low if ≤0.477 and high if >0.477 | Numeric (log mg/kg/day) | |
| hERG I inhibitor | No | No | No | n.a. | Categorical (Yes/No) | |
| hERG II inhibitor | No | No | No | n.a. | Categorical (Yes/No) | |
| Oral Rat Acute Toxicity (LD50) | 2.397 | 1.992 | 2.482 | n.a. | Numeric (mol/kg) | |
| Oral Rat Chronic Toxicity (LOAEL) | 3.466 | 3.526 | 4.397 | n.a. | Numeric (log mg/kg_bw/day) | |
| Hepatotoxicity | No | No | No | n.a. | Categorical (Yes/No) | |
| Skin Sensitisation | No | No | No | n.a. | Categorical (Yes/No) | |
| T. pyriformis toxicity | 0.285 | 0.285 | 0.285 | High if >−0.5 | Numeric (log ug/L) | |
| Minnow toxicity | 8.584 | 6.191 | 14.792 | High if <0.5 | Numérico (log mM) | |
| Drug-Likeness Tests | |||||
|---|---|---|---|---|---|
| Molecule | Lipinski | Ghose | Veber | Egan | Muegge |
| dIC | Violation: H-acceptor > 10 | Violation: WLOGP < −0.4 | Violation: TPSA > 140 | Violation: TPSA > 131.6 | Violations: XLOGP3 < −2, TPSA > 150, H-acceptor > 10 |
| dKC | Violation: H-acceptor > 10 | Violation: WLOGP < −0.4 | Violation: TPSA > 140 | Violation: TPSA > 131.6 | Violations: XLOGP3 < −2, TPSA > 150, H-acceptor > 10 |
| dLC | Violations: MW > 500, H-acceptor > 10 | Violations: MW > 480, WLOGP < −0.4 | Violation: TPSA > 140 | Violation: TPSA > 131.6 | Violations: XLOGP3 < −2, TPSA > 150, H-acceptor > 10 |
| SwissTarget | PPB2 | Probes and Drugs | ||||
|---|---|---|---|---|---|---|
| Target | Uniprot ID | Target Class | dIC Rank | dKC Rank | dLC Rank | Cancer Drugs with the Same Target |
| CA I | P00915 | Lyase | 3 | 2 | 4 | Celecoxib, imatinib, nilotinib, bortezomib |
| CA II | P00918 | Lyase | 1 | 1 | 1 | Celecoxib, imatinib, hydroxyurea, nilotinib, bortezomib, zoledronic acid |
| CA IX | Q16790 | Lyase | 2 | 3 | 2 | Celecoxib, imatinib, hydroxyurea, nilotinib, pazopanib, bortezomib, zoledronic acid |
| CA XII | O43570 | Lyase | 4 | 4 | 3 | Celecoxib, imatinib, nilotinib, bortezomib, zoledronic acid |
| CA XIV | Q9ULX7 | Lyase | 5 | 5 | 5 | Celecoxib, imatinib, nilotinib, bortezomib, zoledronic acid |
| Ligand | Receiver | Docking Score (kcal/mol) |
|---|---|---|
| dIC | CA I | −9.7 |
| CA II | −9.1 | |
| CA IX | −8 | |
| CA XII | −9.3 | |
| CA XIV | −8.4 | |
| dKC | CA I | −9.2 |
| CA II | −8.3 | |
| CA IX | −8.6 | |
| CA XII | −8.8 | |
| CA XIV | −8.5 | |
| dLC | CA I | −10.3 |
| CA II | −9.2 | |
| CA IX | −8.6 | |
| CA XII | −9.8 | |
| CA XIV | −10.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, O.L.T.; Alves, M.G.d.C.F.; Rocha, H.A.O. Exploring the Pharmacological Potential of Carrageenan Disaccharides as Antitumor Agents: An In Silico Approach. Mar. Drugs 2025, 23, 6. https://doi.org/10.3390/md23010006
Silva OLT, Alves MGdCF, Rocha HAO. Exploring the Pharmacological Potential of Carrageenan Disaccharides as Antitumor Agents: An In Silico Approach. Marine Drugs. 2025; 23(1):6. https://doi.org/10.3390/md23010006
Chicago/Turabian StyleSilva, Ohana Leticia Tavares, Monique Gabriela das Chagas Faustino Alves, and Hugo Alexandre Oliveira Rocha. 2025. "Exploring the Pharmacological Potential of Carrageenan Disaccharides as Antitumor Agents: An In Silico Approach" Marine Drugs 23, no. 1: 6. https://doi.org/10.3390/md23010006
APA StyleSilva, O. L. T., Alves, M. G. d. C. F., & Rocha, H. A. O. (2025). Exploring the Pharmacological Potential of Carrageenan Disaccharides as Antitumor Agents: An In Silico Approach. Marine Drugs, 23(1), 6. https://doi.org/10.3390/md23010006







