New Cyclic Pentapeptides from the Mangrove-Derived Aspergillus fumigatus GXIMD 03099
Abstract
1. Introduction
2. Results
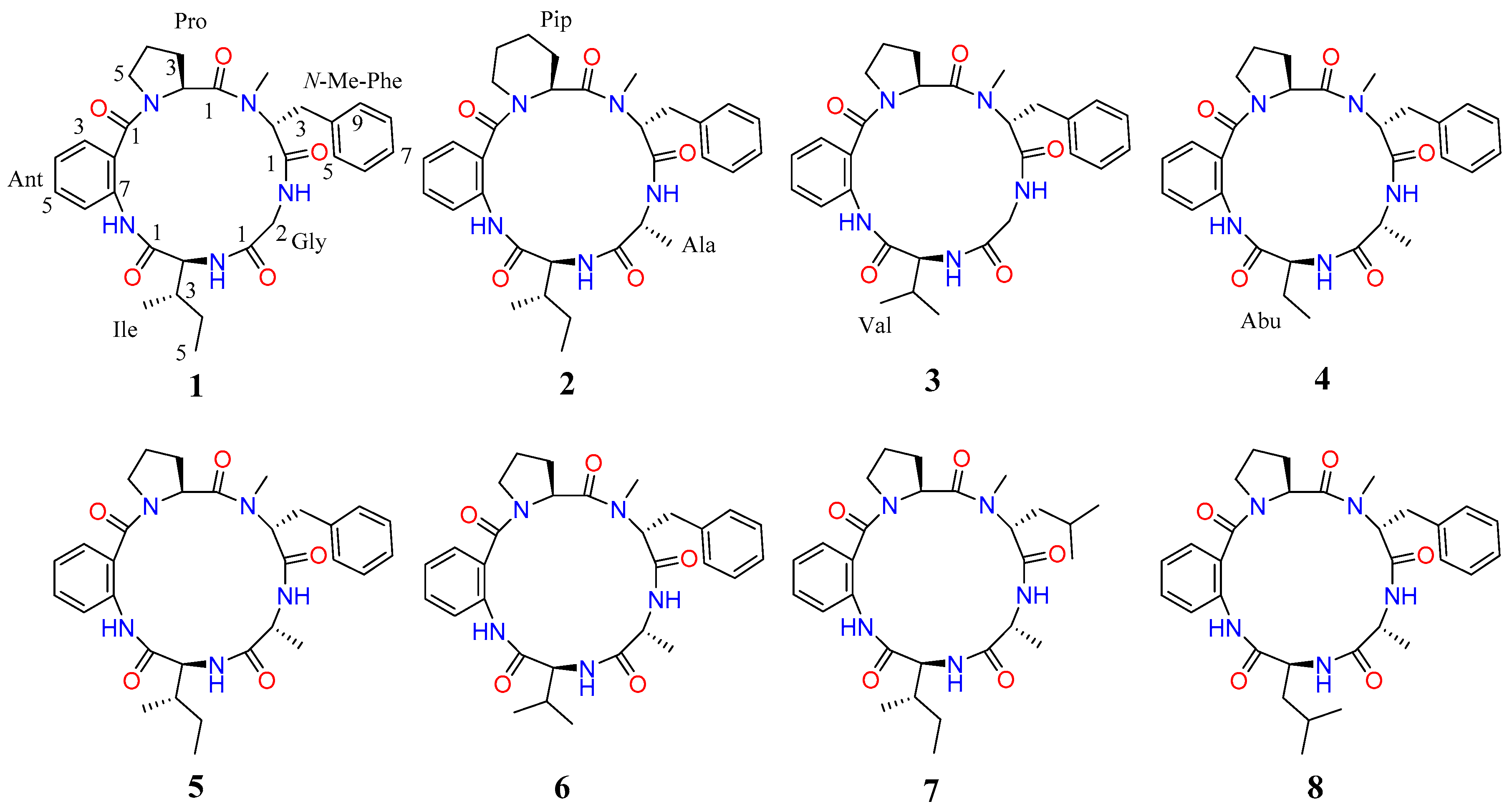
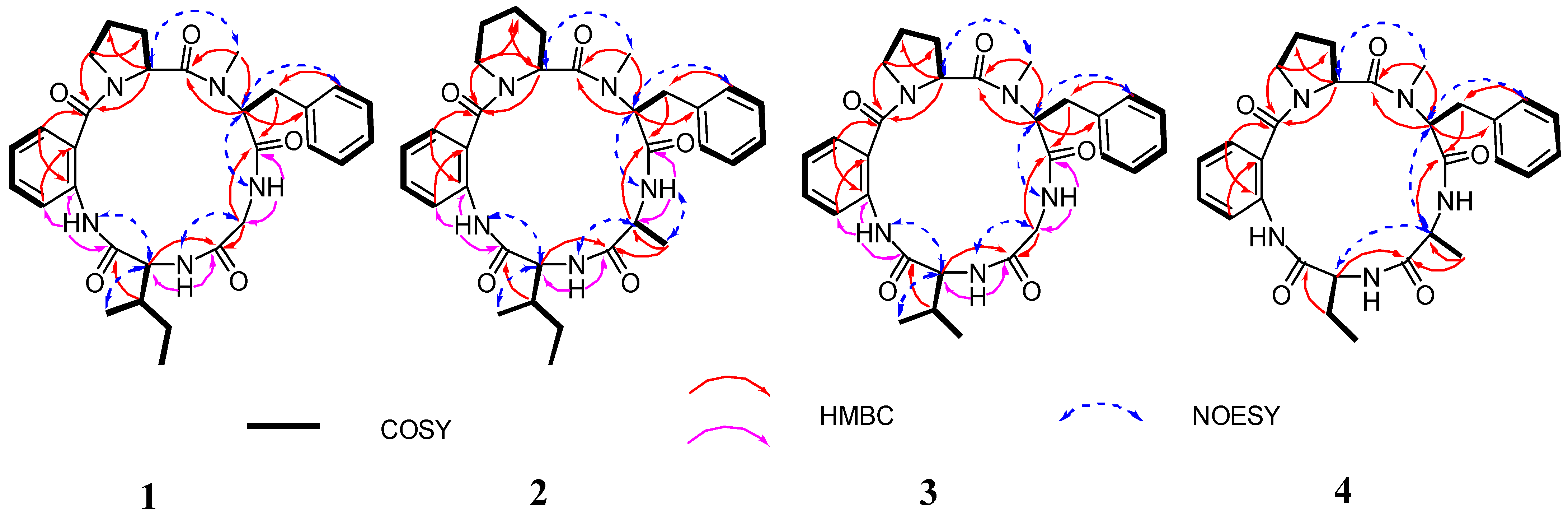
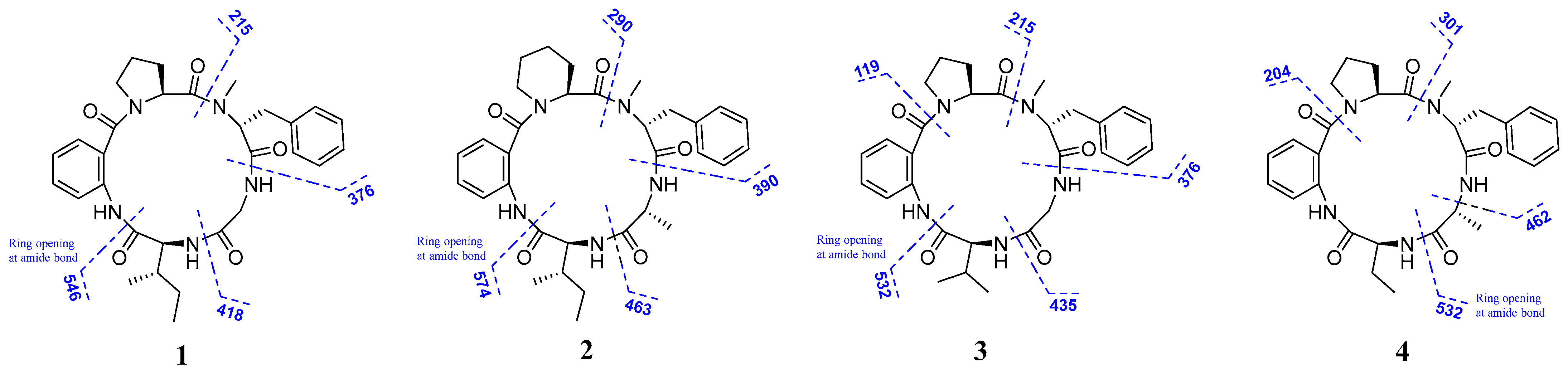
2.1. Structural Elucidation
2.2. Biological Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material, Isolation, and Purification
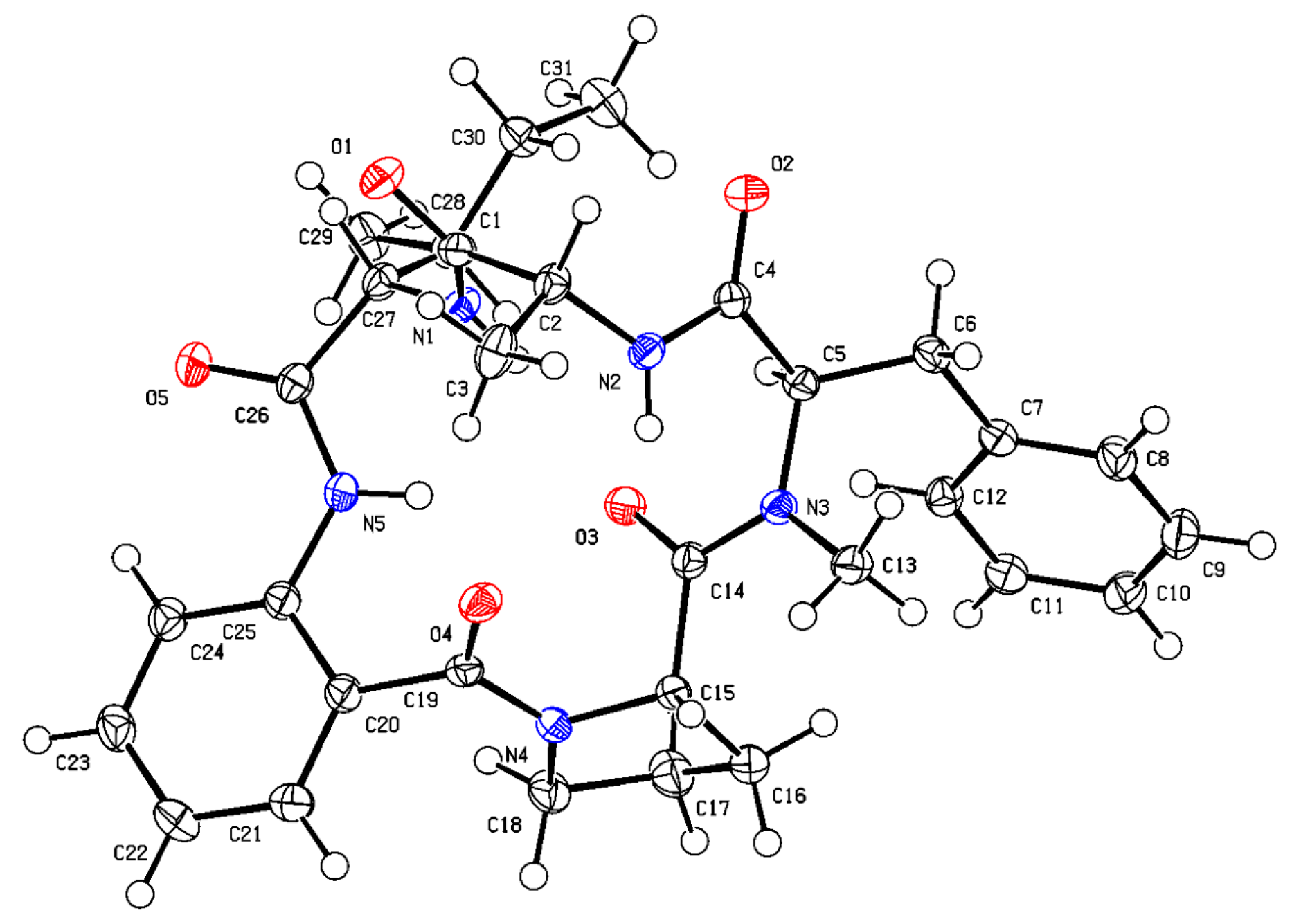
3.3. X-ray Crystallographic Analyses of Compound 5
3.4. Acid Hydrolysis and Marfey’s Analysis Methods
3.5. Insecticidal Activities
3.6. Antibacterial Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hafez Ghoran, S.; Taktaz, F.; Ayatollahi, S.A.; Kijjoa, A. Anthraquinnoes and their analogues from marine-derived fungi: Chemistry and biological activities. Mar. Drugs 2022, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Survase, S.A.; Kagliwal, L.D.; Annapure, U.S.; Singhal, R.S. Cyclosporin A—A review on fermentative production, downstream processing and pharmacological applications. Biotechnol. Adv. 2011, 29, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Moellering, R.C.; Courvalin, P.; Jones, R.N.; Levine, D.P.; Rybak, M.J.; Stevens, D.L.; Saloulas, G. Predicting and defining vancomycin efficacy: Program overview. Clin. Infect. Dis. 2006, 42, S35–S39. [Google Scholar] [CrossRef]
- Bills, G.; Li, Y.; Chen, L.; Yue, Q.; Niu, X.M.; An, Z. New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibiotics. Nat. Prod. Rep. 2014, 31, 1348–1375. [Google Scholar] [CrossRef] [PubMed]
- Humphries, R.M.; Pollett, S.; Sakoulas, G. A current perspective on daptomycin for the clinical microbiologist. Clin. Microbiol. Rev. 2013, 26, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Lin, M.Y.; Xu, D.; Lai, D.W.; Zhou, L.G. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.; Taktaz, F.; Sousa, E.; Fernandes, C.; Kijjoa, A. Peptides from marine-derived fungi: Chemistry and biological activities. Mar. Drugs 2023, 21, 510. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Bao, J.; Zhang, X.Y.; Tu, Z.C.; Shi, Y.M.; Qi, S.H. Asperterrestide A, cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGA0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Chao, R.; Hou, X.M.; Xu, W.F.; Hai, Y.; Wei, M.Y.; Wang, C.L.; Gu, Y.C.; Shao, C.L. Targeted isolation of asperheptatides from a coral-derived fungus using LC-MS/MS-based molecular networking and antitubercular activities of modified cinnamate derivatives. J. Nat. Prod. 2021, 84, 11–19. [Google Scholar] [CrossRef]
- Luo, M.H.; Zang, R.C.; Wang, X.; Chen, Z.M.; Song, X.X.; Ju, J.H.; Huang, H.B. Hydroxamate-containing siderophore acremonpeptides A–D and an aluminum complex of acremonpeptide D from the marine-derived Acremonium persicinum SCSIO 115. J. Nat. Prod. 2019, 82, 2594–2600. [Google Scholar] [CrossRef]
- Ding, W.J.; Tian, D.M.; Chen, M.; Xia, Z.X.; Tang, X.Y.; Zhang, S.H.; Wei, J.H.; Li, X.N.; Yao, X.S.; Wu, B.; et al. Molecular networking-guided isolation of cyclopentapeptides from the hydrothermal vent sediment derived fungus Aspergillus pseudoviridinutans TW58-5 and their anti-inflammatory effects. J. Nat. Prod. 2023, 86, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tan, R.X.; Wang, Q.; Huang, W.Y.; Yin, Y.X. Antifungal cyclopeptides from Halobacillus litoralis YS3106 of marine origin. Tetrahedron Lett. 2002, 43, 6545–6548. [Google Scholar] [CrossRef]
- Tang, W.Z.; Liu, J.T.; Hu, Q.; He, R.J.; Guan, X.Q.; Ge, G.B.; Han, H.; Yang, F.; Lin, H.W. Pancreatic lipase inhibitory cyclohexapeptides from the marine sponge-derived fungus Aspergillus sp. 151304. J. Nat. Prod. 2020, 83, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Deyle, K.; Heinis, C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017, 38, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.H.; Zhou, J. Plant cyclopeptides. Chem. Rev. 2006, 106, 840–895. [Google Scholar] [CrossRef] [PubMed]
- Pomilio, A.B.; Battista, M.E.; Vitale, A.A. Naturally-occurring cyclopeptides: Structures and bioactivity. Curr. Org. Chem. 2006, 10, 2075–2121. [Google Scholar] [CrossRef]
- Yamazaki, M.; Horie, Y.; Bae, K.; Maebayashi, Y.; Jisai, Y.; Fujimoto, H. New fungal metabolites avellanins A and B from Hamigra avellanea with pressor effect. Chem. Pharm. Bull. 1987, 35, 2122–2124. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Gohda, F.; Kadoshima, T.; Fukuda, T.; Hanafusa, T.; Shojima, A.; Nakayama, J.; Bill, G.F.; Peterson, S. Avellanin C, an inhibitor of quorum-sensing signaling in Staphylococcus aureus, from Hamigera ingelheimensis. J. Antibiot. 2015, 75, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.H.; Ma, L.Y.; Shao, Y.H.; Wang, X.; Wang, L.; Liu, W.Z.; Huang, Y.L. A new chromone derivative produced by Aspergillus petraakii. Chin. J. Mar. Drugs 2017, 36, 23–27. [Google Scholar]
- Bai, M.; Huang, G.L.; Mei, R.Q.; Wang, B.; Luo, Y.P.; Nong, X.H.; Chen, G.Y.; Zheng, C.J. Bioactive lactones from the mangrove-derived fungus Penicillium sp. TGM112. Mar. Drugs 2019, 17, 433. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, C.J.; Nong, X.H.; Zhou, X.M.; Luo, Y.P.; Chen, G.Y. Four new insecticidal xanthene derivatives from the mangrove-derived fungus Penicillium sp. JY246. Mar. Drugs 2019, 17, 649. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.P.; Xia, J.L.; Zhao, L.Y.; Tang, Z.Z.; Lin, X.; Liu, Y.H.; Gao, C.H.; Liu, K.; Bai, M. Penicixanthene E, a new xanthene isolated from a mangrove-derived fungus Penicillium sp. J. Antibiot. 2022, 75, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.M.; Xia, J.L.; Zhao, L.Y.; Liu, K.; Tang, Z.Z.; Huang, B.Y.; Liu, Y.H.; Gao, C.H.; Bai, M. Two new isocoumarins isolated from a mangrove-derived Penicillium sp. Phytochem. Lett. 2022, 50, 21–24. [Google Scholar] [CrossRef]
- Igarashi, Y.; Hanafusa, T.; Gohda, F.; Peterson, S.; Bills, G. Species-level assessment of secondary metabolite diversity among Hamigera species and a taxonomic note on the genus. Mycology 2014, 5, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tang, Z.Z.; Gan, Y.M.; Li, Z.Y.; Luo, X.W.; Gao, C.H.; Zhao, L.Y.; Chai, L.; Liu, Y.H. 18-residue peptaibols produced by the songe-derived Trichoderma sp. GXIMD 01001. J. Nat. Prod. 2023, 86, 994–1002. [Google Scholar] [CrossRef]
- Yin, Y.H.; Yang, W.C.; Chen, T.; Tan, Q.; Zou, G.; Zang, Z.M.; Li, J.H.; Wang, B.; She, Z.G. Cytosporones W and X: Two mutually converting epimers from a mangrove endophytic fungus Diaporthe sp. ZJHJYZ-1. ACS Omega 2023, 8, 26628–26634. [Google Scholar] [CrossRef]
| 1 a | 2 b | ||||||
|---|---|---|---|---|---|---|---|
| Unit | Position | δH, (J in Hz) | δC, Type | Unit | Position | δH, (J in Hz) | δC, Type |
| Pro | 1 | 173.5 (C) | Pip | 1 | 173.7 (C) | ||
| 2 | 4.88, dd, (8.5, 3.0) | 56.7 (CH) | 2 | 4.86, t (6.0) | 51.7 (CH) | ||
| 3 | 2.11, m; 1.39, m | 28.1 (CH2) | 3 | 1.69, m; 1.34, m | 23.1 (CH2) | ||
| 4 | 1.85, m | 24.6 (CH2) | 4 | 1.49, m; 1.30, m | 17.6 (CH2) | ||
| 5 | 3.63, m; 3.29, m | 50.1 (CH2) | 5 | 1.62, m | 22.4 (CH2) | ||
| 6 | 3.57, m; 3.40, m | 44.9 (CH2) | |||||
| Ant | 1 | 167.4 (C) | Ant | 1 | 171.9 (C) | ||
| 2 | 135.7 (C) | 2 | 136.4 (C) | ||||
| 3 | 7.52, d, (7.5) | 127.5 (CH) | 3 | 7.35, dd, (8.0, 1.6) | 127.9 (CH) | ||
| 4 | 7.16, d, (7.5) | 123.2 (CH) | 4 | 7.18, dd, (8.0, 8.0) | 123.4 (CH) | ||
| 5 | 7.47, t, (8.0) | 130.9 (CH) | 5 | 7.48, dd, (8.0, 8.0) | 131.2 (CH) | ||
| 6 | 8.38, d, (8.5) | 120.0 (CH) | 6 | 8.40, dd, (8.0, 1.6) | 120.2 (CH) | ||
| 7 | 123.9 (C) | 7 | 122.7 (C) | ||||
| NH | 9.62, s | NH | 9.27, s | ||||
| Ile | 1 | 168.9 (C) | Ile | 1 | 168.8 (C) | ||
| 2 | 4.38, dd, (8.5, 4.5) | 58.1 (CH) | 2 | 4.23, dd, (7.1, 4.2) | 58.4 (CH) | ||
| 3 | 2.01, m | 36.2 (CH) | 3 | 2.01, m | 35.8 (CH) | ||
| 4 | 1.37, m; 1.20, m | 24.1 (CH2) | 4 | 1.39, m; 1.26, m | 24.5 (CH2) | ||
| 5 | 0.83, t, (7.0) | 11.7 (CH3) | 5 | 0.84, t, (9.4) | 11.8 (CH3) | ||
| 3′ | 0.86, d, (7.0) | 15.7 (CH3) | 3′ | 0.85, d, (6.8) | 15.6 (CH3) | ||
| NH | 7.20, d, (9.0) | NH | 7.26, d, (9.0) | ||||
| Gly | 1 | 169.4 (C) | Ala | 1 | 172.2 (C) | ||
| 2 | 4.26, dd, (17.0, 8.0) 3.66, dd, (9.5, 3.5), | 42.6 (CH2) | 2 | 4.56, m | 47.7 (CH) | ||
| 3 | 1.30, d, (7.2) | 17.5 (CH3) | |||||
| NH | 7.78, dd, (7.5, 4.0) | NH | 7.29, d, (7.5) | ||||
| N-Me-Phe | 1 | 169.9 (C) | N-Me-Phe | 1 | 168.9 (C) | ||
| 2 | 5.30, dd, (12.0, 4.5) | 58.8 (CH) | 2 | 5.58, dd, (12.4, 4.4) | 57.2 (CH) | ||
| 3 | 3.40, dd, (15.5, 4.5) 3.10, dd, (15.5, 12.5) | 32.5 (CH2) | 3 | 3.44, m; 3.05, m | 31.9 (CH2) | ||
| 4 | 137.7 (C) | 4 | 137.8 (C) | ||||
| 5, 9 | 7.25, m | 128.3 (CH) | 5, 9 | 7.21, d (12.0) | 128.2 (CH) | ||
| 6, 8 | 7.31, m | 128.3 (CH) | 6, 8 | 7.28, d, (7.2) | 128.3 (CH) | ||
| 7 | 7.22, m | 126.4 (CH) | 7 | 7.21, t, (7.2) | 126.3 (CH2) | ||
| N-Me | 3.00, s | 31.6 (CH3) | N-Me | 2.97, s | 30.6 (CH3) | ||
| 3 c | 4 d | ||||||
|---|---|---|---|---|---|---|---|
| Unit | Position | δH, (J in Hz) | δC, Type | Unit | Position | δH, (J in Hz) | δC, Type |
| Pro | 1 | 173.5 (C) | Pro | 1 | 176.6 (C) | ||
| 2 | 4.90, dd, (9.0, 3.5) | 56.8 (CH) | 2 | 4.88, m | 57.9 (CH) | ||
| 3 | 2.11, m; 1.37, m | 28.1 (CH2) | 3 | 2.03, m; 1.40, m | 29.5 (CH2) | ||
| 4 | 1.84, m | 24.5 (CH2) | 4 | 2.08, m; 1.89, m | 26.1 (CH2) | ||
| 5 | 3.63, m; 3.28, m | 50.1 (CH2) | 5 | 3.80, m; 3.52, m | 52.6 (CH2) | ||
| Ant | 1 | 167.4 (C) | Ant | 1 | 170.4 (C) | ||
| 2 | 135.6 (C) | 2 | 138.8 (C) | ||||
| 3 | 7.52, d, (6.5) | 127.4 (CH) | 3 | 7.58, dd (7.5, 1.5) | 129.6(CH) | ||
| 4 | 7.18, t, (7.0) | 123.2 (CH) | 4 | 7.23, t (7.0) | 127.8(CH) | ||
| 5 | 7.47, td, (8.5, 1.0) | 130.9 (CH) | 5 | 7.48, td (7.5, 1.5) | 132.9(CH) | ||
| 6 | 8.35, d, (8.0) | 120.1 (CH) | 6 | 8.50, dd (8.5, 1.0) | 121.6(CH) | ||
| 7 | 124.1 (C) | 7 | 123.6(C) | ||||
| NH | 9.62, s | Abu | 1 | 171.7 (C) | |||
| Val | 1 | 168.9 (C) | 2 | 4.42, dd (10.0, 4.0) | 57.6 (CH) | ||
| 2 | 5.29, dd, (12.0, 4.5) | 58.7 (CH) | 3 | 2.13, m; 1.73, m | 25.1 (CH2) | ||
| 3 | 2.31, m | 29.4 (CH) | 4 | 1.00, t (7.0) | 11.1 (CH3) | ||
| 4 | 0.88, d, (7.0) | 19.0 (CH3) | Ala | 1 | 174.8 (C) | ||
| 5 | 0.86, d, (7.0) | 16.9 (CH3) | 2 | 4.81, dd (14.5, 7.0) | 49.6 (CH) | ||
| NH | 7.12, d, (9.0) | 3 | 1.44, d (7.0) | 18.2 (CH3) | |||
| Gly | 1 | 169.4 (C) | N-Me-Phe | 1 | 172.1 (C) | ||
| 2 | 4.24, dd, (17.0, 8.0), 3.67, dd, (17.0, 4.5), | 42.6 (CH2) | 2 3 4 | 5.73, dd (12.0, 4.5) 3.63, dd (15.0, 4.0), 3.06, dd (15.0, 12.5) | 59.6 (CH) 33.9 (CH2) 138.2 (C) | ||
| 3 | 3.63, dd (15.0, 4.0), 3.06, dd (15.0, 12.5) | 33.9 (CH2) | |||||
| NH | 7.79, dd, (8.0, 4.5) | 5, 9 | 129.5 (CH) | ||||
| N-Me-Phe | 1 | 170.0 (C) | 7 | 124.4 (CH) | |||
| 2 | 4.36, dd, (9.0, 4.5) | 58.7 (CH) | 6, 8 | 129.5 (CH) | |||
| 3 | 3.41, dd, (15.5, 4.5); 3.12, dd, (15.0, 12.0), | 32.5 (CH2) | N-Me | 32.1 (CH3) | |||
| 4 | 137.6 (C) | ||||||
| 5, 9 | 7.31, m; 7.25, m | 128.3(CH) | |||||
| 6, 8 | 7.31, m; 7.25, m | 128.4(CH) | |||||
| 7 | 7.23, t, (7.5) | 126.4 (CH) | |||||
| N-Me | 3.01, s | 31.5 (CH3) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, G.; Gan, Y.; Lin, X.; Yi, X.; Zhao, L.; Liu, Y.; Gao, C.; Bai, M. New Cyclic Pentapeptides from the Mangrove-Derived Aspergillus fumigatus GXIMD 03099. Mar. Drugs 2024, 22, 282. https://doi.org/10.3390/md22060282
Wang Y, Cao G, Gan Y, Lin X, Yi X, Zhao L, Liu Y, Gao C, Bai M. New Cyclic Pentapeptides from the Mangrove-Derived Aspergillus fumigatus GXIMD 03099. Marine Drugs. 2024; 22(6):282. https://doi.org/10.3390/md22060282
Chicago/Turabian StyleWang, Yu, Guangping Cao, Yuman Gan, Xiao Lin, Xiangxi Yi, Longyan Zhao, Yonghong Liu, Chenghai Gao, and Meng Bai. 2024. "New Cyclic Pentapeptides from the Mangrove-Derived Aspergillus fumigatus GXIMD 03099" Marine Drugs 22, no. 6: 282. https://doi.org/10.3390/md22060282
APA StyleWang, Y., Cao, G., Gan, Y., Lin, X., Yi, X., Zhao, L., Liu, Y., Gao, C., & Bai, M. (2024). New Cyclic Pentapeptides from the Mangrove-Derived Aspergillus fumigatus GXIMD 03099. Marine Drugs, 22(6), 282. https://doi.org/10.3390/md22060282






