Characterization of Three Polysaccharide-Based Hydrogels Derived from Laminaria japonica and Their Hemostatic Properties
Abstract
1. Introduction
2. Results
2.1. Chemical Compositions of Three LJPs
2.2. Characterization of Three Hydrogels
2.2.1. Swelling Properties
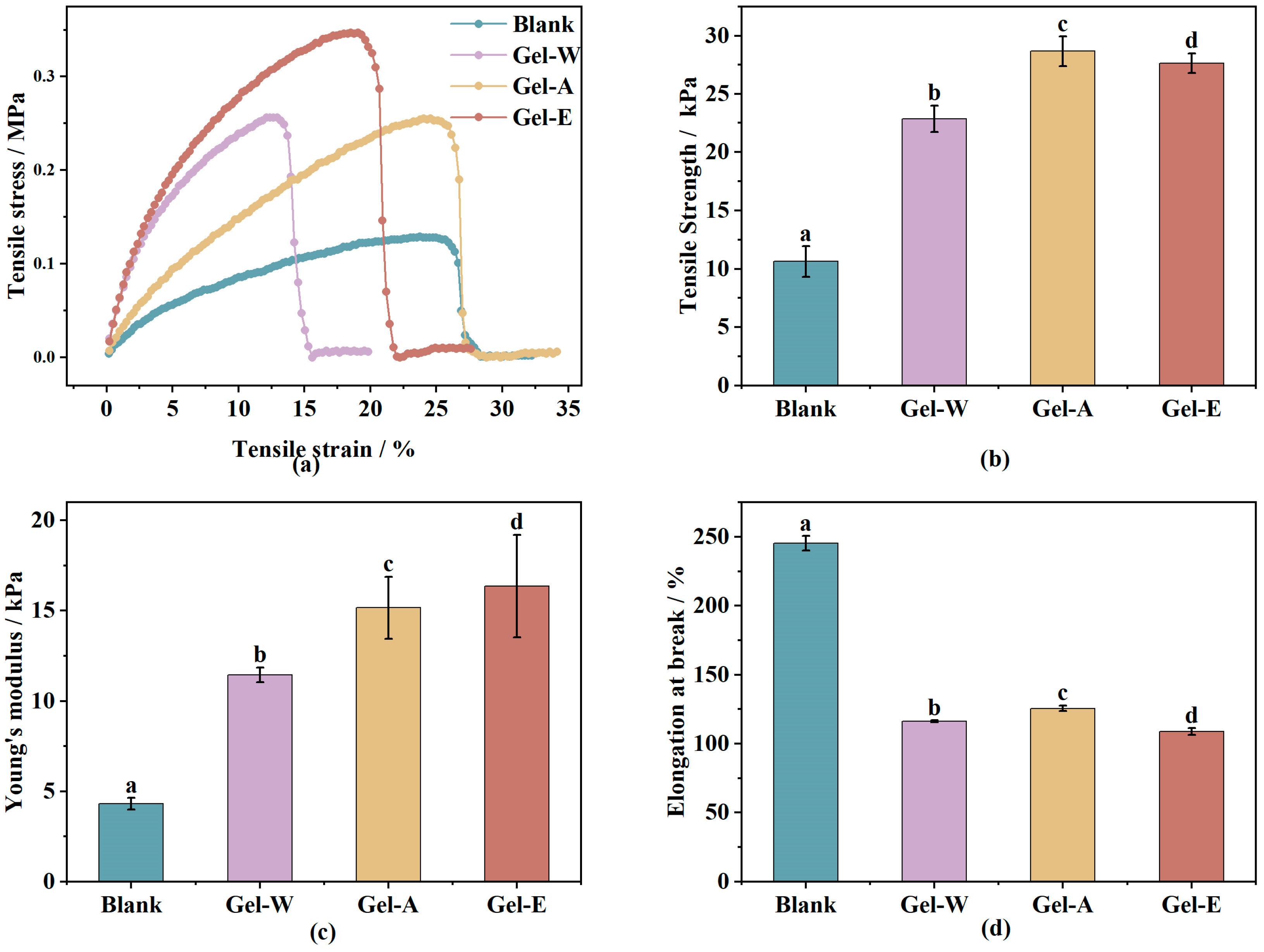
2.2.2. Mechanical Property Analysis
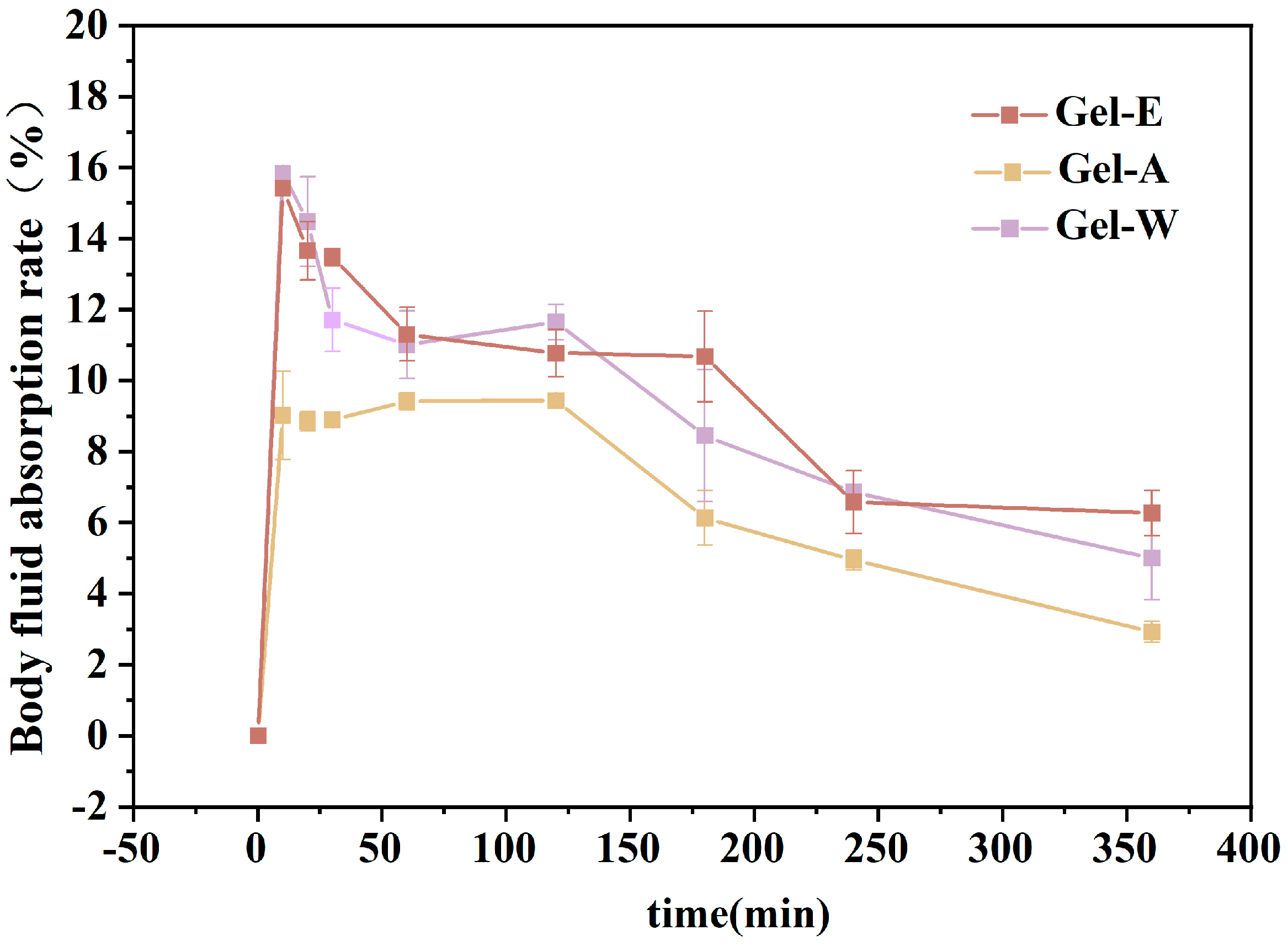
2.3. Absorption Capacity of Simulated Body Fluid (SBF)
2.4. Hemolysis Rate
2.5. Absorption of Whole Blood Properties
2.6. The Whole-Blood Coagulation Index
2.7. Quantitative Platelet Analysis
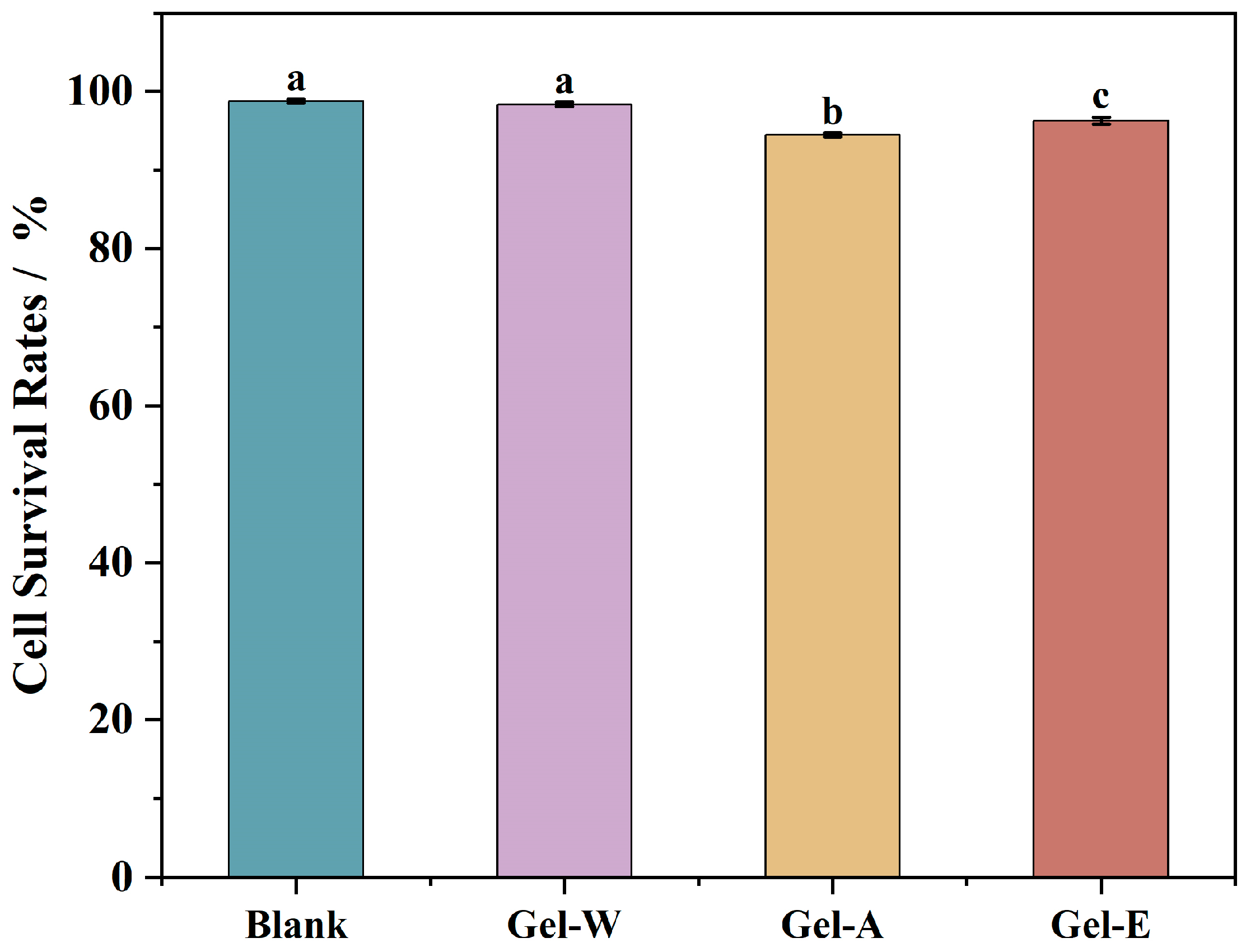
2.8. Cell Cytotoxicity
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Extraction of LJPs via Different Methods
3.2.1. Pre-Treatment of Laminaria japonica
3.2.2. LJP Extraction via Water Extraction
3.2.3. LJP Extraction via Acid Extraction
3.2.4. LJP Extraction via Enzymatic Extraction
3.3. Chemical Compositions of LJPs
3.3.1. Basic Compositions
3.3.2. Monosaccharide Composition
3.4. Preparation of LJP/Chitosan/PVA Composite Hydrogels
3.5. Characterization of Hydrogels
3.5.1. Swelling Property Analysis
3.5.2. Mechanical Property Determination
3.5.3. Rate of Absorption of Simulated Body Fluid
3.6. Hemostatic Properties Analysis
3.6.1. Hemolysis Rate
3.6.2. Absorption of Whole Blood Properties
3.6.3. Blood Coagulation Index (BCI)
3.6.4. Quantitative Platelet Analysis
3.7. Cell Cytotoxicity
3.7.1. Cell Culture
3.7.2. Pretreatments of LJP and LJP-Gels
3.7.3. Cell Cytotoxicity in HaCaT
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, R.; Pang, D.; Wen, L.; You, L.; Huang, R.; Kulikouskaya, V. In Vitro digestibility and prebiotic activities of a sulfated polysaccharide from Gracilaria lemaneiformis. J. Funct. Foods 2020, 64, 103652. [Google Scholar] [CrossRef]
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey loaded alginate/PVA nanofibrous membrane as potential bioactive wound dressing. Carbohydr. Polym. 2019, 219, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Ma, Y.; Tan, H.; Yuan, G.; Li, S.; Li, J.; Jia, Y.; Zhou, T.; Niu, X.; Hu, X. Alginate membrane dressing toughened by chitosan floccule to load antibacterial drugs for wound healing. Polym. Test. 2019, 79, 106039. [Google Scholar] [CrossRef]
- Ma, X.; Bian, Q.; Hu, J.; Gao, J. Stem from nature: Bioinspired adhesive formulations for wound healing. J. Control. Release 2022, 345, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Gorain, B.; Pandey, M.; Leng, N.H.; Yan, C.W.; Nie, K.W.; Kaur, S.J.; Marshall, V.; Sisinthy, S.P.; Panneerselvam, J.; Molugulu, N.; et al. Advanced drug delivery systems containing herbal components for wound healing. Int. J. Pharm. 2022, 617, 121617. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wu, T.; Wang, W.; Li, B.; Wang, M.; Chen, L.; Xia, H.; Zhang, T. Biofunctions of antimicrobial peptide-conjugated alginate/hyaluronic acid/collagen wound dressings promote wound healing of a mixed-bacteria-infected wound. Int. J. Biol. Macromol. 2019, 140, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Park, K.M.; Park, K.D. Oxygen-generating alginate hydrogels as a bioactive acellular matrix for facilitating wound healing. J. Ind. Eng. Chem. 2019, 69, 397–404. [Google Scholar] [CrossRef]
- Wang, P.; Pu, Y.; Ren, Y.; Liu, S.; Yang, R.; Tan, X.; Zhang, W.; Shi, T.; Li, S.; Chi, B. Bio-inspired hydrogel-based bandage with robust adhesive and antibacterial abilities for skin closure. Sci. China Mater. 2021, 65, 246–254. [Google Scholar] [CrossRef]
- Hickman, D.A.; Pawlowski, C.L.; Sekhon, U.D.S.; Marks, J.; Sen Gupta, A. Biomaterials and Advanced Technologies for Hemostatic Management of Bleeding (vol 30, 1700859, 2018). Adv. Mater. 2018, 30, 1804635. [Google Scholar] [CrossRef]
- Long, M.; Zhang, Y.; Huang, P.; Chang, S.; Hu, Y.; Yang, Q.; Mao, L.; Yang, H. Emerging Nanoclay Composite for Effective Hemostasis. Adv. Funct. Mater. 2018, 28, 1704452. [Google Scholar] [CrossRef]
- Purello-D’Ambrosio, F.; Gangemi, S.; La Rosa, G.; Merendino, R.A.; Tomasello, F. Allergy to gelatin. Allergy 2000, 55, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wu, H.; Ge, S.; Huang, L.; Chen, L.; Connor, C.; Guo, Z.; Jiang, Y.; Bin Xu, B.; Peng, W. A Cellulose/Chitosan Dual Cross-Linked Multifunctional and Resilient Hydrogel for Emergent Open Wound Management. Adv. Healthc. Mater. 2024, 13, 2304676. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, Z.; Wang, H.; Zhou, B.; Huang, F.; Dong, S.; Yang, J.; Li, B.; Wang, X. A multi-crosslinking strategy of organic and inorganic compound bio-adhesive polysaccharide-based hydrogel for wound hemostasis. Biomater. Adv. 2023, 152, 213481. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.-Y.; Lei, X.-X.; Hu, J.-J.; Jiang, Y.-L.; Li, Q.-J.; Song, Y.-T.; Zhang, Q.-Y.; Li-Ling, J.; Xie, H.-Q. Multi-crosslinking hydrogels with robust bio-adhesion and pro-coagulant activity for first-aid hemostasis and infected wound healing. Bioact. Mater. 2022, 16, 388–402. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 9, 2902. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Ma, N.; Yang, X.; Ling, G.; Yu, J.; Zhang, P. Preparation of intelligent DNA hydrogel and its applications in biosensing. Eur. Polym. J. 2020, 137, 109951. [Google Scholar] [CrossRef]
- Wang, E.; Wen, H.; Guo, P.; Luo, Y.; Wang, C.; He, Z.; Pan, J.; Chen, X.; Cao, B.; Wang, Y.; et al. Fabrication of methacrylated casein/alginate microspheres crosslinked by UV light coupled with Ca2+ chelation for pH-sensitive drug delivery. Colloid Polym. Sci. 2022, 300, 553–567. [Google Scholar] [CrossRef]
- Li, H.-Y.; Yi, Y.-L.; Guo, S.; Zhang, F.; Yan, H.; Zhan, Z.-L.; Zhu, Y.; Duan, J.-A. Isolation, structural characterization and bioactivities of polysaccharides from Laminaria japonica: A review. Food Chem. 2021, 370, 131010. [Google Scholar] [CrossRef]
- Yin, D.; Sun, X.; Li, N.; Guo, Y.; Tian, Y.; Wang, L. Structural properties and antioxidant activity of polysaccharides extracted from Laminaria japonica using various methods. Process Biochem. 2021, 111, 201–209. [Google Scholar] [CrossRef]
- Van Breda, D.; Lufu, R.; Goosen, N.J. Optimisation of cellulase-assisted extraction of laminarin from the brown seaweed Ecklonia maxima, using response surface methodology. Biomass Convers. Biorefin. 2021, 13, 10399–10412. [Google Scholar] [CrossRef]
- Cui, C.; Lu, J.; Sun-Waterhouse, D.; Mu, L.; Sun, W.; Zhao, M.; Zhao, H. Polysaccharides from Laminaria japonica: Structural characteristics and antioxidant activity. LWT-Food Sci. Technol. 2016, 73, 602–608. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of acidic, water and alkaline extraction on structural features, antioxidant activities of Laminaria japonica polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Shang, M.; Li, X.; Jiang, L.; Chen, L.; Long, J.; Jiao, A.; Ji, H.; Jin, Z.; Qiu, C. Advances in intelligent response and nano-enhanced polysaccharide-based hydrogels: Material properties, response types, action mechanisms, applications. Food Hydrocoll. 2023, 146, 109190. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Cheon, K.-H.; Song, E.-H.; Seong, Y.-J.; Park, J.-U.; Kim, H.-E.; Jeong, S.-H. Fluorine-ion-releasing injectable alginate nanocomposite hydrogel for enhanced bioactivity and antibacterial property. Int. J. Biol. Macromol. 2018, 123, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and characterization of chitosan/gelatin/PVA hydrogel for wound dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, Y.; Hu, J.; Yao, W.; You, L.; Cheung, P.C.-K. Algal sulfated polysaccharide-based hydrogels enhance gelling properties and in vitro wound healing compared to conventional hydrogels. Algal Res. 2022, 65, 102740. [Google Scholar] [CrossRef]
- Liao, J.; Dai, H.; Huang, H. Construction of hydrogels based on the homogeneous carboxymethylated chitin from Hericium erinaceus residue: Role of carboxymethylation degree. Carbohydr. Polym. 2021, 262, 117953. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Bag, D.S.; Kalra, S.J.S. Synthesis of strong and stretchable double network (DN) hydrogels of PVA-borax and P(AM-co-HEMA) and study of their swelling kinetics and mechanical properties. Polymer 2017, 119, 263–273. [Google Scholar] [CrossRef]
- Su, X.; Chen, B. Tough, resilient and pH-sensitive interpenetrating polyacrylamide/alginate/montmorillonite nanocomposite hydrogels. Carbohydr. Polym. 2018, 197, 497–507. [Google Scholar] [CrossRef]
- Matricardi, P.; Di Meo, C.; Coviello, T.; Hennink, W.E.; Alhaique, F. Interpenetrating Polymer Networks polysaccharide hydrogels for drug delivery and tissue engineering. Adv. Drug Deliv. Rev. 2013, 65, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.F.; Barrias, C.C.; Granja, P.L.; Bartolo, P.J. Advanced biofabrication strategies for skin regeneration and repair. Nanomedicine 2013, 8, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xiang, H.; You, L.; Cui, C.; Sun-Waterhouse, D.; Zhao, M. Hypolipidaemic and antioxidant capacities of polysaccharides obtained from Laminaria japonica by different extraction media in diet-induced mouse model. Int. J. Food Sci. Technol. 2017, 52, 2274–2281. [Google Scholar] [CrossRef]
- Zhou, C.; Keshavarz Hedayati, M.; Zhu, X.; Nielsen, F.; Levy, U.; Kristensen, A. Optofluidic Sensor for Inline Hemolysis Detection on Whole Blood. ACS Sens. 2018, 3, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Delvasto-Nuñez, L.; Jongerius, I.; Zeerleder, S. It takes two to thrombosis: Hemolysis and complement. Blood Rev. 2021, 50, 100834. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, K.; Liu, P.; Bai, X.; Li, A.; Lyu, Z.; Li, Q. Preparation and properties of cellulose acetate graft copolymer-coated adsorbent resin for hemoperfusion device. J. Appl. Polym. Sci. 2023, 140, 53895. [Google Scholar] [CrossRef]
- Padoł, A.M.; Draget, K.I.; Stokke, B.T. Effects of added oligoguluronate on mechanical properties of Ca-alginate-oligoguluronate hydrogels depend on chain length of the alginate. Carbohydr. Polym. 2016, 147, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Hanyková, L.; Krakovský, I.; Šestáková, E.; Šťastná, J.; Labuta, J. Poly(N,N′-Diethylacrylamide)-Based Thermoresponsive Hydrogels with Double Network Structure. Polymers 2020, 12, 2502. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-F.; Shau, M.-D.; Chang, M.-Y.; Chiou, S.-K.; Chang, J.-K.; Cherng, J.-Y. Platelet adsorption and hemolytic properties of liquid crystal/composite polymers. Int. J. Pharm. 2006, 327, 117–125. [Google Scholar] [CrossRef]
- Mangin, P.H.; Neeves, K.B.; Lam, W.A.; Cosemans, J.M.E.M.; Korin, N.; Kerrigan, S.W.; Panteleev, M.A. In Vitro flow-based assay: From simple toward more sophisticated models for mimicking hemostasis and thrombosis. J. Thromb. Haemost. 2021, 19, 582–587. [Google Scholar] [CrossRef]
- Heebkaew, N.; Promjantuek, W.; Chaicharoenaudomrung, N.; Phonchai, R.; Kunhorm, P.; Soraksa, N.; Noisa, P. Encapsulation of HaCaT Secretome for Enhanced Wound Healing Capacity on Human Dermal Fibroblasts. Mol. Biotechnol. 2023, 66, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, R.; Li, Y.; Li, X.; You, L.; Kulikouskaya, V.; Hileuskaya, K. Degradation of polysaccharides from Sargassum fusiforme using UV/H2O2 and its effects on structural characteristics. Carbohydr. Polym. 2019, 230, 115647. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ji, D.; You, L.; Zhou, L.; Zhao, Z.; Brennan, C. Structural properties and protective effect of Sargassum fusiforme polysaccharides against ultraviolet B radiation in hairless Kun Ming mice. J. Funct. Foods 2018, 43, 8–16. [Google Scholar] [CrossRef]


| Sample | Total Carbohydrate Content (%) | Galacturonic Acid Content (%) | Glucuronic Acid Content (%) | Protein Content (%) |
|---|---|---|---|---|
| LJP-W | 85.27 ± 0.53 | 25.08 ± 1.78 | 35.92 ± 2.51 | 2.22 ± 0.35 |
| LJP-A | 95.12 ± 0.77 | 24.87 ± 1.04 | 41.81 ± 0.58 | 2.14 ± 0.12 |
| LJP-E | 95.86 ± 0.72 | 26.15 ± 3.62 | 43.69 ± 3.46 | 1.41 ± 0.93 |
| Molar Percentage (%) | LJP-W | LJP-A | LJP-E |
|---|---|---|---|
| fucose | 22.72 | 17.12 | 11.07 |
| arabinose | 1.82 | 1.21 | 0.87 |
| galactose | 13.28 | 9.95 | 7.11 |
| glucose | 1.10 | 5.02 | 8.40 |
| galacturonic acid | 25.08 | 24.87 | 26.15 |
| glucuronic acid | 35.92 | 41.81 | 43.69 |
| xylose | 0.08 | 0.02 | 2.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Shi, J.; Qiu, H.; You, L.; Xu, P.; Rao, R.; Wu, M.; Jia, R. Characterization of Three Polysaccharide-Based Hydrogels Derived from Laminaria japonica and Their Hemostatic Properties. Mar. Drugs 2024, 22, 188. https://doi.org/10.3390/md22040188
Chen Y, Shi J, Qiu H, You L, Xu P, Rao R, Wu M, Jia R. Characterization of Three Polysaccharide-Based Hydrogels Derived from Laminaria japonica and Their Hemostatic Properties. Marine Drugs. 2024; 22(4):188. https://doi.org/10.3390/md22040188
Chicago/Turabian StyleChen, Yang, Jinying Shi, Huamai Qiu, Lijun You, Panqi Xu, Rui Rao, Minqian Wu, and Ruohan Jia. 2024. "Characterization of Three Polysaccharide-Based Hydrogels Derived from Laminaria japonica and Their Hemostatic Properties" Marine Drugs 22, no. 4: 188. https://doi.org/10.3390/md22040188
APA StyleChen, Y., Shi, J., Qiu, H., You, L., Xu, P., Rao, R., Wu, M., & Jia, R. (2024). Characterization of Three Polysaccharide-Based Hydrogels Derived from Laminaria japonica and Their Hemostatic Properties. Marine Drugs, 22(4), 188. https://doi.org/10.3390/md22040188






