Brown Algae Dictyopteris divaricata Attenuates Adipogenesis by Modulating Adipocyte Differentiation and Promoting Lipolysis through Heme Oxygenase-1 Activation in 3T3-L1 Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of Chemical Constituents in D. divaricata
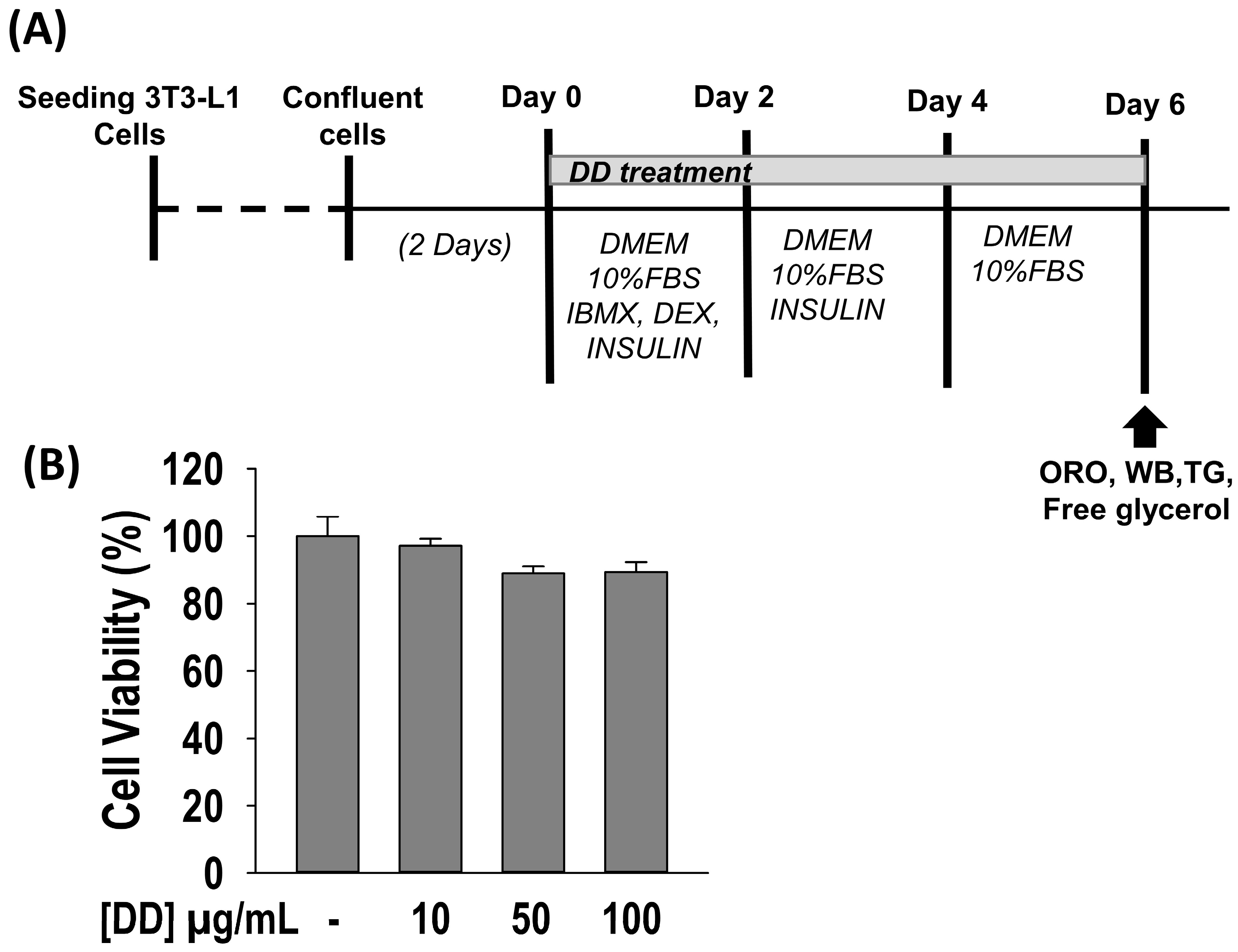
2.2. Effect of D. divaricata on Cell Viability
2.3. D. divaricata Inhibits Lipid Accumulation and TG Content, and Promotes Free Glycerol Release
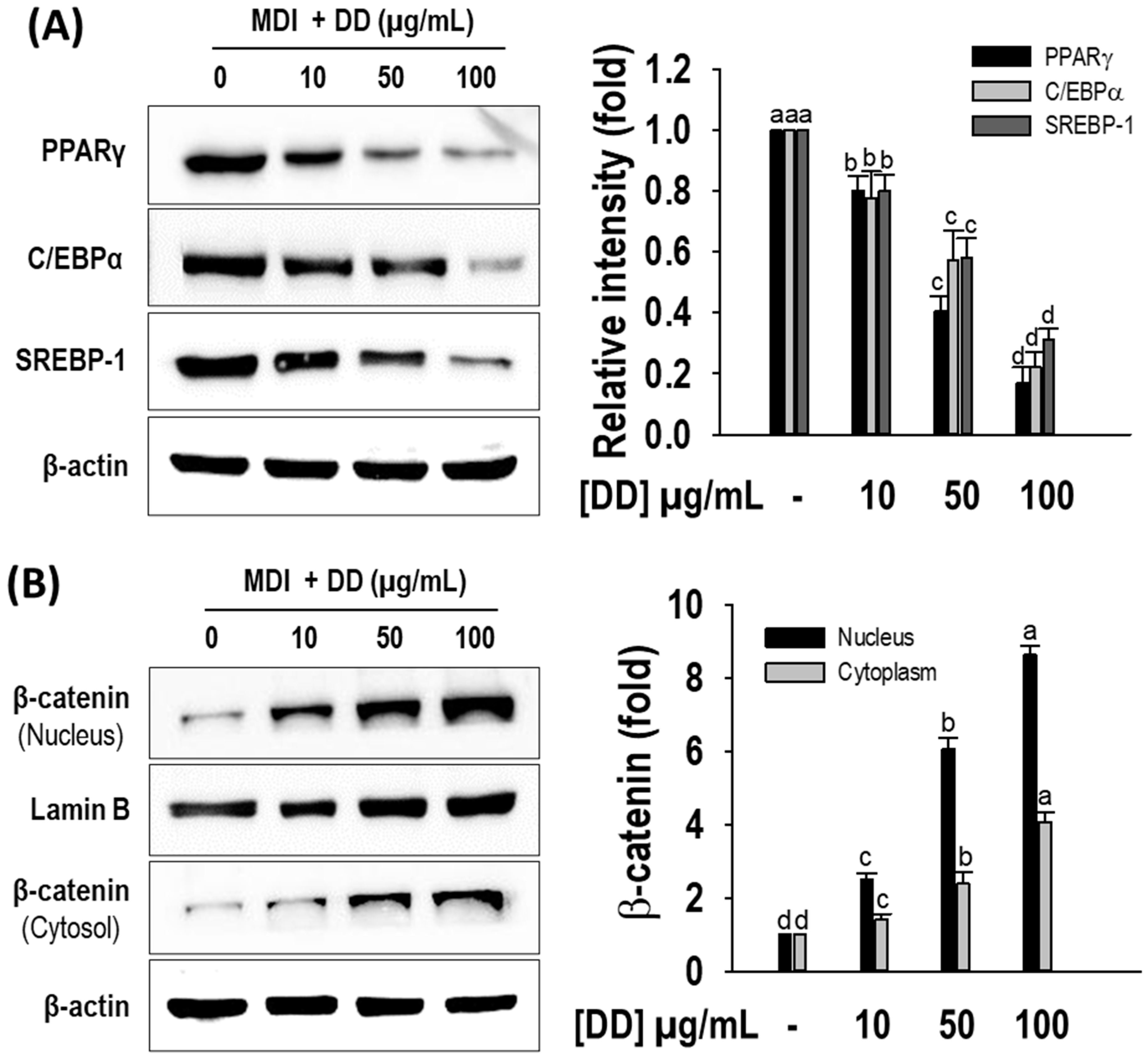
2.4. D. divaricata Inhibits Expression of Key Adipogenic Markers and Stimulates β-Catenin Nuclear Translocation
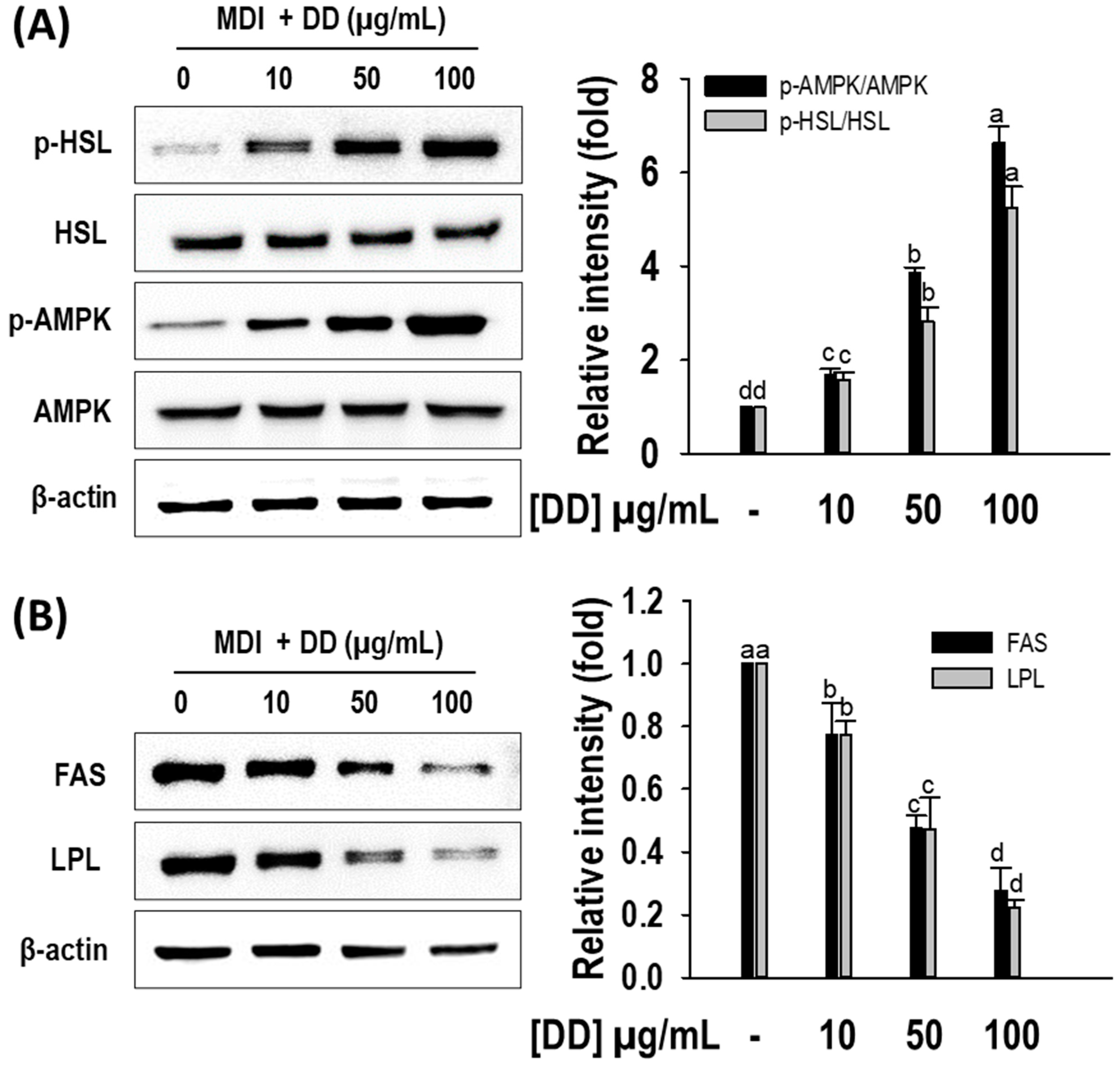
2.5. D. divaricata Regulates Lipolysis and Lipogenesis Target Protein Expression
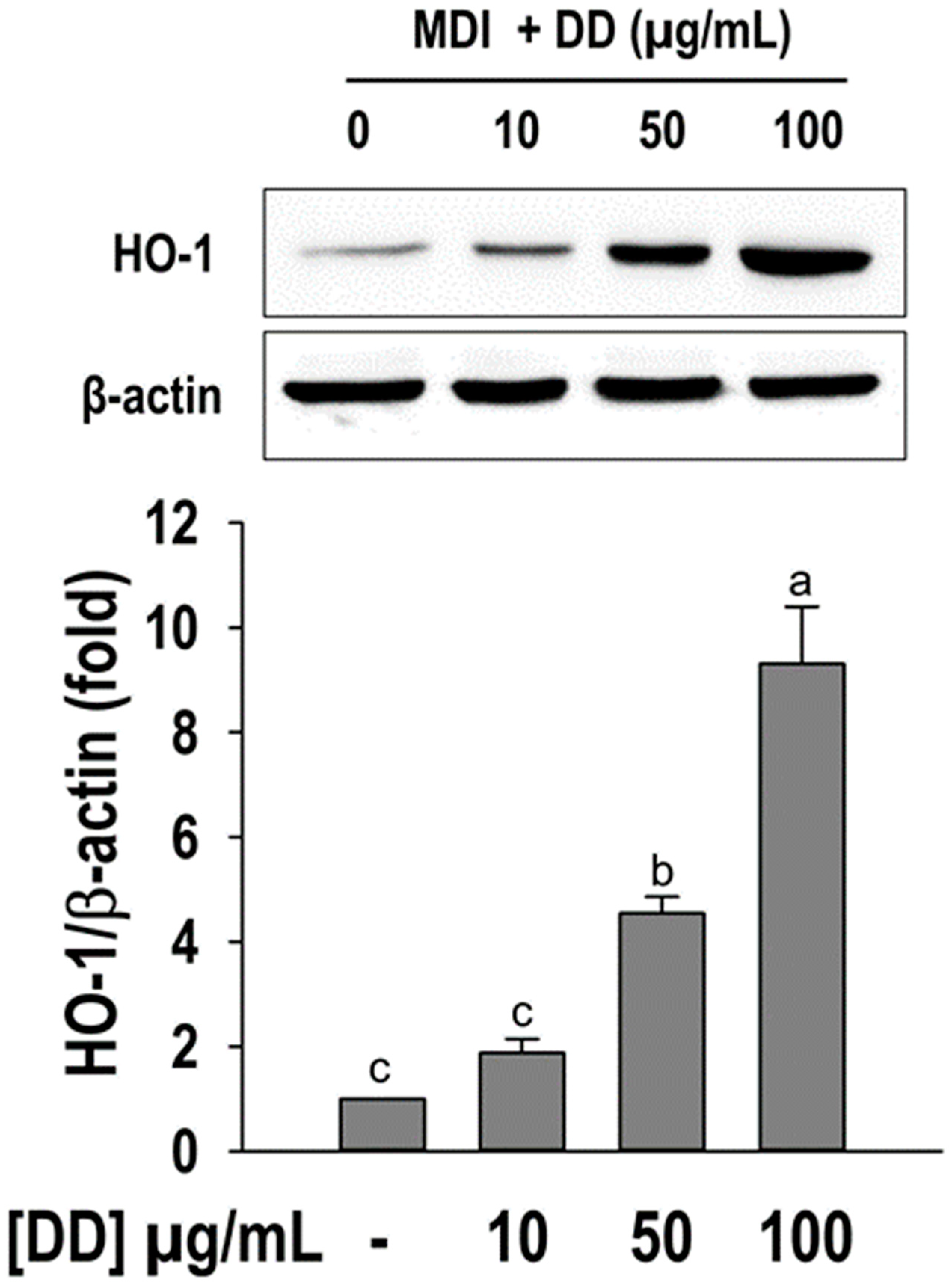
2.6. D. divaricata Promotes HO-1 Activation
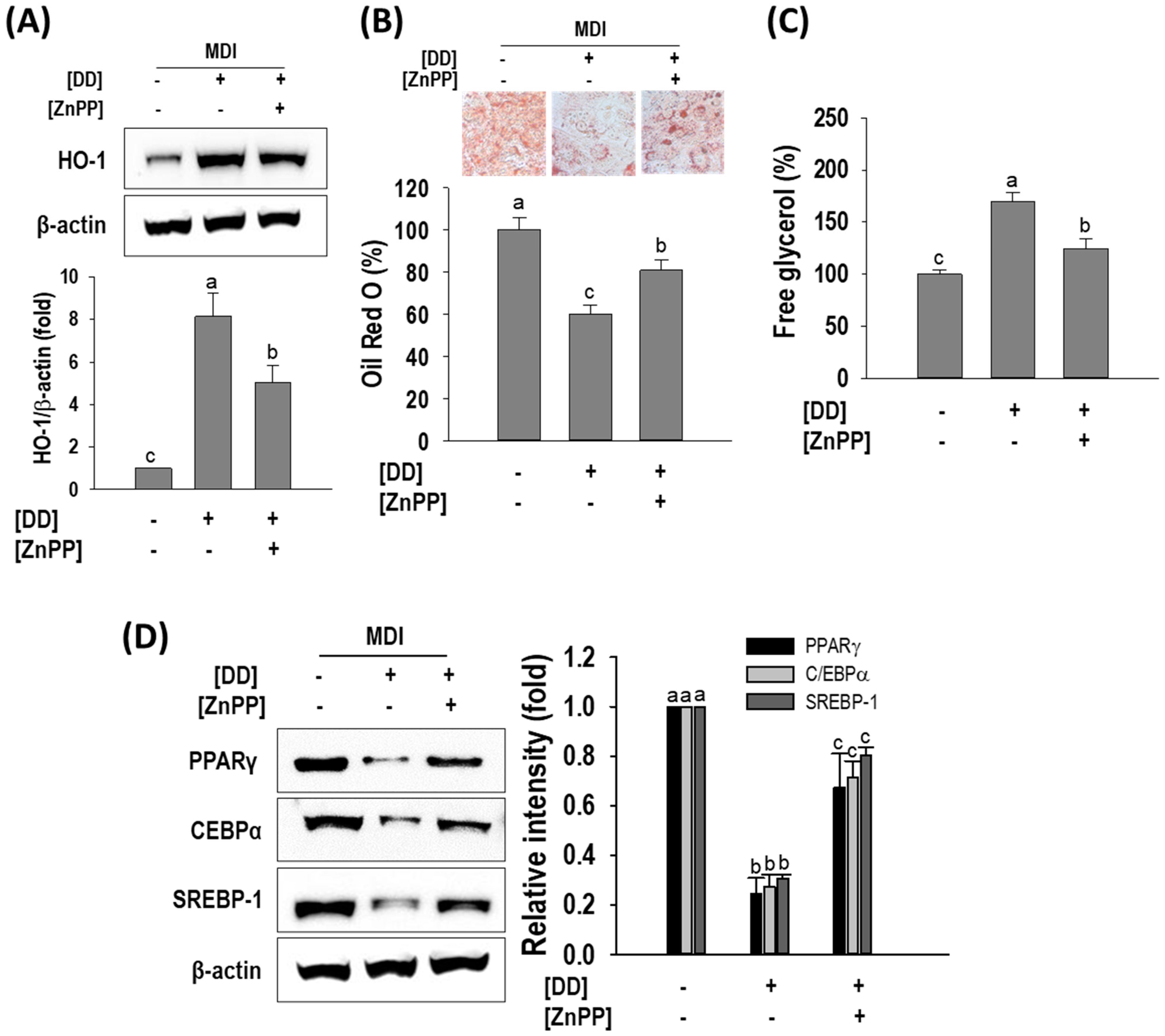
2.7. ZnPP Effect on D. divaricata Mediated Anti-Adipogenic Effect
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of D. divaricata Extract
4.3. Total Flavonoid Content (TFC) and Total Phenol Content (TPC)
4.4. Characterization of Active Compounds
4.5. Cell Culture
4.6. Induction of Adipocyte Differentiation
4.7. Cell Cytotoxicity Evaluation
4.8. Oil Red O Staining
4.9. TG Assay
4.10. Lipolysis Assay
4.11. Western Blot Analysis
4.12. Statistical Analysis
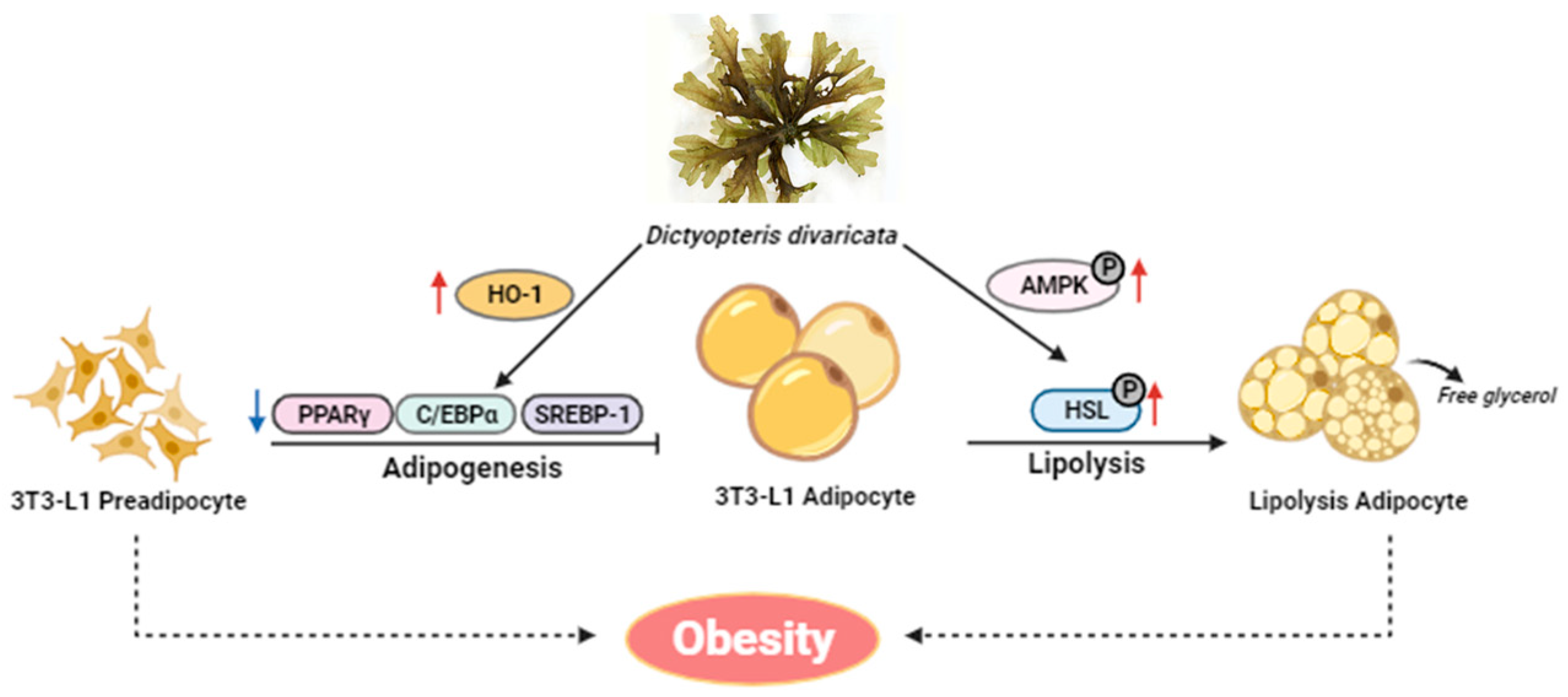
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tsai, Y.C.; Yang, B.C.; Peng, W.H.; Lee, Y.M.; Yen, M.H.; Cheng, P.Y. Heme oxygenase-1 mediates anti-adipogenesis effect of raspberry ketone in 3T3-L1 cells. Phytomedicine 2017, 31, 11–17. [Google Scholar] [CrossRef]
- Song, Y.; Oh, G.H.; Kim, M.B.; Hwang, J.-K. Fucosterol inhibits adipogenesis through the activation of AMPK and Wnt/β-catenin signaling pathways. Food Sci. Biotechnol. 2017, 26, 489–494. [Google Scholar] [CrossRef]
- Ali, A.T.; Hochfeld, W.E.; Myburgh, R.; Pepper, M.S. Adipocyte and adipogenesis. Eur. J. Cell Biol. 2013, 92, 229–236. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, M.; Zhao, H.; Yaqoob, S.; Zheng, M.; Cai, D.; Liu, J. Antiobesity effects of ginsenoside Rg1 on 3T3-L1 preadipocytes and high fat diet-induced obese mice mediated by AMPK. Nutrients 2018, 10, 830. [Google Scholar] [CrossRef]
- Nicolai, A.; Li, M.; Kim, D.H.; Peterson, S.J.; Vanella, L.; Positano, V.; Gastaldelli, A.; Rezzani, R.; Rodella, L.F.; Drummond, G. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 2009, 53, 508–515. [Google Scholar] [CrossRef]
- Hosick, P.A.; Stec, D.E. Heme oxygenase, a novel target for the treatment of hypertension and obesity? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R207–R214. [Google Scholar] [CrossRef]
- Abraham, N.G.; Junge, J.M.; Drummond, G.S. Translational significance of heme oxygenase in obesity and metabolic syndrome. Trends Pharmacol. Sci. 2016, 37, 17–36. [Google Scholar] [CrossRef]
- Oh, Y.; Ahn, C.B.; Je, J.Y. Low molecular weight blue mussel hydrolysates inhibit adipogenesis in mouse mesenchymal stem cells through upregulating HO-1/Nrf2 pathway. Food Res. Int. 2020, 136, 109603. [Google Scholar] [CrossRef]
- Yang, J.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory effects of butein on adipogenesis through upregulation of the Nrf2/HO-1 pathway in 3T3-L1 adipocytes. Prev. Nutr. Food Sci. 2017, 22, 306. [Google Scholar] [CrossRef]
- Park, E.J.; Koo, O.J.; Lee, B.C. Overexpressed human heme Oxygenase-1 decreases adipogenesis in pigs and porcine adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2015, 467, 935–940. [Google Scholar] [CrossRef]
- Kuda, T.; Kunii, T.; Goto, H.; Suzuki, T.; Yano, T. Varieties of antioxidant and antibacterial properties of Ecklonia stolonifera and Ecklonia kurome products harvested and processed in the Noto peninsula, Japan. Food Chem. 2007, 103, 900–905. [Google Scholar] [CrossRef]
- Kadam, S.; Prabhasankar, P. Marine foods as functional ingredients in bakery and pasta products. Food Res. Int. 2010, 43, 1975–1980. [Google Scholar] [CrossRef]
- Ilavenil, S.; Kim, D.H.; Vijayakumar, M.; Srigopalram, S.; Roh, S.G.; Arasu, M.V.; Lee, J.S.; Choi, K.C. Potential role of marine algae extract on 3T3-L1 cell proliferation and differentiation: An in vitro approach. Biol. Res. 2016, 49, 38. [Google Scholar] [CrossRef]
- Brown, E.M.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef]
- Kim, M.-J.; Chang, U.-J.; Lee, J.-S. Inhibitory effects of fucoidan in 3T3-L1 adipocyte differentiation. Mar. Biotechnol. 2009, 11, 557–562. [Google Scholar] [CrossRef]
- Awang, A.N.; Ng, J.L.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B.H. Anti-obesity property of the brown seaweed, Sargassum polycystum using an in vivo animal model. J. Appl. Phycol. 2014, 26, 1043–1048. [Google Scholar] [CrossRef]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.-E. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, X.; Li, S.; Hao, L.; Du, J.; Gao, D.; Kang, Q.; Lu, J. Extraction, characterization and biological activity of sulfated polysaccharides from seaweed Dictyopteris divaricata. Int. J. Biol. Macromol. 2018, 117, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.Z.; Rehman, A.U.; Yousuf, W.; Khan, A.I.; Farooqui, N.A.; Zang, S.; Xin, Y.; Wang, L. Effect of crude polysaccharide from seaweed, Dictyopteris divaricata (CDDP) on gut microbiota restoration and anti-diabetic activity in streptozotocin (STZ)-induced T1DM mice. Gut Pathog. 2022, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Ham, Y.; Moon, J.; Kim, M.; Kim, D.; Lee, W.; Lee, N.; Hyun, C. In vitro cytotoxic activity of Sargassum thunbergii and Dictyopteris divaricata (Jeju seaweeds) on the HL-60 tumour cell line. Int. J. Pharmacol. 2009, 5, 298–306. [Google Scholar] [CrossRef]
- Xiancui, L.; Rongli, N.; Xiao, F.; Lijun, H.; Lixin, Z. Macroalage as a source of alpha-glucosidase inhibitors. Chin. J. Oceanol. Limnol. 2005, 23, 354–356. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Rodeiro, I.; Hernández, I.; Herrera, J.A.; Riera, M.; Donato, M.T.; Tolosa, L.; González, K.; Ansoar, Y.; Gómez-Lechón, M.J.; Vanden Berghe, W. Assessment of the cytotoxic potential of an aqueous-ethanolic extract from Thalassia testudinum angiosperm marine grown in the Caribbean Sea. J. Pharm. Pharmacol. 2018, 70, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Kolanjinathan, K.; Ganesh, P.; Saranraj, P. Pharmacological importance of seaweeds: A review. World J. Fish Mar. Sci. 2014, 6, 1–15. [Google Scholar]
- Lee, H.G.; Lu, Y.A.; Je, J.G.; Jayawardena, T.U.; Kang, M.-C.; Lee, S.-H.; Kim, T.-H.; Lee, D.-S.; Lee, J.-M.; Yim, M.-J. Effects of ethanol extracts from Grateloupia elliptica, a red seaweed, and its chlorophyll derivative on 3T3-L1 adipocytes: Suppression of lipid accumulation through downregulation of adipogenic protein expression. Mar. Drugs 2021, 19, 91. [Google Scholar] [CrossRef]
- Jeon, T.; Hwang, S.G.; Hirai, S.; Matsui, T.; Yano, H.; Kawada, T.; Lim, B.O.; Park, D.K. Red yeast rice extracts suppress adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 cells. Life Sci. 2004, 75, 3195–3203. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, B.; Oh, M.J.; Yoon, J.; Kim, H.Y.; Lee, K.J.; Lee, J.D.; Choi, K.Y. Persicaria hydropiper (L.) spach and its flavonoid components, isoquercitrin and isorhamnetin, activate the Wnt/β-catenin pathway and inhibit adipocyte differentiation of 3T3-L1 cells. Phytother. Res. 2011, 25, 1629–1635. [Google Scholar] [CrossRef]
- Takada, I.; Kouzmenko, A.P.; Kato, S. Wnt and PPARγ signaling in osteoblastogenesis and adipogenesis. Nat. Rev. Rheumatol. 2009, 5, 442–447. [Google Scholar] [CrossRef]
- Lee, H.; Bae, S.; Kim, K.; Kim, W.; Chung, S.-I.; Yang, Y.; Yoon, Y. Shikonin inhibits adipogenesis by modulation of the WNT/β-catenin pathway. Life Sci. 2011, 88, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Kong, C.-S. Anti-adipogenic effect of dioxinodehydroeckol via AMPK activation in 3T3-L1 adipocytes. Chem. Biol. Interac. 2010, 186, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding adipocyte differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed]
- Ilavenil, S.; Arasu, M.V.; Lee, J.-C.; Kim, D.H.; Roh, S.G.; Park, H.S.; Choi, G.J.; Mayakrishnan, V.; Choi, K.C. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Phytomedicine 2014, 21, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Katoh, S.; Morimoto, C.; Sakayama, K.; Shiosaka, T.; Masuno, H.; Okuda, H. The hormonal responses of lipoprotein lipase activity and lipolysis in adipose tissue differ depending on the stage of the estrous cycle in female rats. Int. J. Obes. 2002, 26, 610–617. [Google Scholar] [CrossRef]
- Kim, I.H.; Nam, T.J. Enzyme-treated Ecklonia cava extract inhibits adipogenesis through the downregulation of C/EBPα in 3T3-L1 adipocytes. Int. J. Mol. Med. 2017, 39, 636–644. [Google Scholar] [CrossRef]
- Kim, J.A.; Karadeniz, F.; Ahn, B.N.; Kwon, M.S.; Mun, O.J.; Bae, M.J.; Seo, Y.; Kim, M.; Lee, S.H.; Kim, Y.Y. Bioactive quinone derivatives from the marine brown alga Sargassum thunbergii induce anti-adipogenic and pro-osteoblastogenic activities. J. Sci. Food Agric. 2016, 96, 783–790. [Google Scholar] [CrossRef]
- Kang, M.C.; Kang, N.; Ko, S.C.; Kim, Y.-B.; Jeon, Y.J. Anti-obesity effects of seaweeds of Jeju Island on the differentiation of 3T3-L1 preadipocytes and obese mice fed a high-fat diet. Food Chem. Toxicol. 2016, 90, 36–44. [Google Scholar] [CrossRef]
- Grönke, S.; Müller, G.; Hirsch, J.; Fellert, S.; Andreou, A.; Haase, T.; Jäckle, H.; Kühnlein, R.P. Dual lipolytic control of body fat storage and mobilization in Drosophila. PLoS Biol. 2007, 5, e137. [Google Scholar] [CrossRef]
- Miyoshi, H.; Souza, S.C.; Zhang, H.-H.; Strissel, K.J.; Christoffolete, M.A.; Kovsan, J.; Rudich, A.; Kraemer, F.B.; Bianco, A.C.; Obin, M.S. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and-independent mechanisms. J. Biol. Chem. 2006, 281, 15837–15844. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, S.; Cho, E.J. Flavonoids from Acer okamotoanum inhibit adipocyte differentiation and promote lipolysis in the 3T3-L1 cells. Molecules 2020, 25, 1920. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, S.; Xu, H.; Wang, J.; Xue, C.; Wang, Y. Long-chain bases from Cucumaria frondosa inhibit adipogenesis and regulate lipid metabolism in 3T3-L1 adipocytes. Food Sci. Biotechnol. 2016, 25, 1753–1760. [Google Scholar] [CrossRef]
- Fernández-Galilea, M.; Pérez-Matute, P.; Prieto-Hontoria, P.L.; Martinez, J.A.; Moreno-Aliaga, M.J. Effects of lipoic acid on lipolysis in 3T3-L1 adipocytes. J. Lipid Res. 2012, 53, 2296–2306. [Google Scholar] [CrossRef]
- Habinowski, S.A.; Witters, L.A. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem. Biophys. Res. Commun. 2001, 286, 852–856. [Google Scholar] [CrossRef]
- Jin, H.; Lee, K.; Chei, S.; Oh, H.-J.; Lee, K.P.; Lee, B.-Y. Ecklonia stolonifera extract suppresses lipid accumulation by promoting lipolysis and adipose browning in high-fat diet-induced obese male mice. Cells 2020, 9, 871. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-G.; Kim, H.-S.; Je, J.-G.; Hwang, J.; Sanjeewa, K.A.; Lee, D.-S.; Song, K.-M.; Choi, Y.-S.; Kang, M.-C.; Jeon, Y.-J. Lipid inhibitory effect of (−)-loliolide isolated from Sargassum horneri in 3T3-L1 adipocytes: Inhibitory mechanism of adipose-specific proteins. Mar. Drugs 2021, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 108073. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.; Lee, J.H.; Chung, H.T.; Pae, H.O. Therapeutic roles of heme oxygenase-1 in metabolic diseases: Curcumin and resveratrol analogues as possible inducers of heme oxygenase-1. Oxid. Med. Cell. Longev. 2013, 2013, 639541. [Google Scholar] [CrossRef] [PubMed]
- Eo, H.; Jeon, Y.J.; Lee, M.; Lim, Y. Brown Alga Ecklonia cava polyphenol extract ameliorates hepatic lipogenesis, oxidative stress, and inflammation by activation of AMPK and SIRT1 in high-fat diet-induced obese mice. J. Agric. Food Chem. 2015, 63, 349–359. [Google Scholar] [CrossRef]
- Guan, B.; Chen, K.; Tong, Z.; Chen, L.; Chen, Q.; Su, J. Advances in fucoxanthin research for the prevention and treatment of inflammation-related diseases. Nutrients 2022, 14, 4768. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.J.; Kim, K.J.; Seo, Y.-J.; Choi, J.; Lee, B.Y. Modulation of HO-1 by ferulic acid attenuates adipocyte differentiation in 3T3-L1 cells. Molecules 2017, 22, 745. [Google Scholar] [CrossRef]
- Lee, H.G.; Hur, J.; Won, J.P.; Seo, H.G. Heme oxygenase-1 mediates the inhibitory effect of ginseng (Panax ginseng) leaf extract on differentiation in 3T3-L1 adipocytes. Mol. Cell. Toxicol. 2023, 20, 1–10. [Google Scholar] [CrossRef]
- Seo, Y.J.; Kim, K.J.; Koh, E.J.; Choi, J.; Lee, B.Y. Anti-adipogenesis mechanism of pterostilbene through the activation of heme oxygenase-1 in 3T3-L1 cells. Phytomedicine 2017, 33, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R.; Arora, A. Health promoting functional lipids from microalgae pool: A review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Lee, O.H.; Yoon, K.Y.; Kim, K.J.; You, S.; Lee, B.Y. Seaweed extracts as a potential tool for the attenuation of oxidative damage in obesity-related pathologies 1. J. Phycol. 2011, 47, 548–556. [Google Scholar] [CrossRef]
- Iwai, K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-A y mice. Plant Foods Hum. Nut. 2008, 63, 163–169. [Google Scholar] [CrossRef]
- Funahashi, H.; Imai, T.; Mase, T.; Sekiya, M.; Yokoi, K.; Hayashi, H.; Shibata, A.; Hayashi, T.; Nishikawa, M.; Suda, N. Seaweed prevents breast cancer? Jpn.J. Cancer Res. 2001, 92, 483–487. [Google Scholar] [CrossRef]
- Teas, J.; Vena, S.; Cone, D.L.; Irhimeh, M. The consumption of seaweed as a protective factor in the etiology of breast cancer: Proof of principle. J. Appl. Phycol. 2013, 25, 771–779. [Google Scholar] [CrossRef]
- Song, F.H.; Fan, X.; Xu, X.L.; Zhao, J.L.; Han, L.J.; Shi, J.G. Chemical constituents of the brown alga Dictyopteris divaricata. J. Asian Nat. Prod. Res. 2005, 7, 777–781. [Google Scholar] [CrossRef]
- Zatelli, G.A.; Philippus, A.C.; Falkenberg, M. An overview of odoriferous marine seaweeds of the Dictyopteris genus: Insights into their chemical diversity, biological potential and ecological roles. Rev. Bras. Farmacogn. 2018, 28, 243–260. [Google Scholar] [CrossRef]
- Miyashita, K. Function of marine carotenoids. Food Factors Health Promot. 2009, 61, 136–146. [Google Scholar]
- Sharma, P.P.; Baskaran, V. Polysaccharide (laminaran and fucoidan), fucoxanthin and lipids as functional components from brown algae (Padina tetrastromatica) modulates adipogenesis and thermogenesis in diet-induced obesity in C57BL6 mice. Algal Res. 2021, 54, 102187. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Kim, M.-S.; Choi, J.S. Anti-adipogenic activity of the edible brown alga Ecklonia stolonifera and its constituent fucosterol in 3T3-L1 adipocytes. Arch. Pharm. Res. 2014, 37, 713–720. [Google Scholar] [CrossRef]
- Han, J.; Guo, X.; Koyama, T.; Kawai, D.; Zhang, J.; Yamaguchi, R.; Zhou, X.; Motoo, Y.; Satoh, T.; Yamada, S. Zonarol protected liver from methionine-and choline-deficient diet-induced nonalcoholic fatty liver disease in a mouse model. Nutrients 2021, 13, 3455. [Google Scholar] [CrossRef]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef]
- Rosyantari, A.; Prasedya, E.; Ilhami, B.; Martyasari, N.; Padmi, H.; Abidin, A.; Ambana, Y.; Kirana, I.; Sunarwidhi, A. Total Phenolic Content (TPC), Total Flavonoid Content (TFC) and Antioxidants Activity of Marine Sponge Stylissa flabelliformis Ethanol Extract. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012109. [Google Scholar] [CrossRef]
- Farasat, M.; Khavari-Nejad, R.-A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant properties of two edible green seaweeds from northern coasts of the Persian Gulf. J. Nat. Pharm. Prod. 2013, 8, 47. [Google Scholar]
- Rizzatti, V.; Boschi, F.; Pedrotti, M.; Zoico, E.; Sbarbati, A.; Zamboni, M. Lipid droplets characterization in adipocyte differentiated 3T3-L1 cells: Size and optical density distribution. Eur. J. Histochem. 2013, 57, e24. [Google Scholar] [CrossRef]
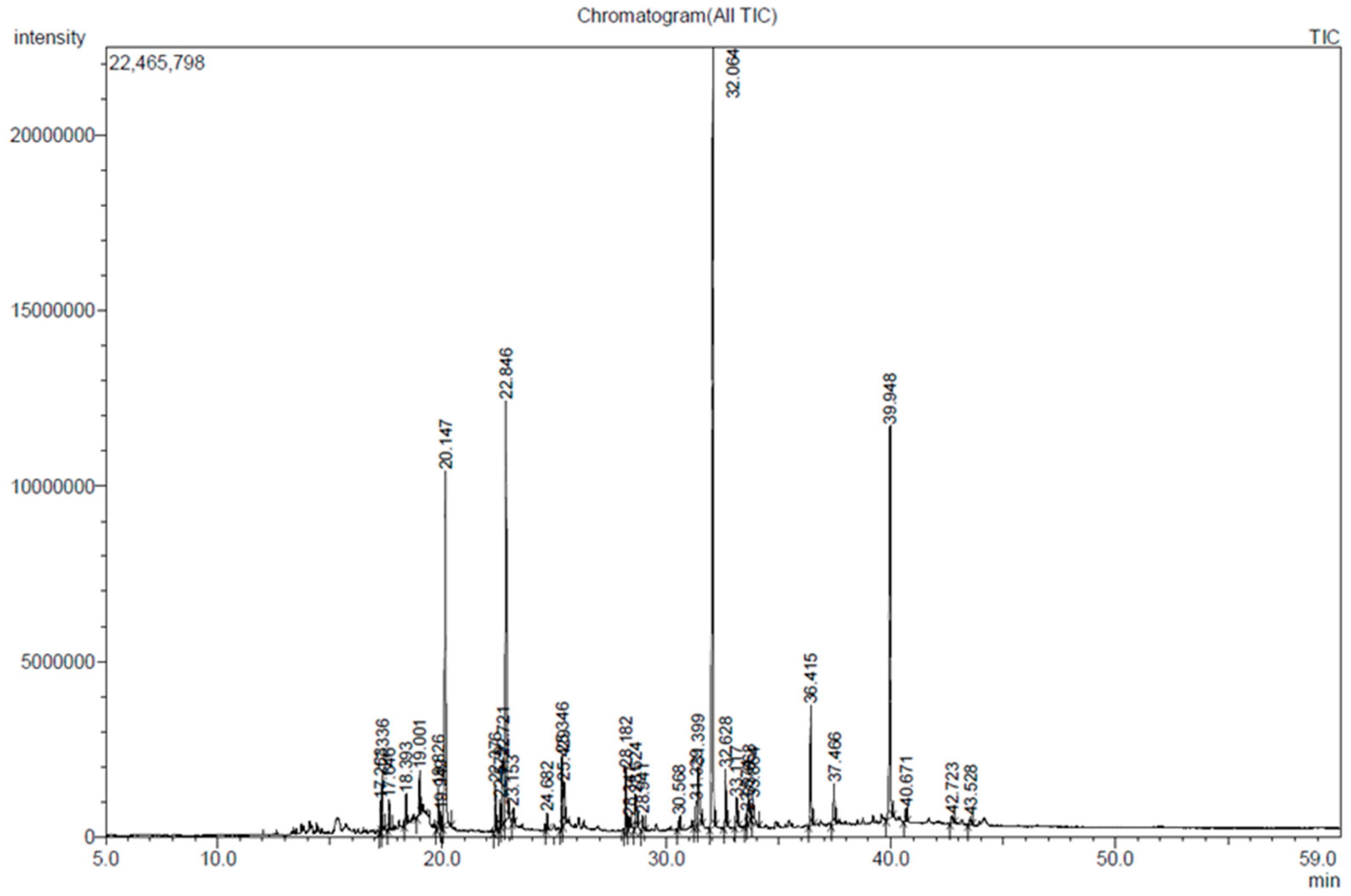

| R. Time | Compound Name | Molecular Formula | Peak Area (%) |
|---|---|---|---|
| 17.253 | (-)-Loliolide | C11H16O3 | 0.64 |
| 17.336 | Tetradecanoic acid | C14H28O2 | 1.13 |
| 18.393 | Neophytadiene | C20H38 | 0.73 |
| 19.826 | 9-Hexadecenoic acid | C16H30O2 | 1.01 |
| 19.949 | Hexadecenoic acid, Z-11- | C16H30O2 | 0.38 |
| 20.147 | n-Hexadecanoic acid | C16H32O2 | 11.46 |
| 22.376 | 2-Hexadecen-1-ol,3,7,11,15-tetramethyl | C20H40O | 1.15 |
| 22.721 | Linoelaidic acid | C18H32O2 | 2.20 |
| 22.846 | 7-Tetradecenal | C14H26O | 14.79 |
| 23.153 | Octadecanoic acid | C18H36O2 | 0.35 |
| 25.346 | Arachidonic acid | C20H32O2 | 2.17 |
| 25.429 | Doconexent | C22H32O2 | 1.27 |
| 28.624 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) ethyl ester | C19H38O4 | 1.02 |
| 31.399 | 9-Octadecenoic acid (Z)-, 2,3-dihydroxypropyl ester | C21H40O4 | 3.02 |
| 32.064 | 1,4-Benzenediol,2-(decahydro-5,5,8a-trimethyl-2-methylene-1-[1R-(1.alpha.,4a.beta.,8a.alpha.)] | C21H30O2 | 28.59 |
| 32.628 | 13-Docosenamide | C22H43NO | 1.52 |
| 36.415 | γ-Tocopherol | C28H48O2 | 3.22 |
| 37.466 | Vitamin E | C29H50O2 | 1.53 |
| 39.948 | Fucosterol | C29H48O | 12.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dayarathne, L.A.; Ko, S.-C.; Yim, M.-J.; Lee, J.M.; Kim, J.-Y.; Oh, G.-W.; Kim, C.H.; Kim, K.W.; Lee, D.-S.; Je, J.-Y. Brown Algae Dictyopteris divaricata Attenuates Adipogenesis by Modulating Adipocyte Differentiation and Promoting Lipolysis through Heme Oxygenase-1 Activation in 3T3-L1 Cells. Mar. Drugs 2024, 22, 91. https://doi.org/10.3390/md22020091
Dayarathne LA, Ko S-C, Yim M-J, Lee JM, Kim J-Y, Oh G-W, Kim CH, Kim KW, Lee D-S, Je J-Y. Brown Algae Dictyopteris divaricata Attenuates Adipogenesis by Modulating Adipocyte Differentiation and Promoting Lipolysis through Heme Oxygenase-1 Activation in 3T3-L1 Cells. Marine Drugs. 2024; 22(2):91. https://doi.org/10.3390/md22020091
Chicago/Turabian StyleDayarathne, Lakshi A., Seok-Chun Ko, Mi-Jin Yim, Jeong Min Lee, Ji-Yul Kim, Gun-Woo Oh, Chul Hwan Kim, Kyung Woo Kim, Dae-Sung Lee, and Jae-Young Je. 2024. "Brown Algae Dictyopteris divaricata Attenuates Adipogenesis by Modulating Adipocyte Differentiation and Promoting Lipolysis through Heme Oxygenase-1 Activation in 3T3-L1 Cells" Marine Drugs 22, no. 2: 91. https://doi.org/10.3390/md22020091
APA StyleDayarathne, L. A., Ko, S.-C., Yim, M.-J., Lee, J. M., Kim, J.-Y., Oh, G.-W., Kim, C. H., Kim, K. W., Lee, D.-S., & Je, J.-Y. (2024). Brown Algae Dictyopteris divaricata Attenuates Adipogenesis by Modulating Adipocyte Differentiation and Promoting Lipolysis through Heme Oxygenase-1 Activation in 3T3-L1 Cells. Marine Drugs, 22(2), 91. https://doi.org/10.3390/md22020091






