Calcium Transport Activity of UV/H2O2-Degraded Fucoidans and Their Structural Characterization
Abstract
1. Introduction
2. Results and Discussion
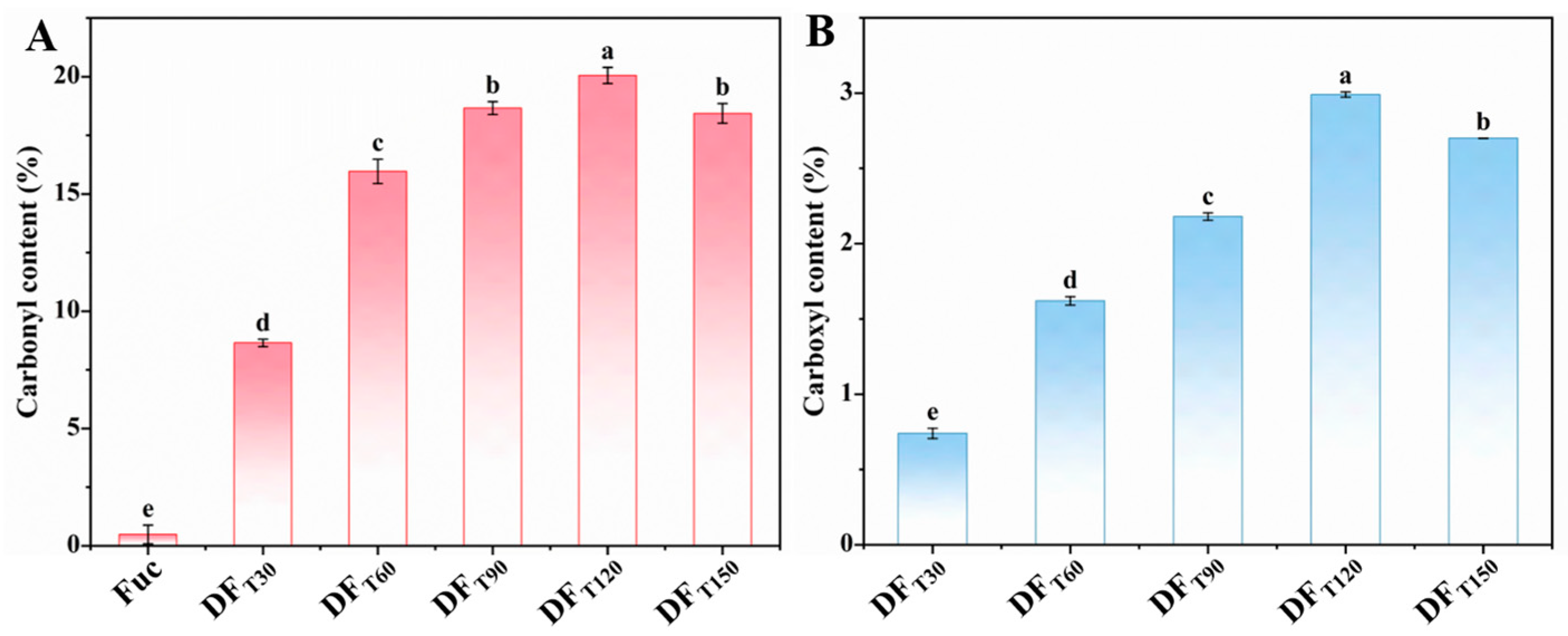
2.1. Preparation of DF with High Carbonyl and Carboxyl Contents
2.2. Characterization of DF
2.2.1. Molecular Weight (Mw)
2.2.2. Chain Conformation
2.3. Calcium-Chelating Capacity of DF-Ca Chelates
2.4. Characterization of DF-Ca Chelates
2.4.1. Thermodynamic Mechanism of Calcium Binding to DF
2.4.2. Microstructural Analysis
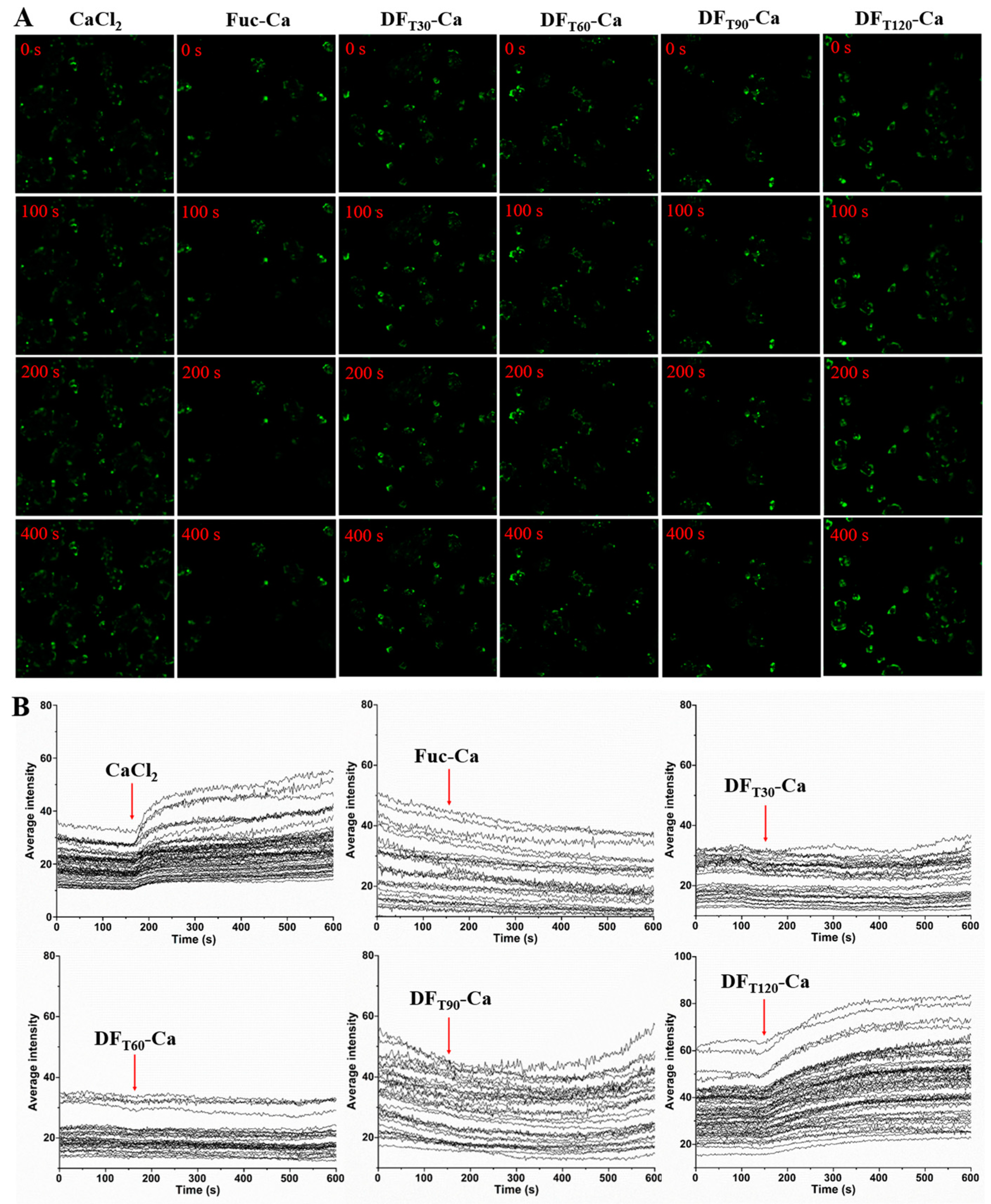
2.5. Transport Capacities of Different DFs Across Caco-2 Cell Monolayers
2.6. Changes in Intracellular Calcium Level by DF-Ca Chelates
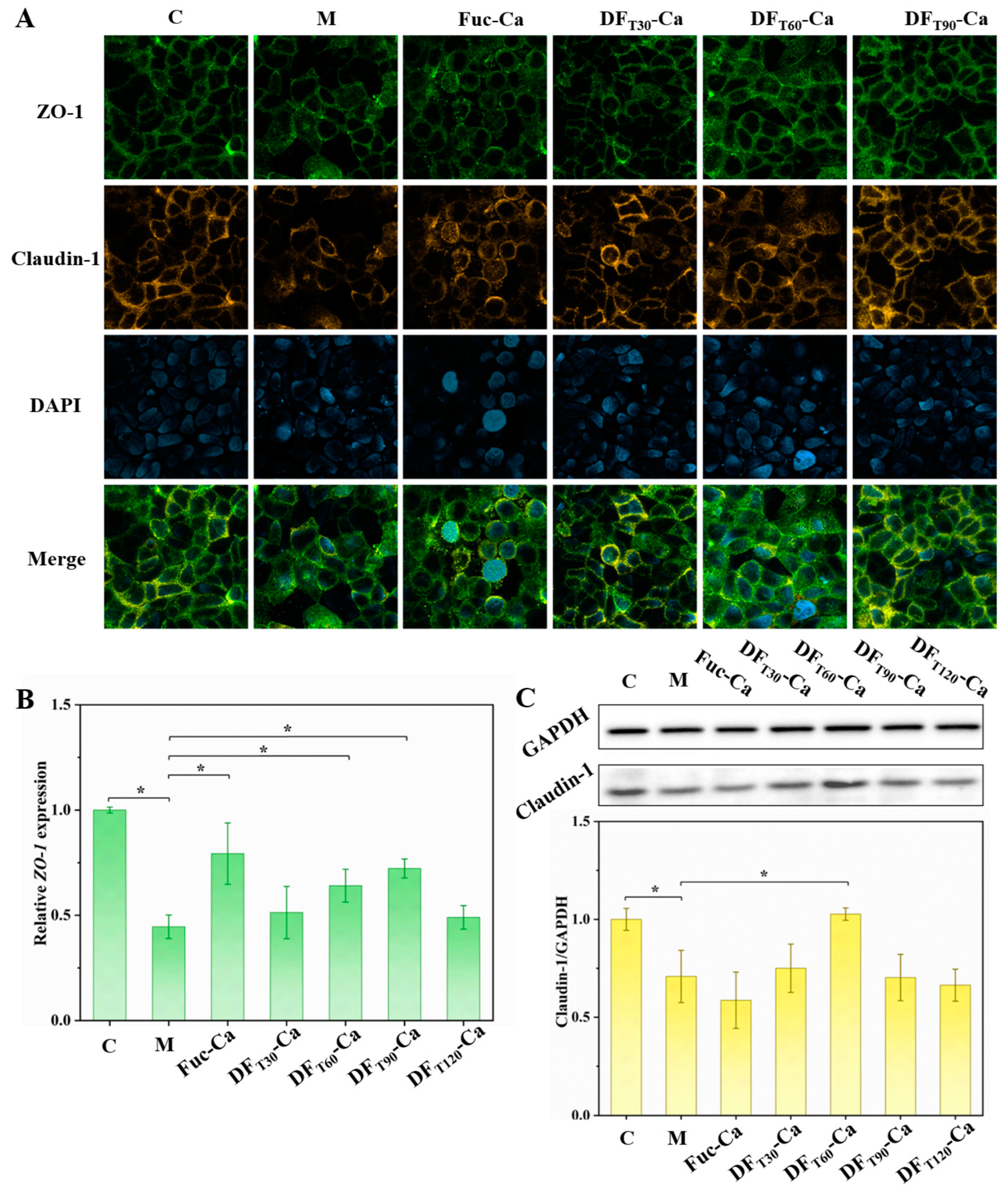
2.7. Influence of Different DF-Ca Chelates on Caco-2 Cell Barrier
3. Materials and Methods
3.1. Materials and Reagents
3.2. Degradation of Fucoidan Using UV/H2O2 System
3.3. Contents of Carbonyl and Carboxyl Groups
3.4. Characterization of Degraded Fucoidan
3.4.1. Molecular Weight (Mw) Distributions
3.4.2. Dynamic Light Scattering (DLS)
3.4.3. Small-Angle X-Ray Scattering (SAXS)
3.5. Preparation of Degraded Fucoidan–Calcium (DF-Ca) Chelates
3.6. Characterization of DF-Ca Chelates
3.6.1. Isothermal Titration Calorimetry (ITC)
3.6.2. Scanning Electron Microscopy (SEM)
3.7. Transport Studies of Different DFs Across Caco-2 Cell Monolayers
3.7.1. Fluorescent Labeling of DF
3.7.2. Cell Cultivation and Viability
3.7.3. Transport Capacity of DF-FITC Across Caco-2 Cell Monolayers
3.8. Calcium Transport-Promoting Activity of DF-Ca Chelate
3.8.1. Effects of DF-Ca Chelates on Intracellular Calcium Level
3.8.2. Effects of DF-Ca on Caco-2 Cell Barrier
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Krupa-Kozak, U.; Swiatecka, D.; Baczek, N.; Brzoska, M.M. Inulin and fructooligosaccharide affect in vitro calcium uptake and absorption from calcium-enriched gluten-free bread. Food Funct. 2016, 7, 1950–1958. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; He, H.; Hou, T. Purification and characterization of positive allosteric regulatory peptides of calcium sensing receptor (CaSR) from desalted duck egg white. Food Chem. 2020, 325, 126919. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, B.; Luo, M.; Sun, S.; Lin, Q.; Kan, Q.; He, Z.; Miao, J.; Du, H.; Xiao, H.; et al. A comprehensive review on preparation, structure-activities relationship, and calcium bioavailability of casein phosphopeptides. Crit. Rev. Food Sci. Nutr. 2024, 64, 996–1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, Y.; Zhang, X.; Li, Y.; Wang, R.; Luo, X.; Li, Y.; Li, J.; Chen, Z. Isolation of a novel calcium-binding peptide from wheat germ protein hydrolysates and the prediction for its mechanism of combination. Food Chem. 2018, 239, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Liu, W.; Shi, W.; Ma, Z.; He, H. Desalted duck egg white peptides promote calcium uptake by counteracting the adverse effects of phytic acid. Food Chem. 2017, 219, 428–435. [Google Scholar] [CrossRef]
- Li, X.; Jiang, F.; Liu, M.; Qu, Y.; Lan, Z.; Dai, X.; Huang, C.; Yue, X.; Zhao, S.; Pan, X. Synthesis, characterization, and bioactivities of polysaccharide metal complexes: A review. J. Agric. Food Chem. 2022, 3, 70–79. [Google Scholar] [CrossRef]
- Chi, Y.; Li, H.; Fan, L.; Du, C.; Zhang, J.; Guan, H.; Wang, P.; Li, R. Metal-ion-binding properties of ulvan extracted from Ulva clathrata and structural characterization of its complexes. Carbohydr. Polym. 2021, 272, 118508. [Google Scholar] [CrossRef]
- Zhou, J.; Cheng, J.; Liu, L.; Luo, J.; Peng, X. Lactobacillus acidophilus (LA) fermenting astragalus polysaccharides (APS) improves calcium absorption and osteoporosis by altering gut microbiota. Foods 2023, 12, 275. [Google Scholar] [CrossRef]
- Ren, L.; Li, J.; Xiao, Y.; Zhang, Y.; Fan, J.; Zhang, B.; Wang, L.; Shen, X. Polysaccharide from Lycium barbarum L. leaves enhances absorption of endogenous calcium, and elevates cecal calcium transport protein levels and serum cytokine levels in rats. J. Funct. Foods 2017, 33, 227–234. [Google Scholar] [CrossRef]
- Zheng, Z.; Pan, X.; Luo, L.; Zhang, Q.; Huang, X.; Liu, Y.; Wang, K.; Zhang, Y. Advances in oral absorption of polysaccharides: Mechanism, affecting factors, and improvement strategies. Carbohydr. Polym. 2022, 282, 119110. [Google Scholar] [CrossRef]
- Yin, J.Y.; Nie, S.P.; Guo, Q.B.; Cui, S.; Wang, Q.; Xie, M.Y. Effect of calcium on solution and conformational characteristics of polysaccharide from seeds of Plantago asiatica L. Carbohydr. Polym. 2015, 124, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Marin, E.; Martinez, A. Carbohydrates and their free radical scavenging capability: A theoretical study. J. Phys. Chem. B 2012, 116, 9668–9675. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Shao, C.; Piao, Y.; Hu, H.; Lu, K.; Zhang, T.; Zhang, X.; Jia, S.; Wang, M.; Man, S. The mechanism for cleavage of three typical glucosidic bonds induced by hydroxyl free radical. Carbohydr. Polym. 2017, 178, 34–40. [Google Scholar] [CrossRef]
- Simões, J.; Moreira, A.S.P.; Costa, E.; Evtyugin, D.; Domingues, P.; Nunes, F.M.; Coimbra, M.A.; Domingues, M.R.M. Oxidation of amylose and amylopectin by hydroxyl radicals assessed by electrospray ionisation mass spectrometry. Carbohydr. Polym. 2016, 148, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Tudella, J.; Nunes, F.M.; Paradela, R.; Evtuguin, D.V.; Domingues, P.; Amado, F.; Coimbra, M.A.; Barros, A.I.; Domingues, M.R.M. Oxidation of mannosyl oligosaccharides by hydroxyl radicals as assessed by electrospray mass spectrometry. Carbohydr. Res. 2011, 346, 2603–2611. [Google Scholar] [CrossRef]
- Zhu, B.; Ma, C.; You, L. Degradation mechanisms of six typical glucosidic bonds of disaccharides induced by free radicals. J. Agric. Food Chem. 2024, 72, 5439–5451. [Google Scholar] [CrossRef]
- Guay, D.F.; Cole, B.J.W.; Fort, R.C.; Genco, J.M.; Hausman, M.C. Mechanisms of oxidative degradation of carbohydrates during oxygen delignktcation. I. Reaction of methyl β-D-glucopyranoside with photochemically generated hydroxyl radicals. J. Wood Chem. Technol. 2000, 20, 375–394. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Sun-Waterhouse, D.; You, L.J. Insights into the mechanisms underlying the degradation of xylooligosaccharides in UV/H2O2 system. Carbohydr. Polym. 2023, 317, 121091. [Google Scholar] [CrossRef]
- Boulos, S.; Nystrm, L. UPLC-MS/MS investigation of β-glucan oligosaccharide oxidation. Analyst 2016, 141, 6533–6548. [Google Scholar] [CrossRef]
- Li, Y.X.; Wang, J.X.; Peng, S.Q.; Lu, G.X.; Li, S.B. Photocatalytic hydrogen generation in the presence of glucose over ZnS-coated ZnIn2S4 under visible light irradiation. Int. J. Hydrog. Energy 2010, 35, 7116–7126. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, R.F.; Li, Y.Z.; Li, X.; You, L.J.; Kulikouskaya, V.; Hileuskaya, K. Degradation of polysaccharides from Sargassum fusiforme using UV/H2O2 and its effects on structural characteristics. Carbohydr. Polym. 2020, 230, 115647. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.F.; Ma, Y.X.; Cheung, C.; You, L.J.; Liao, L.; Pedisic, S.; Kulikouskaya, V. Structural characteristics and anti-inflammatory activity of UV/H2O2 -treated algal sulfated polysaccharide from Gracilaria lemaneiformis. Food Chem. Toxicol. 2021, 152, 112157. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yang, J.; Wang, Z.; Lu, M.; Yue, K. Modified citrus pectins by UV/H2O2 oxidation at acidic and basic conditions: Structures and in vitro anti-inflammatory, anti-proliferative activities. Carbohydr. Polym. 2020, 247, 116742. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Tao, L.; Wang, Z.-L.; Li, L.-F.; Zhao, C.-C.; Shi, C.-Y.; Sheng, J.; Tian, Y. Effects of UV/H2O2 degradation on Moringa oleifera Lam. leaves polysaccharides: Composition, in vitro fermentation and prebiotic properties on gut microorganisms. Food Chem. X 2024, 22, 101272. [Google Scholar] [CrossRef]
- Narrainen, A.P.; Lovell, P.A. Mechanism and kinetics of free-radical degradation of xyloglucan in aqueous solution. Polymer 2010, 51, 6115–6122. [Google Scholar] [CrossRef]
- Cai, W.D.; Qiu, W.Y.; Ding, Z.C.; Wu, L.X.; Yan, J.K. Conformational and rheological properties of a quaternary ammonium salt of curdlan. Food Chem. 2019, 280, 130–138. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Q.; Kang, Y.; Liu, Y.; Luo, W. Different molecular sizes and chain conformations of water-soluble yeast β-glucan fractions and their interactions with receptor Dectin-1. Carbohydr. Polym. 2021, 273, 118568. [Google Scholar] [CrossRef]
- Ma, H.; Huang, Q.; Ren, J.; Zheng, Z.; Xiao, Y. Structure characteristics, solution properties and morphology of oxidized yeast β-glucans derived from controlled TEMPO-mediated oxidation. Carbohydr. Polym. 2020, 250, 116924. [Google Scholar] [CrossRef]
- Yuguchi, Y.; Tran, V.T.T.; Bui, L.M.; Takebe, S.; Suzuki, S.; Nakajima, N.; Kitamura, S.; Thanh, T.T.T. Primary structure, conformation in aqueous solution, and intestinal immunomodulating activity of fucoidan from two brown seaweed species Sargassum crassifolium and Padina australis. Carbohydr. Polym. 2016, 147, 69–78. [Google Scholar] [CrossRef]
- Ye, J.; Hua, X.; Zhao, Q.; Zhao, W.; Chu, G.; Zhang, W.; Yang, R. Chain conformation and rheological properties of an acid-extracted polysaccharide from peanut sediment of aqueous extraction process. Carbohydr. Polym. 2020, 228, 115410. [Google Scholar] [CrossRef]
- Bulgariu, L.; Bulgariu, D.; Rusu, C. Marine algae biomass for removal of heavy metal Ions. In Springer Handbook of Marine Biotechnology; Kim, S.-K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 11, pp. 611–648. [Google Scholar]
- Xiao, M.; Ren, X.; Yu, Y.; Gao, W.; Zhu, C.; Sun, H.; Kong, Q.; Fu, X.; Mou, H. Fucose-containing bacterial exopolysaccharides: Sources, biological activities, and food applications. Food Chem. X 2022, 13, 100233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Guo, S.; Cheung, P.C.-K.; Hileuskaya, K.; You, L. Protection and delivery of probiotics by degraded fucoidan-chitosan nanogel-based W1/O/W2 double emulsion incorporated in self-assembled hydrogel particles. Food Hydrocoll. 2024, 153, 109999. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Q.; Song, S.; Wang, H.; Tan, M. Enhanced cytotoxicity of cadmium by a sulfated polysaccharide from abalone. J. Agric. Food Chem. 2020, 68, 14996–15004. [Google Scholar] [CrossRef]
- Sun, N.; Hu, S.; Wang, D.; Jiang, P.; Zhang, S.; Lin, S. Calcium delivery systems assembled using antarctic krill derived heptapeptides: Exploration of the assembly mechanism, in vitro digestion profile, and calcium absorption behavior. J. Agric. Food Chem. 2022, 70, 2018–2028. [Google Scholar] [CrossRef]
- Astasov-Frauenhoffer, M.; Varenganayil, M.M.; Decho, A.W.; Waltimo, T.; Braissant, O. Exopolysaccharides regulate calcium flow in cariogenic biofilms. PLoS ONE 2017, 12, e0186256. [Google Scholar] [CrossRef]
- Chen, X.Y.; You, L.J.; Ma, Y.X.; Zhao, Z.G.; Kulikouskaya, V. Influence of UV/H2O2 treatment on polysaccharides from Sargassum fusiforme: Physicochemical properties and RAW 264.7 cells responses. Food Chem. Toxicol. 2021, 153, 112246. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wei, Y.; Zhao, J.; Yu, G.; Li, Q. Transport mechanism and subcellular localization of polysaccharides from Cucurbia Moschata across Caco-2 cells model. Int. J. Biol. Macromol. 2021, 182, 1003–1014. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Z.; Yang, X.; Pan, X.; Yin, L.; Huang, X.; Li, Q.; Shu, Y.; Zhang, Q.; Wang, K. A sensitive and rapid radiolabelling method for the in vivo pharmacokinetic study of lentinan. Food Funct. 2018, 9, 3114–3125. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2012, 403, 37–45. [Google Scholar] [CrossRef]
- Zhu, B.; He, H.; Guo, D.; Zhao, M.; Hou, T. Two novel calcium delivery systems fabricated by casein phosphopeptides and chitosan oligosaccharides: Preparation, characterization, and bioactive studies. Food Hydrocoll. 2020, 102, 105567. [Google Scholar] [CrossRef]
- Vavrusova, M.; Munk, M.B.G.; Skibsted, L.H. Aqueous solubility of calcium L-lactate, calcium D-gluconate, and calcium D-lactobionate: Importance of complex formation for solubility increase by hydroxycarboxylate mixtures. J. Agric. Food Chem. 2013, 61, 8207–8214. [Google Scholar] [CrossRef] [PubMed]
- Ogulur, I.; Pat, Y.; Aydin, T.; Yazici, D.; Ruckert, B.; Peng, Y.; Kim, J.; Radzikowska, U.; Westermann, P.; Sokolowska, M.; et al. Gut epithelial barrier damage caused by dishwasher detergents and rinse aids. J. Allergy Clin. Immunol. 2023, 151, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, X.; Liu, J.; Zhong, F.; Zhang, C.; Chen, Y.; Sun, T.; Ji, C.; Ma, D. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat. Commun. 2022, 9, 2522. [Google Scholar] [CrossRef] [PubMed]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern recognition receptor signaling and cytokine networks in microbial defenses and regulation of intestinal barriers: Implications for inflammatory bowel disease. Gastroenterology 2022, 162, 1602–1616. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, X.; Sun-Waterhouse, D.X.; Zhu, B.Y.; You, L.J.; Hileuskaya, K. Polysaccharides from Sargassum fusiforme after UV/H2O2 degradation effectively ameliorate dextran sulfate sodium-induced colitis. Food Funct. 2021, 12, 11747–11759. [Google Scholar] [CrossRef]
- Dias, A.R.G.; Zavareze, E.d.R.; Helbig, E.; Moura, F.A.d.; Vargas, C.G.; Ciacco, C.F. Oxidation of fermented cassava starch using hydrogen peroxide. Carbohydr. Polym. 2011, 86, 185–191. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, Q.; Weng, H.; Zhang, Y.; Yang, Q.; Xiao, A. Extraction of sulfated agar from Gracilaria lemaneiformis using hydrogen peroxide-assisted enzymatic method. Carbohydr. Polym. 2020, 232, 115790. [Google Scholar] [CrossRef]



| Samples | α value | Rh (nm) | Rg (nm) | Rg/Rh |
|---|---|---|---|---|
| Fuc | −1.06 ± 0.03 | 64.21 ± 1.69 | 142.36 ± 13.65 | 2.22 |
| DFT60 | −1.48 ± 0.02 | 21.07 ± 0.77 | 60.90 ± 11.50 | 2.80 |
| DFT120 | −1.71 ± 0.03 | 5.74 ± 0.09 | 19.71 ± 1.64 | 3.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Wang, J.; You, L.; Lin, L.; Lin, K.; Hileuskaya, K. Calcium Transport Activity of UV/H2O2-Degraded Fucoidans and Their Structural Characterization. Mar. Drugs 2024, 22, 499. https://doi.org/10.3390/md22110499
Zhu B, Wang J, You L, Lin L, Lin K, Hileuskaya K. Calcium Transport Activity of UV/H2O2-Degraded Fucoidans and Their Structural Characterization. Marine Drugs. 2024; 22(11):499. https://doi.org/10.3390/md22110499
Chicago/Turabian StyleZhu, Biyang, Jiacheng Wang, Lijun You, Lianzhu Lin, Kuncheng Lin, and Kseniya Hileuskaya. 2024. "Calcium Transport Activity of UV/H2O2-Degraded Fucoidans and Their Structural Characterization" Marine Drugs 22, no. 11: 499. https://doi.org/10.3390/md22110499
APA StyleZhu, B., Wang, J., You, L., Lin, L., Lin, K., & Hileuskaya, K. (2024). Calcium Transport Activity of UV/H2O2-Degraded Fucoidans and Their Structural Characterization. Marine Drugs, 22(11), 499. https://doi.org/10.3390/md22110499







