Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity
Abstract
1. Introduction
2. Structural and Biological Activity Studies
2.1. Terpenoids
2.1.1. Monoterpenoids
2.1.2. Sesquiterpenes
2.1.3. Diterpenoids
2.1.4. Meroterpenoids
2.2. Polyketides
2.2.1. Lactones
2.2.2. Azaphilones
2.2.3. Xanthones
2.2.4. Other Polyketides
2.3. Nitrogen-Containing Compounds
2.3.1. Alkaloids
2.3.2. Peptides
2.4. Steroids
2.5. Other Classes
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Ghallab, D.S.; Ibrahim, R.S.; Mohyeldin, M.M.; Shawky, E. Marine algae: A treasure trove of bioactive anti-inflammatory compounds. Mar. Pollut. Bull. 2024, 199, 116023. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P. Inflammation and its role in regeneration and repair. Circ. Res. 2019, 124, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. An inflammation-centric view of neurological disease: Beyond the neuron. Front. Cell. Neurosci. 2018, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yi, M.; Ding, L.; He, S. A review of anti-inflammatory compounds from marine fungi, 2000–2018. Mar. Drugs 2019, 17, 636. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Leroy, X.; Bland, E.J.; Montero-Melendez, T. Resolution pharmacology: Opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 2015, 36, 737–755. [Google Scholar] [CrossRef]
- Zhuo, Y.; Li, D.; Cui, L.; Li, C.; Zhang, S.; Zhang, Q.; Zhang, L.; Wang, X.; Yang, L. Treatment with 3,4-dihydroxyphenylethyl alcohol glycoside ameliorates sepsis-induced ALI in mice by reducing inflammation and regulating M1 polarization. Biomed. Pharmacother. 2019, 116, 109012. [Google Scholar] [CrossRef]
- Peerapornratana, S.; Manrique-Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Vo, T.; Ngo, D.; Kim, S. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: An overview. Inflamm. Allergy-Drug Targets 2012, 11, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Dray, A. Inflammatory mediators of pain. Br. J. Anaesth. 1995, 75, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867.11. [Google Scholar] [CrossRef] [PubMed]
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Nah, J.; Jeon, Y. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Wang, Y.; Li, W.; Zhang, H.; Wang, X.; Mu, Q.; He, Z.; Yao, H. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int. Immunopharmacol. 2015, 29, 779–786. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.F.; Wu, N.N.; Wu, Y.W.; Qi, Y.X.; Wei, M.Y.; Pineda, L.M.; Ng, M.G.; Spadafora, C.; Zheng, J.Y.; Lu, L.; et al. Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar. Life Sci. Technol. 2022, 4, 88–97. [Google Scholar] [CrossRef]
- Haque, N.; Parveen, S.; Tang, T.T.; Wei, J.E.; Huang, Z.N. Marine Natural Products in Clinical Use. Marine Drugs. 2022, 20, 528. [Google Scholar] [CrossRef]
- Belgiovine, C.; Bello, E.; Liguori, M.; Craparotta, I.; Mannarino, L.; Paracchini, L.; Beltrame, L.; Marchini, S.; Galmarini, C.M.; Mantovani, A.; et al. Lurbinectedin reduces tumour-associated macrophages and the inflammatory tumour microenvironment in preclinical models. Br. J. Cancer 2017, 117, 628–638. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine polyhydroxynaphthoquinone, echinochrome a: Prevention of atherosclerotic inflammation and probable molecular targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.Q.; Zhang, Q.; Xu, W.F.; Hai, Y.; Chao, R.; Wang, C.F.; Hou, X.M.; Wei, M.Y.; Gu, Y.C.; Wang, C.Y.; et al. Targeted isolation of antitubercular cycloheptapeptides and an unusual pyrroloindoline-containing new analog, asperpyrroindotide A, using LC-MS/MS-based molecular networking. Mar. Life Sci. Technol. 2023, 5, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hai, Y.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987–2020). Mar. Life Sci. Technol. 2021, 3, 488–518. [Google Scholar] [CrossRef]
- Niu, S.W.; Yang, L.H.; Chen, T.T.; Hong, B.H.; Pei, S.X.; Shao, Z.Z.; Zhang, G.Y. New monoterpenoids and polyketides from the deep-sea sediment-derived fungus Aspergillus sydowii MCCC 3A00324. Mar. Drugs 2020, 18, 561. [Google Scholar] [CrossRef]
- Sun, B.; Wang, D.; Ren, J.; Wang, C.; Yan, P.; Gustafson, K.R.; Jiang, W. Paraconulones A–G: Eremophilane sesquiterpenoids from the marine-derived fungus Paraconi othyrium sporulosum DL-16. J. Nat. Prod. 2023, 86, 1360–1369. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.J.; Zou, G.; Yang, W.C.; Zhang, L.S.; Yan, Z.Y.; Long, Y.H.; She, Z.G. Bioactive sesquiterpene derivatives from mangrove endophytic fungus Phomopsis sp. SYSU-QYP- Structures and nitric oxide inhibitory activities. Bioorg. Chem. 2021, 107, 104530. [Google Scholar] [CrossRef]
- Hu, Z.B.; Chen, J.J.; Liu, Q.Q.; Wu, Q.L.; Chen, S.H.; Wang, J.J.; Li, J.; Liu, L.; Gao, Z.Z. Cyclohexenone derivative and drimane sesquiterpenes from the seagrass-derived fungus Aspergillus insuetus. Chem. Biodivers. 2023, 20, e202300424. [Google Scholar] [CrossRef]
- Ning, Y.D.; Gu, Q.W.F.; Zheng, T.; Xu, Y.; Li, S.; Zhu, Y.P.; Hu, B.; Yu, H.B.; Liu, X.Y.; Zhang, Y.; et al. Genome mining leads to diverse sesquiterpenes with anti-inflammatory activity from an arctic-derived fungus. J. Nat. Prod. 2024, 87, 1426–1440. [Google Scholar] [CrossRef]
- Wang, G.S.; Yuan, Y.L.; Li, Z.K.; Liu, X.G.; Chu, Y.H.; She, Z.G.; Kang, W.Y.; Chen, Y. Pleosmaranes A–R, isopimarane and 20-nor isopimarane diterpenoids with anti-inflammatory activities from the mangrove endophytic fungus Pleosporales sp. HNQQJ. J. Nat. Prod. 2024, 87, 304–314. [Google Scholar] [CrossRef]
- He, J.X.; Zou, Q.H.; Deng, H.M.; He, S.T.; Yan, D.; Pan, K.H.; Zhou, Y.W.; Zhao, Z.X.; Cui, H.; Liu, Y.N. Novel 6/7/6 ring system diterpenoids and cytochalasins from the fungus Eutypella scoparia GZU-4-19Y and their anti-inflammatory activity. Fitoterapia 2024, 173, 105804. [Google Scholar] [CrossRef]
- Wang, G.S.; Wu, J.Y.; Li, Z.K.; Chen, T.; Liu, Y.F.; Wang, B.; Chen, Y.; She, Z.G. Talaroacids A–D and talaromarane A, diterpenoids with anti-Inflammatory activities from mangrove endophytic fungus Talaromyces sp. JNQQJ-4. Int. J. Mol. Sci. 2024, 25, 6691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, X.C.; Pan, W.C.; Liu, X.; Tan, S.L.; Cui, H.; Zhao, Z.X. Meroterpenoids from the fungus penicillium sclerotiorum GZU-XW03-2 and their anti-inflammatory activity. Phytochemistry 2022, 202, 113307. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Liu, Y.N.; Ruan, Q.F.; Zhao, M.; Zhao, Z.X.; Cui, H. Aspermeroterpenes A–C: Three meroterpenoids from the marine-derived fungus Aspergillus terreus GZU-31-1. Org. Lett. 2020, 22, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.J.; Cui, X.; Xiong, B.; Yang, M.S.; Zhang, Y.X.; Liu, X.M. Terretonin D1, a new meroterpenoid from marine-derived Aspergillus terreus ML-44. Nat. Prod. Res. 2019, 33, 2262–2265. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Luo, L.; Liu, Y.C.; Bo, X.L.; Wu, F.R.; Wang, F.F.; Tan, M.J.; Wei, Y.Q.; Dou, X.B.; Wang, C.Y.; et al. Diisoprenyl-cyclohexene-type meroterpenoids from a mangrove endophytic fungus Aspergillus sp. GXNU-Y65 and their anti-nonalcoholic steatohepatitis activity in AML12 cells. Phytochemistry 2024, 218, 113955. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.M.; Ye, Y.X.; Xu, Z.Y.; Zhao, Q.L.; Luo, X.W.; Liu, Y.H.; Guo, P. Anti-inflammatory compounds from the mangrove endophytic fungus Amorosia sp. SCSIO 41026. Front. Microbiol. 2022, 13, 976399. [Google Scholar] [CrossRef]
- Yuan, S.W.; Chen, L.T.; Wu, Q.L.; Jiang, M.H.; Guo, H.; Hu, Z.B.; Chen, S.H.; Liu, L.; Gao, Z.Z. Genome mining of α-pyrone natural products from ascidian-derived fungus Amphichorda felina SYSU-MS7908. Mar. Drugs 2022, 20, 294. [Google Scholar] [CrossRef]
- Zhai, G.; Chen, S.; Shen, H.; Guo, H.; Jiang, M.; Liu, L. Bioactive monoterpenes and polyketides from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 553. [Google Scholar] [CrossRef]
- Ding, W.J.; Wang, F.F.; Li, Q.W.; Xue, Y.X.; Dong, Z.T.; Tian, D.M.; Chen, M.; Zhang, Y.W.; Hong, K.; Tang, J.S. Isolation and characterization of anti-inflammatory sorbicillinoids from the mangrove-derived fungus Penicillium sp. DM815. Chem. Biodivers. 2021, 18, e2100229. [Google Scholar] [CrossRef]
- Chen, C.; Ye, G.T.; Tang, J.; Li, J.L.; Liu, W.B.; Wu, L.; Long, Y.H. New polyketides from mangrove endophytic fungus Penicillium sp. BJR-P2 and their anti-inflammatory activity. Mar. Drugs 2022, 20, 583. [Google Scholar] [CrossRef]
- Guo, H.; Wu, Q.L.; Chen, D.N.; Jiang, M.H.; Chen, B.; Lu, Y.J.; Li, J.; Liu, L.; Chen, S.H. Absolute configuration of polypropionate derivatives: Decempyrones A–J and their MptpA inhibition and anti-inflammatory activities. Bioorg. Chem. 2021, 115, 105156. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Ren, X.; Tao, H.M.; Cai, W.T.; Chen, Y.C.; Luo, X.W.; Guo, P.; Liu, Y.H. Anti-inflammatory polyketides from an alga-derived fungus Aspergillus ochraceopetaliformis SCSIO 41020. Mar. Drugs 2022, 20, 295. [Google Scholar] [CrossRef]
- Liu, Z.M.; Qiu, P.; Liu, H.J.; Li, J.; Shao, C.L.; Yan, T.; Cao, W.H.; She, Z.G. Identification of anti-inflammatory polyketides from the coral-derived fungus Penicillium sclerotiorin: In vitro approaches and molecular-modeling. Bioorg. Chem. 2019, 88, 102973. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhang, M.; Fan, H.; Chen, Y.; Dong, S.; Zhou, F.; Wang, B.; Liu, J.; Jin, J.; Luo, Y.; et al. The marine Penicillium sp. GGF16-1-2 metabolite dicitrinone G inhibits pancreatic angiogenesis by regulating the activation of NLRP3 inflammasome. J. Nat. Med. 2024, 78, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.T.; Suping Xiao, S.P.; Huiyi Liao, H.Y.; Jiang, Q.J.; Chen, G.; Wen, L. A new chloro-containing γ-butyrolactone from the mangrove endophytic fungus Neofusicoccum parvum Y2NBKZG1016. Chem. Nat. Comp. 2023, 59, 424–427. [Google Scholar] [CrossRef]
- Zou, Z.B.; Wu, T.Z.; Yang, L.H.; He, X.W.; Liu, W.Y.; Zhang, K.; Xie, C.L.; Xie, M.M.; Zhang, Y.; Yang, X.W.; et al. Hepialiamides A–C: Aminated fusaric acid derivatives and related metabolites with anti-inflammatory activity from the deep-sea-derived fungus Samsoniella hepiali W7. Mar. Drugs 2023, 21, 596. [Google Scholar] [CrossRef]
- Dong, L.; Kim, H.J.; Cao, T.Q.; Liu, Z.; Lee, H.; Ko, W.; Kim, Y.C.; Sohn, J.H.; Kim, T.K.; Yim, J.H.; et al. Anti-inflammatory effects of metabolites from antarctic fungal strain Pleosporales sp. SF-7343 in HaCaT human keratinocytes. Int. J. Mol. Sci. 2021, 22, 9674. [Google Scholar] [CrossRef]
- Hsiao, G.; Chi, W.C.; Chang, C.H.; Chiang, Y.R.; Fu, Y.J.; Lee, T.H. Bioactive pulvinones from a marine algicolous fungus Aspergillus terreus NTU243. Phytochemistry 2022, 200, 113229. [Google Scholar] [CrossRef]
- Tilvi, S.; Parvatkar, R.; Singh, K.S.; Devi, P. Chemical investigation of marine-derived fungus Aspergillus flavipes forpotential anti-inflammatory agents. Chem. Biodivers. 2021, 18, e2000956. [Google Scholar] [CrossRef]
- Chen, S.W.; Zhang, Y.; Niu, X.T.; Mohyuddin, S.G.; Wen, J.Y.; Bao, M.L.; Ju, X.H. Coral-derived endophytic fungal product, butyrolactone-I, alleviates LPS induced intestinal epithelial cell inflammatory response through TLR4/NF-κB and MAPK signaling pathways: An in vitro and in vivo studies. Front. Nutr. 2021, 8, 748118. [Google Scholar] [CrossRef]
- Wu, W.; Liu, L.Y.; Zhu, H.R.; Sun, Y.T.; Wu, Y.; Liao, H.Z.; Gui, Y.H.; Li, L.; Liu, L.; Sun, F.; et al. Butyrolactone-I, an efficient α-glucosidase inhibitor, improves type 2 diabetes with potent TNF-α–lowering properties through modulating gut microbiota in db/db mice. FASEB J. 2019, 33, 12616. [Google Scholar] [CrossRef] [PubMed]
- Gou, X.S.; Tian, D.M.; Wei, J.H.; Ma, Y.H.; Zhang, Y.X.; Chen, M.; Ding, W.J.; Wu, B.; Tang, J.S. New drimane sesquiterpenes and polyketides from marine-derived fungus Penicillium sp. TW58-16 and their anti-inflammatory and α-glucosidase inhibitory effects. Mar. Drugs 2021, 19, 416. [Google Scholar] [CrossRef]
- Hu, Y.W.; Zhao, X.Y.; Song, Y.; Jiang, J.H.; Long, T.; Cong, M.J.; Miao, Y.H.; Liu, Y.Y.; Yang, Z.Y.; Zhu, Y.G.; et al. Anti-inflammatory and neuroprotective α-Pyrones from a marine-derived strain of the fungus Arthrinium arundinis and their heterologous expression. J. Nat. Prod. 2024, 87, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.B.; Wang, Z.; Chang, W.J.; Zhao, W.B.; Wang, H.; Chen, H.Q.; Dai, H.F.; Lv, F. New azaphilones from the marine-derived fungus Penicillium sclerotiorum E23Y-1A with their anti-inflammatory and antitumor activities. Mar. Drugs 2023, 21, 75. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, Y.B.; Yin, J.J.; Chang, W.J.; Zhao, X.L.; Mao, Y. Two new azaphilones from the marine-derived fungus Penicillium sclerotiorum E23Y-1A. Phytochem. Lett. 2022, 47, 76–80. [Google Scholar] [CrossRef]
- Li, J.L.; Li, Z.X.; Chen, T.; Ye, G.T.; Qiu, L.Y.; Long, Y.H. New azaphilones from mangrove endophytic fungus Penicillium sclerotiorin SCNU-F0040. Nat. Prod. Res. 2023, 37, 296–304. [Google Scholar] [CrossRef]
- Wang, H.C.; Ke, T.Y.; Ko, Y.C.; Lin, J.J.; Chang, J.S.; Cheng, Y.B. Anti-inflammatory azaphilones from the edible alga-derived fungus Penicillium sclerotiorum. Mar. Drugs 2021, 19, 529. [Google Scholar] [CrossRef]
- Chen, S.; Guo, H.; Jiang, M.; Wu, Q.; Li, J.; Shen, H.; Liu, L. Mono-and dimeric xanthones with anti-glioma and anti-inflammatory activities from the ascidian-derived fungus Diaporthe sp. SYSU-MS4722. Mar. Drugs 2022, 20, 51. [Google Scholar] [CrossRef]
- Chu, Y.C.; Chang, C.H.; Liao, H.R.; Fu, S.L.; Chen, J.J. Anti-cancer and anti-inflammatory activities of three new chromone derivatives from the marine-derived Penicillium citrinum. Mar. Drugs 2021, 19, 408. [Google Scholar] [CrossRef]
- Lee, Y.S.; Wu, H.C.; Huang, S.J.; Hsiao, G.; Chi, W.C.; Lee, T.H. Anti-inflammatory constituents from a sea anemone-derived fungus Arthrinium arundinis MA30. Phytochemistry 2024, 219, 113998. [Google Scholar] [CrossRef]
- Koopklang, K.; Choodej, S.; Hantanong, S.; Intayot, R.; Jungsuttiwong, S.; Insumran, Y.; Ngamrojanavanich, N.; Pudhom, K. Anti-Inflammatory properties of oxygenated isocoumarins and xanthone from thai mangrove-associated endophytic fungus Setosphaeria rostrata. Molecules 2024, 29, 603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Zhang, Y.; Li, G.; Dong, K.; Wang, J.L.; Xiao, S.J.; Lou, H.X.; Peng, X.P. Anti-inflammatory monomeric sorbicillinoids from the marine-fish-derived fungus Trichoderma sp. G13. Fitoterapia 2024, 175, 105963. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Deng, Y.L.; Lin, X.J.; Chen, B.; Li, J.; Liu, H.J.; Chen, S.H.; Liu, L. Anti-inflammatory mono-and dimeric sorbicillinoids from the marine-derived fungus Trichoderma reesei 4670. J. Nat. Prod. 2019, 82, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Chen, T.; Sun, B.; Tan, Q.; Ouyang, H.; Wang, B.; Yu, H.J.; She, Z.G. Mono-and dimeric sorbicillinoid inhibitors targeting IL-6 and IL-1β from the mangrove-derived fungus Trichoderma reesei BGRg-3. Int. J. Mol. Sci. 2023, 24, 16096. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Meng, Q.Y.; Liu, D.; Fan, A.; Huang, J.; Lin, W.H. Targeted isolation of sorbicilinoids from a deep-sea derived fungus with anti-neuroinflammatory activities. Phytochemistry 2024, 219, 113976. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.X.; Song, X.S.; Pan, W.C.; Cui, H.; Zhao, Z.X. New chromone compounds from the marine derived fungus Diaporthe sp. XW12-1. Fitoterapia 2023, 164, 105384. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, W.; Liao, Q.; She, Z. Pyrone derivatives from a mangrove endophytic fungus Phomopsis asparagi LSLYZ-87. Chem. Biodivers. 2022, 19, e202200491. [Google Scholar] [CrossRef]
- Qin, X.Y.; Huang, J.G.; Zhou, D.X.; Zhang, W.X.; Zhang, Y.J.; Li, J.; Yang, R.Y.; Huang, X.S. Polyketide derivatives, guhypoxylonols A–D from a mangrove endophytic fungus Aspergillus sp. GXNU-Y45 that inhibit nitric oxide production. Mar. Drugs 2021, 20, 5. [Google Scholar] [CrossRef]
- Lei, H.; Bi, X.X.; Lin, X.P.; She, J.L.; Luo, X.W.; Niu, H.; Zhang, D.; Yang, B. Heterocornols from the sponge-derived fungus Pestalotiopsis heterocornis with anti-inflammatory activity. Mar. Drugs 2021, 19, 585. [Google Scholar] [CrossRef]
- Cong, M.J.; Zhang, Y.; Feng, X.Y.; Pang, X.Y.; Liu, Y.H.; Zhang, X.Y.; Yang, Z.Y.; Wang, J.F. Anti-inflammatory alkaloids from the cold-seep-derived fungus Talaromyces helicus SCSIO41311. 3 Biotech. 2022, 12, 161. [Google Scholar] [CrossRef]
- Lu, C.J.; Liang, L.F.; Zhang, G.S.; Li, H.Y.; Fu, C.Q.; Yu, Q.; Zhou, D.M.; Su, Z.W.; Liu, K.; Gao, C.H.; et al. Carneusones A–F, benzophenone derivatives from sponge-derived fungus Aspergillus carneus GXIMD00543. Mar. Drugs 2024, 22, 63. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Quang, T.H.; Tien, N.T.; Kim, K.W.; Kim, Y.C.; Ngan, N.T.T.; Cuong, N.X.; Nam, N.H.; Oh, H. Anti-neuroinflammatory effect of oxaline, isorhodoptilometrin, and 5-hydroxy-7-(2′-hydroxypropyl)-2-methyl-chromone obtained from the marine fungal strain Penicillium oxalicum CLC-MF05. Arch. Pharm. Res. 2022, 45, 90–104. [Google Scholar] [CrossRef]
- Li, X.J.; Chen, Y.C.; Li, S.N.; Zhang, W.Y.; Yan, H.J.; Liu, H.X.; Zhang, W.M. 3-Carboxy-indole derivatives from the deep-sea-derived fungus Phomopsis tersa FS441. Fitoterapia 2024, 172, 105772. [Google Scholar] [CrossRef]
- Song, Y.Y.; She, J.L.; Chen, W.H.; Wang, J.M.; Tan, Y.H.; Pang, X.Y.; Zhou, X.F.; Wang, J.F.; Liu, Y.H. New fusarin derivatives from the marine algicolous fungus Penicillium steckii SCSIO41040. Mar. Drugs 2023, 21, 532. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.Q.; Zheng, Y.Y.; Wang, C.Y.; Liu, Y.; Yao, G.S. Sclerotioloids A–C: Three new alkaloids from the marine-derived fungus Aspergillus sclerotiorum ST0501. Mar. Drugs 2023, 21, 219. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Guo, X.; Wu, J.S.; Liu, D.; Gu, Y.C.; Huang, J.; Fan, A.; Lin, W.H. Prenylated notoamide-type alkaloids isolated from the fungus Aspergillus sclerotiorum and their inhibition of NLRP3 inflammasome activation and antibacterial activities. Phytochemistry 2022, 203, 113424. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.T.; Wang, J.Z.; Tian, Y.; Li, M.; Xu, S.H.; Zhang, L.J.; Luo, X.W.; Tan, Y.H.; Liang, H.; Chen, M. Equisetin protects from atherosclerosis in vivo by binding to STAT3 and inhibiting its activity. Pharmacol. Res. 2024, 206, 107289. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.T.; Yang, L.; Guo, J.C.; Ma, Q.Y.; Xie, Q.Y.; Jiang, L.; Yu, Z.F.; Dai, H.F.; Zhao, Y.X. Anti-diabetic and anti-inflammatory indole diterpenes from the marine-derived fungus Penicillium sp. ZYX-Z-143. Bioorg. Chem. 2024, 145, 107205. [Google Scholar] [CrossRef] [PubMed]
- Niveditha, L.; Fu, P.; Leao, T.F.; Li, T.; Wang, T.; Poulin, R.X.; Gaspar, L.R.; Naman, C.B.; Thavarool, P.S. Targeted isolation of two new anti-inflammatory and UV-A protective dipyrroloquinones from the sponge-associated fungus Aspergillus tamarii MCCF102. Planta Med. 2022, 88, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Anh, C.V.; Yoon, Y.D.; Kang, J.S.; Lee, H.S.; Heo, C.S.; Shin, H.J. Nitrogen-containing secondary metabolites from a deep-sea fungus Aspergillus unguis and their anti-inflammatory activity. Mar. Drugs 2022, 20, 217. [Google Scholar] [CrossRef]
- Yao, G.S.; Ma, Z.L.; Zheng, Y.Y.; Lv, L.; Mao, J.Q.; Wang, C.Y. Bioactive alkaloids from the marine-derived fungus Metarhizium sp. P2100. J. Fungi 2022, 8, 1218. [Google Scholar] [CrossRef]
- Liu, Z.M.; Chen, Y.C.; Li, S.N.; Hu, C.Y.; Liu, H.X.; Zhang, W.M. Indole diketopiperazine alkaloids from the deep-sea-derived fungus Aspergillus sp. FS445. Nat. Prod. Res. 2022, 36, 5213–5221. [Google Scholar] [CrossRef]
- Li, P.H.; Zhang, M.Q.; Li, H.N.; Wang, R.C.; Hou, H.R.; Li, X.B.; Liu, K.C.; Chen, H. New prenylated indole homodimeric and pteridine alkaloids from the marine-derived fungus Aspergillus austroafricanus Y32-2. Mar. Drugs 2021, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Liu, Z.M.; Tan, H.B.; Chen, Y.C.; Zhu, S.; Liu, H.X.; Zhang, W.M. Photeroids A and B, unique phenol–sesquiterpene meroterpenoids from the deep-sea-derived fungus Phomopsis tersa. Org. Biomol. Chem. 2020, 18, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Wu, L.; Liu, R.R.; Li, J.L.; Liu, L.L.; Chen, C.; Li, J.S.; Zhang, K.; Liao, J.J.; Long, Y.H. Penifuranone A: A novel alkaloid from the mangrove endophytic fungus Penicillium crustosum SCNU-F0006. Int. J. Mol. Sci. 2024, 25, 5032. [Google Scholar] [CrossRef]
- Chen, S.H.; Jiang, M.H.; Chen, B.; Salaenoi, J.; Niaz, S.I.; He, J.G.; Liu, L. Penicamide A, a unique N, N′-ketal quinazolinone alkaloid from ascidian-derived fungus Penicillium sp. 4829. Mar. Drugs 2019, 17, 522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Du, H.F.; Liu, Y.F.; Cao, F.; Luo, D.Q.; Wang, C.Y. Novel anti-inflammatory diketopiperazine alkaloids from the marine-derived fungus Penicillium brasilianum. Appl. Microbiol. Biot. 2024, 108, 194. [Google Scholar] [CrossRef]
- Chen, Y.H.; Zhu, Q.; Li, J.; Yang, R.; Zhang, J.; You, M.; Luo, L.; Yang, B. Optimization of Fermentation Process for New Anti-Inflammatory Glycosylceramide Metabolite from Aspergillus sp. Metabolites 2024, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wu, X.; Xu, L.; El-Shazly, M.; Ma, C.; Yuan, S.; Wang, P.; Luo, L. Two new cerebroside metabolites from the marine fungus Hortaea werneckii. Chem. Biodivers. 2022, 19, e202200008. [Google Scholar] [CrossRef]
- Hsiao, G.; Wang, S.W.; Chiang, Y.R.; Chi, W.C.; Kuo, Y.H.; Phong, D.A.; Chen, C.Y.; Lee, T.H. Anti-inflammatory effects of peptides from a marine algicolous fungus Acremonium sp. NTU492 in BV-2 microglial cells. J. Food. Drug Anal. 2020, 28, 283. [Google Scholar] [CrossRef]
- Ding, W.J.; Tian, D.M.; Chen, M.; Xia, Z.X.; Tang, X.Y.; Zhang, S.H.; Wei, J.H.; Li, X.N.; Yao, X.S.; Wu, B.; et al. Molecular networking-guided isolation of cyclopentapeptides from the hydrothermal vent sediment derived fungus Aspergillus pseudoviridinutans TW58-5 and their anti-inflammatory effects. J. Nat. Prod. 2023, 86, 1919–1930. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Chen, Y.H.; Bian, H.H.; Zhang, J.P.; Su, L.; Han, H.; Zhang, W. Anti-inflammatory ergosteroid derivatives from the coral-associated fungi Penicillium oxalicum HL-44. Molecules 2023, 28, 7784. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dong, Z.; Qiu, P.; Wang, Q.; Yan, J.; Lu, Y.; Wasu, P.A.; Hong, K.; She, Z. Two new bioactive steroids from a mangrove-derived fungus Aspergillus sp. Steroids 2018, 140, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liang, J.; Wang, Y.; Liu, Y.; Zhou, C.; Hong, P.; Zhang, Y.; Qian, Z.J. A new benzaldehyde from the coral-derived fungus Aspergillus terreus C23-3 and its anti-inflammatory effects via suppression of MAPK signaling pathway in RAW264. 7 cells. J. Zhejiang Univ. Sci. B 2022, 23, 230–240. [Google Scholar] [CrossRef]
- Cai, J.; Zhou, X.M.; Yang, X.; Tang, M.M.; Liao, Q.Y.; Meng, B.Z.; Liao, S.; Chen, G.Y. Three new bioactive natural products from the fungus Talaromyces assiutensis JTY2. Bioorg. Chem. 2020, 94, 103362. [Google Scholar] [CrossRef]
- Wen, H.L.; Chen, C.M.; Sun, W.G.; Zang, Y.; Li, Q.; Wang, W.X.; Zeng, F.R.; Liu, J.J.; Zhou, Y.; Zhou, Q.; et al. Phenolic C-glycosides and aglycones from marine-derived Aspergillus sp. and their anti-inflammatory activities. J. Nat. Prod. 2019, 82, 1098–1106. [Google Scholar] [CrossRef]
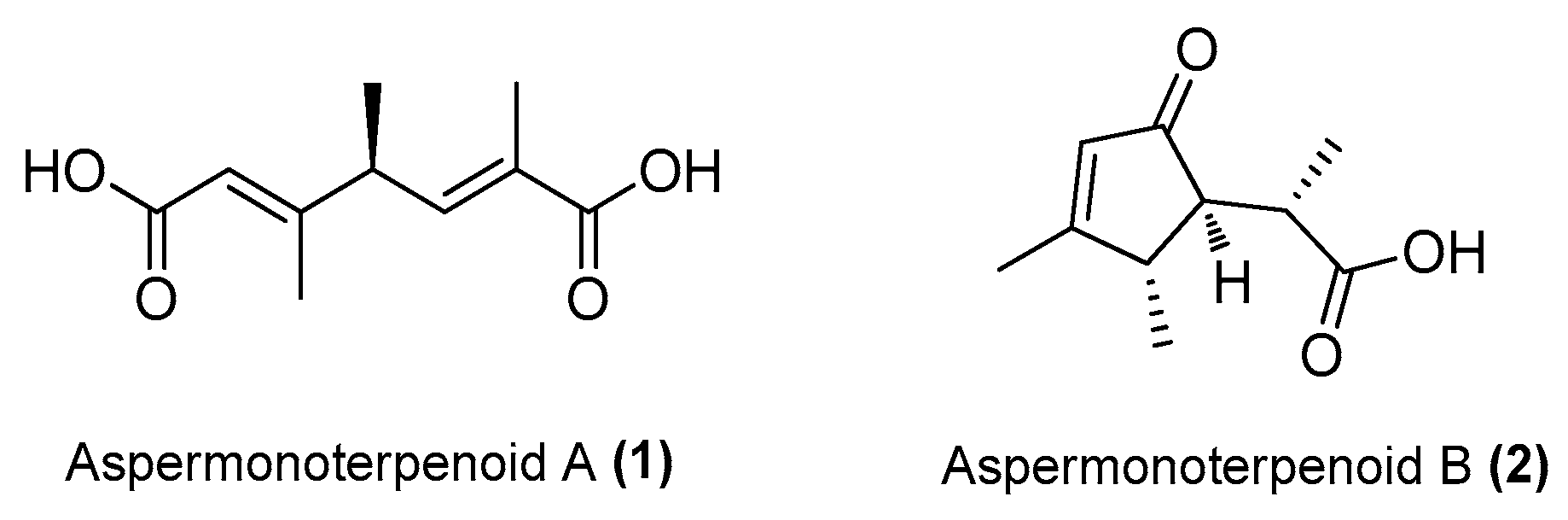

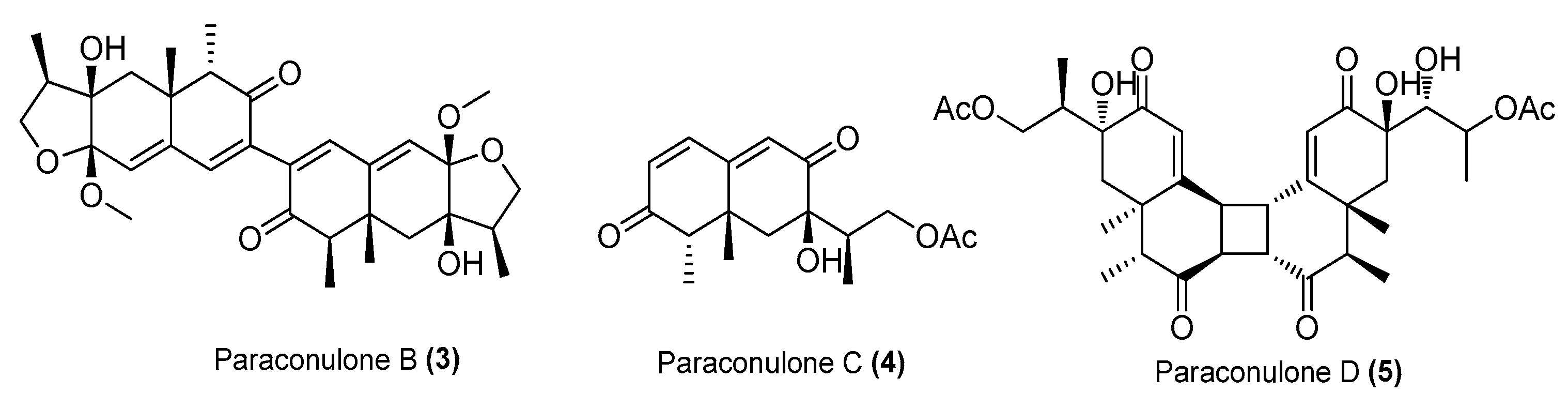
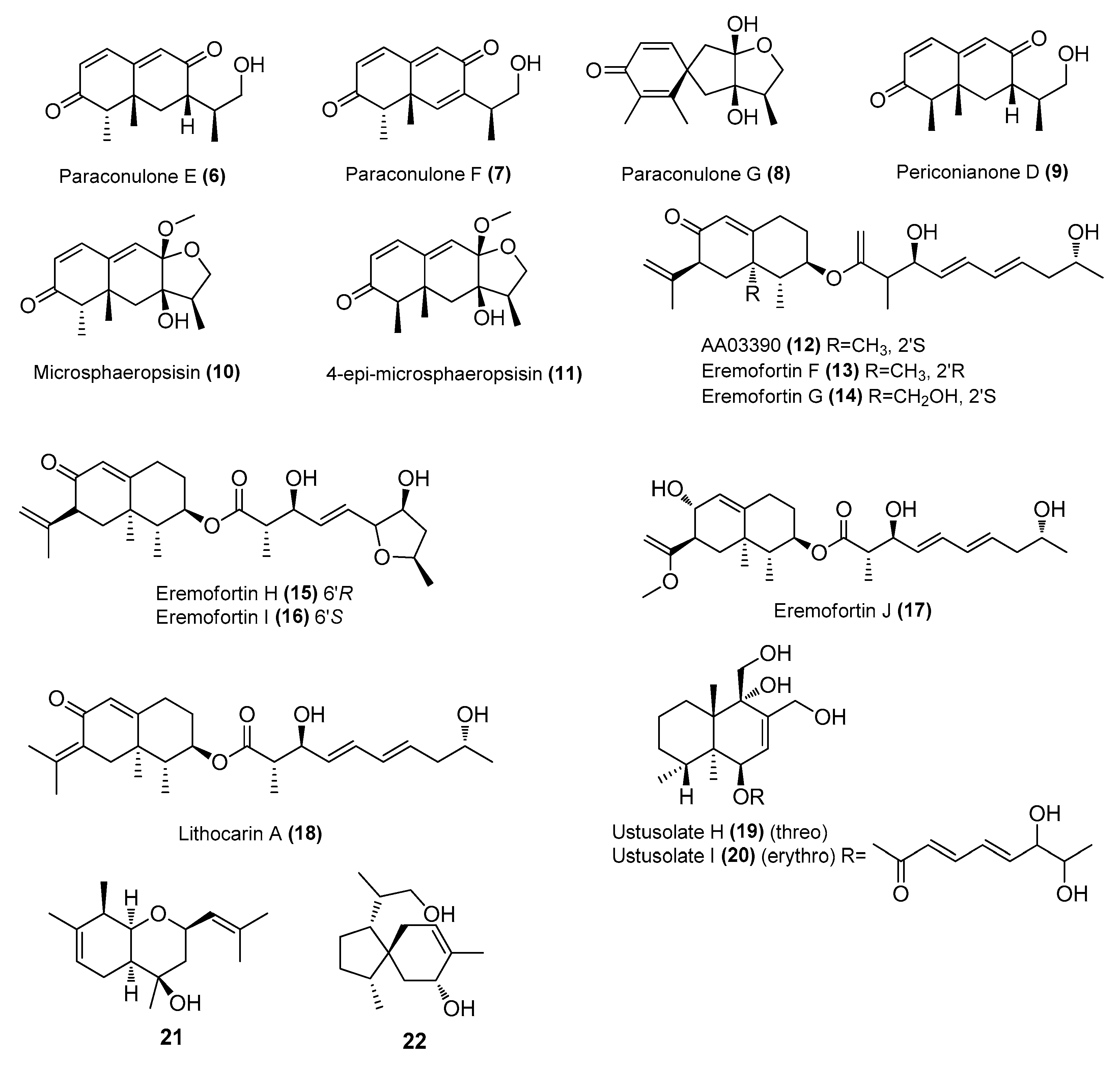
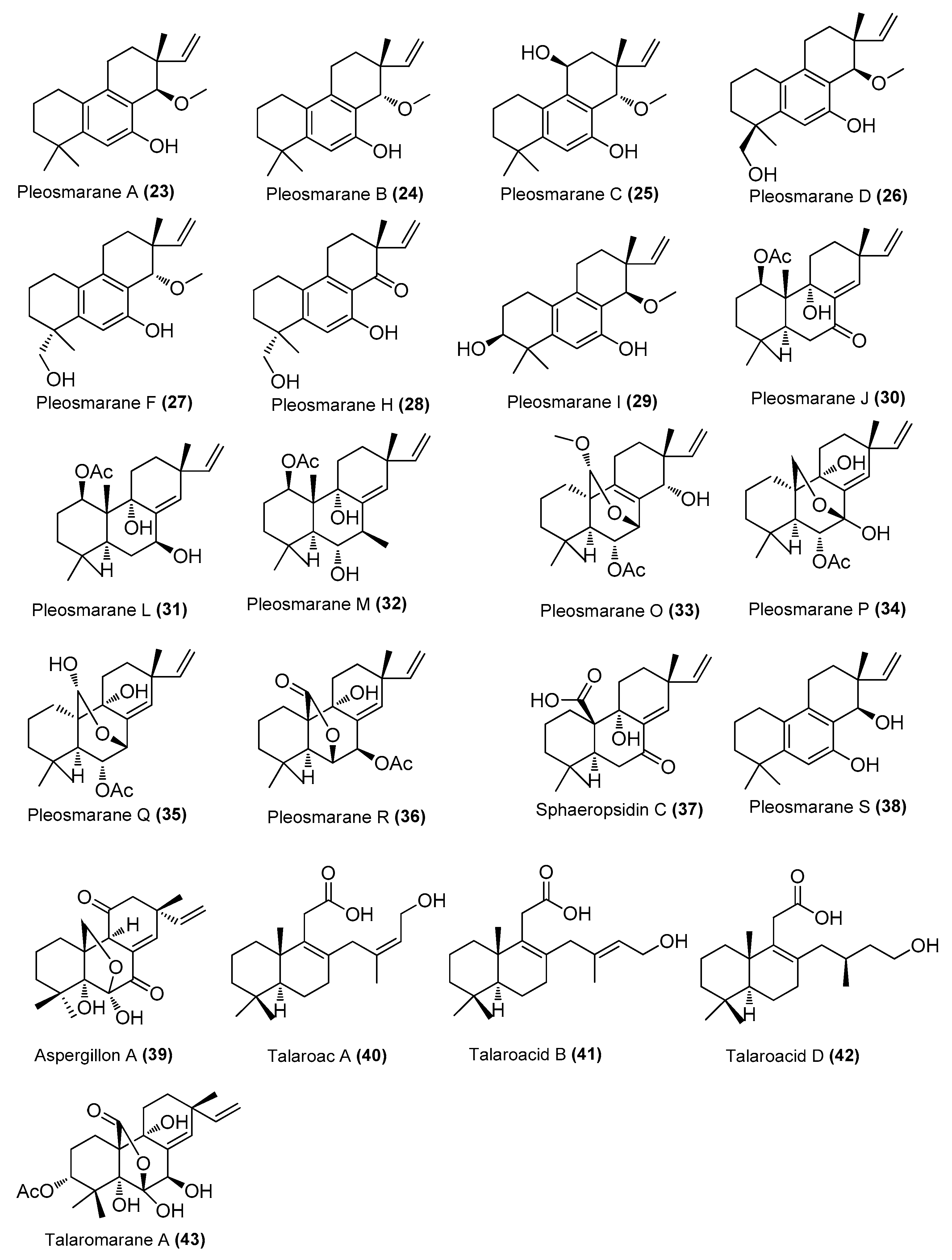






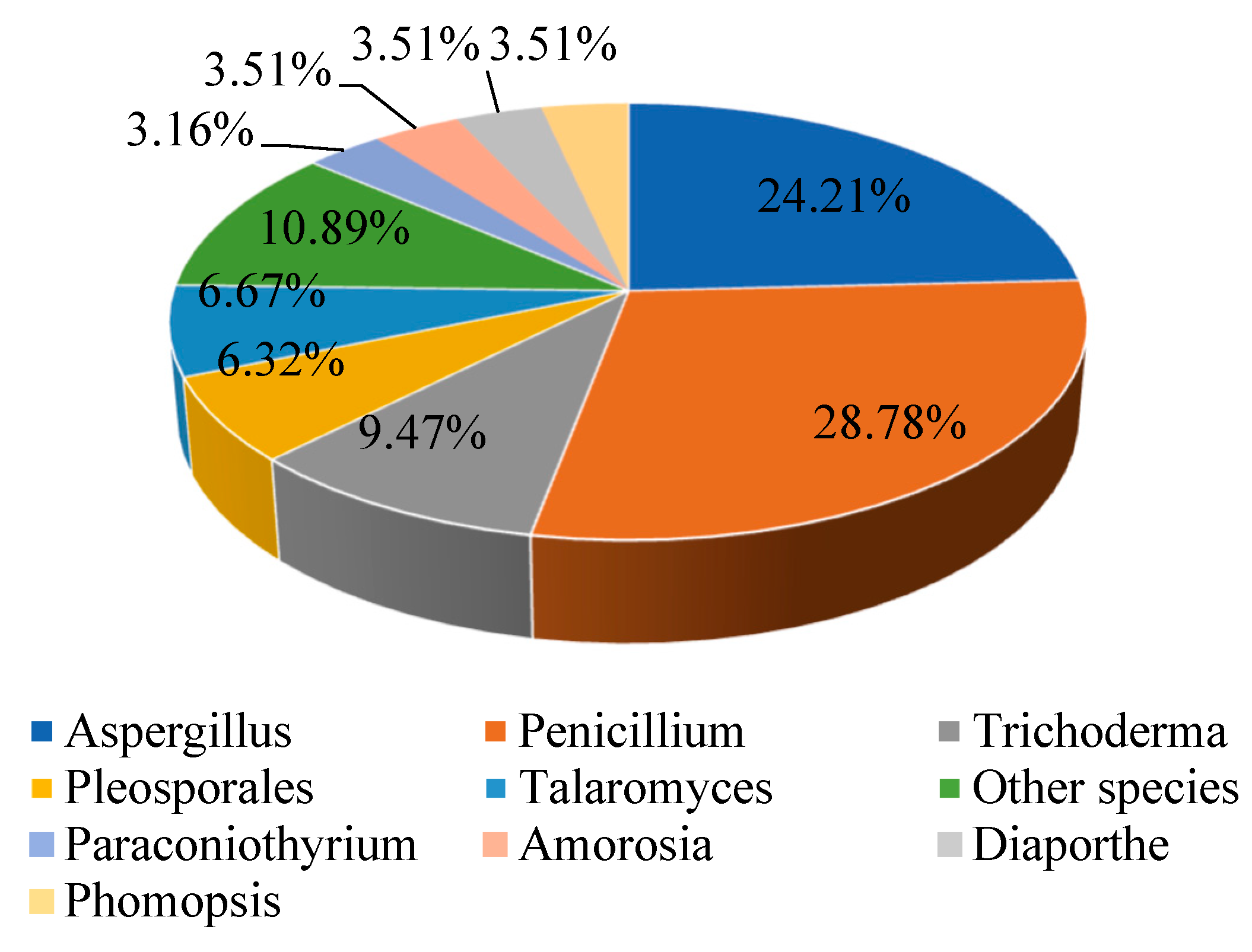
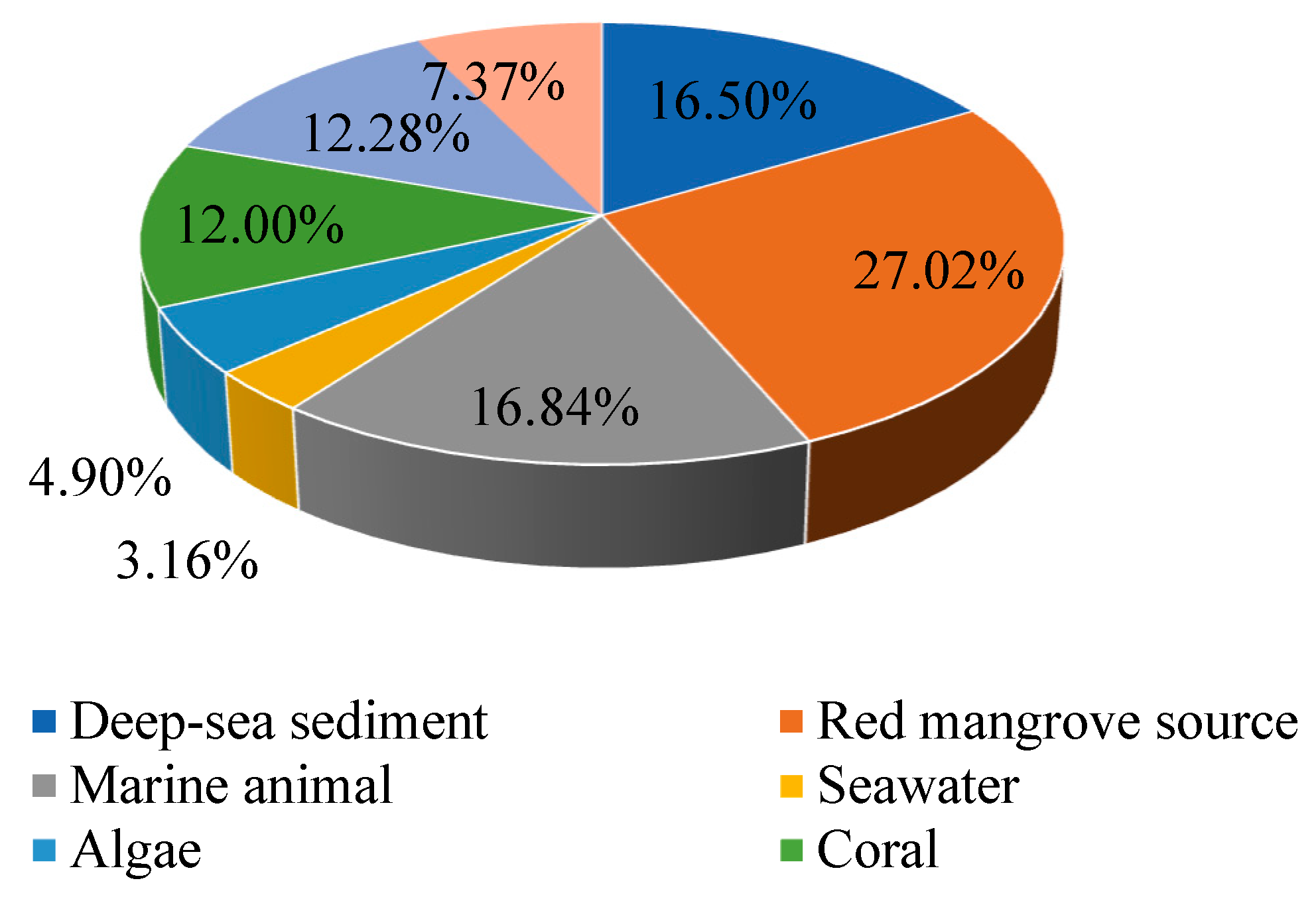
| Compounds | Producing Strains | Habitats | Genbank Accession Number | Bioactivities | References |
|---|---|---|---|---|---|
| Aspermonoterpenoid A (1) | Aspergillus sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced RAW 264.7 cells at 20 µM | [24] |
| Aspermonoterpenoid B (2) | A. sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced RAW 264.7 cells at 10 µM | [24] |
| Paraconulones B−E (3−6) | Paraconiothyrium sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.9 ± 2.6, 7.7 ± 2.0, 2.8 ± 0.5, 8.1 ± 2.9 μΜ, respectively | [25] |
| Paraconulone F (7) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 40 ± 15 μΜ | [25] |
| Paraconulone G (8) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced BV2 cells, IC50 = 8.1 ± 3.5 μΜ | [25] |
| Periconianone D (9) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced BV2 cells, IC50 = 98 ± 17 μΜ | [25] |
| Microsphaeropsisin (10) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced BV2 cells, IC50 = 80 ± 38 μΜ | [25] |
| 4-epi-microsphaeropsisin (11) | P. sporulosum DL-16 | Coastal sediment, Bohai Bay, Liaoning, China | MZ505391 | Inhibited NO production in LPS-induced BV2 cells, IC50 = 4.6 ± 3.5 μΜ | [25] |
| AA03390 (12) | Phomopsis sp. SYSU-QYP-23 | Mangrove, East Harbour National Nature Reserve, Hainan, China | MN871866 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 14.5 μΜ | [26] |
| Eremofortins G-J (13-17) | Phomopsis sp. SYSU-QYP-23 | Mangrove, East Harbour National Nature Reserve, Hainan, China | MN871866 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 8.6−13.5 μΜ | [26] |
| lithocarin A (18) | Phomopsis sp. SYSU-QYP-23 | Mangrove, East Harbour National Nature Reserve, Hainan, China | MN871866 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 10.5 μΜ | [26] |
| Ustusolates H-J (19-20) | Aspergillus insuetus SYSU6925 | Seagrass, Zhuhai, Guangdong, China | MZ411391 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 21.5 and 32.6 μΜ, respectively | [27] |
| 21 and 22 | Eutypella sp. D-1 | London Island, Arctic | FJ430580 | Modulated the MAPK and NLRP3/caspase-1 signaling pathways | [28] |
| Pleosmaranes A-D (23-26), F (27), H–J (28–30), L (31), M (32), and O–R (33–36); sphaeropsidin C (37), pleosmarane S (38) | Pleosporales sp. HNQQJ-1 | Mangrove, Dongzhai Harbor Mangrove Nature Reserve, Hainan, China | OR616722 | Inhibited NO production in LP S induced RAW 264.7 cells, IC50 = 30, 37, 38, 42, 42, 19, 35, 33, 25, 35, 37, 30, 33, 35, 31 and 40 μM, compared with the positive control (L-NMMA, 33 μM). | [29] |
| Aspergillon A (39) | Eutypella scoparia GZU-4-19Y | Xuwen, Guangdong, China | OM920979 | Inhibited NO production, IC50 = 2.0 μM, suppressed the protein expression of iNOS and COX-2 at 2.5 μM | [30] |
| Talaroacids A (40) and D (42), Talaromarane A (43) | Talaromyces sp. JNQQJ-4 | Mangrove, Jinniu Island Mangrove Nature Reserve, Guangzhou, China | MK450749.1 | Inhibited NO production, IC50 = 15.78, 21.60, and 13.38 μM, respectively | [31] |
| Talaroacid B (41) | Talaromyces sp. JNQQJ-4 | Jinniu Island Mangrove Nature Reserve, Guangzhou, China | MK450749.1 | Inhibited NO production, IC50 = 21.60 μΜ, positive control quercetin (IC50, 11.33 μM) | [31] |
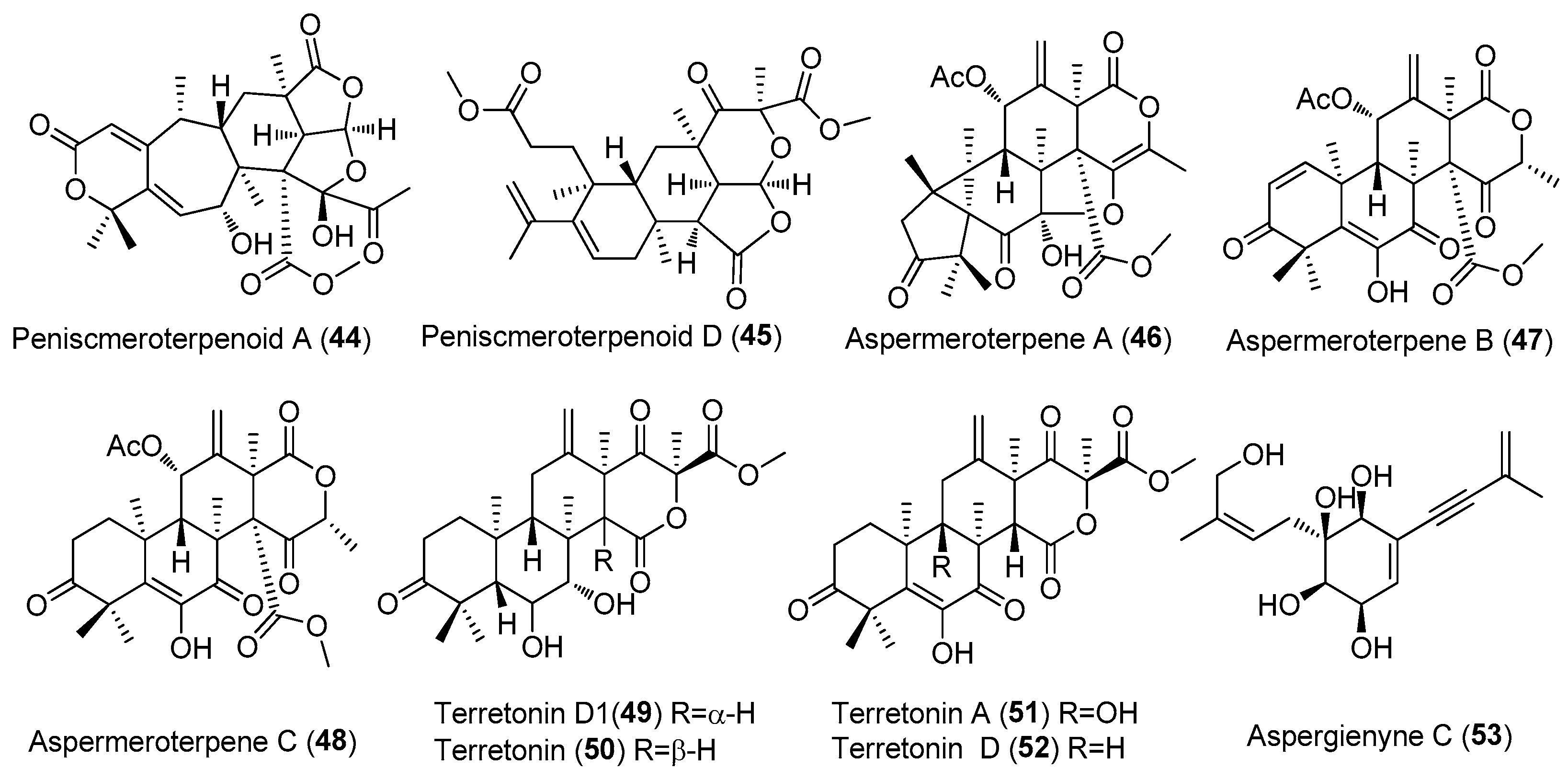
| Peniscmeroterpenoids A and D (44 and 45) | Penicillium sclerotiorum GZU-XW03-2. | Onchidium sp., Guangdong, China. | MT071304) | Inhibited NO production, IC50 = 26.60 and 8.79 μM, respectively | [32] |
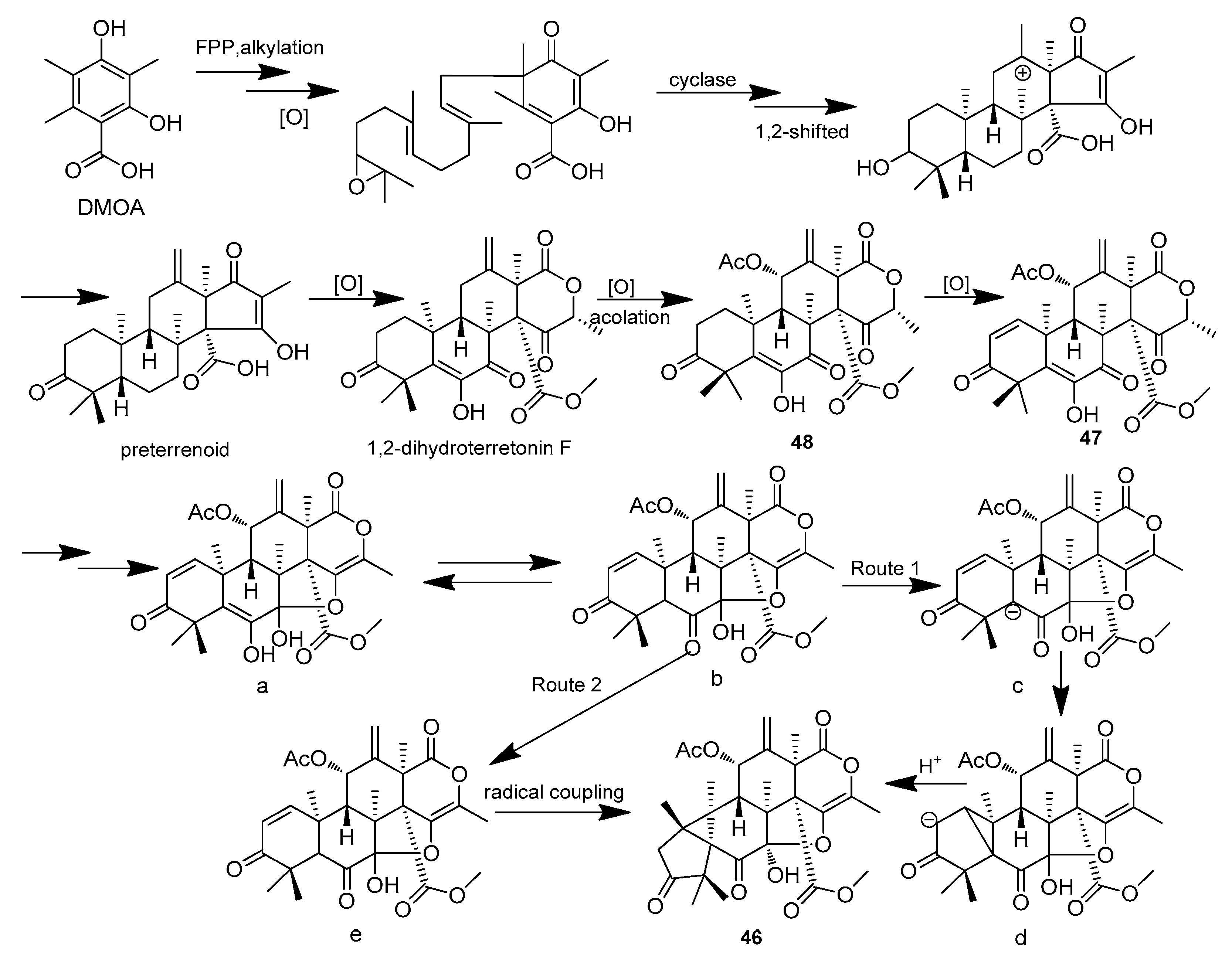
| Aspermeroterpene A-C (46–48) | Aspergillus terreus GZU 31-1 | Guangdong province (Zhanjiang, Xuwen), China | MN860009 | Inhibited NO production, IC50 (anti-inflammatory)17.8,14.1 and 13.4 μM | [33] |
| Terretonin D1(49), Terretonin (50), Terretonins A and D (51 and 52) | Aspergillus terreus ML-44 | Pacific oyster, Yangma Island in Yantai, China | CGMCC 15664 | Inhibited NO production, inhibitory rates of 30.2%, 34.0%, 22.5% and 23.5%, at 50 μg/mL | [34] |
| Aspergienyne C (53) | Aspergillus sp. GXNU-Y65 | Mangrove Kandelia cande, Beihai, China | MT626087 | Aspergienyne C had strong anti-nonalcoholic steatohepatitis activity against AML12 cells treated with PA (palmitic acid) + OA (oleic acid). | [35] |
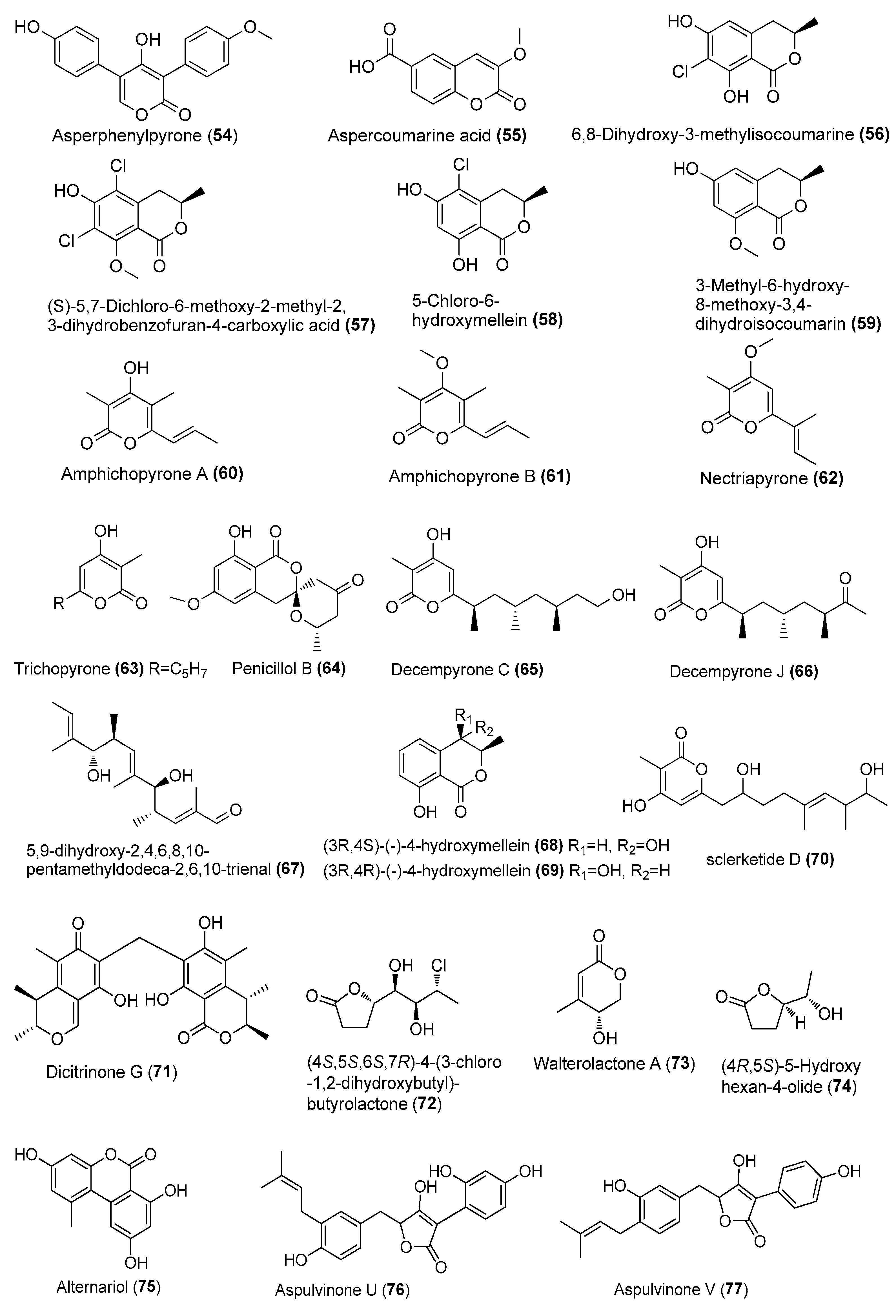
| Asperphenylpyrone (54) | Aspergillus sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced RAW 264.7 cells at 20 µM | [24] |
| Aspercoumarine acid (55) | A. sydowii MCCC 3A00324 | Deep-sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced RAW 264.7 cells at 10 µM | [24] |
| 6,8-dihydroxy-3-methylisocoumarine (56) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited the production of inflammatory factors in both mRNA and protein levels | [36] |
| (S)-5,7-dichloro-6-methoxy-2-methyl-2,3-dihydrobenzofuran-4-carboxylic acid (57) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited the production of inflammatory factors in both mRNA and protein levels | [36] |
| 5-chloro-6-hydroxymellein (58) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited the production of inflammatory factors in both mRNA and protein levels | [36] |
| 3-methyl-6-hydroxy-8-methoxy-3,4-dihydroisocoumarin (59) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited the production of inflammatory factors in both mRNA and protein levels | [36] |
| Amphichopyrones A (60) and B (61) | Amphichorda felina SYSU-MS7908 | Culturing ascidian, Xisha Islands, South China Sea, China. | MT786206 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 18.09 and 7.18 μΜ, respectively | [37] |
| Nectriapyrone (62) | Diaporthe sp. SYSU-MS4722 | Shenzhen City, Guangdong, Province, China | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 35.4 μΜ, positive control indomethacin, IC50 = 35.8 µM | [38] |
| Trichopyrone (63) | Penicillium sp. DM815 | Qinglan, Wenchang, Hainan Province | MW497629 | Weakly inhibited LPS-induced NO release at 10 μM | [39] |
| Penicillol B (64) | Penicillium sp. BJR-P2 | Mangrove Avicennia marinav, Yangjiang Hailing Island Mangrove Wetland Park, China | PRJNA793386 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 12 μΜ | [40] |
| Decempyrones C (65) and J (66) | Fusarium decemcellulare SYSU-MS6716 | Sea grass, Lingshui Xincungang and Li’angang Special Protected Area, Hainan, China | MW851212 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 22.4 and 21.7 μΜ, respectively | [41] |
| 5,9-dihydroxy-2,4,6,8,10-pentamethyldodeca-2,6,10-trienal (67) | A. ochraceopetaliformis SCSIO 41020 | Hypnea pannosa, Sanya city, Hainan province, China | OL884728 | Blocked the release of pro-inflammatory cytokines (IL-6, MCP-1, and TNF-α) induced by LPS both in vivo and in vitro | [42] |
| (3R,4S)-(−)-4-hydroxymellein (68) | A. ochraceopetaliformis SCSIO 41020 | Hypnea pannosa, Sanya city, Hainan province, China | OL884728 | Inhibited NO production in LPS-induced RAW 264.7 cells | [42] |
| (3R,4R)-(−)-4-hydroxymellein (69) | A.ochraceopetaliformis SCSIO 41020 | Hypnea pannosa, Sanya city, Hainan province, China | OL884728 | Inhibited NO production in LPS-induced RAW 264.7 cells | [42] |
| sclerketide D (70) | Penicillium sclerotiorum CHNSCLM-0013 | Gorgonian, Weizhou coral reef, South China Sea | KT695601 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 5.5 μΜ | [43] |
| Dicitrinone G (71) | Penicillium sp. GGF 16-1-2 | in the South China Sea | _ | Regulated the activation of NLRP3 infammasome | [44] |
| (4S,5S,6S,7R)-4-(3-chloro-1,2-dihydroxybutyl)-butyrolactone (72) | Neofusicoccum parvum Y2NBKZG1016 | Mangrove Sonneratia glauca, Nansha District, Guangzhou, China | _ | Weak anti-inflammatory activity at concentrations ≥ 6.25 μM | [45] |
| Walterolactone A (73) | Samsoniella hepiali W7 | Deep-sea sulfide sample, South Atlantic | NR_160318.1 | Inhibited NO production in LPS-activated BV-2 microglia cells, with inhibition rates of 38.6% at 1 µM | [46] |
| (4R,5S)-5-hydroxyhexan-4-olide (74) | Samsoniella hepiali W7 | Deep-sea sulfide sample, South Atlantic | NR_160318.1 | Inhibited NO production in LPS-activated BV-2 microglia cells, IC50 = 426.2 nM | [46] |
| Alternariol (75) | Pleosporales sp. SF-7343 | King George Island, Antarctica | MK785420 | Inhibited inflammatory factors | [47] |
| Aspulvinone U (76) | Aspergillus terreus NTU243 | Marine alga Ulva lactuca, northeastern coast, Taiwan, China | PRJNA611016 | Inhibited LPS-induced MMP-9-mediated gelatinolysis, inhibition rate of 56.0% at 10 µM | [48] |
| Aspulvinone V (77) | A. terreus NTU243 | Marine alga Ulva lactuca, northeastern coast, Taiwan, China | PRJNA611016 | Inhibited NO production in LPS-induced RAW 264.7 cells, and LPS-induced MMP-9-mediated gelatinolysis, with inhibition rates of 45.0% and 67.8%, 10 µM | [48] |
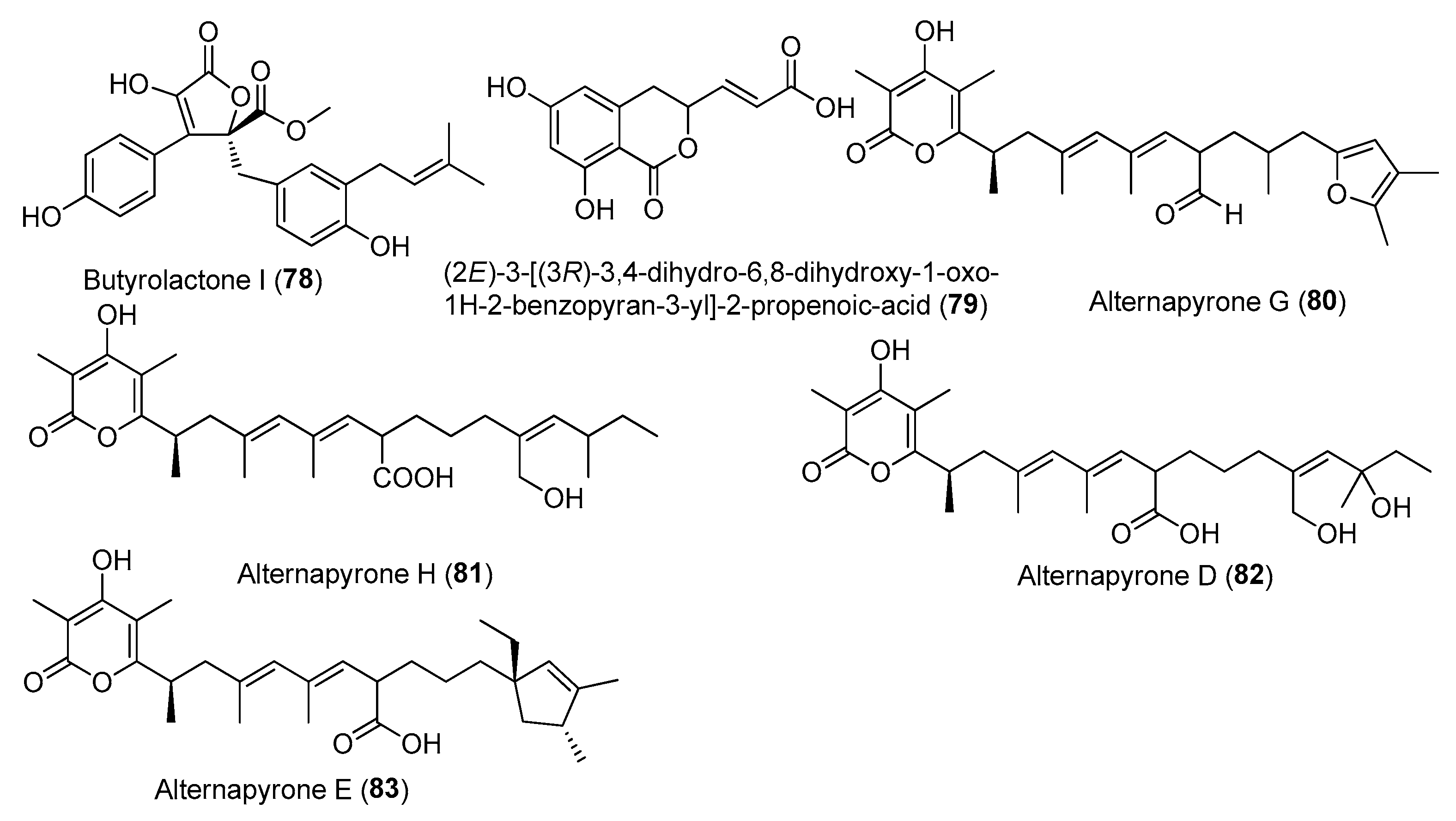
| Butyrolactone I (78) | Aspergillus flavipes MTCC 5220 | Mangrove plant Acanthus illicifolius, Goa, India | PRJNA611016 | IC50 (anti-inflammatory) 12.03 µM (IL-6), 43.29 µM (TNF-α) | [49] |
| Butyrolactone I (78) | Aspergillus terreus XWC21-10 | Coral Porites pukoensis, Zhanjiang seawaters of the South China Sea | PRJNA611016 | Inhibited the expression of iNOS and COX-2 | [50] |
| Butyrolactone I (78) | A. terreus var. africanus IFO 8835 | _ | _ | Regulating inflammation by regulating the gut microbiota | [51] |
| (2E)-3-[(3R)-3,4-dihydro-6,8-dihydroxy-1-oxo-1H-2-benzopyran-3-yl]-2-propenoic-acid (79) | Penicillium sp. TW58-16 | Deep-sea hydrothermal vent sediment, Kueishantao, Taiwan, China | MZ558028 | The regulation of gut microbiota contributes to anti-inflammatory effects | [52] |
| Alternapyrones G (80) and H (81) | Arthrinium arundinis ZSDS-F3 | Phakellia fusca, Xisha Islands of China | KF693784 | Inhibited NO release stronger than 50% at 20 µM | [53] |
| Alternapyrone D (82) | A. arundinis ZSDS-F3 | Phakellia fusca, Xisha Islands of China | KF693784 | Inhibited NO release stronger than 50% at 20 µM | [53] |
| 6-alkenylpyrone polyketides alternapyrones E (83) | A. arundinis ZSDS-F3 | The Xisha Islands of China | KF693784 | Inhibited NO release stronger than 50% at 20 µM | [53] |
| Penicilazaphilones F (84) and G (85) | Penicillium sclerotiorum E23Y-1A | Sponge Holoxea sp., Quanfu Island, Hainan, China | MW090660 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 31.7 ± 1.5 and 34.5 ± 1.4, respectively | [54,55] |
| Penicilazaphilones I, K, L and N (86, 87, 88 and 89) | P. sclerotiorum E23Y-1A | Sponge, Quanfu Island, Hainan, China | MW090660 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 22.63–65.30 µM | [54,55] |
| Hypocrellone A (90) | P. sclerotiorum E23Y-1A | Sponge, Quanfu Island, Hainan, China | MW090660 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 25.3 ± 2.2 μΜ | [55] |
| Penicillazaphilone D (91) | P. sclerotiorum E23Y-1A | Sponge, Quanfu Island, Hainan, China | MW090660 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 34.8 ± 1.9 μΜ | [55] |
| Sclerketide F (92) | Penicillium sclerotiorin SCNU-F0040 | Mangrove Bruguiera gymnorhiza, Zhanjiang Mangrove Nature Reserve, Guangdong, China | MW-541637 | COX-2 inhibitory activity, IC50 = 47.8 μΜ | [56] |
| 8a-epi-hypocrellone A (93) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Inhibited the TNF-α-induced NF-κB phosphorylation | [57] |
| 8a-epi-eupenicilazaphilone C (94) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Promote both TGF-β/Smad signaling and transcriptional function | [57] |
| Hypocrellone A (95) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Inhibited the TNF-α-induced NF-κB phosphorylation | [57] |
| Sclerotiorin (96) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Inhibited both TGF-β/Smad-mediated signaling and transcriptional function | [57] |
| Isochromophilone IV (97) | P. sclerotiorum | Alga Grateloupia sp., Yilan County, Taiwan | KM265451.1 | Inhibited the TNF-α-induced NF-κB phosphorylation | [57] |
| Sclerketide B (98) and Sclerketide C (99) | P. sclerotiorum CHNSCLM-0013 | Gorgonian, Weizhou coral reef, South China Sea | KT695601 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 3.4 and 17.6 μΜ, respectively | [43] |
| Isochromophilone IX (100) | P. sclerotiorum CHNSCLM-0013 | Gorgonian, Weizhou coral reef, South China Sea | KT695601 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 2.7 μΜ, respectively | [43] |
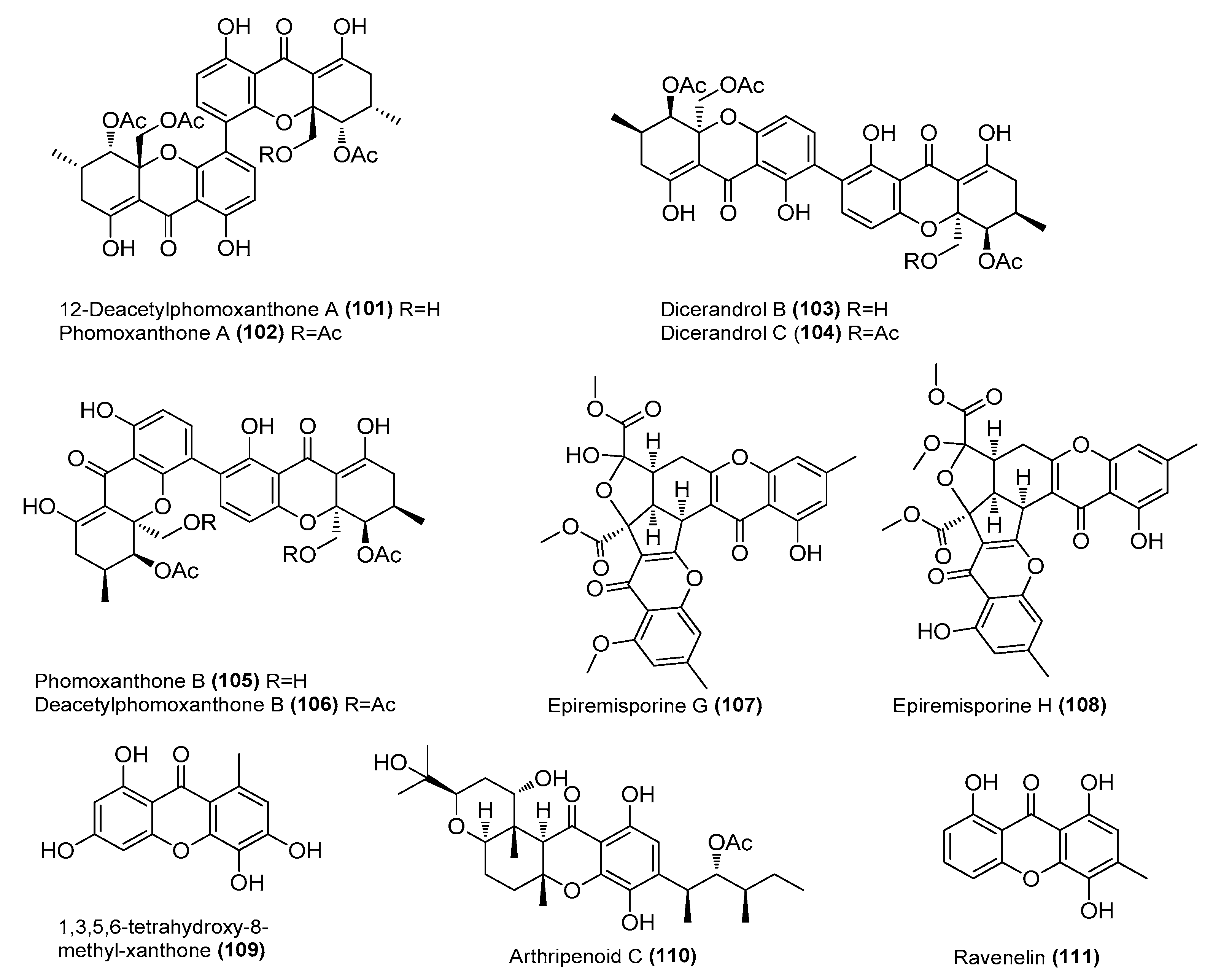
| 12-Deacetylphomoxanthone A (101) | Diaporthe sp. SYSU-MS4722 | Ascidian, Bay of Da’ao, Guangdong Province, China | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.3 μΜ | [58] |
| Phomoxanthones A (102) and B (105) | Diaporthe sp. SYSU-MS4722 | Ascidian, Bay of Da’ao, Guangdong Province, Chin | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 7.5 and 8.0 μΜ, respectively | [58] |
| Dicerandrols B (103) and C (104) | Diaporthe sp. SYSU-MS4722 | Ascidian, Bay of Da’ao, Guangdong Province, Chin | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.3 and 7.6 μΜ, respectively | [58] |
| Deacetylphomoxanthone B (106) | Diaporthe sp. SYSU-MS4722 | Ascidian, Bay of Da’ao, Guangdong Province, Chin | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 7.8 μΜ | [58] |
| Epiremisporines G (107) and H (108) | Penicillium citrinum BCRC 09F458 | Hazailiao, Dongshi, Chiayi, Taiwan, China | _ | Significantly inhibited the production of superoxide ions by fMLP, IC50 = 31.68 ± 2.53, and 33.52 ± 0.42 µM, respectively. Positive control ibuprofen, IC50 = 28.56 µM | [59] |
| 1,3,5,6-tetrahydroxy-8-methyl-xanthone (109) | Arthrinium arundinis MA30 | Sea anemone, Badouzi | OM761170 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 5.3 μΜ | [60] |
| Arthripenoid C (110) | A. arundinis MA30 | Sea anemone, Badouzi | OM761170 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 5.3 μΜ | [60] |
| Ravenelin (111) | Setosphaeria rostrata | Mangrove, Prachuap Kiri Khan Province, Thailand | OK047731 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.27 μΜ Suppressed iNOS and COX-2 expression | [61] |
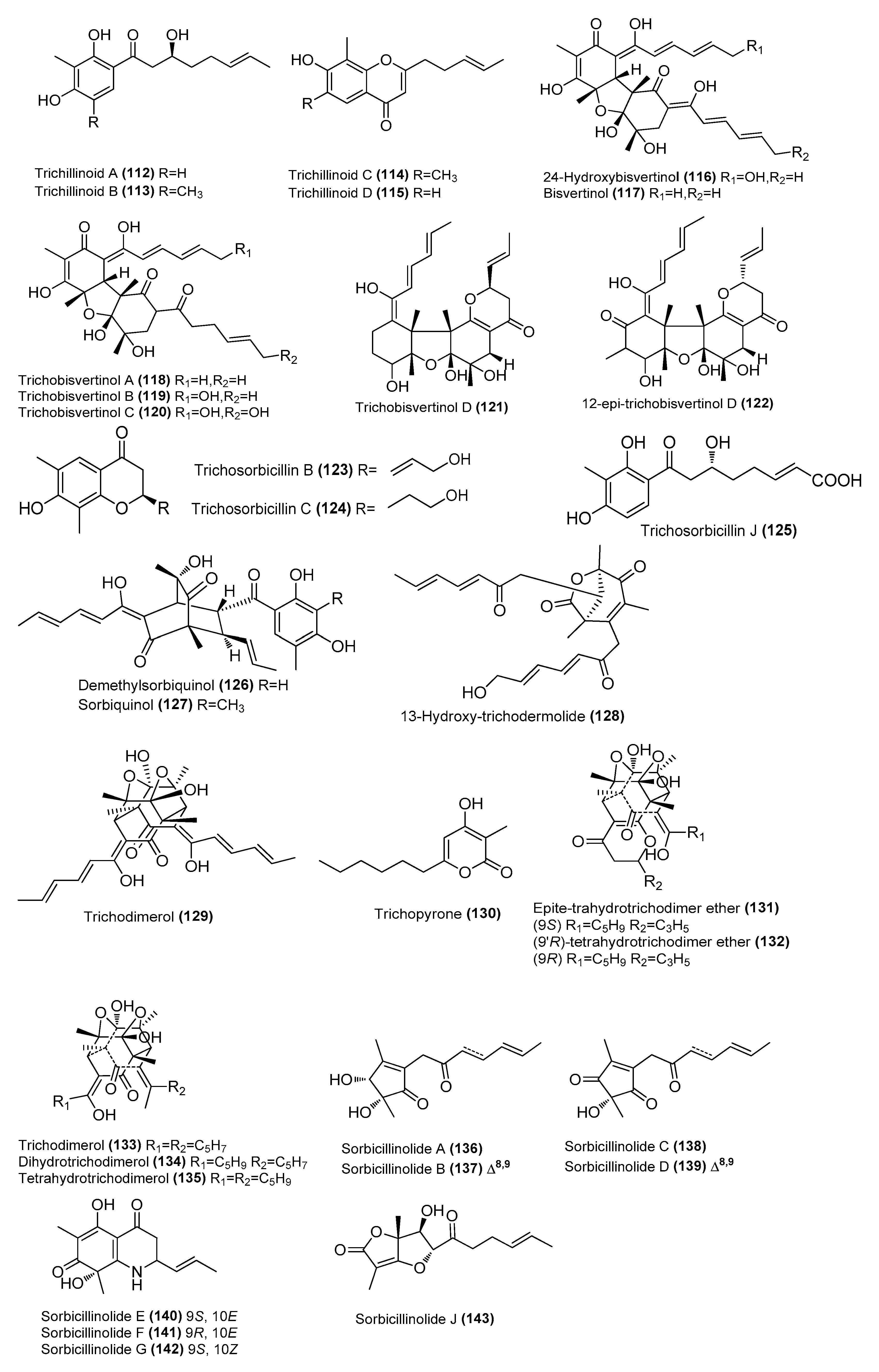
| Trichillinoids A-D (112–115) | Trichoderma sp. G13 | Marine fish Sebastes schlegelii, Yangma Island, Yantai, China | OQ781262 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 14, 14, 16, and 20 µM, respectively | [62] |
| 24-Hydroxybisvertinol (116) | Trichoderma reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.1 μΜ | [63] |
| Bisvertinol (117) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 9.9 μΜ | [63] |
| Trichobisvertinols A-D (118-121) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 5.9, 22 and 24 μΜ, respectively | [63] |
| 12-epi-trichobisvertinol D (122) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 32 μΜ | [63] |
| Trichosorbicillins B (123) and C (124) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 8.5 and 38 μΜ, respectively | [63] |
| Trichosorbicillin J (125) | Trichoderma reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | Potent inhibition of IL-6 45%, and IL-1β 21%, respectively, at 25 µM | [64] |
| Demethylsorbiquinol (126) | T. reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | Potent inhibition of IL-6 27%, and IL-1β 75%, respectively, at 25 µM | [64] |
| Sorbiquinol (127) | T. reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | Potent inhibition of IL-6 35%, and IL-1β, 58%, respectively, at 25 µM | [64] |
| 13-hydroxy-trichodermolide (128) | T. reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | 47% inhibition of IL-6, 85% inhibition of IL-1β at 25 µM | [64] |
| Trichodimerol (129) | T. reesei BGRg-3 | Mangrove plant Avicennia marina, Guangdong Province, China | OR353740 | 67% inhibition of IL-6, 87% inhibition of IL-1β at 25 µM | [64] |
| Trichopyrone (130) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [36] |
| Epite-trahydrotrichodimer ether (131) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| (9′R)-tetrahydrotrichodimer ether (132) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| Trichodimerol (133) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| Dihydrotrichodimerol (134) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
| Tetrahydrotrichodimerol (135) | Penicillium sp. DM815 | Mangrove Hibiscus tiliaceus Linnn, Qinglan, Wenchang, Hainan, China | NR_111815.1 | Inhibition of LPS-induced iNOS expression in a dose-dependent manner | [39] |
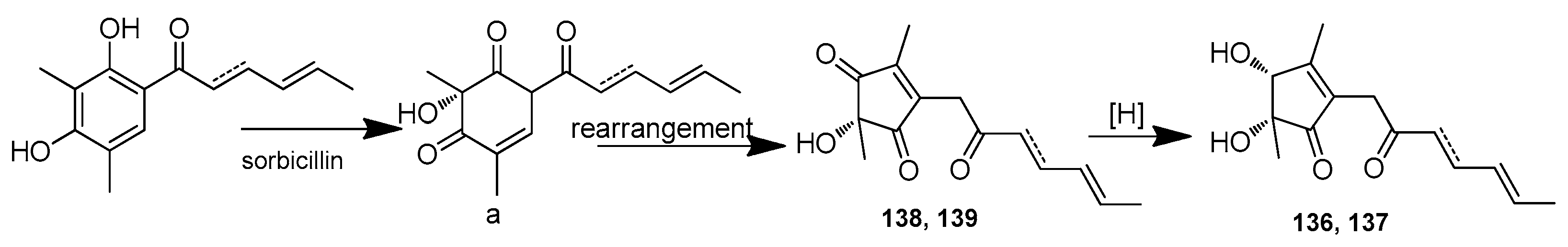
| Sorbicillinolides A–G (136–142) | Penicillium rubens F54 | Deep-sea sediment, Pacific Ocean | OR016127 | Inhibitory effects on the production of NO and PGE2, inhibition rates of 68.6%, 36.6%, 64.7%, 44.5%, 54.9%, 41.9%, and 44.5%, respectively, at 10 μM | [65] |
| Sorbicillinolide J (143) | Penicillium rubens F54 | Deep-sea sediment, Pacific Ocean | OR016127 | Inhibitory effects on the production of NO and PGE2, inhibition rate of 33.4%, at 10 μM | [65] |
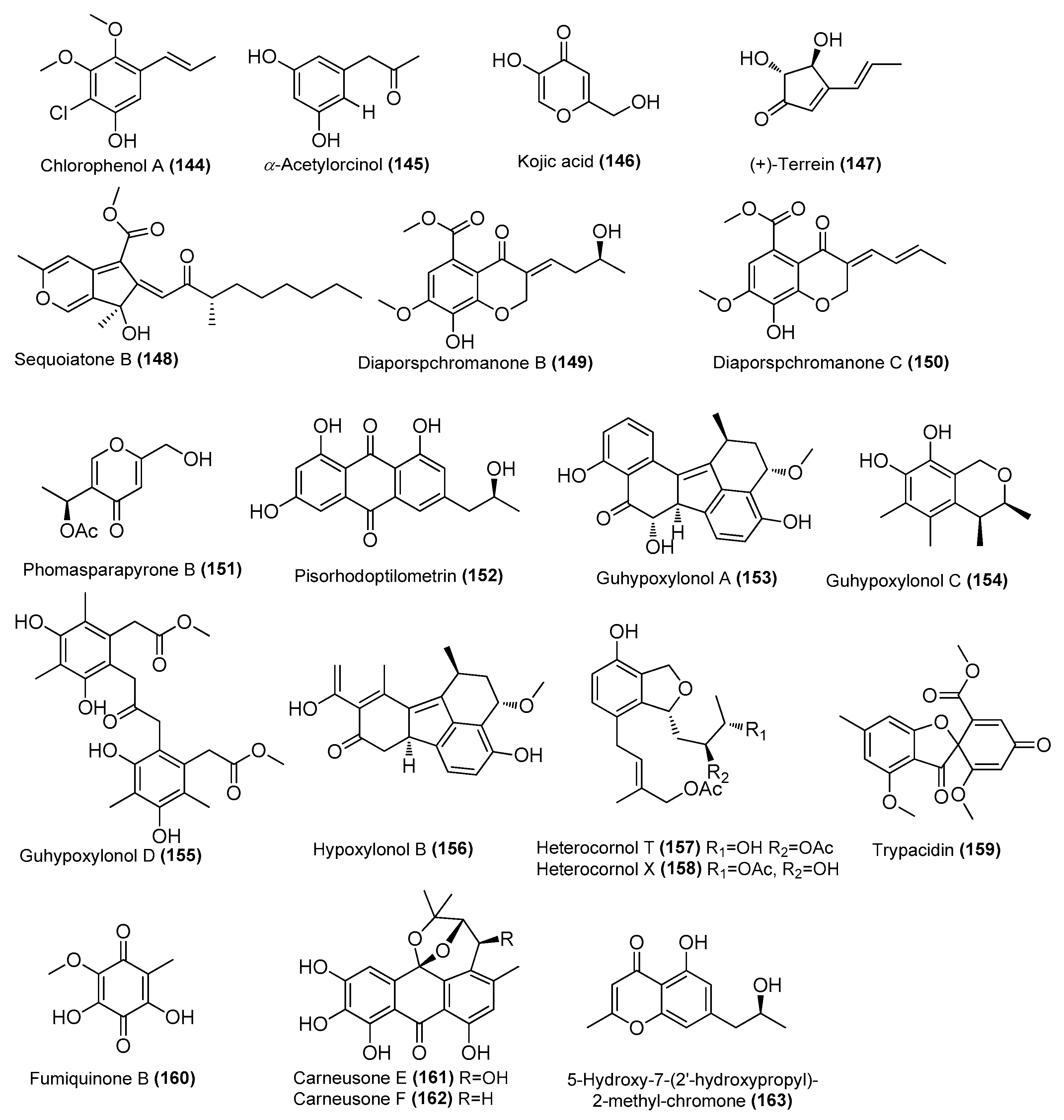
| Chlorophenol A (144) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| α-acetylorcinol (145) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| Kojic acid (146) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| (+)-Terrein (147) | Aspergillus flavipes MTCC 5220 | Alga Ulva lactuca, Goa, India | PRJNA611016 | Inhibitory activity against IL-6 and TNF-α, IC50 = 8.5 ± 0.68 and 15.76 ± 0.18 µM, respectively | [48] |
| Sequoiatone B (148) | Penicillium sclerotiorum CHNSCLM-0013 | Gorgonian, Weizhou coral reef, South China Sea | KT695601 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 5.2 μΜ | [43] |
| Diaporspchromanones B–C (149–150) | Diaporthe sp. XW12-1 | Mangrove, Xuwen, Guangdong, China | MW566595.1 | IC50 (anti-inflammatory) = 19.06 ± 3.60 and 9.56 ± 0.18 μM, respectively, positive control (indomethacin, IC50 = 70.33 ± 0.95 μM) | [66] |
| Phomasparapyrone B (151) | Phomopsis asparagi LSLYZ-87 | Mangrove Acanthus ilicifolius, Huizhou Mangrove National Nature Reserve, Guangdong, China | ON341023 | Inhibition of LPS-induced NO accumulation on BV-2 cells in a dose-dependent manner | [67] |
| Pisorhodoptilometrin (152) | Penicillium oxalicum CLC-MF05 | Sponge, Cu Lao Cham islands, Quang Nam, Vietnam | MT597864.1 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 15.2 µM | [58] |
| Guhypoxylonols A (153), C (154), D (155) | Aspergillus sp. GXNU-Y45 | Mangrove Acanthus ilicifolius, Beihai City, China | MT626059 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 14.42 ± 0.11, 18.03 ± 0.14, 16.66 ± 0.21, and 21.05 ± 0.13 µM, respectively | [68] |
| Hypoxylonol B (156) | Aspergillus sp. GXNU-Y45 | Mangrove Acanthus ilicifolius, Beihai City, China | MT626059 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 21.05 ± 0.13 µM | [68] |
| Heterocornols T (157) and X (158) | Pestalotiopsis heterocornis XWS03F09 | Xisha Islands, China | JN943628.1 | Inhibited NOS protein expression in a concentration-dependent manner | [69] |
| Trypacidin (159) | Talaromyces helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 9.65 μΜ | [70] |
| Fumiquinone B (160) | T. helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 15.54 μΜ | [70] |
| Carneusones E-F (161–162) | Aspergillus carneus GXIMD00543 | Sponge, Weizhou islands coral reef, China | OR501447 | Inhibited NO production in LPS-induced RAW 264.7 cells, EC50 = 34.6 and 20.2 μΜ, respectively | [71] |
| 5-Hydroxy-7-(2′-hydroxypropyl)-2-methyl-chromone (163) | Penicillium oxalicum CLC-MF05 | Sponge, Cu Lao Cham islands, Quang Nam, Vietnam | NR 121232.1 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 75.5 μΜ | [72] |
| Phomtersine A (164) | Phomopsis tersa FS441 | Deep sea in the Indian Ocean | MK592793 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 83.57 μΜ | [73] |
| Steckfusarin A (165) | Penicillium steckii SCSIO 41040 | Green algae Botryocladia sp., South China Sea | OP349656 | Anti-inflammatory activity at 20 µM | [74] |
| 5-O-acetyladenosine (166) | Samsoniella hepiali W7 | Deep-sea sulfide sample, South Atlantic | OR398925 | Inhibited NO production in LPS-induced BV-2 cells, inhibition rates of 34.2%, at 1 µM | [46] |
| Uridine (167) | S. hepiali W7 | Deep-sea sulfide sample, South Atlantic | OR398925 | Inhibited NO production in LPS-induced BV-2 cells, inhibition rates of 30.7%, at 1 µM | [46] |
| Sclerotioloid B (168) | Aspergillus sclerotiorum ST0501 | Guangdong, China | MT534582 | Inhibited NO production in LPS-induced RAW 264.7 cells, inhibition rate of 28.92%, postive control dexamethasone (25.87%) | [75] |
| Sclerotiamide J (169) | Aspergillus sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Sclerotiamides K (170) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Sclerotiamides O-Q (171–173) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Notamide X (174) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Notamide Z (175) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Notamide R (176) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| (−)-notamide A (177) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Notamide I (178) | |||||
| Notamide F (179) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Sclerotiamide (180) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Sclerotiamide B (181) | A. sclerotiorum LZDX-33-4 | Gorgonian, South China Sea | OK012383.1 | Inhibitory effect on the expression of LDH and IL-1β in BV-2 cells | [76] |
| Equisetin (182) | Fusarium equiseti | Sponge, Xuwen County, Zhanjiang, China | SCSIO 41019 | EQST inhibits macrophage inflammatory response in vitro | [77] |
| Penpaxilloids A (183), C (184), D (185) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 7.11, 27.25 and 33.09 μΜ, respectively | [78] |
| 7-methoxypaxilline-13-ene (186) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 38.79 μΜ | [78] |
| Schipenindolene A (187) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 11.87 μΜ | [78] |
| 21-isopentenylpaxilline (188) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 32.95 μΜ | [78] |
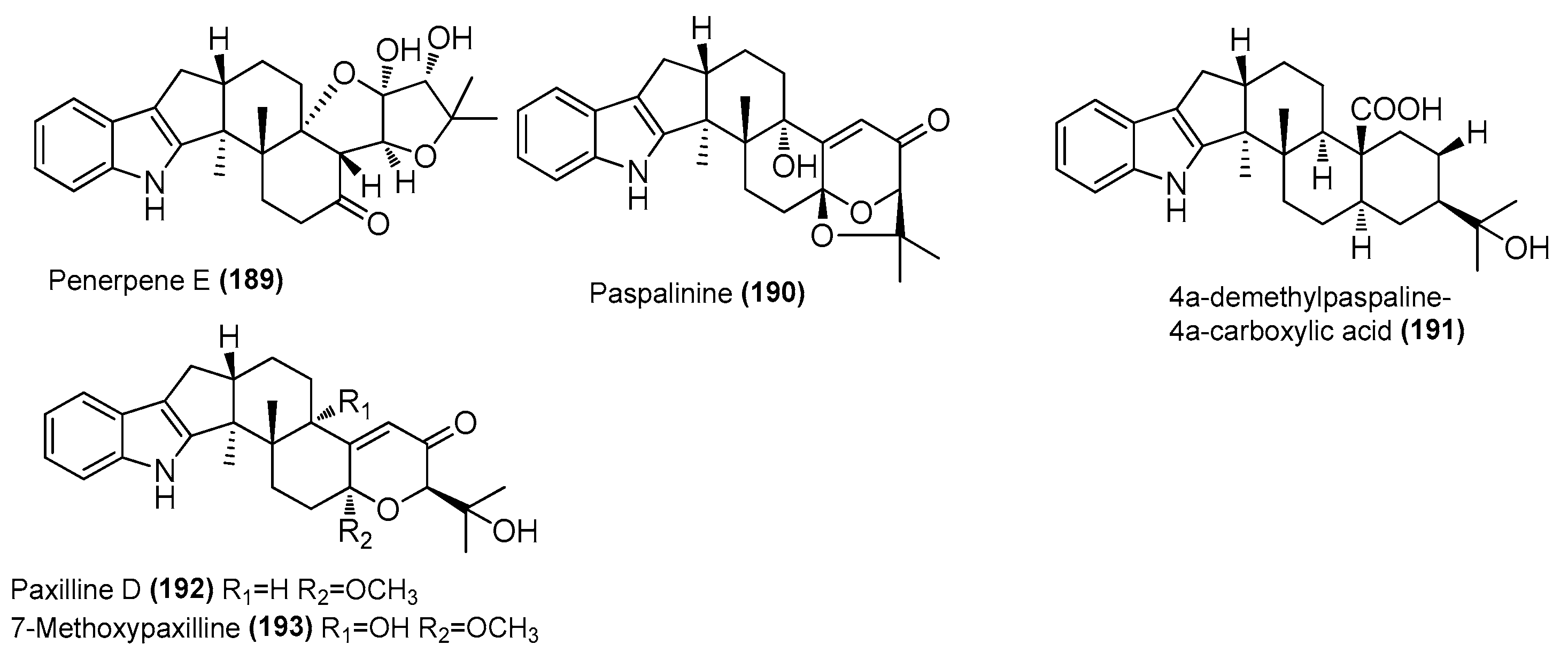
| Penerpene E (189) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 23.89 μΜ | [78] |
| Paspalinine (190) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 19.34 μΜ | [78] |
| 4a-demethylpaspaline-4a-carboxylic acid (191) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 28.22 μΜ | [78] |
| Paxilline D (192) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 4.46 μΜ | [78] |
| Arthropod Dardanus scutellatus, Yinyu Island in South China’s Hainan province | |||||
| 7-methoxypaxilline (193) | Penicillium sp. ZYX-Z-143 | Arthropod Dardanus scutellatus, Yinyu Island, Hainan | ON386189 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 22.88 μΜ | [78] |
| Terreusinones B (194) and C (195) | Aspergillus tamarii MCCF102 | Sponge, Vizhinjam, Southwest coast of India | JAGJCD000000000 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 0.032, 0.046 and 0.096μΜ. respectively | [79] |
| Terreusinone (196) | A. tamarii MCCF102 | Sponge, Vizhinjam, Southwest coast of India | JAGJCD000000000 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 0.032 μΜ | [79] |
| Chaetominine (197) | Talaromyces helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 103.2 μΜ | [70] |
| Isotryptoquivaline F (198) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 26.51 μΜ | [70] |
| Fumiquinazoline F (199) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 21.35 μΜ | [70] |
| 12,13-dihydroxyfumitremorgin C (200) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 24.95 μΜ | [70] |
| Cyclotryprostatin B (201) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 29.58 μΜ | [70] |
| Azaspirofurans A (202) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 9.65 μΜ | [70] |
| 14-norpseurotin A (203) | T. Helicus SCSIO41311 | Cold seep, South China Sea Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 32.37 μΜ | [70] |
| 11-O methylpseurotin A (204) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 32.22 μΜ | [70] |
| Fumigaclavine C (205) | T. Helicus SCSIO41311 | Cold seep, South China Sea | KT224828 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 23.46 μΜ | [70] |
| Oxaline (206) | Penicillium oxalicum CLC-MF05 | Sponge, Cu Lao Cham islands, Quang Nam, Vietnam | MT597864.1 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 9.2 μΜ | [72] |
| Variotin B (207) | Aspergillus unguis IV17-109 | Deep sea, Indian Ocean | OL700797 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 20.0 μΜ | [80] |
| Benzomalvin E (208) | Metarhizium sp. P2100 | Seawater, Qingdao Huiquan Bay, Yellow Sea | OP028052 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 37.08 μΜ | [81] |
| Methylviridicatin (209) | Metarhizium sp. P2100 | Seawater, Qingdao Huiquan Bay, Yellow Sea | OP028052 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 37.48 μΜ | [81] |
| Isoechinulin B (210) | Aspergillus sp. nFS445 | Deep sea, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 16 ± 1.3 µM, positive control aminoguanidine (IC50, 23.7 µM) | [82] |
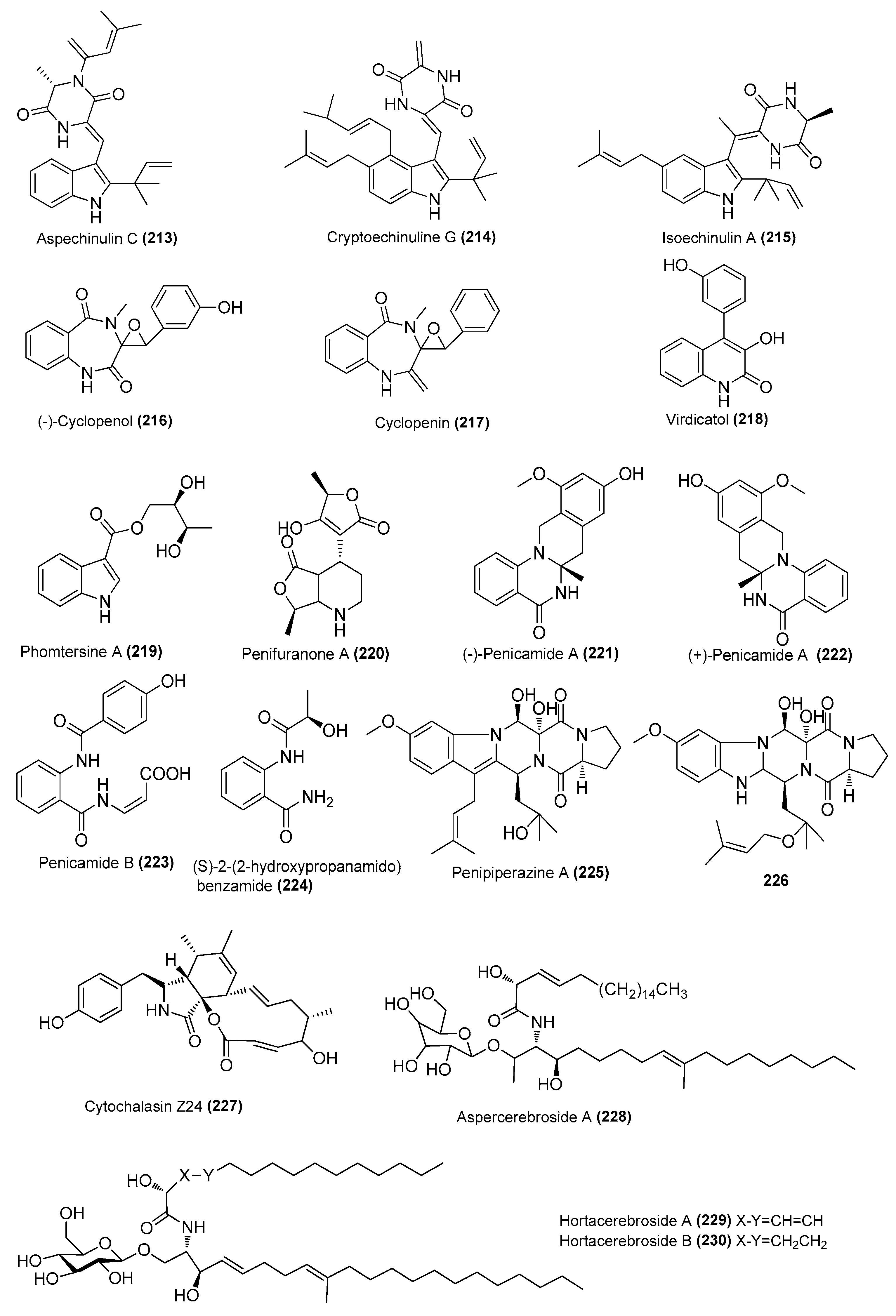
| Aspechinulins B (211) and C (213) | Aspergillus sp. nFS445 | Sponge, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 20 ± 0.28 and 25 ± 1.7 µM, respectively | [82] |
| Neoechinulin B (212) | Aspergillus sp. nFS445 | Sponge, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 89 ± 2.0 µM | [82] |
| Cryptoechinuline G (214) | Aspergillus sp. nFS445 | Sponge, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 37 ± 0.75 µM | [82] |
| Isoechinulin A (215) | Aspergillus sp. nFS445 | Sponge, Indian Ocean | MW386823 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 57 ± 2.3 µM | [82] |
| (−)-cyclopenol (216) | Aspergillus austroafricanus Y32-2 | Indian Ocean | MK267449 | Inhibited NO production in LPS-induced RAW 264.7 cells at 70 µg/mL | [83] |
| Cyclopenin (217) | A. austroafricanus Y32-2 | Indian Ocean | MK267449 | Inhibited NO production in LPS-induced RAW 264.7 cells at 130 µg/mL | [83] |
| Virdicatol (218) | austroafricanus Y32-2 | Indian Ocean | MK267449 | Inhibited NO production in LPS-induced RAW 264.7 cells at 30 µg/mL | [83] |
| Phomtersine A (219) | Phomopsis tersa FS441 | Deep sea, Indian Ocean | MK592793 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 83.57 μΜ | [84] |
| Penifuranone A (220) | Penicillium crustosum SCNU-F0006 | Mangrove, Yangjiang Hailing Island Mangrove Wetland Park, China | MH345907 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 42.2 μΜ | [85] |
| (±)-penicamide A [(−)-221 and (+)-222] | Penicillium sp. 4829 | Styela plicata, Bay of Da’ao, Guangdong, China | MH465534 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 27.2 and 47.5 μΜ, respectively | [86] |
| Penicamide B (223) | Penicillium sp. 4829 | Styela plicata, Bay of Da’ao, Guangdong, China | MH465534 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 45.9 ± 2.0 μΜ | [86] |
| (S)-2-(2-hydroxypropanamido) benzamide (224) | Penicillium sp. 4829 | Styela plicata, Bay of Da’ao, Guangdong, China | MH465534 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 21.8 ± 1.3 µM, respectively | [86] |
| Penipiperazine A (225) | Penicillium brasilianum HBU-136 | Bohai Sea, China | MH377073 | Inhibited the expression of inflammatory factors at 25.0 µM | [87] |
| Metabolite (226) | P. brasilianum HBU-136 | Bohai Sea, China | MH377073 | Inhibited the expression of inflammatory factors at 25.0 µM | [87] |
| Cytochalasins Z24 (227) | Eutypella scoparia GZU-4-19Y | Xuwen, Guangdong province, China | OM920979 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 17.1 μΜ | [30] |
| Aspercerebroside A (228) | Aspergillus sp. | Dongshan Island, Fujian Province | 2167894 | Inhibited NO production in LPS-induced RAW 264.7 cells, at 30 and 40 μg/mL | [88] |
| Hortacerebrosides A (229) and B (230) | Hortaea werneckii | Sponge, Danzhou, Hainan, China | HN-YPG-2-5 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 7 and 5 μΜ, respectively | [89] |
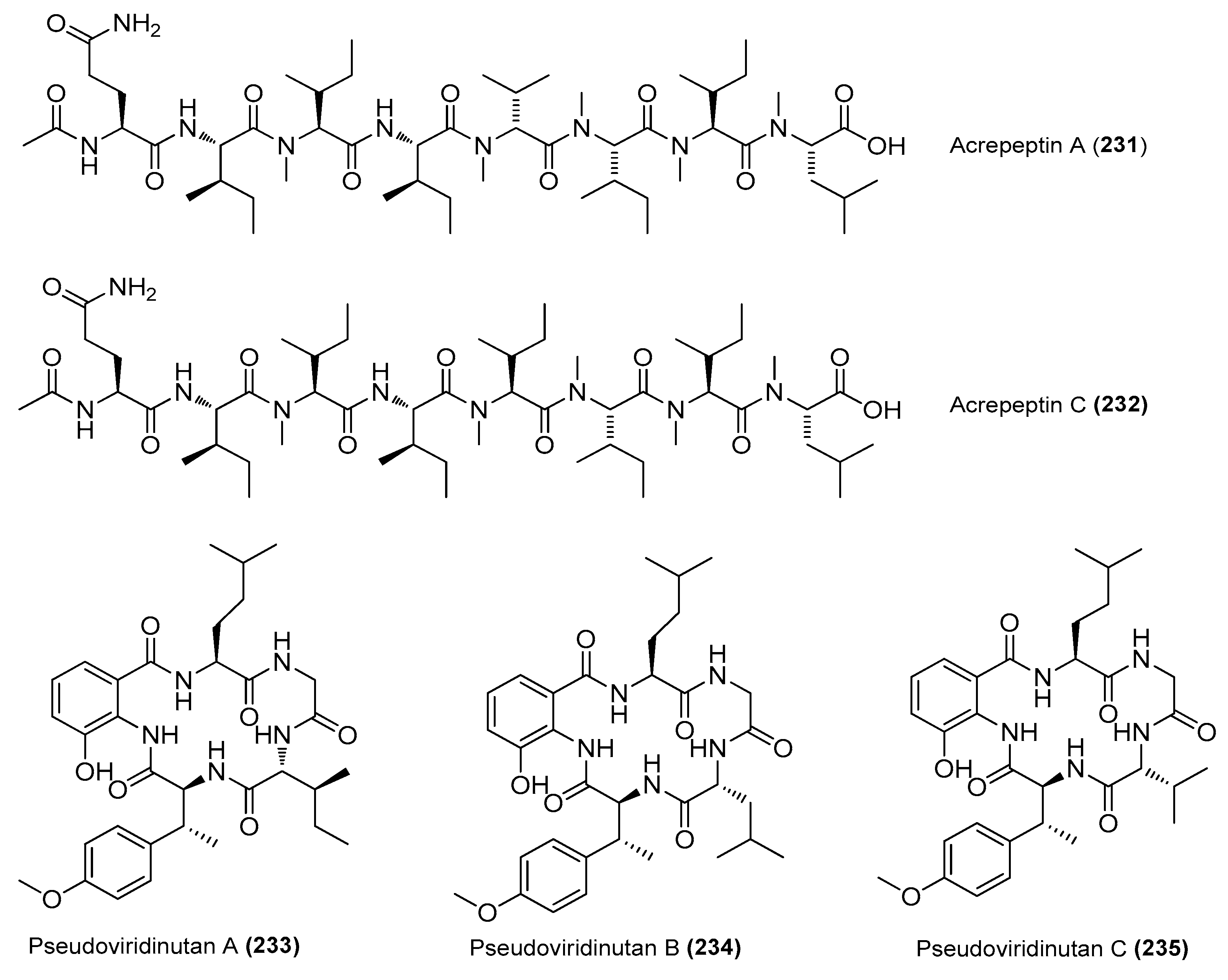
| Acrepeptins A (231) and C (232) | Acremonium sp. NTU492 | Red alga Mastophora rosea, Taiwan, China | KY753131 | Inhibited NO production in LPS-induced BV-2 cells, IC50 = 12.0 and 10.6 μΜ, respectively | [90] |
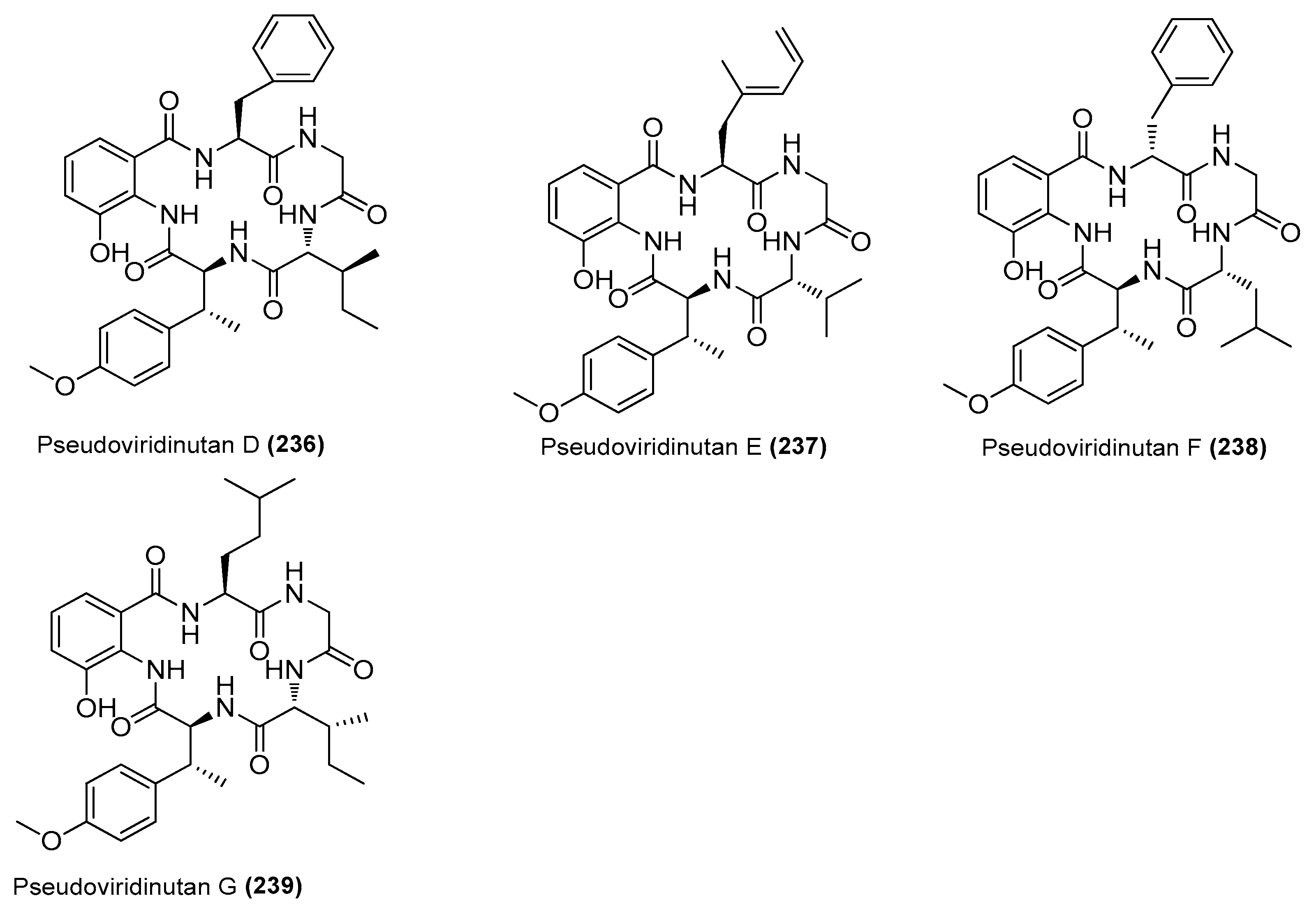
| Pseudoviridinutans A−G (233−239) | Aspergillus pseudoviridinutans TW58-5 | Ahydrothermal vent sediment, Kueishantao, Taiwan, China | OQ405296 | Inhibited NO production in LPS-induced RAW 264.7 cells | [91] |
| Sterolester (240) | Penicillium oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| 22-Tetraen-3-one (241) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Ganodermaside (242) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| 22-Tetraen-3-one (243) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Isocyathisterol (244) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Herbarulide (245) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Dankasterone A (246) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-ergosta-7,22-dien-3β,5α-diol-6-one (247) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-ergosta-7,22-dien-3β,5α,9α-trihydroxy-6-one (248) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-3β-hydroxyergosta-5,8,22-trien-7-one (249) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| 22-triene-3β-ol (250) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-7α-methoxy-5α,6α-epoxyergosta-8(14),22-dien-3β-ol (251) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-6-acetoxy-ergosta-7,22-dien-3β,5α,6β-triol (252) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-5α,8α-epidioxyergosta-6,9(11),22-trien-3β-ol (253) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| (22E,24R)-5α,8α-epidioxyergosta-6,22-dien-3β-ol (254) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Demethylincisterol A3 (255) | P. oxalicum HL-44 | Soft coral Sinularia gaweli, Xisha, South China Sea | MG585101.1 | Strong anti-inflammatory activity at 20 μΜ | [92] |
| Ergosterdiacids A and B (256 and 257) | Aspergillus sp. | Mangrove Aegiceras corniculatum, Thailand | 2167894 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 4.5 and 3.6 μΜ, respectively | [93] |
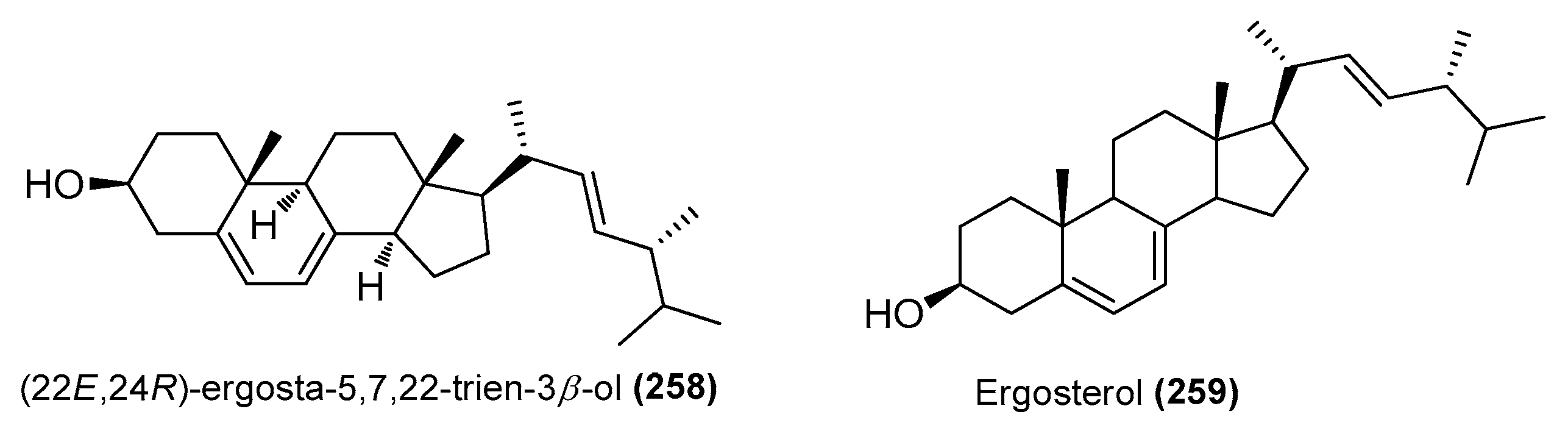
| (22E,24R)-ergosta-5,7,22-trien-3β-ol (258) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited excessive LPS-induced production of NO and pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| Ergosterol (259) | Samsoniella hepiali W7 | Deep-sea sulfide sample, South Atlantic | NR_160318.1 | Inhibited NO production in LPS-induced BV-2 cells, inhibition rate of 32.9% (1 μΜ) | [46] |
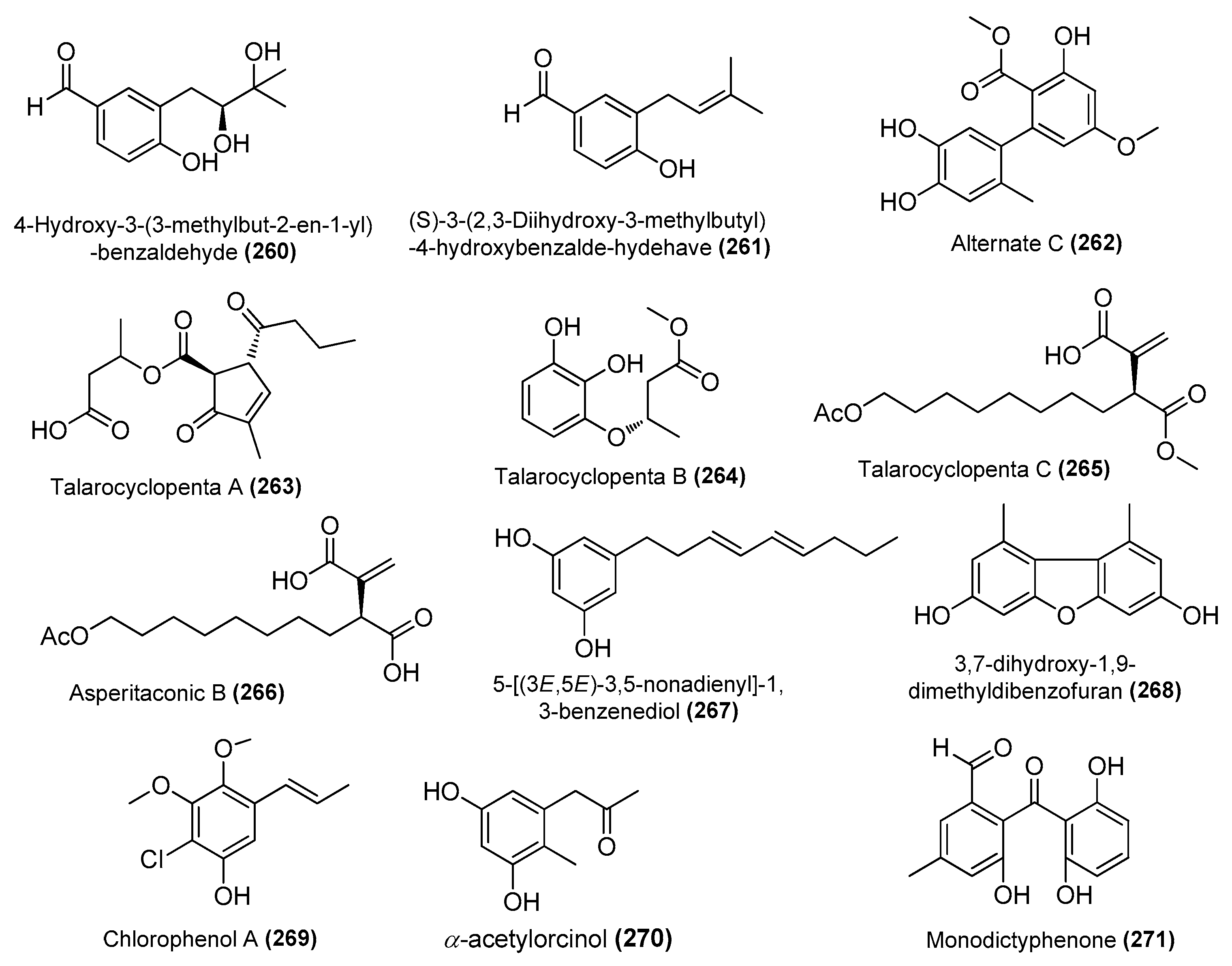
| 4-hydroxy-3-(3-methylbut-2-en-1-yl)-benzaldehyde (260) | Aspergillus terreus C23-3 | Coral, Xuwen natural reserve located, South China Sea | _ | Inhibited the MAPK signaling pathway in RAW264.7 cells | [94] |
| (S)-3-(2,3-dihydroxy-3-methylbutyl)-4-hydroxybenzalde-hydehave (261) | Aspergillus terreus C23-3 | Coral, Xuwen natural reserve located, South China Sea | _ | Inhibited the MAPK signaling pathway in RAW264.7 cells | [94] |
| Alternate C (262) | Pleosporales sp. SF-7343 | King George Island, Antarctica | MK785420 | Inhibition of IL-6 and IL-8 | [47] |
| Talarocyclopentas A (263), B (264) and C (265) | Talaromyces assiutensis JTY2 | Mangrove Ceriops tagal, South China Sea | JN899320.1 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 3.38, 6.26, and 12.56 μΜ, respectively | [95] |
| Asperitaconic B (266) | T. assiutensis JTY2 | Mangrove Ceriops tagal, South China Sea | JN899320.1 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 15.63 μΜ | [95] |
| 5-[(3E,5E)-3,5-nonadienyl]-1,3-benzenediol (267) | Aspergillus sp. | Brown alga Saccharina cichorioides, South China Sea | 2167894 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.0 μΜ | [96] |
| 3,7-dihydroxy-1,9-dimethyldibenzofuran (268) | Aspergillus sydowii MCCC 3A00324 | Deep sea sediment, South Atlantic Ocean | MN918102 | Inhibited NO production in LPS-induced BV-2 cells, 94.4% (10 μΜ) | [24] |
| Chlorophenol A (269) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited excessive LPS-induced production of NO and pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| α-acetylorcinol (270) | Amorosia sp. SCSIO 4102 | Mangrove Avicennia marina, Zhanjiang, Guangdong, China | OL826791 | Inhibited excessive LPS-induced production of NO and pro-inflammatory cytokines at the mRNA and protein levels | [36] |
| Monod-ictyphenone (271) | Diaporthe sp. SYSU-MS4722 | Shenzhen City, Guangdong, Province, China | OK623372 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 40.8 μΜ | [38] |
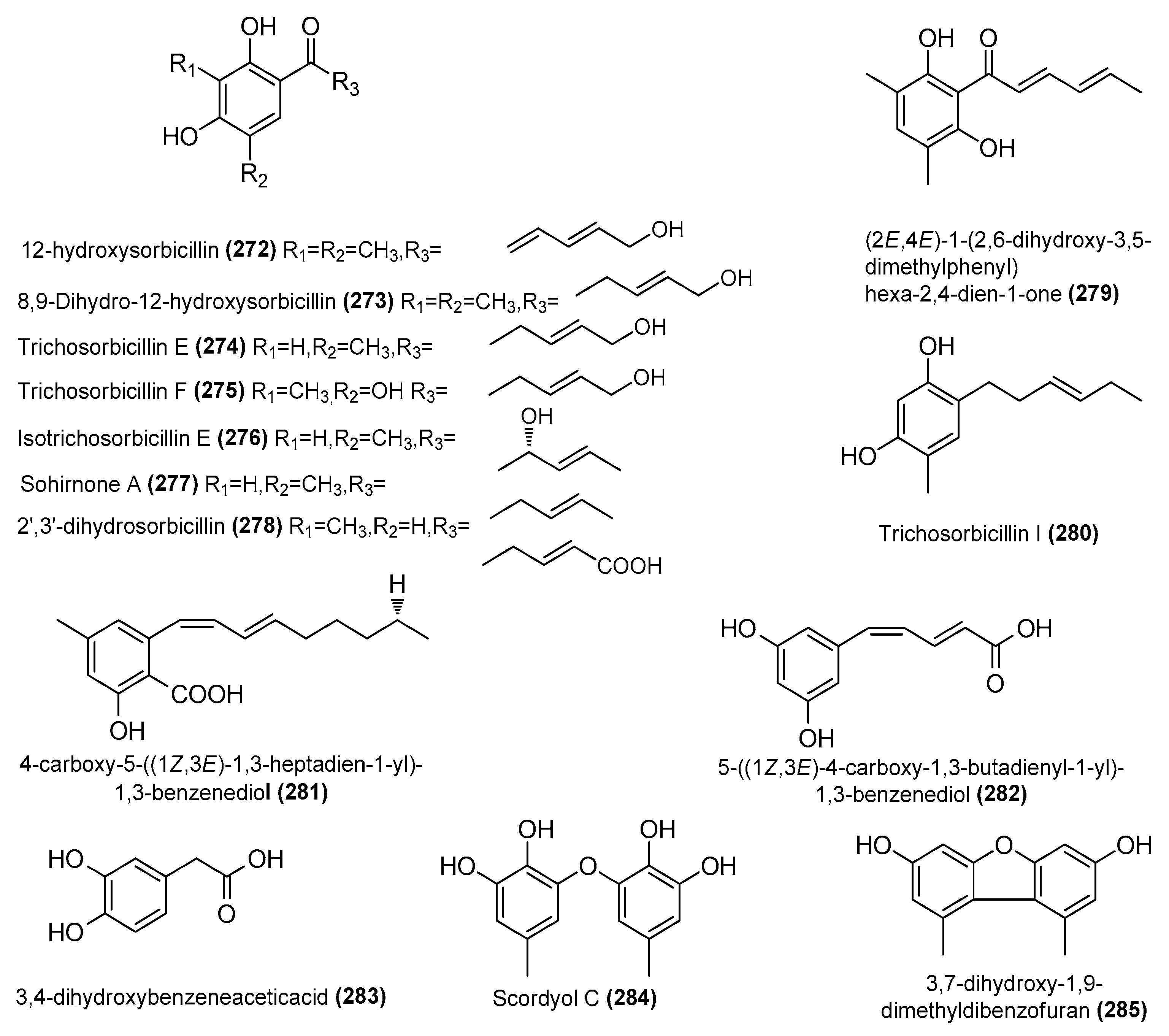
| 12-hydroxysorbicillin (272) | Trichoderma reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.8 μΜ | [63] |
| 8,9-Dihydro-12-hydroxysorbicillin (273) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 2.9 μΜ | [63] |
| Trichosorbicillin E (274) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 0.94 μΜ | [63] |
| Trichosorbicillin F (275) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 6.1 μΜ | [63] |
| Isotrichosorbicillin E (276) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 12 μΜ | [63] |
| Sohirnone A (277) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 14 μΜ | [63] |
| 2′,3′-dihydrosorbicillin (278) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 13 μΜ | [63] |
| (2E,4E)-1-(2,6-dihydroxy-3,5-dimethylphenyl) hexa-2,4-dien-1-one (279) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 3.3 μΜ | [63] |
| Trichosorbicillin I (280) | T. reesei 4670 | Sponge, Shantou, Guangdong Province, China | MH542677 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 13 μΜ | [63] |
| 4-carboxy-5-((1Z,3E)-1,3-heptadien-1-yl)-1,3-benzenediol (281) | Penicillium sp. TW58-16 | Deep-sea hydrothermal vent sediment, Kueishantao, Taiwan, China | MZ558028 | Regulation of gut microbiota contributes to anti-inflammatory effects | [52] |
| 5-((1Z,3E)-4-carboxy-1,3-butadienyl-1-yl)-1,3-benzenediol (282) | Penicillium sp. TW58-16 | Deep-sea hydrothermal vent sediment, Kueishantao, Taiwan, China | MZ558028 | Regulation of gut microbiota contributes to anti-inflammatory effects | [52] |
| 3,4-dihydroxybenzeneaceticacid (283) | Penicillium sp. TW58-16 | Deep-sea hydrothermal vent sediment, Kueishantao, Taiwan, China | MZ558028 | Regulation of gut microbiota contributes to anti-inflammatory effects | [52] |
| Scordyol C (284) | Aspergillus carneus GXIMD00543 | Sponge, Weizhou islands coral reef, China | OR501447 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 26.8 ± 1.7μΜ | [72] |
| 3,7-dihydroxy-1,9-dimethyldibenzofuran (285) | Aspergillus carneus GXIMD00543 | Sponge, Weizhou islands coral reef, China | OR501447 | Inhibited NO production in LPS-induced RAW 264.7 cells, IC50 = 2.9 ± 0.1 μΜ | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Chen, S.; Yu, M.; Shi, J.; Liu, J.; Li, X.; Chen, J.; Sun, X.; Huang, G.; Zheng, C. Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Mar. Drugs 2024, 22, 433. https://doi.org/10.3390/md22100433
Qiu Y, Chen S, Yu M, Shi J, Liu J, Li X, Chen J, Sun X, Huang G, Zheng C. Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Marine Drugs. 2024; 22(10):433. https://doi.org/10.3390/md22100433
Chicago/Turabian StyleQiu, Yikang, Shiji Chen, Miao Yu, Jueying Shi, Jiayu Liu, Xiaoyang Li, Jiaxing Chen, Xueping Sun, Guolei Huang, and Caijuan Zheng. 2024. "Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity" Marine Drugs 22, no. 10: 433. https://doi.org/10.3390/md22100433
APA StyleQiu, Y., Chen, S., Yu, M., Shi, J., Liu, J., Li, X., Chen, J., Sun, X., Huang, G., & Zheng, C. (2024). Natural Products from Marine-Derived Fungi with Anti-Inflammatory Activity. Marine Drugs, 22(10), 433. https://doi.org/10.3390/md22100433






