Lipidome of the Brown Macroalga Undaria pinnatifida: Influence of Season and Endophytic Infection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Lipid Composition of Undaria pinnatifida
2.1.1. Lipid Classes’ Composition of Undaria pinnatifida
2.1.2. Fatty Acids Composition of Undaria pinnatifida
2.1.3. Lipidome of Undaria pinnatifida
2.2. Seasonal Dynamics of the Lipid Composition of Undaria pinnatifida
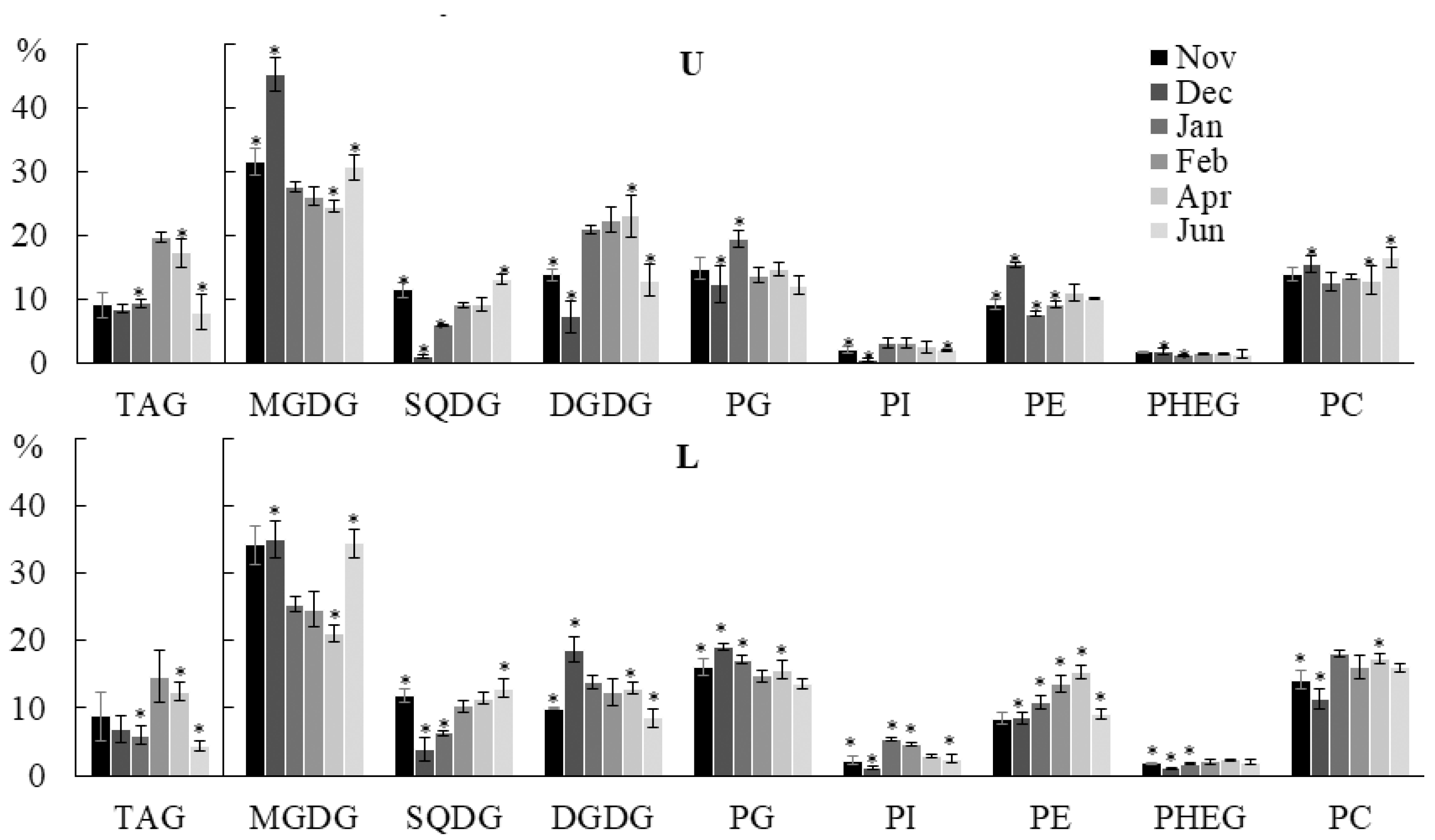
2.2.1. Lipid Classes Composition
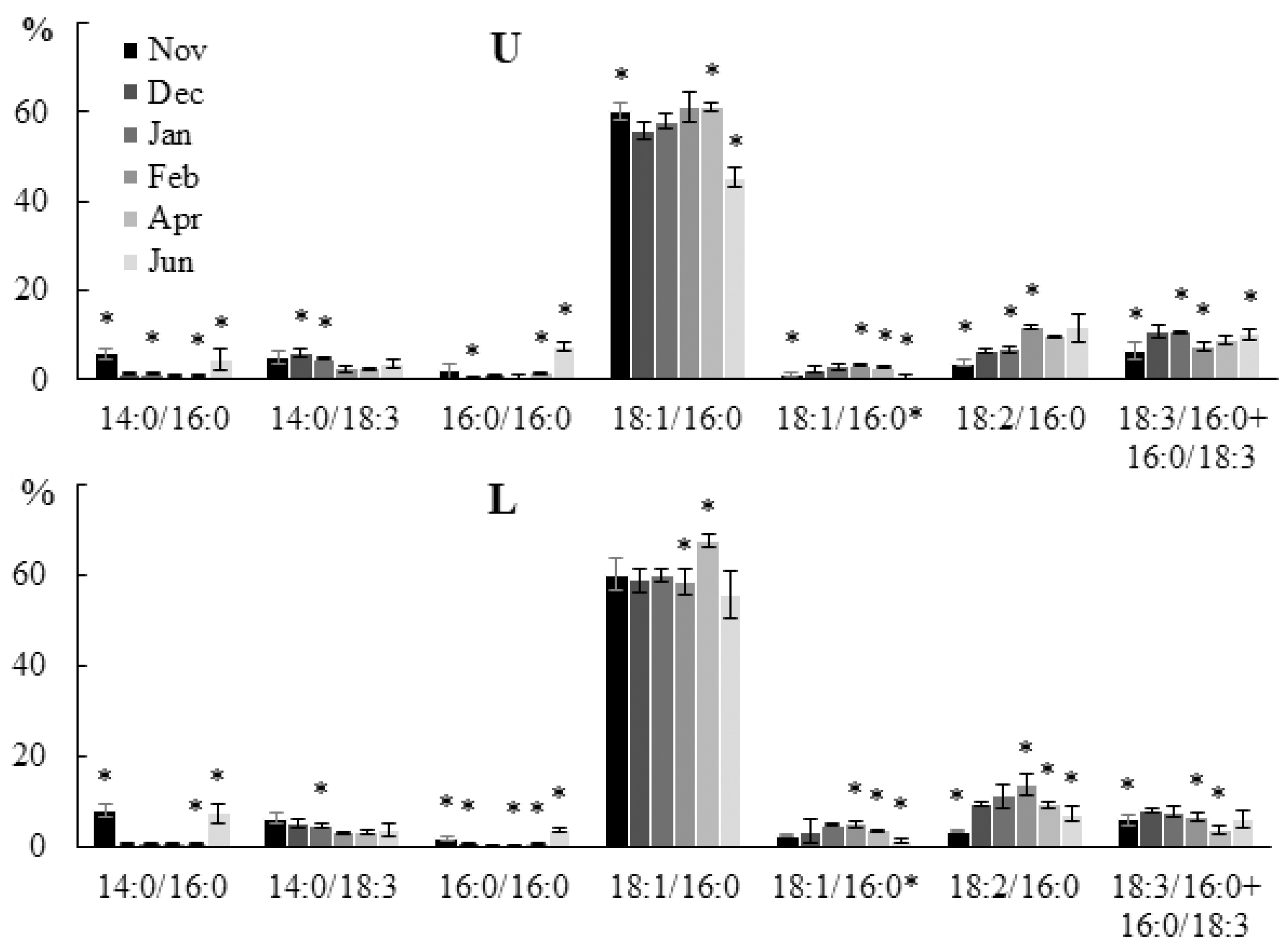
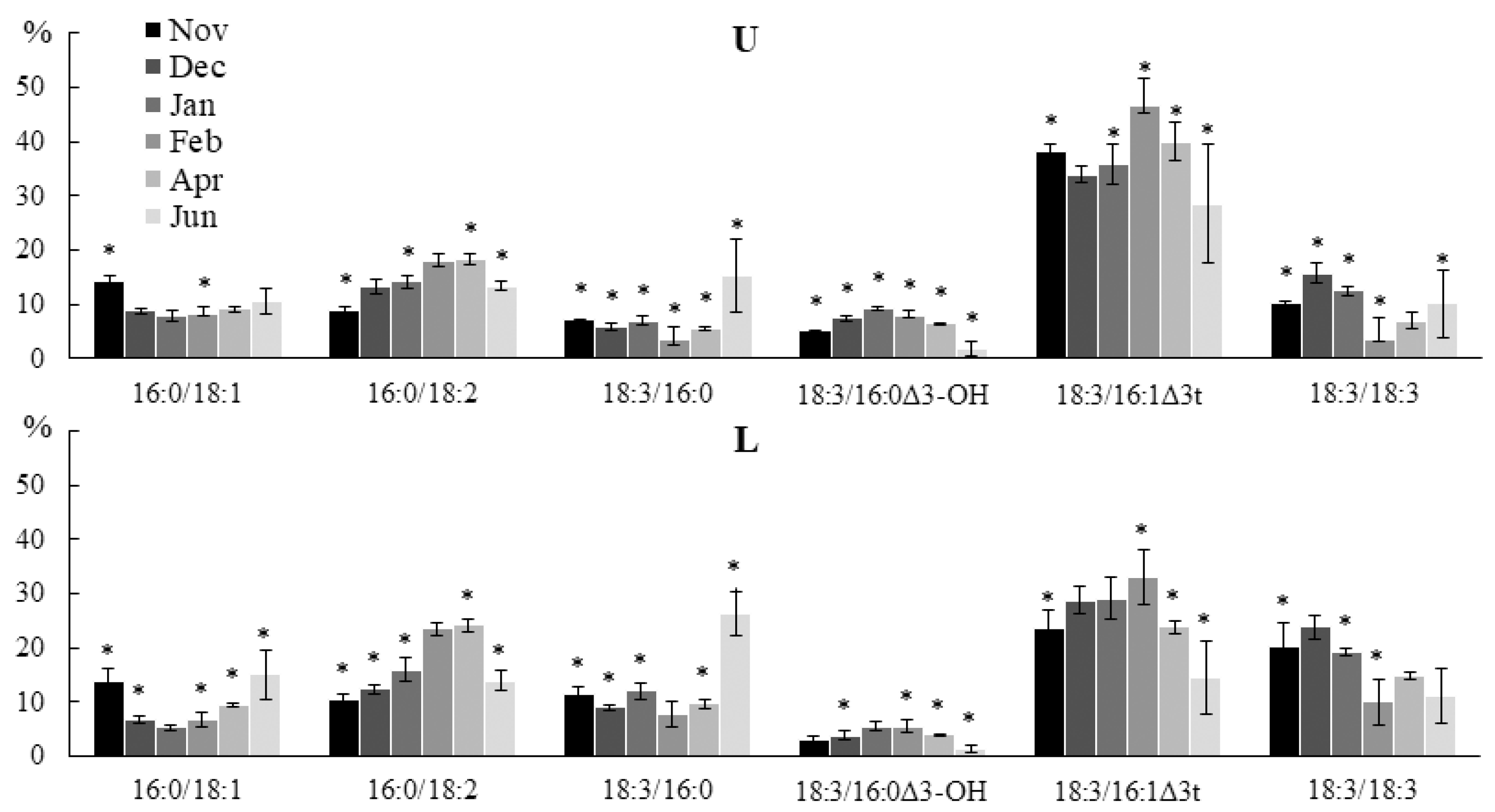
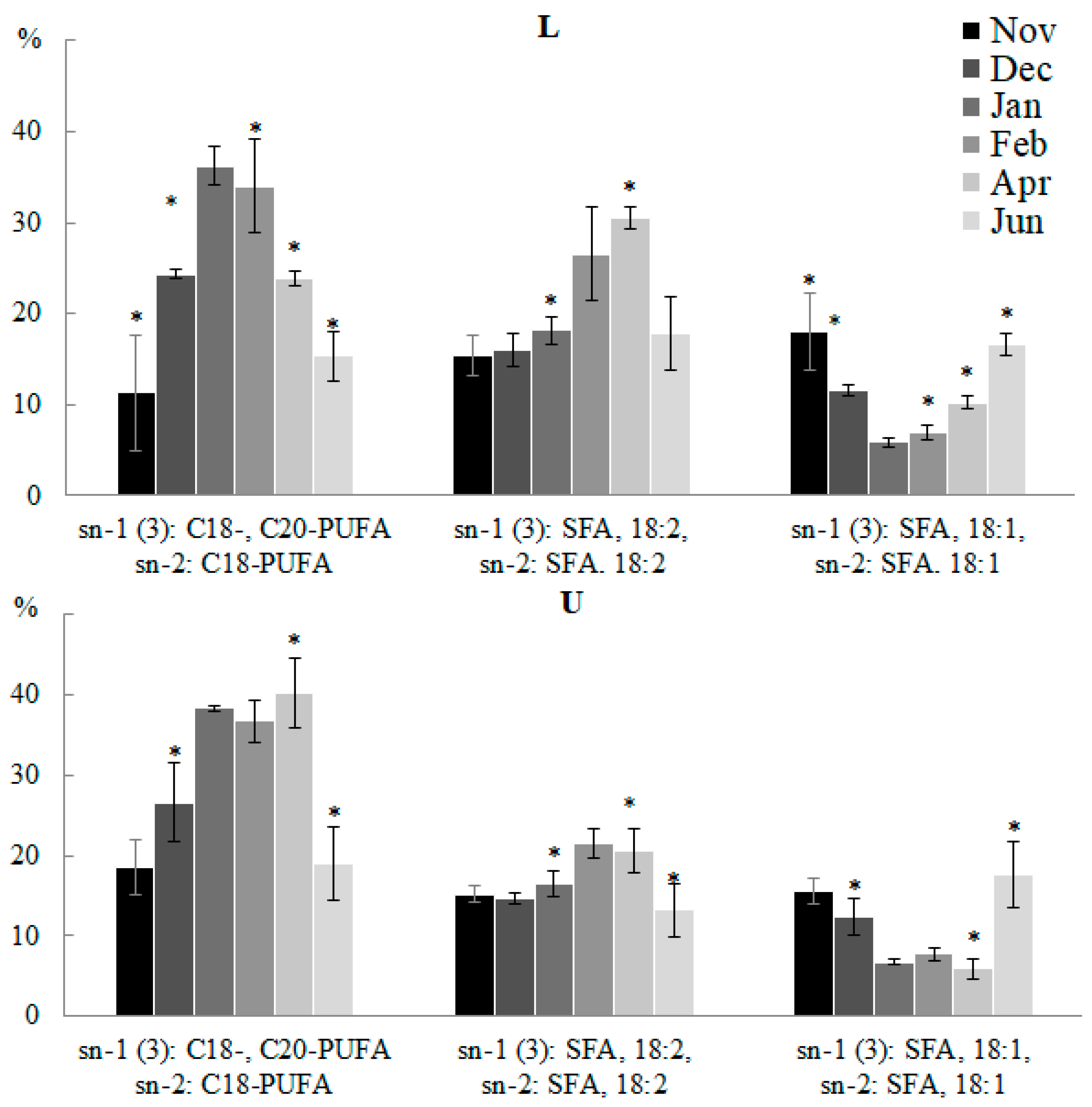
2.2.2. Molecular Species Composition
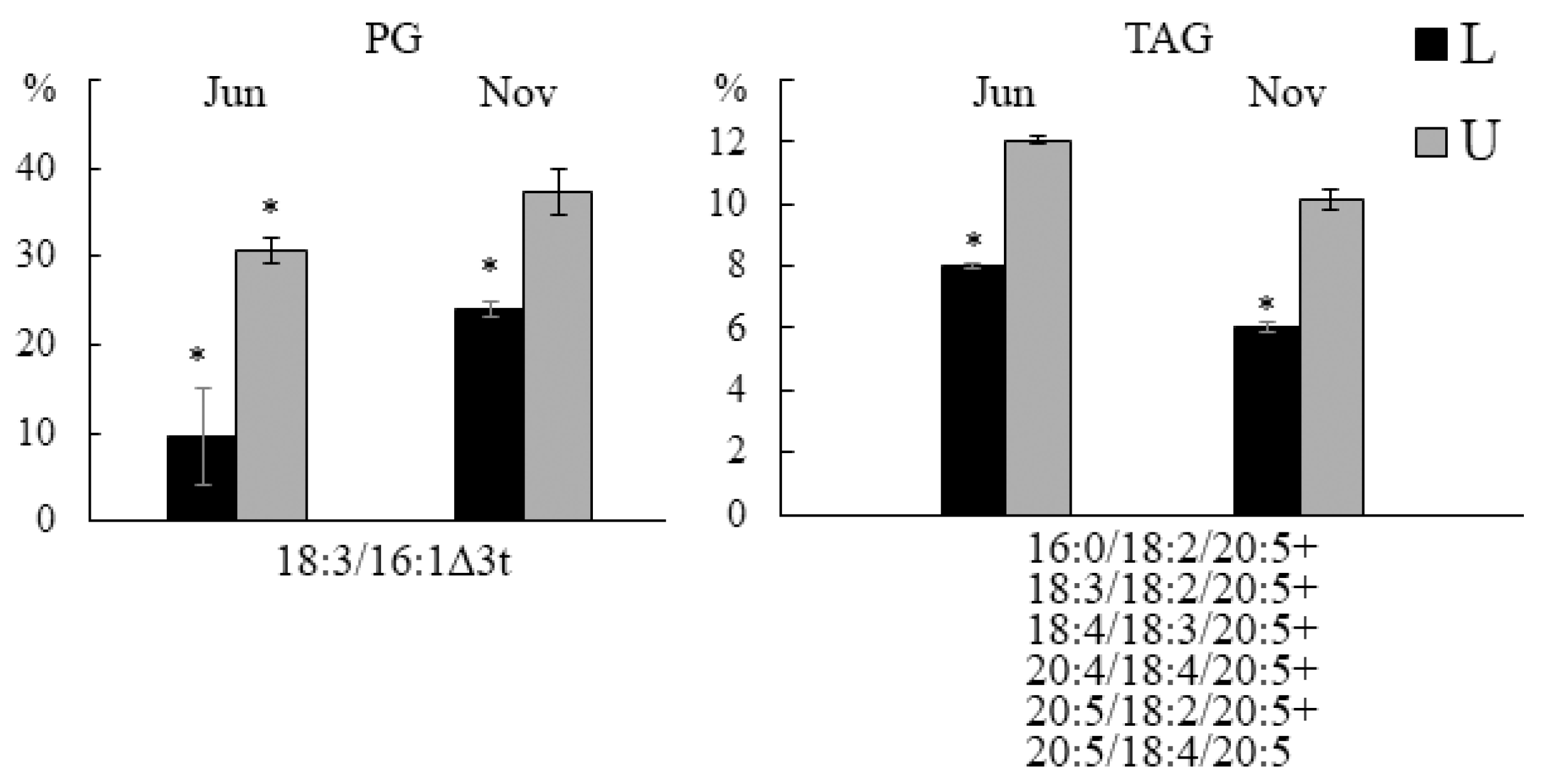
2.3. Effect of Infection with the Endophyte Laminariocolax aecidioides on the Lipid Composition of Undaria pinnatifida
2.3.1. Lipid Classes Composition
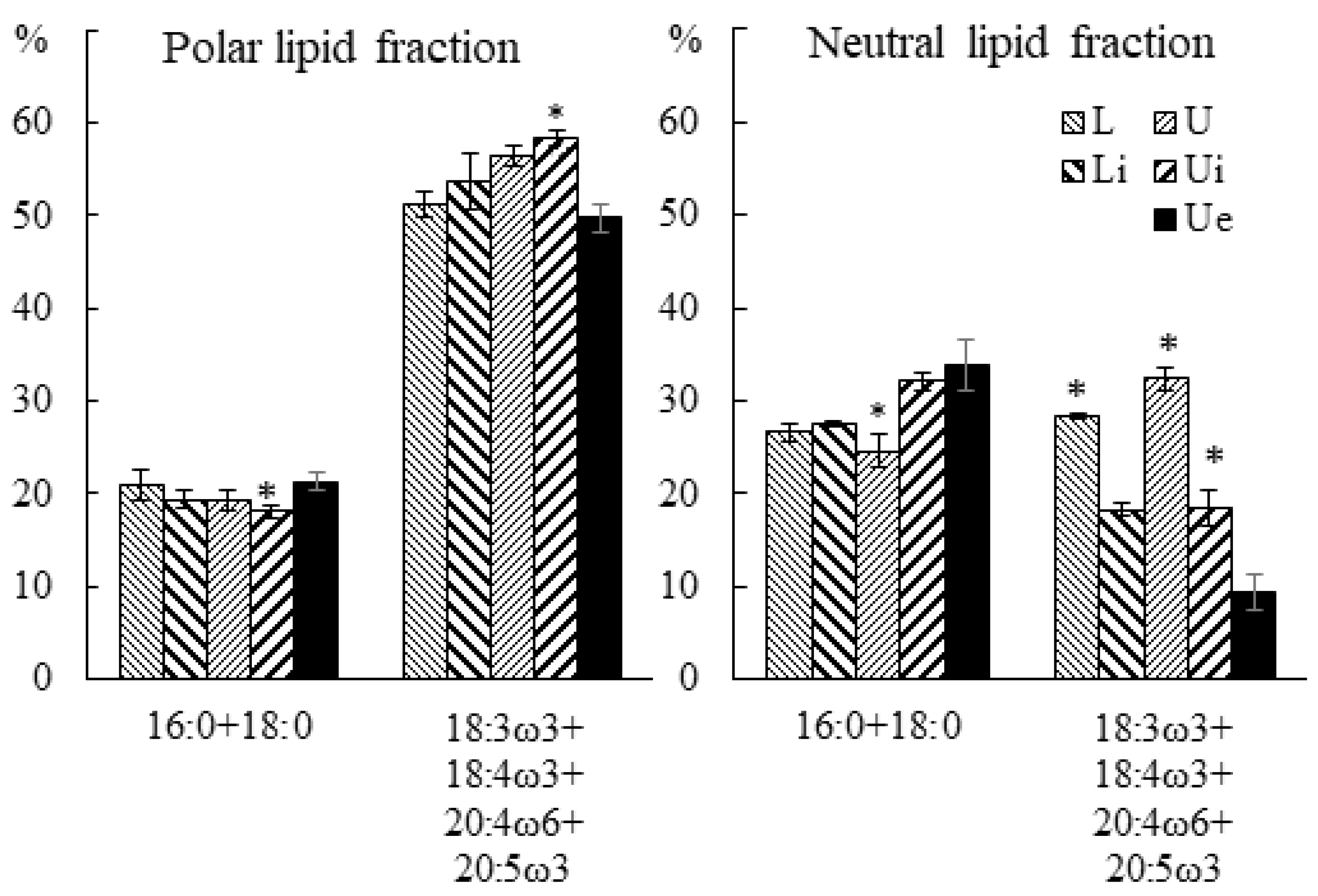
2.3.2. Fatty Acid Composition
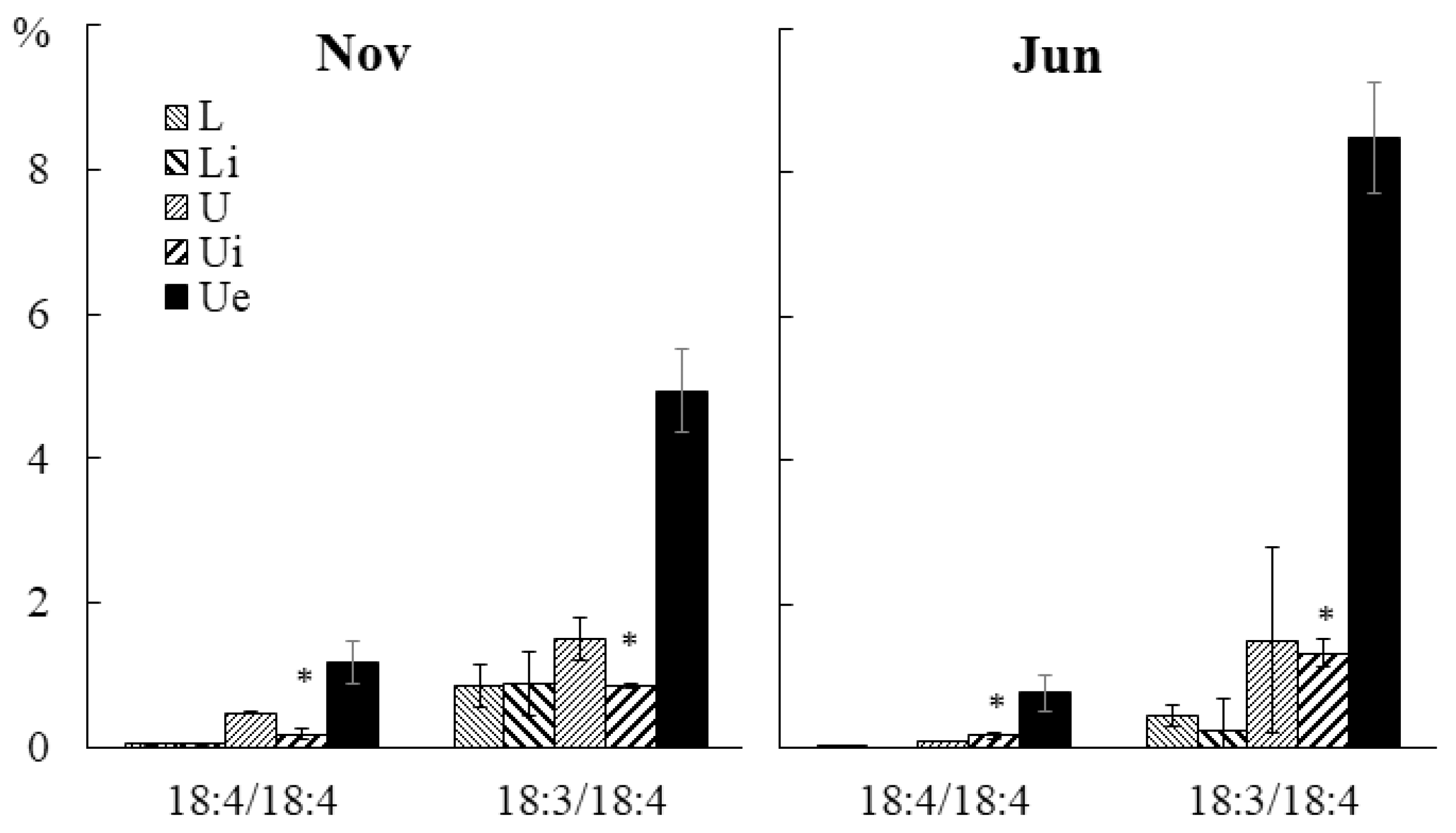
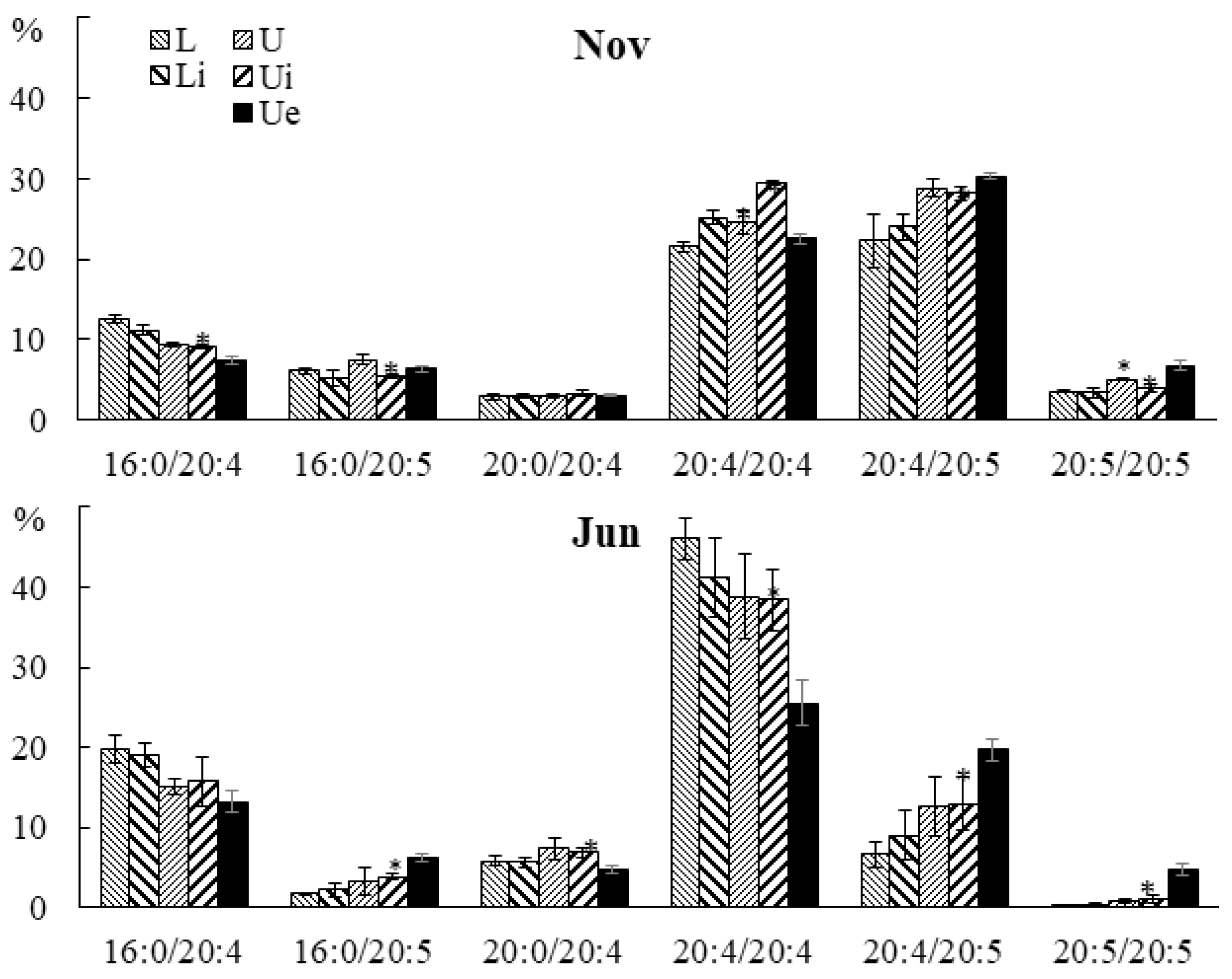
2.3.3. Molecular Species Composition
3. Materials and Methods
3.1. Algae Material
3.2. Lipid Extraction
3.3. Fatty Acids Analysis by GC and GC-MS
3.4. HPLC-MS/MS Analysis of Molecular Species
3.5. HPLC-MS/MS Analysis of Lipid Classes
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BL | Betaine lipid |
| DAG | Diacylglyceride |
| DGDG | Digalactosyldiacylglycerol |
| DGTS | Diacylglyceryl-N,N,N-trimethylhomoserine |
| ER | Endoplasmic reticulum |
| FA | Fatty acid |
| GL | Glycoglycerolipid |
| GlcADG | Glucuronosyldiacylglycerol |
| LHC II | Light-harvesting complex II |
| MGDG | Monogalactosyldiacylglycerol |
| MUFA | Monounsaturated fatty acid |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| PG | Phosphatidylglycerol |
| PHEG | Phosphatidylhydroxyethylglycine |
| PI | Phosphatidylinositol |
| PL | Phosphoglycerolipid |
| PSII | Photosystem II |
| PUFA | Polyunsaturated fatty acid |
| SFA | Saturated fatty acid |
| SQDG | Sulfoquinovosyldiacylglycerol |
| TAG | Triacylglyceride |
References
- Pereira, R.; Yarish, C. Mass Production of Marine Macroalgae. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Oxford, UK, 2008; pp. 2236–2247. ISBN 9780080914565. [Google Scholar]
- Skriptsova, A.; Khomenko, V.; Isakov, V. Seasonal Changes in Growth Rate, Morphology and Alginate Content in Undaria pinnatifida at the Northern Limit in the Sea of Japan (Russia). J. Appl. Phycol. 2004, 16, 17–21. [Google Scholar] [CrossRef]
- Ambika, H.D. Positive and Negative Environmental Impacts on Algae. In Algae Materials; Arunkumar, K., Arun, A., Raja, R., Palaniappan, R., Eds.; Developments in Applied Microbiology and Biotechnology; Academic Press: Oxford, UK, 2023; pp. 343–353. ISBN 978-0-443-18816-9. [Google Scholar]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal Lipids, Fatty Acids and Sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Dominguez, H., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 87–134. ISBN 9780857095121. [Google Scholar]
- Gerasimenko, N.I.; Skriptsova, A.V.; Busarova, N.G.; Moiseenko, O.P. Effects of the Season and Growth Stage on the Contents of Lipids and Photosynthetic Pigments in Brown Alga Undaria pinnatifida. Russ. J. Plant Physiol. 2011, 58, 885–891. [Google Scholar] [CrossRef]
- Boulom, S.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Seasonal Changes in Lipid, Fatty Acid, α-Tocopherol and Phytosterol Contents of Seaweed, Undaria pinnatifida, in the Marlborough Sounds, New Zealand. Food Chem. 2014, 161, 261–269. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Kalita, T.L. The First Record of the Brown Endophytic Alga Laminariocolax aecidioides (Rosenvinge) A.F. Peters, 1998 in the Russian Far-Eastern Seas. Russ. J. Mar. Biol. 2020, 46, 42–48. [Google Scholar] [CrossRef]
- Gauna, M.C.; Parodi, E.R.; Cáceres, E.J. Epi-Endophytic Symbiosis between Laminariocolax aecidioides (Ectocarpales, Phaeophyceae) and Undaria pinnatifida (Laminariales, Phaeophyceae) Growing on Argentinian Coasts. J. Appl. Phycol. 2009, 21, 11–18. [Google Scholar] [CrossRef]
- Xing, Q.; Bernard, M.; Rousvoal, S.; Corre, E.; Markov, G.V.; Peters, A.F.; Leblanc, C. Different Early Responses of Laminariales to an Endophytic Infection Provide Insights About Kelp Host Specificity. Front. Mar. Sci. 2021, 8, 742469:1–742469:14. [Google Scholar] [CrossRef]
- Bouarab, K.; Adas, F.; Gaquerel, E.; Kloareg, B.; Salaün, J.P.; Potin, P. The Innate Immunity of a Marine Red Alga Involves Oxylipins from Both the Eicosanoid and Octadecanoid Pathways. Plant Physiol. 2004, 135, 1838–1848. [Google Scholar] [CrossRef]
- Lion, U.; Wiesemeier, T.; Weinberger, F.; Beltrán, J.; Flores, V.; Faugeron, S.; Correa, J.; Pohnert, G. Phospholipases and Galactolipases Trigger Oxylipin-Mediated Wound-Activated Defence in the Red Alga Gracilaria chilensis against Epiphytes. ChemBioChem 2006, 7, 457–462. [Google Scholar] [CrossRef]
- Kumari, P.; Reddy, C.R.K.; Jha, B. Methyl Jasmonate-Induced Lipidomic and Biochemical Alterations in the Intertidal Macroalga Gracilaria dura (Gracilariaceae, Rhodophyta). Plant Cell Physiol. 2015, 56, 1877–1889. [Google Scholar] [CrossRef]
- Chadova, O.A.; Velansky, P.V. The Influence of Endophyte Laminariocolax aecidioides (Rosenvinge) A.F. Peters, 1998 (Phaeophyceae, Ectocarpales) on the Lipid Composition of the Brown Alga Undaria pinnatifida (Harvey) Suringar, 1873 (Phaeophyceae: Laminariales). Russ. J. Mar. Biol. 2022, 48, 373–379. [Google Scholar] [CrossRef]
- Khotimchenko, S.V.; Kulikova, I.V. Lipids of Different Parts of the Lamina of Laminaria japonica Aresch. Bot. Mar. 2000, 43, 87–91. [Google Scholar] [CrossRef]
- Vyssotski, M.; Lagutin, K.; MacKenzie, A.; Mitchell, K.; Scott, D. Phospholipids of New Zealand Edible Brown Algae. Lipids 2017, 52, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W.; Lester, R.L. Inositol Phosphorylceramide, a Novel Substance and the Chief Member of a Major Group of Yeast Sphingolipids Containing a Single Inositol Phosphate. J. Biol. Chem. 1974, 249, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, F.M.; Key, P.N.; Hickerson, S.M.; Turk, J.; Hsu, F.F.; Beverley, S.M. Inositol Phosphorylceramide Synthase Null Leishmania Are Viable and Virulent in Animal Infections Where Salvage of Host Sphingomyelin Predominates. J. Biol. Chem. 2022, 298, 102522:1–102522:33. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.A.; Brennan, P.J. The Isolation and Biosynthesis of the Ceramide-Phosphoinositol of Aspergillus niger. FEBS Lett. 1977, 74, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Khotimchenko, S.V.; Kulikova, I.V.; Vas’kovskii, V.E. Distribution of Ceramidephosphoinositol in Red Seaweeds. Russ. J. Mar. Biol. 2000, 26, 286–288. [Google Scholar] [CrossRef]
- Eichenberger, W.; Araki, S.; Müller, D.G. Betaine Lipids and Phospholipids in Brown Algae. Phytochemistry 1993, 34, 1323–1333. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L.; Wang, X.; Cong, P.; Xu, J.; Xue, C. Comprehensive Lipidomic Analysis of Three Edible Brown Seaweeds Based on Reversed-Phase Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2022, 70, 4138–4151. [Google Scholar] [CrossRef]
- Rey, F.; Lopes, D.; Maciel, E.; Monteiro, J.; Skjermo, J.; Funderud, J.; Raposo, D.; Domingues, P.; Calado, R.; Domingues, M.R. Polar Lipid Profile of Saccharina latissima, a Functional Food from the Sea. Algal Res. 2019, 39, 101473:1–101473:8. [Google Scholar] [CrossRef]
- Okazaki, Y.; Otsuki, H.; Narisawa, T.; Kobayashi, M.; Sawai, S.; Kamide, Y.; Kusano, M.; Aoki, T.; Hirai, M.Y.; Saito, K. A New Class of Plant Lipid Is Essential for Protection against Phosphorus Depletion. Nat. Commun. 2013, 4, 1510:1–1510:10. [Google Scholar] [CrossRef]
- Eichenberger, W.; Gribi, C. Diacylglyceryl-α-D-Glucuronide from Ochromonas danica (Chrysophyceae). J. Plant Physiol. 1994, 144, 272–276. [Google Scholar] [CrossRef]
- Koelmel, J.P.; Campbell, J.E.; Guingab-Cagmat, J.; Meke, L.; Garrett, T.J.; Stingl, U. Re-Modeling of Foliar Membrane Lipids in a Seagrass Allows for Growth in Phosphorus-Deplete Conditions. PLoS ONE 2019, 14, e0218690:1–e0218690:10. [Google Scholar] [CrossRef] [PubMed]
- Hölzl, G.; Dörmann, P. Structure and Function of Glycoglycerolipids in Plants and Bacteria. Prog. Lipid Res. 2007, 46, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, Y.; Nishizawa, T.; Takano, K.; Ohnishi, M.; Mimura, T.; Saito, K. Induced Accumulation of Glucuronosyldiacylglycerol in Tomato and Soybean under Phosphorus Deprivation. Physiol. Plant. 2015, 155, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Tabakaeva, O.V.; Tabakaev, A.V. Compositions of Lipids and Fatty Acids from Various Parts of the Brown Alga Undaria pinnatifida. Chem. Nat. Compd. 2017, 53, 843–848. [Google Scholar] [CrossRef]
- Dubertret, G.; Gerard-Hirne, C.; Trémolières, A. Importance of Trans-Δ3-Hexadecenoic Acid Containing Phosphatidylglycerol in the Formation of the Trimeric Light-Harvesting Complex in Chlamydomonas. Plant Physiol. Biochem. 2002, 40, 829–836. [Google Scholar] [CrossRef]
- Loll, B.; Kern, J.; Saenger, W.; Zouni, A.; Biesiadka, J. Towards Complete Cofactor Arrangement in the 3.0 Å Resolution Structure of Photosystem II. Nature 2005, 438, 1040–1044. [Google Scholar] [CrossRef]
- Jordan, P.; Fromme, P.; Witt, H.T.; Klukas, O.; Saenger, W.; Krauß, N. Three-Dimensional Structure of Cyanobacterial Photosystem I at 2.5 Å Resolution. Nature 2001, 411, 909–917. [Google Scholar] [CrossRef]
- Los, D.A.; Mironov, K.S.; Allakhverdiev, S.I. Regulatory Role of Membrane Fluidity in Gene Expression and Physiological Functions. Photosynth. Res. 2013, 116, 489–509. [Google Scholar] [CrossRef]
- Garab, G.; Ughy, B.; Goss, R. Role of MGDG and Non-Bilayer Lipid Phases in the Structure and Dynamics of Chloroplast Thylakoid Membranes. In Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2016; Volume 86, pp. 127–157. ISBN 978-3-319-25977-2. [Google Scholar]
- Williams, W.P. The Physical Properties of Thylakoid Membrane Lipids and Their Relation to Photosynthesis. In Lipids in Photosynthesis: Structure, Function and Genetics; Siegenthaler, P.-A., Murata, N., Eds.; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 2006; pp. 103–118. [Google Scholar]
- Moon, B.Y.; Higashi, S.; Gombos, Z.; Murata, N. Unsaturation of the Membrane Lipids of Chloroplasts Stabilizes the Photosynthetic Machinery against Low-Temperature Photoinhibition in Transgenic Tobacco Plants. Proc. Natl. Acad. Sci. USA 1995, 92, 6219–6223. [Google Scholar] [CrossRef]
- Chadova, O.; Skriptsova, A.; Velansky, P. Effect of Temperature and Light Intensity on the Polar Lipidome of Endophytic Brown Algae Streblonema Corymbiferum and Streblonema Sp. in Vitro. Mar. Drugs 2022, 20, 428. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.R.; Ivanov, A.G.; Król, M.; Williams, J.P.; Kahn, M.U.; Myscich, E.G.; Huner, N.P.A. Temperature and Light Modulate the Trans-Δ3-Hexadecenoic Acid Content of Phosphatidylglycerol: Light-Harvesting Complex II Organization and Non-Photochemical Quenching. Plant Cell Physiol. 2005, 46, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-Q.; Zhao, H.; Liang, J.-S.; Ji, B.-H.; Jiao, D.-M. Relationships between Phosphatidylglycerol Molecular Species of Thylakoid Membrane Lipids and Sensitivities to Chilling-Induced Photoinhibition in Rice. J. Integr. Plant Biol. 2008, 50, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Honya, M.; Kinoshita, T.; Ishikawa, M.; Mori, H.; Nisizawa, K. Seasonal Variation in the Lipid Content of Cultured Laminaria japonica: Fatty Acids, Sterols, β-Carotene and Tocopherol. J. Appl. Phycol. 1994, 6, 25–29. [Google Scholar] [CrossRef]
- Balla, T. Phosphoinositides: Tiny Lipids with Giant Impact on Cell Regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gügel, I.L.; Giavalisco, P.; Zeisler, V.; Schreiber, L.; Soll, J.; Philippar, K. FAX1, a Novel Membrane Protein Mediating Plastid Fatty Acid Export. PLoS Biol. 2015, 13, e1002053:1–e1002053:38. [Google Scholar] [CrossRef] [PubMed]
- Bates, P.D.; Ohlrogge, J.B.; Pollard, M. Incorporation of Newly Synthesized Fatty Acids into Cytosolic Glycerolipids in Pea Leaves Occurs via Acyl Editing. J. Biol. Chem. 2007, 282, 31206–31216. [Google Scholar] [CrossRef]
- Wang, Z.; Benning, C. Chloroplast Lipid Synthesis and Lipid Trafficking through ER-Plastid Membrane Contact Sites. Biochem. Soc. Trans. 2012, 40, 457–463. [Google Scholar] [CrossRef]
- Warakanont, J.; Tsai, C.H.; Michel, E.J.S.; Murphy, G.R.; Hsueh, P.Y.; Roston, R.L.; Sears, B.B.; Benning, C. Chloroplast Lipid Transfer Processes in Chlamydomonas reinhardtii Involving a TRIGALACTOSYLDIACYLGLYCEROL 2 (TGD2) Orthologue. Plant J. 2015, 84, 1005–1020. [Google Scholar] [CrossRef]
- Khozin, I.; Adlerstein, D.; Bigongo, C.; Heimer, Y.M.; Cohen, Z. Elucidation of the Biosynthesis of Eicosapentaenoic Acid in the Microalga Porphyridium cruentum. Plant Physiol. 1997, 114, 223–230. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and Lipid Metabolism in Eukaryotic Algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Beisson, F.; Riekhof, W. Metabolism of Acyl-lipids in Chlamydomonas reinhardtii. Plant J. 2015, 82, 504–522. [Google Scholar] [CrossRef] [PubMed]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The Lipid Biochemistry of Eukaryotic Algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Hayashi, R. Effects of High Pressure on Lipids and Biomembranes for Understanding High-Pressure-Induced Biological Phenomena. Biosci. Biotechnol. Biochem. 1999, 63, 1321–1328. [Google Scholar] [CrossRef]
- Del Campo, E.; García-Reina, G.; Correa, J.A. Degradative Disease in Ulva rigida (Chlorophyceae) Associated with Acrochaete geniculata (Chlorophyceae). J. Phycol. 1998, 34, 160–166. [Google Scholar] [CrossRef]
- Malitsky, S.; Ziv, C.; Rosenwasser, S.; Zheng, S.; Schatz, D.; Porat, Z.; Ben-Dor, S.; Aharoni, A.; Vardi, A. Viral Infection of the Marine Alga Emiliania huxleyi Triggers Lipidome Remodeling and Induces the Production of Highly Saturated Triacylglycerol. New Phytol. 2016, 210, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, V.S.; Bandyopadhyay, P.; Rajacharya, G.H.; Sarkar, S.; Poluri, K.M.; Kumar, S. Amelioration of Biomass and Lipid in Marine Alga by an Endophytic Fungus Piriformospora indica. Biotechnol. Biofuels 2019, 12, 176:1–176:19. [Google Scholar] [CrossRef]
- Christie, W.W. Equivalent Chain-Lengths of Methyl Ester Derivatives of Fatty Acids on Gas Chromatography A Reappraisal. J. Chromatogr. A 1988, 447, 305–314. [Google Scholar] [CrossRef]
- Li, Y.; Lou, Y.; Mu, T.; Xu, J.; Zhou, C.; Yan, X. Simultaneous Structural Identification of Diacylglyceryl-N-Trimethylhomoserine (DGTS) and Diacylglycerylhydroxymethyl-N,N,N-Trimethyl-β-Alanine (DGTA) in Microalgae Using Dual Li+/H+ Adduct Ion Mode by Ultra-Performance Liquid Chromatography/Quadrupole Ti. Rapid Commun. Mass Spectrom. 2017, 31, 457–468. [Google Scholar] [CrossRef]
- Malone, M.; Evans, J.J. Determining the Relative Amounts of Positional Isomers in Complex Mixtures of Triglycerides Using Reversed-Phase High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Lipids 2004, 39, 273–284. [Google Scholar] [CrossRef]
- Li, X.; Evans, J.J. Examining the Collision-Induced Decomposition Spectra of Ammoniated Triglycerides as a Function of Fatty Acid Chain Length and Degree of Unsaturation. I. The OXO/YOY Series. Rapid Commun. Mass Spectrom. 2005, 19, 2528–2538. [Google Scholar] [CrossRef]
- Li, X.; Collins, E.J.; Evans, J.J. Examining the Collision-Induced Decomposition Spectra of Ammoniated Triglycerides as a Function of Fatty Acid Chain Length and Degree of Unsaturation. II. The PXP/YPY Series. Rapid Commun. Mass Spectrom. 2006, 20, 171–177. [Google Scholar] [CrossRef]
- Gakwaya, R.; Li, X.; Wong, Y.L.; Chivukula, S.; Collins, E.J.; Evans, J.J. Examining the Collision-Induced Decomposition Spectra of Ammoniated Triglycerides. III. The Linoleate and Arachidonate Series. Rapid Commun. Mass Spectrom. 2007, 21, 3262–3268. [Google Scholar] [CrossRef]



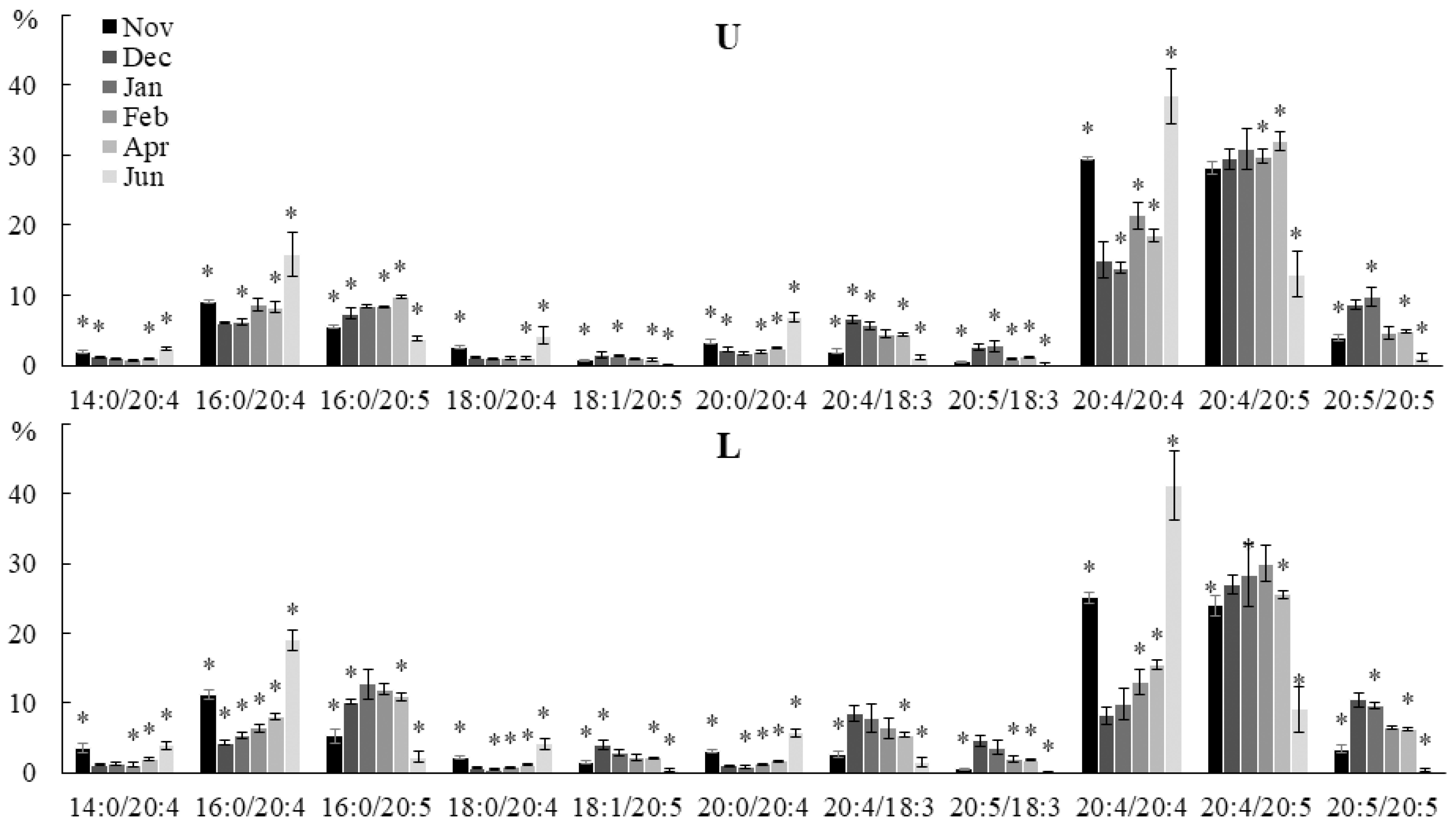
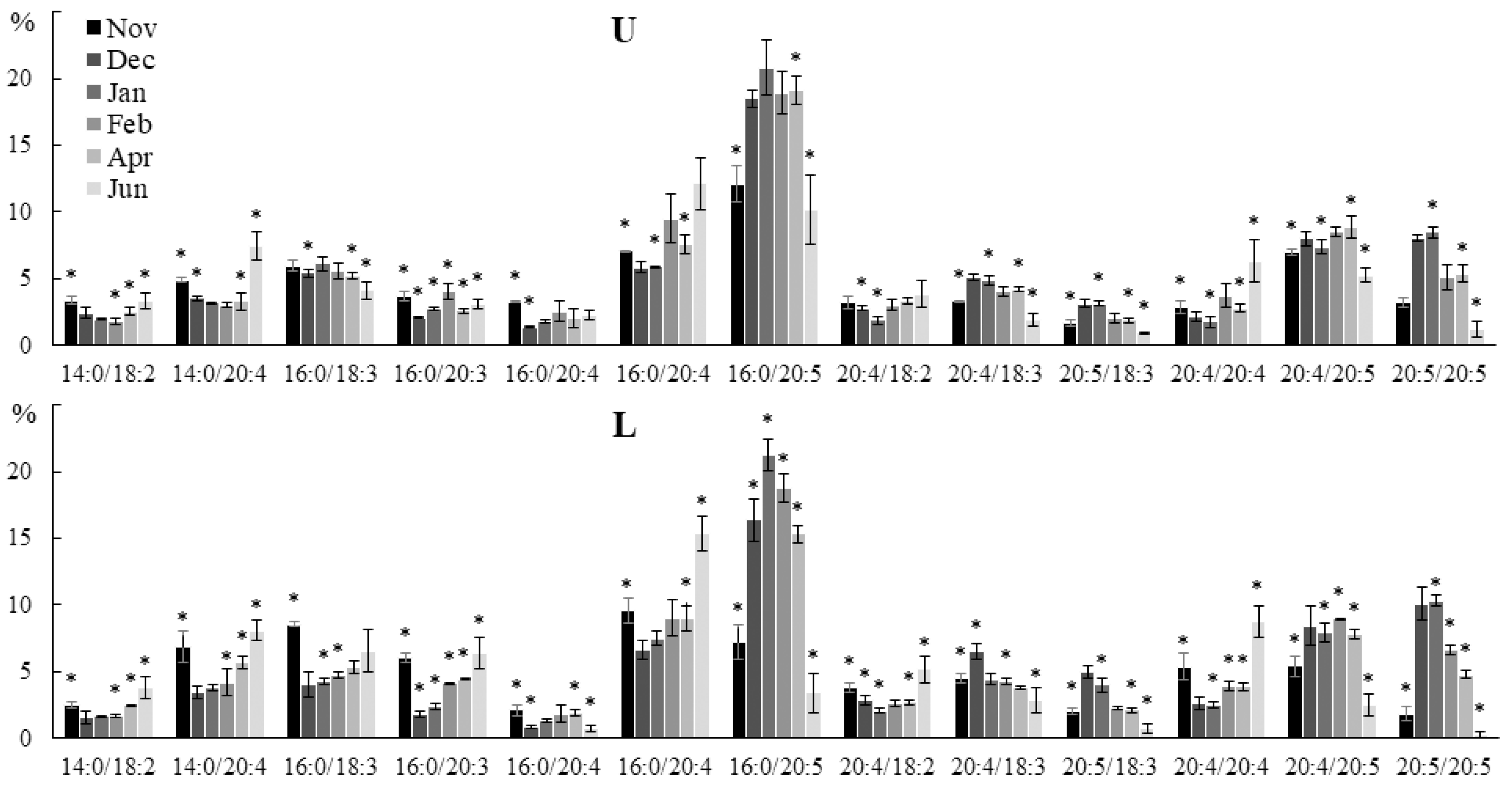
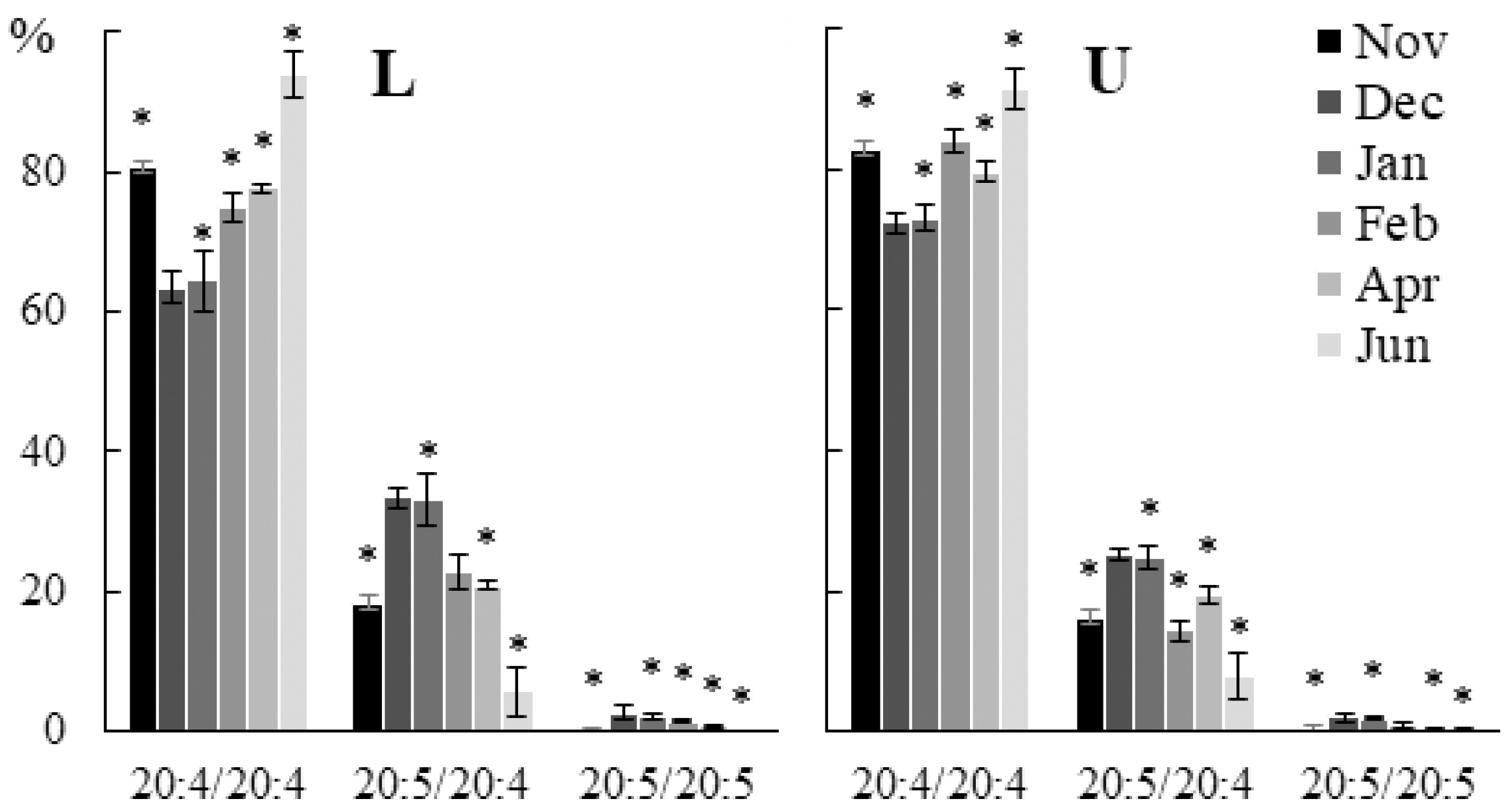
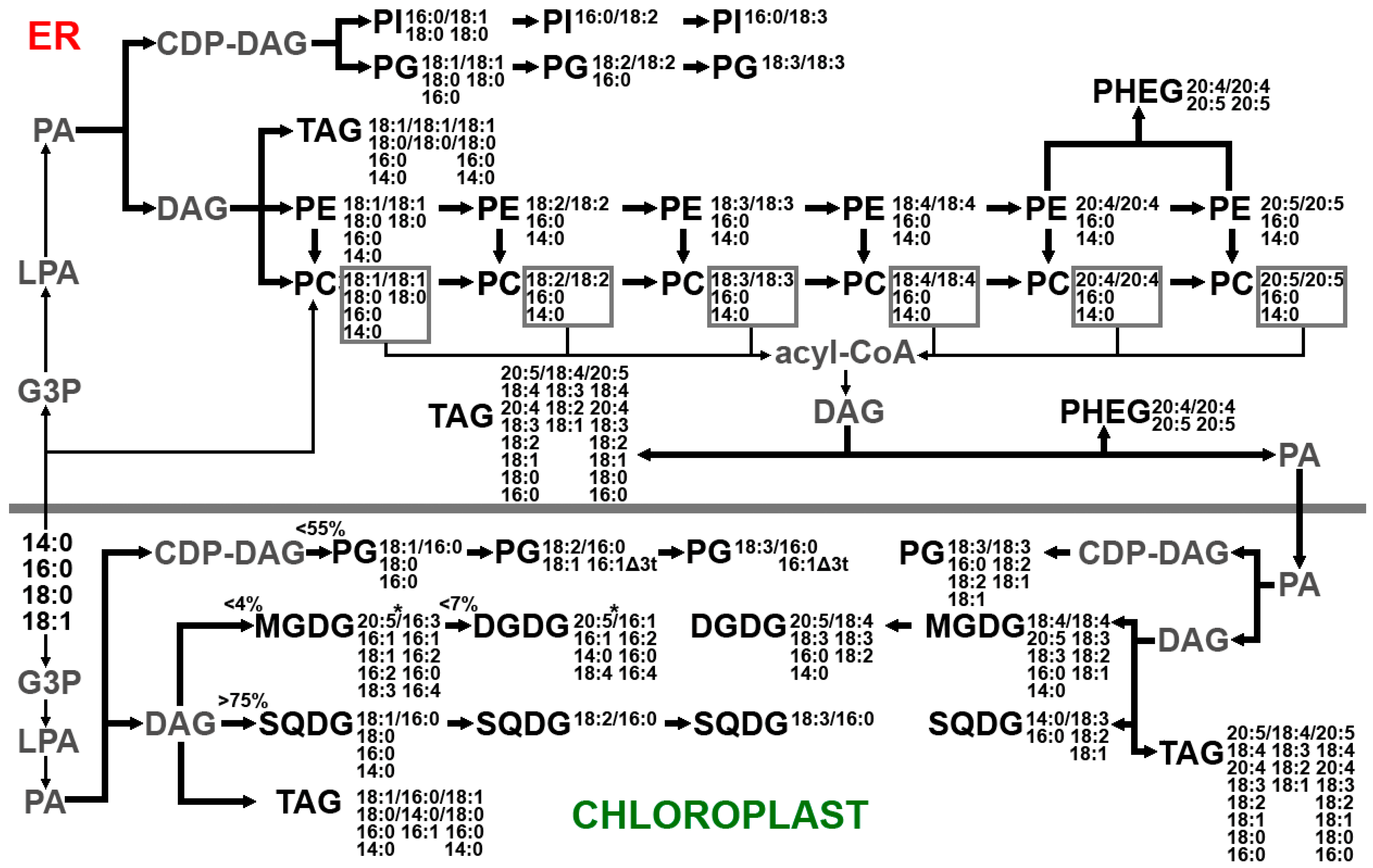


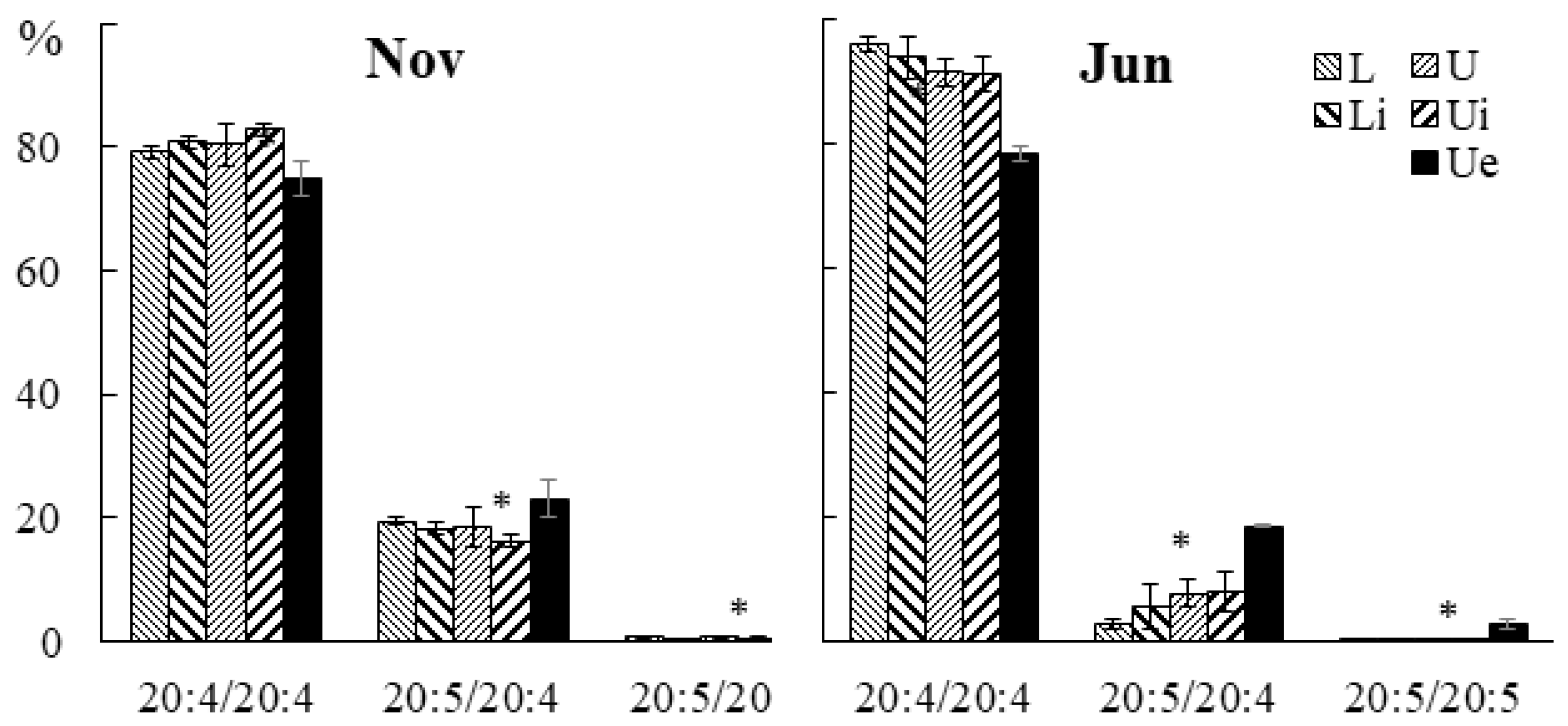
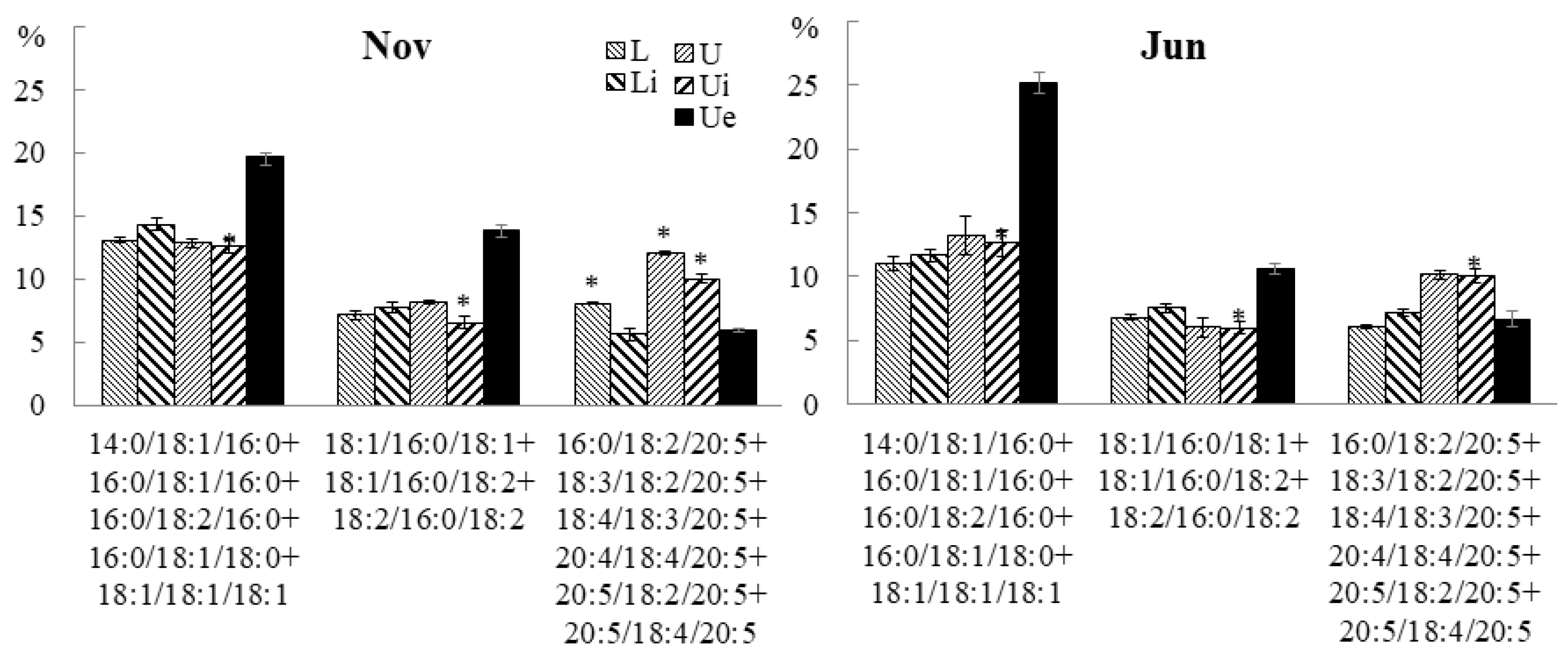

| Lipid Class | Total Number of Molecular Species | Main Molecular Species |
|---|---|---|
| Polar lipids | ||
| Glycoglycerolipids | ||
| MGDG | 146 | 18:4/18:4, 20:5/18:4, 18:3/18:4, 20:4/18:4 |
| DGDG | 66 | 20:5/18:4, 20:4/18:4, 16:0/18:3, 14:0/18:3, 14:0/18:3, 18:3/18:4, 20:5/18:3 |
| SQDG | 47 | 18:1/16:0, 18:2/16:0, 18:3/16:0+16:0/18:3, 14:0/18:2, 14:0/16:0, 16:0/16:0 |
| GlcADG | 36 | 14:0/18:2, 14:0/18:3, 14:0/18:1, 16:1/18:1, 16:0/18:3, 16:0/18:1, 18:1/18:2, 18:1/18:1 |
| Phosphoglycerolipids | ||
| PG | 105 | 18:3/16:0, 16:0/18:2, 16:0/18:1, 18:3/18:3 |
| PE | 96 | 20:4/20:4, 20:4/20:5, 16:0/20:4, 16:0/20:5, 20:5/20:5 |
| PC | 82 | 16:0/20:5, 16:0/20:4, 16:0/18:2, 16:0/18:3, 14:0/20:4, 14:0/20:5, 20:4/20:4, 20:4/20:5 |
| PI | 21 | 16:0/18:1, 16:0/18:2, 16:0/18:0, 16:0/18:3 |
| PHEG | 5 | 20:4/20:4, 20:5/20:4 |
| Betaine lipids | ||
| DGTA | 38 | 18:4/14:0, 20:5/14:0, 18:4/20:5, 20:5/20:5, 18:2/14:0, 16:1/14:0 |
| DGTS | 47 | 18:4/16:0, 18:3/16:0, 16:1/14:0, 18:4/14:0, 18:2/14:0, 16:1/16:0, 18:3/18:1, 18:1/18:1 |
| Phosphosphingolipids | ||
| CPI | 28 | 22:1d/14:0, 22:0d/14:0, 22:1d/13:0, 22:0d/16:0, 22:1d/16:0 |
| Neutral lipids | ||
| TAG | 190 | 14:0/18:1/16:0, 16:0/18:1/16:0, 16:0/18:1/18:0, 18:1/16:0/18:1, 16:0/18:2/20:5, 18:4/18:3/20:5, 20:4/18:4/20:5, 20:5/18:4/20:5 |
| Month | Site of Collection | Water Temperature |
|---|---|---|
| November | Sobol Bay (Ussuriisky Bay, Peter the Great Bay, Sea of Japan) | 5 °C |
| December | Lazurnaya Bay (Ussuriisky Bay, Peter the Great Bay, Sea of Japan) | 0.5 °C |
| January | Lazurnaya Bay (Ussuriisky Bay, Peter the Great Bay, Sea of Japan) | 0–1 °C |
| February | Lazurnaya Bay (Ussuriisky Bay, Peter the Great Bay, Sea of Japan) | 0–1 °C |
| April | Cape Zeleny (Amur Bay, Peter the Great Bay, Sea of Japan) | 0 °C |
| June | Cape Zeleny (Amur Bay, Peter the Great Bay, Sea of Japan) | 18 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chadova, K.; Velansky, P. Lipidome of the Brown Macroalga Undaria pinnatifida: Influence of Season and Endophytic Infection. Mar. Drugs 2023, 21, 466. https://doi.org/10.3390/md21090466
Chadova K, Velansky P. Lipidome of the Brown Macroalga Undaria pinnatifida: Influence of Season and Endophytic Infection. Marine Drugs. 2023; 21(9):466. https://doi.org/10.3390/md21090466
Chicago/Turabian StyleChadova, Ksenia, and Peter Velansky. 2023. "Lipidome of the Brown Macroalga Undaria pinnatifida: Influence of Season and Endophytic Infection" Marine Drugs 21, no. 9: 466. https://doi.org/10.3390/md21090466
APA StyleChadova, K., & Velansky, P. (2023). Lipidome of the Brown Macroalga Undaria pinnatifida: Influence of Season and Endophytic Infection. Marine Drugs, 21(9), 466. https://doi.org/10.3390/md21090466





