Five Years Monitoring the Emergence of Unregulated Toxins in Shellfish in France (EMERGTOX 2018–2022)
Abstract
1. Introduction
2. Results
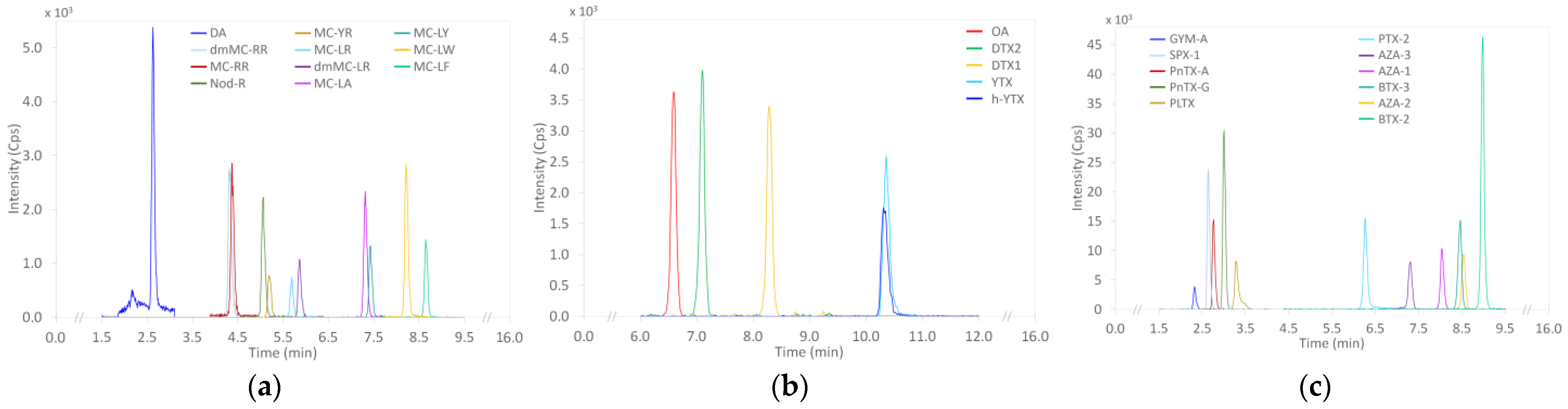
2.1. LC-MS/MS Lipophilic Multi-Toxin Quantification in Shellfish
2.1.1. Optimization and Internal Validation of the LC-MS/MS Lipophilic Toxin Analysis Method in Shellfish
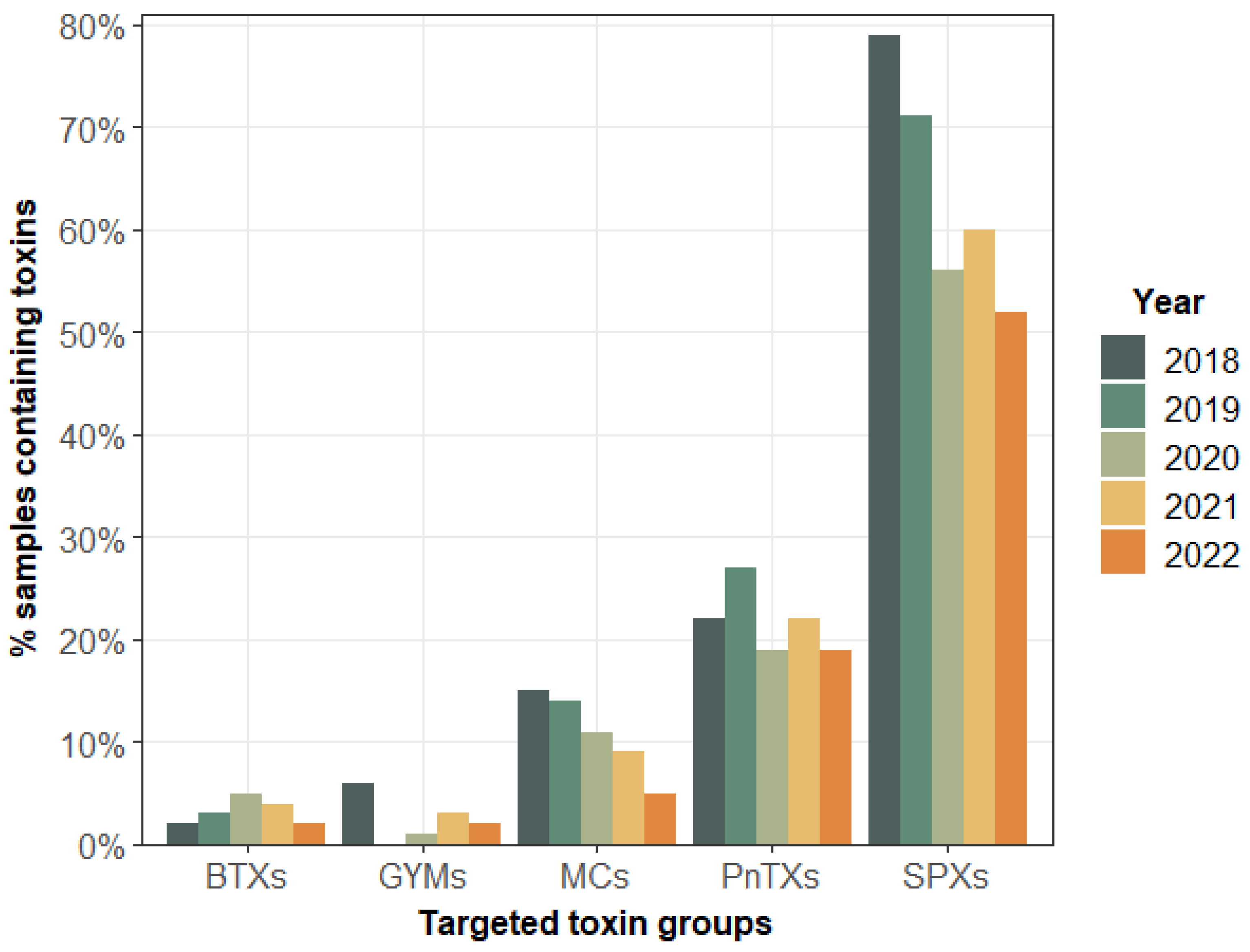
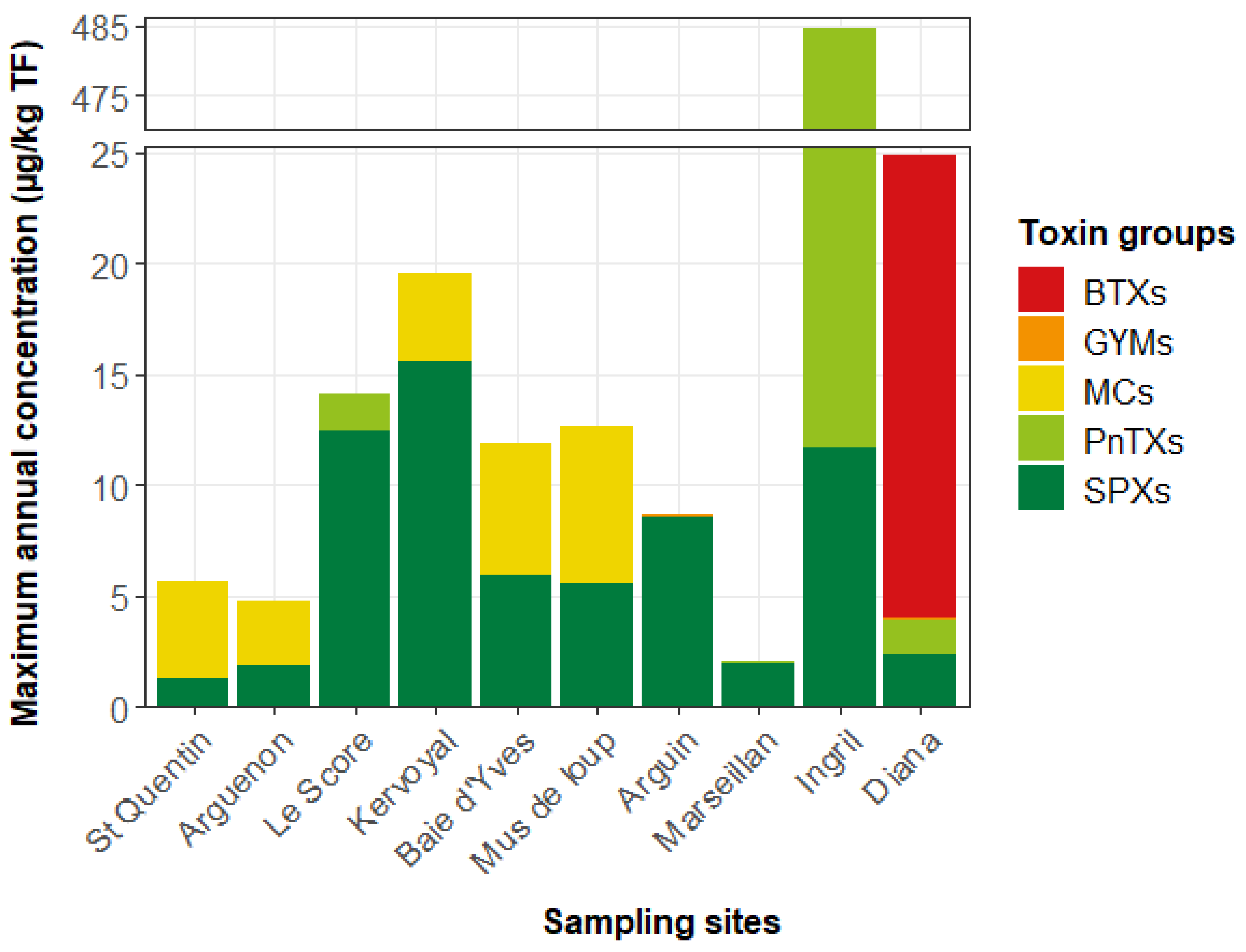
2.1.2. Targeted LC-MS/MS Screening of Lipophilic Toxin Groups Monitored by the EMERGTOX Network
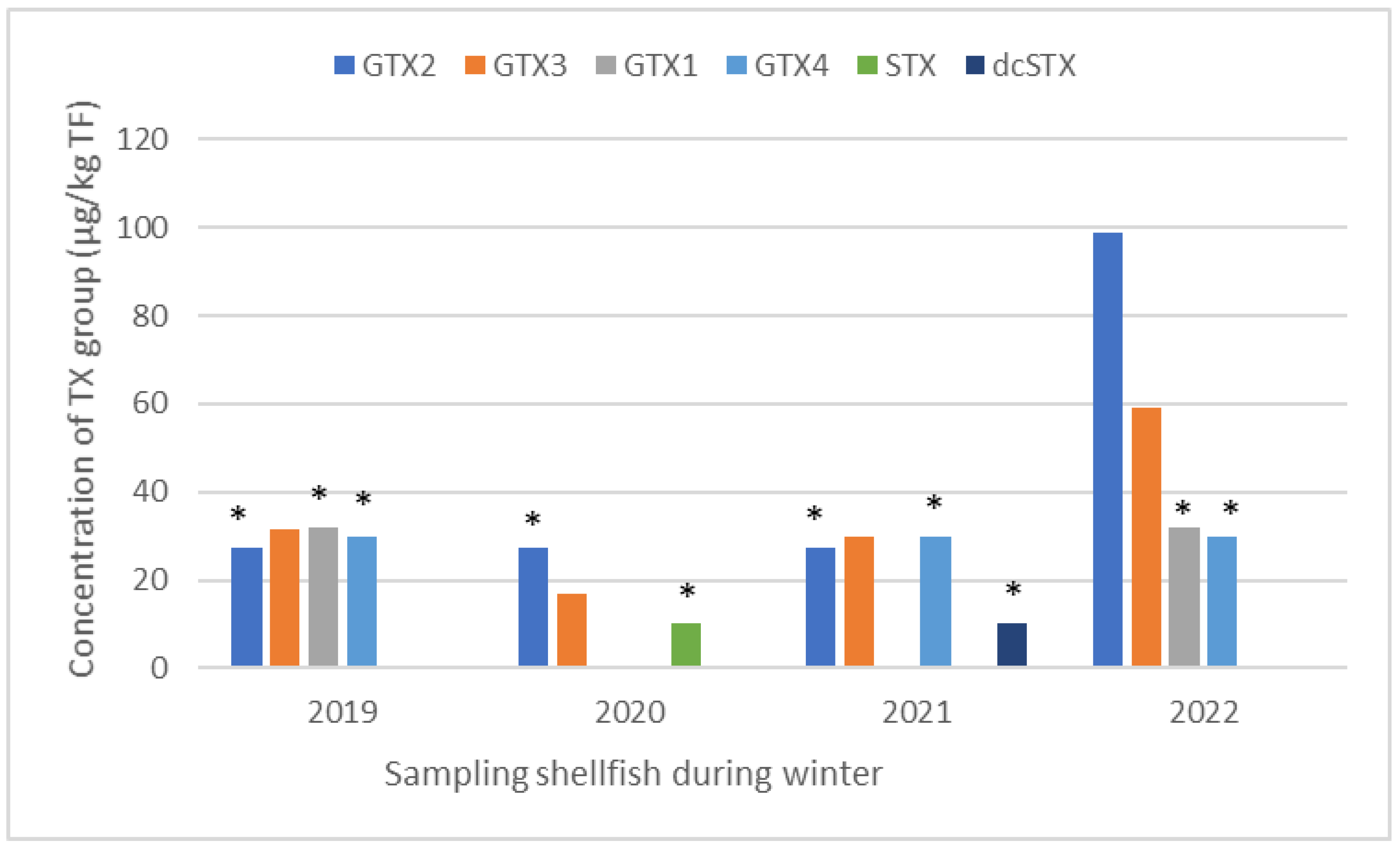
2.2. HILIC-MS/MS Quantification of Saxitoxin, Tetrodotoxin, Anatoxin, and Cylindrospermopsin Groups in Shellfish
2.2.1. Optimization and Internal Validation of the HILIC-MS/MS Analysis Method
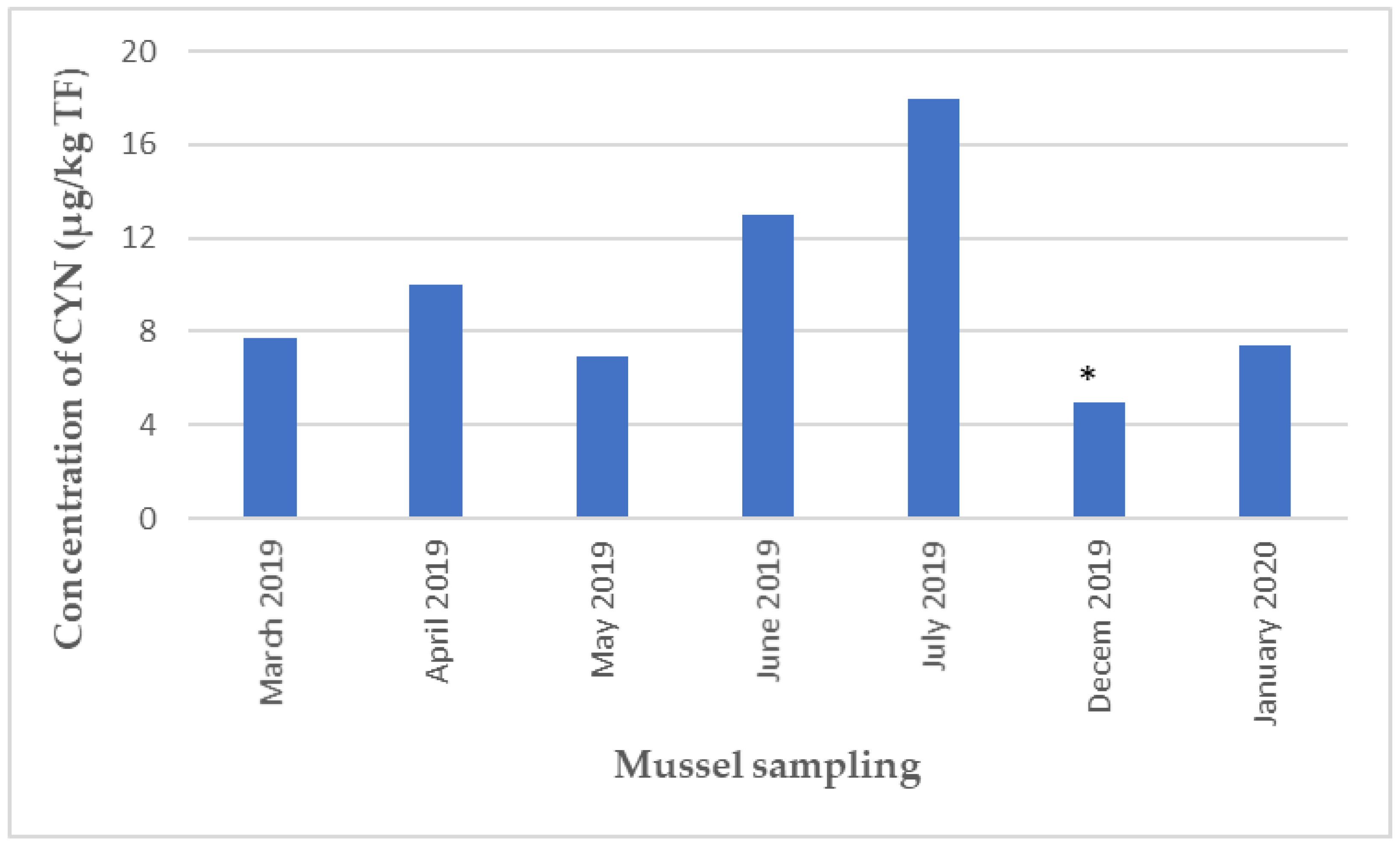
2.2.2. HILIC-MS/MS Analysis of Saxitoxins, Tetrodotoxins, Anatoxins, and Cylindrospermopsin in Shellfish
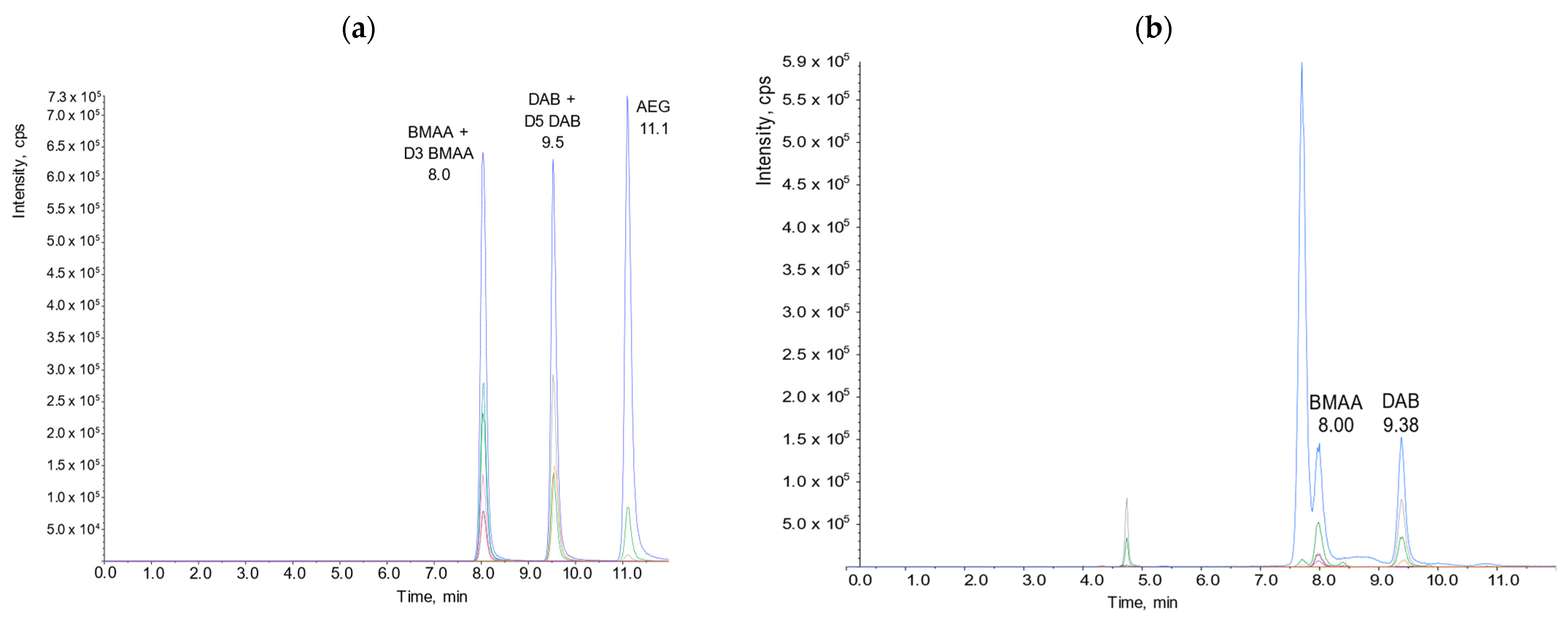
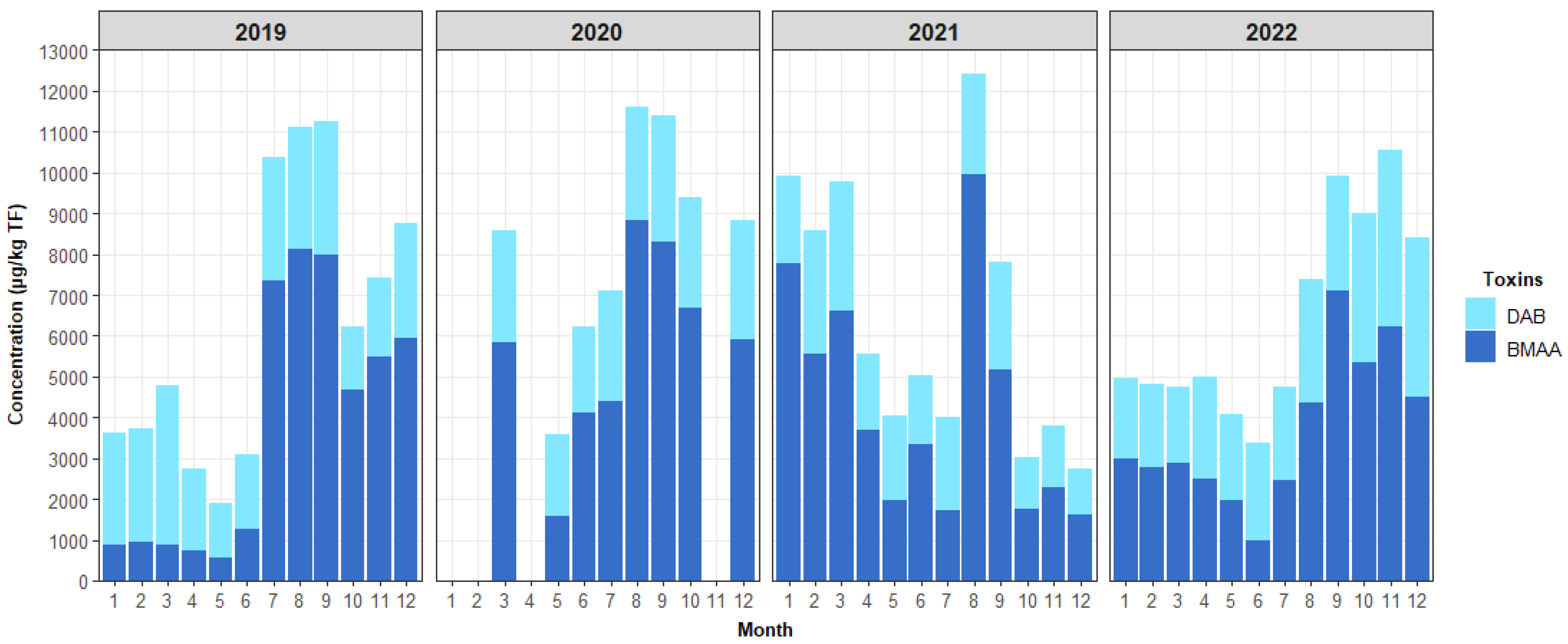
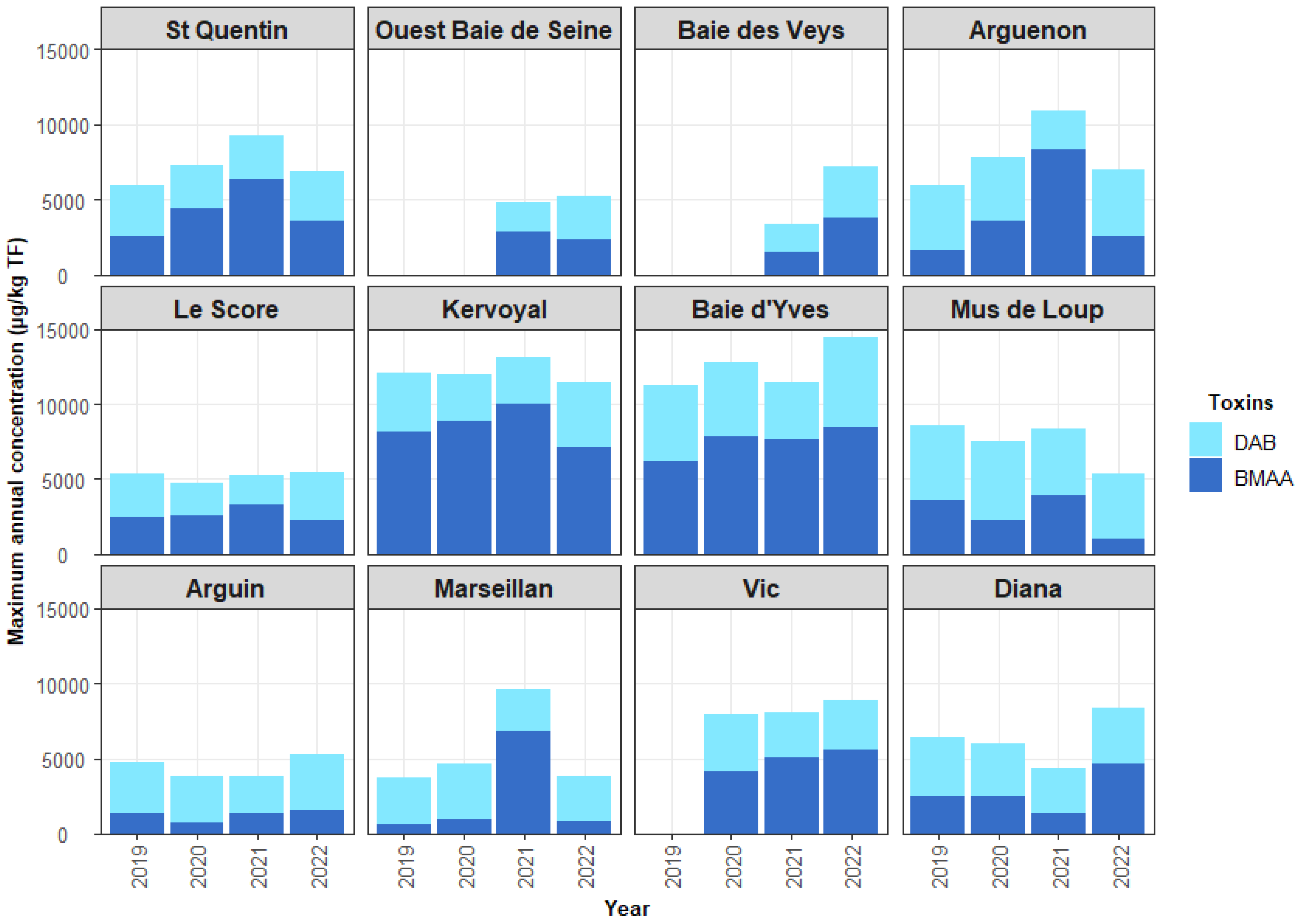
2.3. HILIC-MS/MS Quantification of BMAA and Its Analogues in Shellfish
Optimization and Internal Validation of the HILIC-MS/MS Analysis
3. Discussion
3.1. Contamination of Shellfish by Unregulated Lipophilic Toxins
3.2. Contamination of Shellfish by Unregulated Hydrophilic Toxins (ATXs, CYNs)
3.3. Contamination of Shellfish by BMAA
3.4. Guidance Level for Unregulated Toxins in French Shellfish
4. Materials and Methods
4.1. Materials
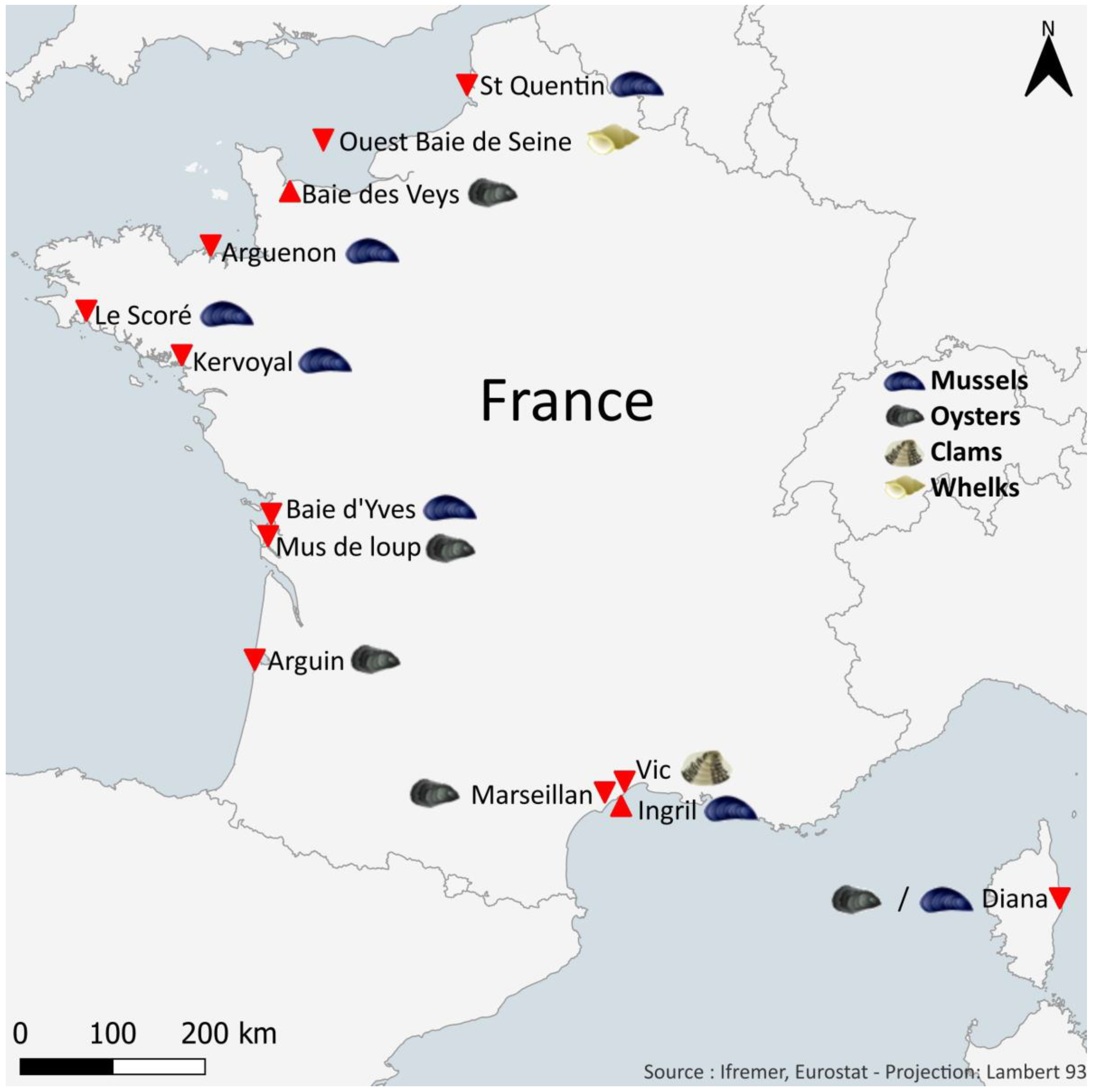
4.2. Sampling Shellfish
- Their location in shellfish harvesting areas (mussels, oysters, clams, or whelks) that are active all year round;
- An equal geographical distribution over the French coastlines;
- The existence of historical data for the sampling points, with the aim to obtain long time series;
- Previous mouse bioassay results for lipophilic toxins (which was the reference test before 2010) that remain unexplained (short survival time and/or neurological symptoms);
- Their location outside areas affected but not immune to possible contamination by potentially toxic microalgae.
4.3. Methods
4.3.1. LC-MS/MS Analysis of Lipophilic Toxins and Domoic Acid
Sample Preparation
LC-MS/MS Conditions
4.3.2. HILIC-MS/MS Analysis of STXs, TTXs, ATXs, and CYN Toxins Groups
Sample Preparation
HILIC-MS/MS Conditions
4.3.3. HILIC-MS/MS Analysis of BMAA and Its Analogues (DAB, AEG)
Sample Preparation
HILIC-MS/MS Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Official Journal of the European Union L 297 of 20 August 2021. Corrigendum to Commission Delegated Regulation (EU) 2021/1374 of 12 April 2021 amending Annex III to Regulation (EC) No 853/2004 of the European Parliament and of the Council as regards specific hygiene requirements for food of animal origin. Off. J. Eur. Union 2021. Available online: http://data.europa.eu/eli/reg_del/2021/1374/oj (accessed on 1 July 2023).
- Peacock, M.B.; Gibble, C.M.; Senn, D.B.; Cloern, J.E.; Kudela, R.M. Blurred lines: Multiple freshwater and marine algal toxins at the land-sea interface of san Francisco bay, California. Harmful Algae 2018, 73, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Lance, E.; Arnich, N.; Maignien, T.; Biré, R. Occurrence of β-N-methylamino-L-alanine (BMAA) and Isomers in Aquatic Environments and Aquatic Food Sources for Humans. Toxins 2018, 10, 83. [Google Scholar] [CrossRef]
- Lance, E.; Lepoutre, A.; Savar, V.; Robert, E.; Bormans, M.; Amzil, Z. In situ use of bivalves and passive samplers to reveal water contamination by microcystins along a freshwater-marine continuum in France. Water Res. 2021, 204, 117620. [Google Scholar] [CrossRef]
- Hu, T.; Curtis, J.M.; Walter, J.A.; Wright, J.L.C. Characterization of biologically inactive spirolides E and F: Identification of the spirolides pharmacophore. Tetrahedron Lett. 1996, 37, 7671–7674. [Google Scholar] [CrossRef]
- Seki, T.; Satake, M.; Mackenzie, L.; Kaspar, H.F.; Yasumoto, T. Gymnodimine, a new marine toxin of unprecedented structure isolated from New-Zealand oysters and the dinoflagellate, Gymnodinium sp. Tetrahedron Lett. 1995, 36, 7093–7096. [Google Scholar] [CrossRef]
- Uemura, D.; Chou, T.; Haino, T.; Nagatsu, A.; Fukuzawa, S.; Zheng, S.; Chen, H. Pinnatoxin A: A toxic amphoteric macrocycle from the Okinawan bivalve Pinna muricata. J. Am. Chem. Soc. 1995, 117, 1155–1156. [Google Scholar] [CrossRef]
- Molgo, J.; Girard, B.; Benoit, E. Cyclic imines: An Insight into this Emerging Group of Bioactive Marine Toxins. In Phycotoxins, Chemistry and Biochemistry; Botana, L.M., Ed.; Blackwell Publishing: Oxford, UK, 2007; pp. 319–335. [Google Scholar]
- Aasen, J.; Mackinnon, S.L.; Le Blanc, P.; Walter, J.A.; Hovgaard, P.; Aune, T.; Quilliam, M.A. Detection and identification of spirolides in Norwegian shellfish and plankton. Chem. Res. Toxicol. 2005, 18, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Amzil, Z.; Sibat, M.; Royer, F.; Masson, N.; Abadie, E. Report on the first detection of pectenotoxin-2, spirolide-A and their derivatives in French shellfish. Mar. Drugs 2007, 5, 168–179. [Google Scholar] [CrossRef]
- Medhioub, W.; Gueguen, M.; Lassus, P.; Bardouil, M.; Truquet, P.; Sibat, M.; Medhioub, N.; Soudant, P.; Kraiem, M.; Amzil, Z. Detoxification enhancement in the gymnodimine-contaminated grooved carpet shell, Ruditapes decussatus (Linne). Harmful Algae 2010, 9, 200–207. [Google Scholar]
- Selwood, A.I.; Miles, C.O.; Wilkins, A.L.; Van Ginkel, R.; Munday, R.; Rise, F.; McNabb, P. Isolation, structural determination and acute toxicity of pinnatoxins E, F and G. J. Agric. Food Chem. 2010, 58, 6532–6542. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on marine biotoxins in shellfish—Cyclic imines (spirolides, gymnodimines, pinnatoxins and pteriatoxins). EFSA J. 2010, 8, 1628. [Google Scholar] [CrossRef]
- Hess, P.; Abadie, E.; Herve, F.; Berteaux, T.; Sechet, V.; Araoz, R.; Molgo, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef]
- Uemura, D. Bioorganic studies on marine natural products—Diverse chemical structures and bioactivities. Chem. Rec. 2006, 6, 235–248. [Google Scholar] [CrossRef]
- Arnich, N.; Abadie, E.; Delcourt, N.; Fessard, V.; Fremy, J.M.; Hort, V.; Lagrange, E.; Maignien, T.; Molgó, J.; Peyrat, M.B.; et al. Health risk assessment related to pinnatoxins in French shellfish. Toxicon 2020, 180, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E.; Sheuer, P. Palytoxin: A new marine toxin from a coelenterate. Science 1971, 172, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E. Structure of palytoxin. Fortschr. Chem. Org. Naturst. 1985, 48, 81–202. [Google Scholar] [PubMed]
- Habermann, E. Review article: Palytoxin acts through Na+/K+ ATPase. Toxicon 1989, 27, 1175–1187. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Forino, M.; Grauso, L.; Tartaglione, L.; Guerrini, F.; Pistocchi, R. Complex palytoxin-like profile of Ostreopsis ovata. Identification of four new ovatoxins by high-resolution liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2735–2744. [Google Scholar] [CrossRef]
- Ciminiello, P.; Dell’Aversano, C.; Dello Iacovo, E.; Fattorusso, E.; Tartaglione, L.F.; Battocchi, C.; Crinelli, R.; Carloni, E.; Magnani, M.; Penna, A. Unique toxin profile of a Mediterranean Ostreopsis cf. ovata strain: HR LC–MSn characterization of ovatoxin-f, a new palytoxin congener. Chem. Res. Toxicol. 2012, 25, 1243–1252. [Google Scholar] [CrossRef]
- Amzil, Z.; Sibat, M.; Chomerat, N.; Grossel, H.; Marco-Miralles, F.; Lemee, R.; Nezan, E.; Sechet, V. Ovatoxin-a and Palytoxin Accumulation in Seafood in Relation to Ostreopsis cf. ovata Blooms on the French Mediterranean Coast. Mar. Drugs 2012, 10, 477–496. [Google Scholar] [CrossRef]
- Brissard, C.; Hervé, F.; Sibat, M.; Séchet, V.; Hess, P.; Amzil, Z.; Herrenknecht, C. Characterization of ovatoxin-h, a new ovatoxin analog, and evaluation of chromatographic columns for ovatoxin analysis and purification. J. Chromatogr. A 2015, 1388, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Accoroni, S.; Romagnoli, T.; Penna, A.; Capellacci, S.; Ciminiello, P.; Dell’Aversano, C.; Tartaglione, L.; Abboud-Abi Saab, M.; Giussani, V.; Asnaghi, V.; et al. Ostreopsis fattorussoi sp. nov. (Dinophyceae), a new benthic toxic Ostreopsis species from the eastern Mediterranean Sea. J. Phycol. 2016, 52, 1064–1084. [Google Scholar] [CrossRef] [PubMed]
- Tartaglione, L.; Dello Iacovo, E.; Mazzeo, A.; Casabianca, S.; Ciminiello, P.; Penna, A.; Dell’Aversano, C. Variability in Toxin Profiles of the Mediterranean Ostreopsis cf. ovata and in Structural Features of the Produced Ovatoxins. Environ. Sci. Technol. 2017, 51, 13920–13928. [Google Scholar] [CrossRef]
- Gémin, M.P.; Réveillon, D.; Hervé, F.; Pavaux, A.S.; Tharaud, M.; Séchet, V.; Bertrand, S.; Lemée, R.; Amzil, Z. Toxin content of Ostreopsis cf. ovata depends on bloom phases, depth and macroalgal substrate in the NW Mediterranean Sea. Harmful Algae 2020, 92, 101727. [Google Scholar] [CrossRef]
- Vila, M.; Abòs-Herràndiz, R.; Isern-Fontanet, J.; Àlvarez, J.; Berdalet, E. Establishing the link between Ostreopsis cf. ovata blooms and human health impacts using ecology and epidemiology. Sci. Mar. 2016, 80, 107–115. [Google Scholar]
- Tichadou, L.; Glaizal, M.; Armengaud, A.; Grossel, H.; Lemee, R.; Kantin, R.; Lasalle, J.L.; Drouet, G.; Rambaud, L.; Malfait, P.; et al. Health impact of unicellular algae of the Ostreopsis genus blooms in the Mediterranean Sea: Experience of the French Mediterranean coast surveillance network from 2006 to 2009. Clin. Toxicol. 2010, 48, 839–844. [Google Scholar] [CrossRef]
- Aligizaki, K.; Katikou, P.; Nikolaidis, G.; Panou, A. First episode of shellfish contamination by palytoxin-like compounds from Ostreopsis species (Aegean Sea, Greece). Toxicon 2008, 51, 418–427. [Google Scholar] [CrossRef]
- Brissard, C.; Herrenknecht, C.; Sechet, V.; Herve, F.; Pisapia, F.; Harcouet, J.; Lemee, R.; Chomerat, N.; Hess, P.; Amzil, Z. Complex Toxin Profile of French Mediterranean Ostreopsis cf. ovata Strains, Seafood Accumulation and Ovatoxins Prepurification. Mar. Drugs 2014, 12, 2851–2876. [Google Scholar] [CrossRef]
- Biré, R.; Trotereau, S.; Lemée, R.; Oregioni, D.; Delpont, C.; Krys, S.; Guérin, T. Hunt for palytoxins in a wide variety of marine organisms harvested in 2010 on the French Mediterranean coast. Mar. Drugs 2015, 13, 5425–5446. [Google Scholar] [CrossRef] [PubMed]
- Chomérat, N.; Antajan, E.; Auby, I.; Bilien, G.; Carpentier, L.; De Ganthy, F.; Hervé, F.; Labadie, M.; Méteigner, C.; Paradis, C.; et al. First Characterization of Ostreopsis cf. ovata (Dinophyceae) and Detection of Ovatoxins during a Multispecific and Toxic Ostreopsis Bloom on French Atlantic Coast. Mar. Drugs 2022, 20, 461. [Google Scholar] [CrossRef] [PubMed]
- Lago, J.; Rodríguez, L.P.; Blanco, L.; Vieites, J.M.; Cabado, A.G. Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses. Mar. Drugs 2015, 13, 6384–6406. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.K.; Alexander, J.; Barregard, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Scientific opinion on the risks for publichealth related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves andgastropods. EFSA J. 2017, 15, 4752. [Google Scholar] [CrossRef]
- Boundy, M.J.; Biessy, L.; Roughan, B.; Nicolas, J.; Harwood, D.T. Survey of Tetrodotoxin in New Zealand Bivalve Molluscan Shellfish over a 16-Month Period. Toxins 2020, 12, 512. [Google Scholar] [CrossRef]
- Guardone, L.; Maneschi, A.; Meucci, V.; Gasperetti, L.; Nucera, D.; Armani, A. A Global Retrospective Study on Human Cases of Tetrodotoxin (TTX) Poisoning after Seafood Consumption. Food Rev. Int. 2020, 36, 645–667. [Google Scholar] [CrossRef]
- Hort, V.; Arnich, N.; Guérin, T.; Lavison-Bompard, G.; Nicolas, M. First Detection of Tetrodotoxin in Bivalves and Gastropods from the French Mainland Coasts. Toxins 2020, 12, 599. [Google Scholar] [CrossRef]
- Réveillon, D.; Savar, V.; Schaefer, E.; Chevé, J.; Halm-Lemeille, M.P.; Hervio-Heath, D.; Travers, M.A.; Abadie, E.; Rolland, J.L. Tetrodotoxins in French Bivalve Mollusks—Analytical Methodology, Environmental Dynamics and Screening of Bacterial Strain Collections. Toxins 2021, 13, 740. [Google Scholar] [CrossRef]
- Abraham, A.; Wang, Y.; El Said, K.R.; Plakas, S.M. Characterisation of brevetoxin metabolisms in Karenia brevis bloom-exposed clams (Mercenaria sp.) by LC-MS/MS. Toxicon 2012, 60, 1030–1040. [Google Scholar] [CrossRef]
- Lassus, P.; Chomérat, N.; Hess, P.; Nézan, E. Toxic and Harmful Microalgae of the World Ocean/Micro-Algues Toxiques et Nuisibles de L’océan Mondial. Denmark, International Society for the Study of Harmful Algae/Intergovernmental Oceanographic Commission of UNESCO. IOC Man. Guid. 2016, 68, 523. [Google Scholar]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef]
- Fleming, L.E.; Backer, L.C.; Baden, D.G. Overview of aerosolized Florida red tide toxins: Exposures and effects. Environ. Health Perspect. 2005, 113, 618–620. [Google Scholar] [CrossRef]
- Abraham, A.; Plakas, S.M.; Wang, Z.; Jester, E.L.E.; El Said, K.R.; Granade, H.R.; Henry, M.S.; Blum, P.C.; Pierce, R.H.; Dickey, R.W. Characterization of polar brevetoxin derivatives isolated from Karenia brevis cultures and natural blooms. Toxicon 2006, 48, 104–115. [Google Scholar] [CrossRef]
- Hort, V.; Abadie, E.; Arnich, N.; Dechraoui-Bottein, M.Y.; Amzil, Z. Chemodiversity of Brevetoxins and Other Potentially Toxic Metabolites Produced by Karenia spp. and Their Metabolic Products in Marine Organisms. Mar. Drugs 2021, 19, 656. [Google Scholar] [CrossRef]
- Amzil, Z.; Derrien, A.; Terre Terrillon, A.; Duval, A.; Connes, C.; Marco-Miralles, F.; Nézan, E.; Mertens, K.N. Monitoring the Emergence of Algal Toxins in Shellfish: First Report on Detection of Brevetoxins in French Mediterranean Mussels. Mar. Drugs 2021, 19, 393. [Google Scholar] [CrossRef]
- Gibble, C.M.; Peacock, M.B.; Kudela, R.M. Evidence of freshwater algal toxins in marine shellfish: Implications for human and aquatic health. Harmful Algae 2016, 59, 59–66. [Google Scholar] [CrossRef]
- Gibble, K. Detection of persistent microcystin toxins at the land-sea interface in Monterey Bay, California. Harmful Algae 2014, 39, 146–153. [Google Scholar] [CrossRef]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in estuarine and marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Bormans, M.; Amzil, Z.; Mineaud, E.; Brient, L.; Savar, V.; Robert, E.; Lance, E. Demonstrated transfer of cyanobacteria and cyanotoxins along a freshwater-marine continuum in France. Harmful Algae 2019, 87, 101639. [Google Scholar] [CrossRef]
- Overlingėa, D.; Kataržytėa, M.; Vaičiūtėa, D.; Gyraitea, B.; Gečaitėa, B.I.; Jonikaitėa, E.; Mazur-Marzec, H. Are there concerns regarding cHAB in coastal bathing waters affected by freshwater-brackish continuum? Mar. Pollut. Bull. 2020, 159, 111500. [Google Scholar] [CrossRef]
- Kruk, C.; Martínez, A.; De la Escalera, G.M.; Trinchin, R.; Manta, G.; Segura, A.M.; Calliari, D. Rapid freshwater discharge on the coastal ocean as a mean of long distance spreading of an unprecedented toxic cyanobacteria bloom. Stoten 2021, 754, 142362. [Google Scholar] [CrossRef]
- Testai, E.; Scardala, S.; Vichi, S.; Buratti, F.M.; Funari, E. Risk to human health associated with the environmental occurrence of cyanobacterial neurotoxic alkaloids anatoxins and saxitoxins. Crit. Rev. Toxicol. 2016, 46, 385–419. [Google Scholar] [CrossRef]
- Biré, R.; Bertin, T.; Dom, I.; Hort, V.; Schmitt, C.; Diogène, J.; Lemée, R.; De Haro, L.; Nicolas, M. First Evidence of the Presence of Anatoxin-A in Sea Figs Associated with Human Food Poisonings in France. Mar. Drugs 2020, 18, 285. [Google Scholar] [CrossRef]
- Reveillon, D.; Abadie, E.; Sechet, V.; Brient, L.; Savar, V.; Bardouil, M.; Hess, P.; Amzil, Z. Beta-N-Methylamino-L-Alanine: LC-MS/MS Optimization, Screening of Cyanobacterial Strains and Occurrence in Shellfish from Thau, a French Mediterranean Lagoon. Mar. Drugs 2014, 12, 5441–5467. [Google Scholar] [CrossRef]
- Spencer, P.S.; Nunn, P.B.; Hugon, J.; Ludolph, A.C.; Ross, S.M.; Roy, D.N.; Robertson, R.C. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science 1987, 237, 517–522. [Google Scholar] [CrossRef]
- Delcourt, N.; Claudepierre, T.; Maignien, T.; Arnich, N.; Mattei, C. Cellular and Molecular Aspects of the β-N-Methylamino-l-alanine (BMAA) Mode of Action within the Neurodegenerative Pathway: Facts and Controversy. Toxins 2017, 10, 6. [Google Scholar] [CrossRef]
- Karamyan, V.T.; Speth, R.C. Animal models of BMAA neurotoxicity: A critical review. Life Sci. 2008, 82, 233–246. [Google Scholar] [CrossRef]
- Chiu, A.S.; Gehringer, M.M.; Welch, J.H.; Neilan, B.A. Does α-amino-β-methylaminopropionic acid (BMAA) play a role in neurodegeneration? Int. J. Environ. Res. Public Health 2011, 8, 3728–3746. [Google Scholar] [CrossRef]
- Masseret, E.; Banack, S.; Boumédiène, F.; Abadie, E.; Brient, L.; Pernet, F.; Juntas-Morales, R.; Pageot, N.; Metcalf, J.; Cox, P.; et al. The French Network on, A.L.S.C.D., Investigatio Dietary BMAA Exposure in an Amyotrophic Lateral Sclerosis Cluster from Southern France. PLoS ONE 2013, 8, e83406. [Google Scholar] [CrossRef]
- ANSES. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the Acute and Chronic Toxicity of BMAA (Beta-Methylamino-L-Alanine); ANSES: Maisons-Alfort, France, 2017; pp. 1–17. Available online: https://www.anses.fr/en/system/files/ERCA2016SA0012EN.pdf (accessed on 4 September 2017).
- Anonymous. Protocole de Caractérisation en vue de la Validation d’une Méthode d’analyse Quantitative par Construction du Profil d’exactitude. NF V03-110 Mai, 57 Pages. French Association for Standardization (AFNOR). Collections: National Standards and National Normative Documents. 2010. Available online: https://norminfo.afnor.org/norme/nf-v03-110/analyse-des-produits-agricoles-et-alimentaires-protocole-de-caracterisation-en-vue-de-la-validation-dune-methode-danalyse-quantitative-par-construction-du-profil-dexactitude/83631 (accessed on 31 August 2011).
- Derrien, A.; Terre-Terrillon, A.; Duval, A.; Martin, C.; Sibat, M.; Rovillon, G.A.; Reveillon, D.; Amzil, Z. From development to implementation of a new LC-MS/MS approach to quantify 43 lipophilic toxins within the French monitoring program. In Proceedings of the ICHA 2018—18th International Conference on Harmful Algae “From Ecosystems to Socio-Ecosystems”, Nantes, France, 21–26 October 2018. [Google Scholar]
- Villar González, A.; Rodríguez-Velasco, M.L.; Ben-Gigirey, B.; Botana, L.M. First evidence of spirolides in Spanish shellfish. Toxicon 2006, 48, 1068–1074. [Google Scholar] [CrossRef]
- Blanco, J.; Arévalo, F.; Moroño, Á.; Correa, J.; Rossignoli, A.E.; Lamas, J.P. Spirolides in Bivalve Mollusk of the Galician (NW Spain) Coast: Interspecific, Spatial, Temporal Variation and Presence of an Isomer of 13-Desmethyl Spirolide C. Toxins 2023, 15, 13. [Google Scholar] [CrossRef]
- Pigozzi, S.; Bianchi, L.; Boschetti, L.; Cangini, M.; Ceredi, A.; Magnani, F.; Milandri, A.; Montanari, S.; Pompei, M.; Riccardi, E.; et al. First evidence of spirolide accumulation in northwestern Adriatic shellfish. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2008; pp. 319–322. [Google Scholar]
- Miles, C.O.; Rundberget, T.; Sandvik, M.; Aasen, J.A.B.; Selwood, A.I. The Presence of Pinnatoxins in Norwegian Mussels. Report 07b—2010, Veterinærinstituttet, National Veterinary Institute. Available online: https://www.academia.edu/download/42073102/The_presence_of_pinnatoxins_in_Norwegian20160204-28712-15xizpo.pdf (accessed on 8 April 2010).
- España Amórtegui, J.C.; Pekar, H.; Retrato, M.D.C.; Persson, M.; Karlson, B.; Bergquist, J.; Zuberovic-Muratovic, A. LC-MS/MS Analysis of Cyanotoxins in Bivalve Mollusks—Method Development, Validation and First Evidence of Occurrence of Nodularin in Mussels (Mytilus edulis) and Oysters (Magallana gigas) from the West Coast of Sweden. Toxins 2023, 15, 329. [Google Scholar] [CrossRef] [PubMed]
- Biré, R.; Trotereau, S.; Lemée, R.; Delpont, C.; Chabot, B.; Aumond, Y.; Krys, S. Occurrence of palytoxins in marine organisms from different trophic levels of the French Mediterranean coast harvested in 2009. Harmful Algae 2013, 28, 10–22. [Google Scholar] [CrossRef]
- Bolotaolo, M.; Kurobe, T.; Puschner, B.; Hammock, B.G.; Hengel, M.J.; Lesmeister, S.; Teh, S.J. Analysis of covalently bound microcystins in sediments and clam tissue in the Sacramento–san Joaquin river delta, California, USA. Toxins 2020, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Bormans, M.; Savar, V.; Legrand, B.; Mineaud, E.; Robert, E.; Lance, E.; Amzil, Z. Cyanobacteria and cyanotoxins in estuarine water and sediment. Aquat. Ecol. 2020, 54, 625–640. [Google Scholar] [CrossRef]
- Campos, A.; Vasconcelos, V. Molecular mechanisms of microcystin toxicity in animal cells. Int. J. Mol. Sci. 2010, 11, 268–287. [Google Scholar] [CrossRef]
- Lance, E.; Neffling, M.R.; G’erard, C.; Meriluoto, J.; Bormans, M. Accumulation of free and covalently bound microcystins in tissues of Lymnaea stagnalis (Gastropoda) following toxic cyanobacteria or dissolved microcystin-LR exposure. Environ. Pollut. 2010, 158, 674–680. [Google Scholar] [CrossRef]
- Lepoutre, A.; Grillot, T.; Jean, S.; Geffard, A.; Lance, E. Bioaccumulation of free and total MCs by freshwater bivalves as indicator of water contamination by microcystin-producing cyanobacteria? Appl. Sci. 2020, 10, 3426. [Google Scholar] [CrossRef]
- Abraham, A.; Flewelling, L.J.; El Said, K.R.; Odom, W.; Geiger, S.P.; Granholm, A.A.; Jackson, J.T.; Bodager, D. An Occurrence of Neurotoxic Shellfish Poisoning by Consumption of Gastropods Contaminated with Brevetoxins. Toxicon 2021, 191, 9–17. [Google Scholar] [CrossRef]
- Plakas, S.M.; El Said, K.R.; Jester, E.L.E.; Ray Granade, H.; Musser, S.M.; Dickey, R.W. Confirmation of Brevetoxin Metabolism in the Eastern Oyster (Crassostrea Virginica) by Controlled Exposures to Pure Toxins and to Karenia Brevis Cultures. Toxicon 2002, 40, 721–729. [Google Scholar] [CrossRef]
- ANSES. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the State of Knowledge on Brevetoxins in Shellfish, Data on Toxicity, Occurrence and Brevetoxin-Producing Microalgae; ANSES: Maisons-Alfort, France, 2021; pp. 1–18. Available online: https://www.anses.fr/en/system/files/ERCA2020SA0020EN.pdf (accessed on 2 March 2021).
- Gerssen, A.; Bovee, T.H.F.; Klijnstra, M.D.; Poelman, M.; Portier, L.; Hoogenboom, R.A.P. First Report on the Occurrence of Tetrodotoxins in Bivalve Mollusks in The Netherlands. Toxins 2018, 10, 450. [Google Scholar] [CrossRef]
- Anderson, L.; Fabbro, L.D.; Cowden, K. Assessment of Blue-Green Algal Toxins in Barramundi, Red Clay and Mussels from Awoonga Dam; Central Queensland University: Gladstone, Australia, 2003. [Google Scholar]
- Saker, M.L.; Metcalf, J.S.; Codd, G.A.; Vasconcelos, V.M. Accumulation and depuration of the cyanobacterial toxin cylindrospermopsin in the freshwater mussel Anodonta cygnea. Toxicon 2004, 43, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Reveillon, D.; Abadie, E.; Sechet, V.; Masseret, E.; Hess, P.; Amzil, Z. β-N-methylamino-l-alanine (BMAA) and isomers: Distribution in different food web compartments of Thau lagoon, French Mediterranean Sea. Mar. Environ. Res. 2015, 110, 8–18. [Google Scholar] [CrossRef]
- Reveillon, D.; Sechet, V.; Hess, P.; Amzil, Z. Systematic detection of BMAA (β-N-methylamino-L-alanine) and DAB (2,4-diaminobutyric acid) in mollusks collected in shellfish production areas along the French coasts. Toxicon 2016, 110, 35–46. [Google Scholar] [CrossRef]
- Salomonsson, M.L.; Hansson, A.; Bondesson, U. Development and in-House Validation of a Method for Quantification of Bmaa in Mussels Using Dansyl Chloride Derivatization and Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. Anal. Methods 2013, 5, 4865–4874. [Google Scholar] [CrossRef]
- Lage, S.; Costa, P.R.; Moita, T.; Eriksson, J.; Rasmussen, U.; Rydberg, S.J. Bmaa in Shellfish from Two Portuguese TransitionalWater Bodies Suggests the Marine Dinoflagellate Gymnodinium Catenatum as a Potential Bmaa Source. Aquat. Toxicol. 2014, 152, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Brand, L.E.; Pablo, J.; Compton, A.; Hammerschlag, N.; Mash, D.C. Cyanobacterial Blooms and the Occurrence of the Neurotoxin Beta-N-Methylamino-L-Alanine (Bmaa) in South Florida Aquatic Food Webs. Harmful Algae 2010, 9, 620–635. [Google Scholar] [CrossRef]
- Jonasson, S.; Eriksson, J.; Berntzon, L.; Spacil, Z.; Ilag, L.L.; Ronnevi, L.O.; Rasmussen, U.; Bergman, B. Transfer of a Cyanobacterial Neurotoxin within a Temperate Aquatic Ecosystem Suggests Pathways forHuman Exposure. Proc. Natl. Acad. Sci. USA 2010, 107, 9252–9257. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on marine biotoxins in shellfish—Palytoxin group. EFSA J. 2009, 7, 1393. [CrossRef]
- Arnich, N.; Abadie, E.; Amzil, Z.; Dechraoui-Bottein, M.-Y.; Comte, K.; Chaix, E.; Delcourt, N.; Hort, V.; Mattei, C.; Molgó, J. Guidance Level for Brevetoxins in French Shellfish. Mar. Drugs 2021, 19, 520. [Google Scholar] [CrossRef]
- Anses. Avis Relatif Aux Risques Pour la Santé Humaine Liés aux Proliférations d’Ostreopsis spp. sur le Littoral Basque (Saisine 2021-SA-0212). Maisons-Alfort: Anses, 40p. 2023. Available online: https://www.anses.fr/fr/system/files/EAUX2021SA0212.pdf (accessed on 12 May 2023).
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on marine biotoxins in shellfish—Emerging toxins: Brevetoxin group. EFSA J. 2010, 8, 1677. [CrossRef]
- Anonymous; New Zealand Government. Regulated Control Scheme—Bivalve Molluscan Shellfish for Human Consumption; New Zealand Government: Wellington, New Zealand, 2021; pp. 1–68. Available online: https://www.mpi.govt.nz/dmsdocument/30282-Animal-Products-Notice-Regulated-Control-Scheme-Bivalve-Molluscan-Shellfish-for-Human-Consumption-2018 (accessed on 22 November 2021).
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.C. Ultrahigh-performance hydrophilic interaction liquid chromatography with tandem mass spectrometry method for the determination of paralytic shellfish toxins and tetrodotoxin in mussels, oysters, clams, cockles, and scallops: Collaborative study. J. AOAC Int. 2020, 103, 533–562. [Google Scholar] [CrossRef] [PubMed]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography-mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amzil, Z.; Derrien, A.; Terre Terrillon, A.; Savar, V.; Bertin, T.; Peyrat, M.; Duval, A.; Lhaute, K.; Arnich, N.; Hort, V.; et al. Five Years Monitoring the Emergence of Unregulated Toxins in Shellfish in France (EMERGTOX 2018–2022). Mar. Drugs 2023, 21, 435. https://doi.org/10.3390/md21080435
Amzil Z, Derrien A, Terre Terrillon A, Savar V, Bertin T, Peyrat M, Duval A, Lhaute K, Arnich N, Hort V, et al. Five Years Monitoring the Emergence of Unregulated Toxins in Shellfish in France (EMERGTOX 2018–2022). Marine Drugs. 2023; 21(8):435. https://doi.org/10.3390/md21080435
Chicago/Turabian StyleAmzil, Zouher, Amélie Derrien, Aouregan Terre Terrillon, Véronique Savar, Thomas Bertin, Marion Peyrat, Audrey Duval, Korian Lhaute, Nathalie Arnich, Vincent Hort, and et al. 2023. "Five Years Monitoring the Emergence of Unregulated Toxins in Shellfish in France (EMERGTOX 2018–2022)" Marine Drugs 21, no. 8: 435. https://doi.org/10.3390/md21080435
APA StyleAmzil, Z., Derrien, A., Terre Terrillon, A., Savar, V., Bertin, T., Peyrat, M., Duval, A., Lhaute, K., Arnich, N., Hort, V., & Nicolas, M. (2023). Five Years Monitoring the Emergence of Unregulated Toxins in Shellfish in France (EMERGTOX 2018–2022). Marine Drugs, 21(8), 435. https://doi.org/10.3390/md21080435





