Wound Restorative Power of Halimeda macroloba/ Mesenchymal Stem Cells in Immunocompromised Rats via Downregulating Inflammatory/Immune Cross Talk
Abstract
1. Introduction
2. Results
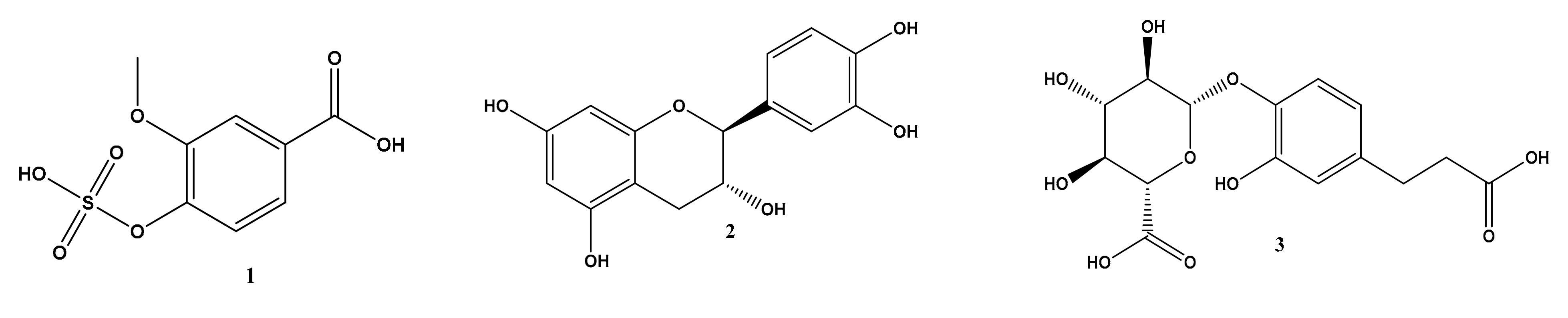
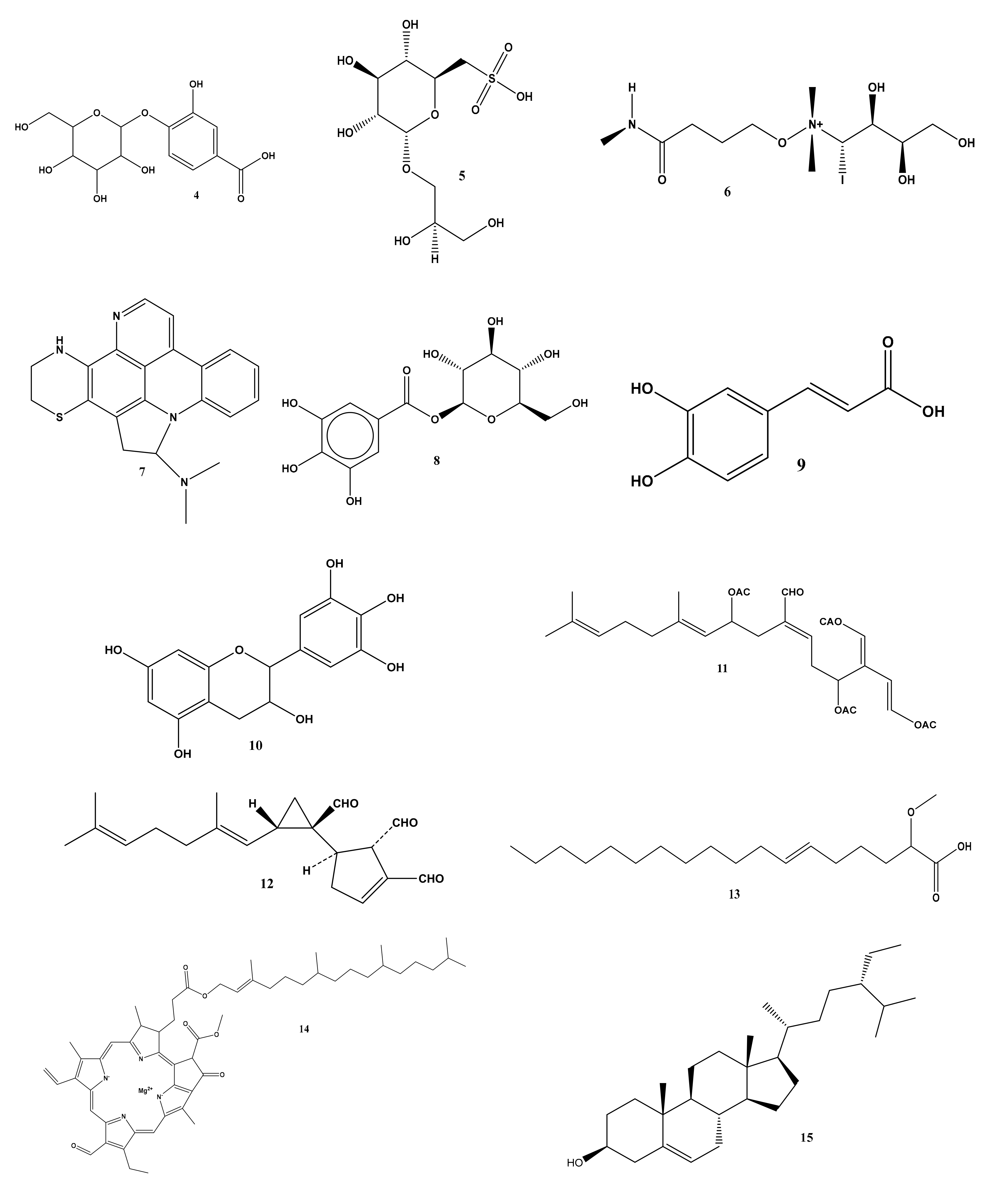
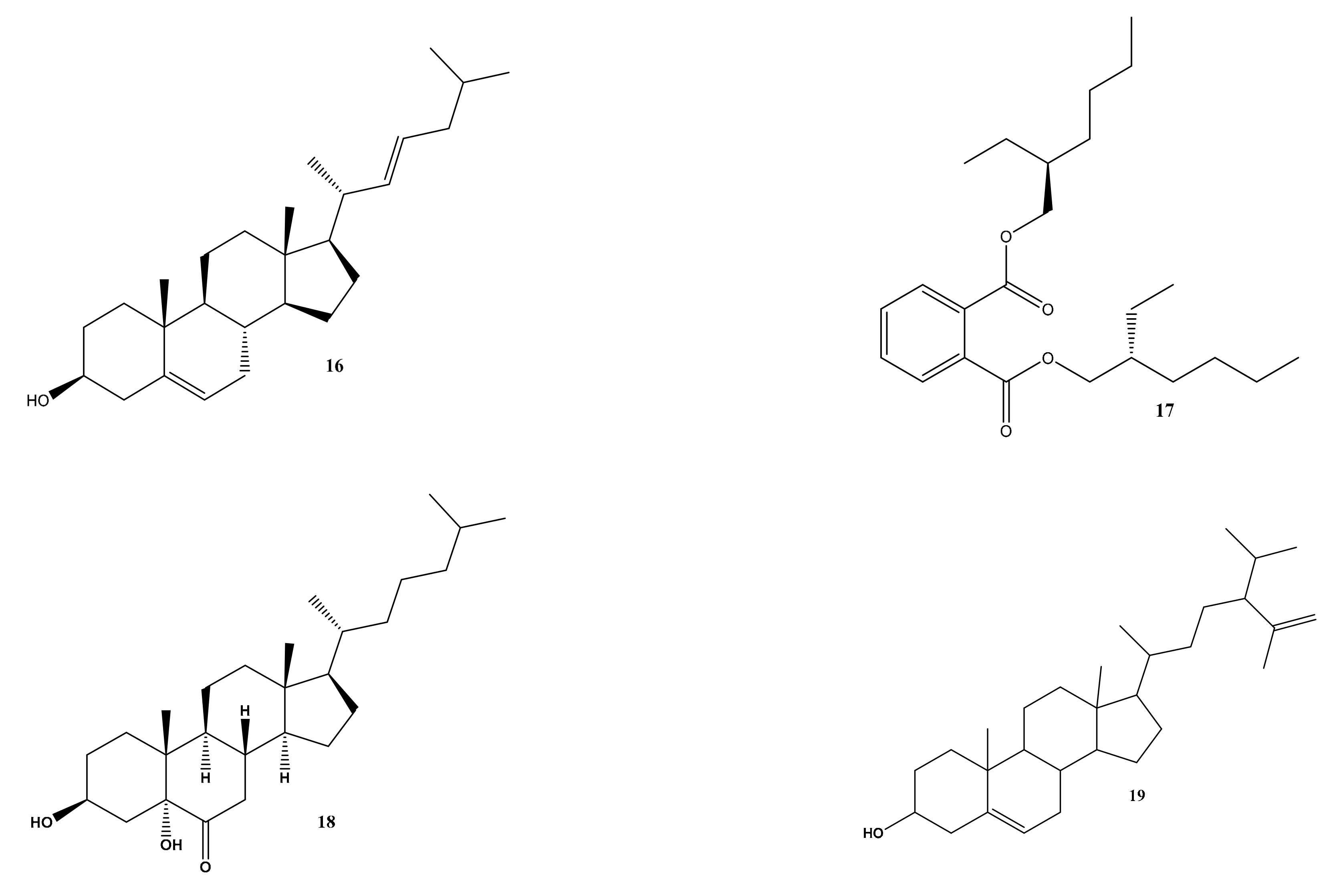
2.1. Metabolomic Profiling of Halimeda macroloba Algae
2.2. Biological Study
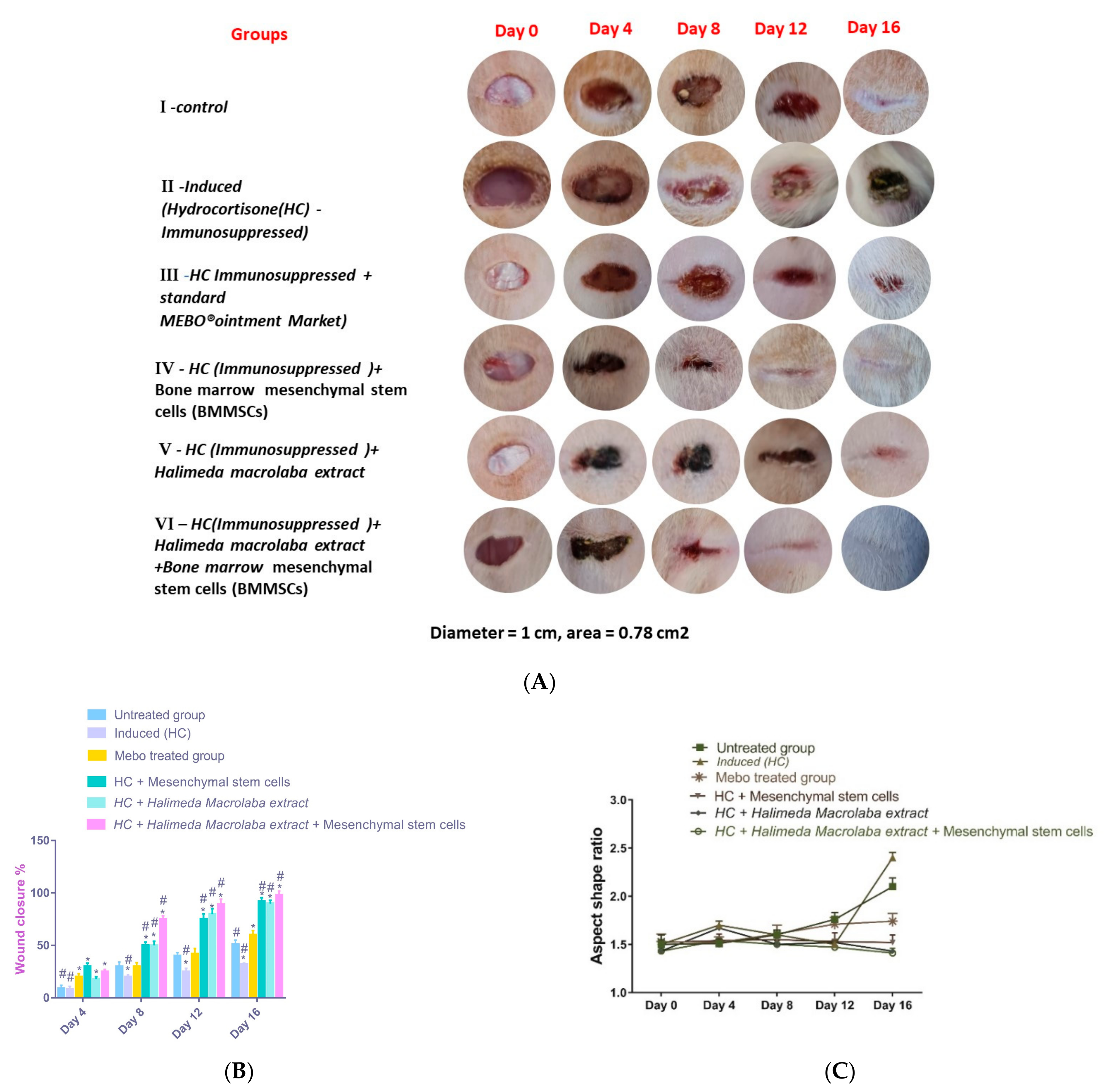
2.2.1. Gross Evaluation and Estimation of Wound Closure Rate
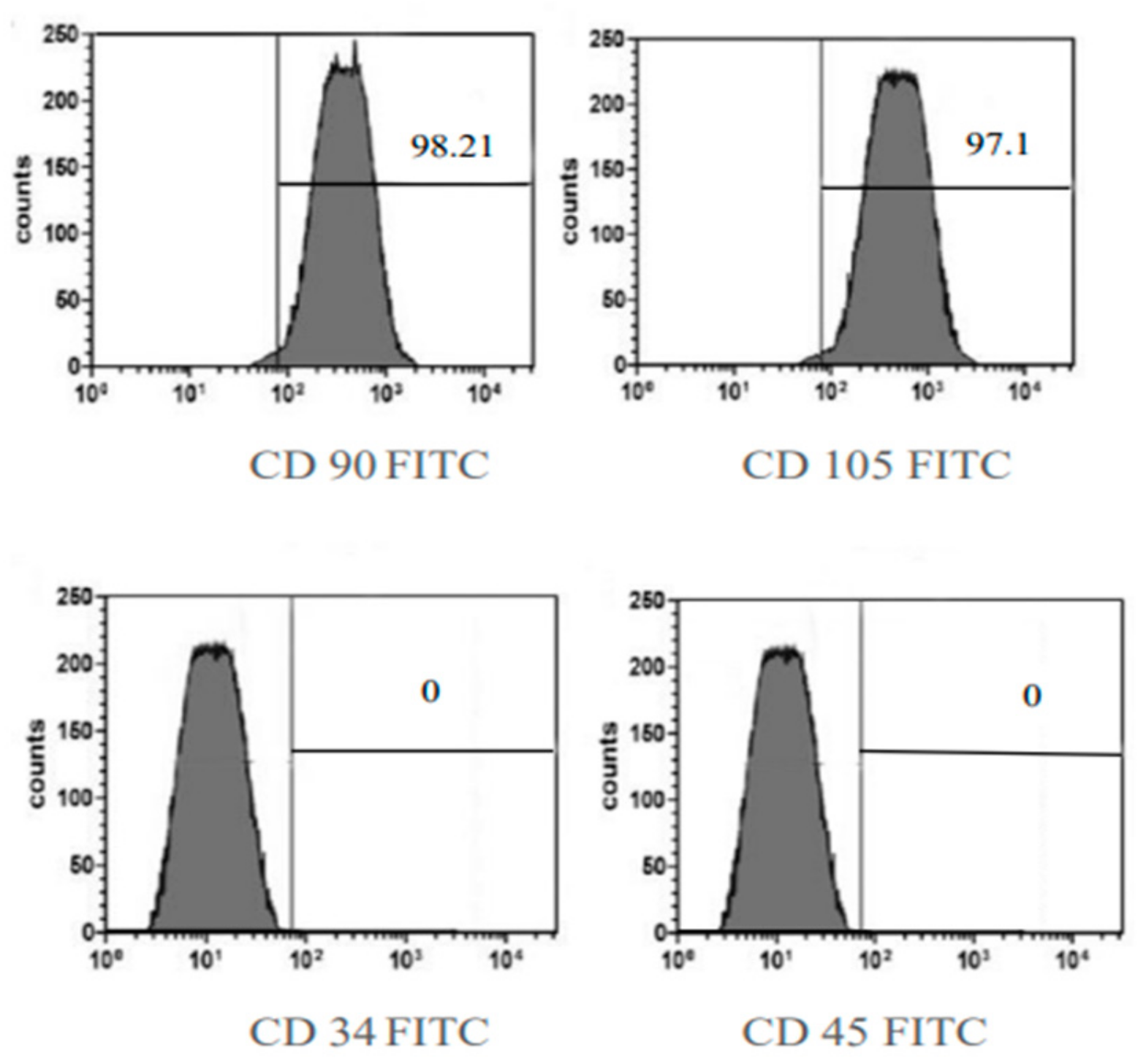
2.2.2. BMMSC Immunophenotyping
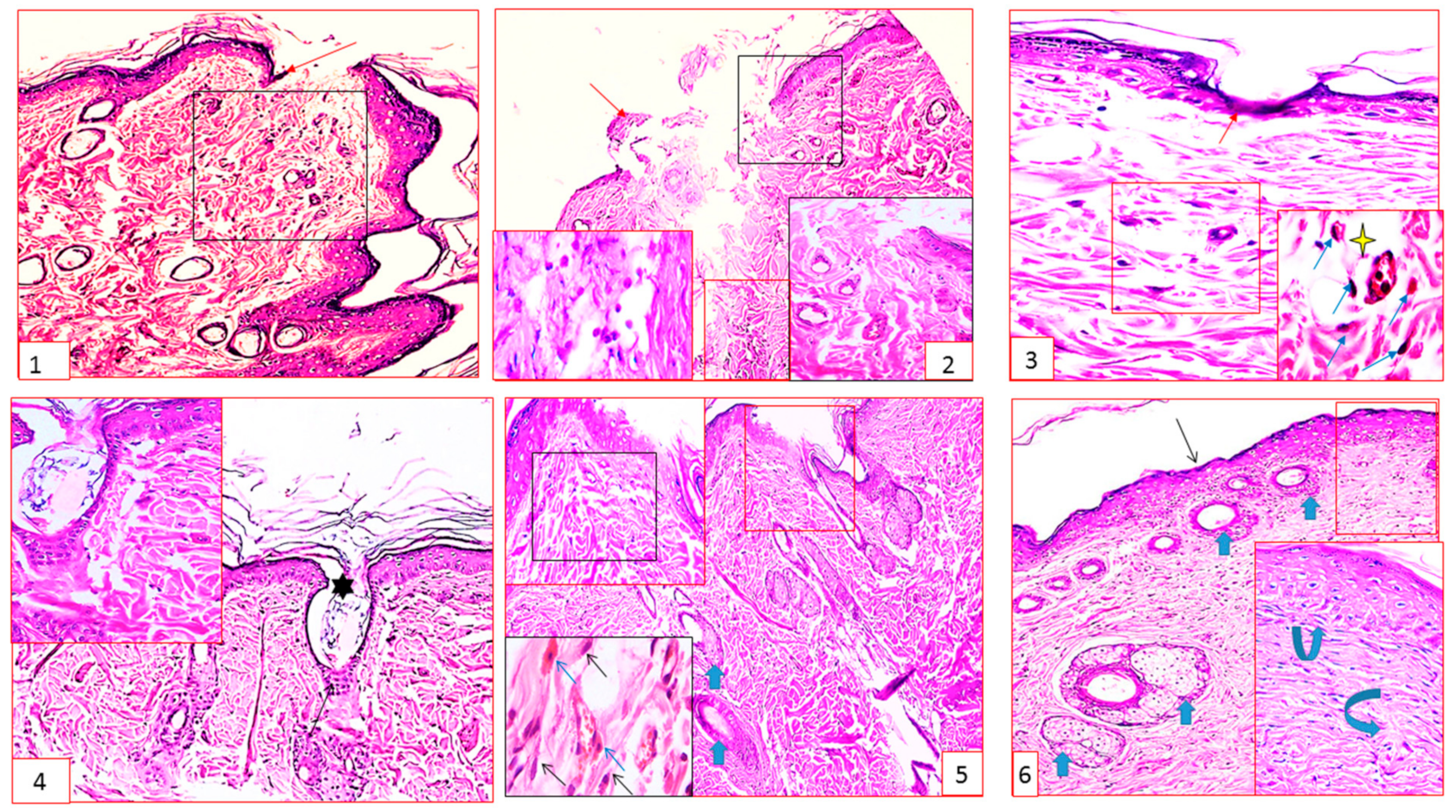
2.2.3. Histopathology Results
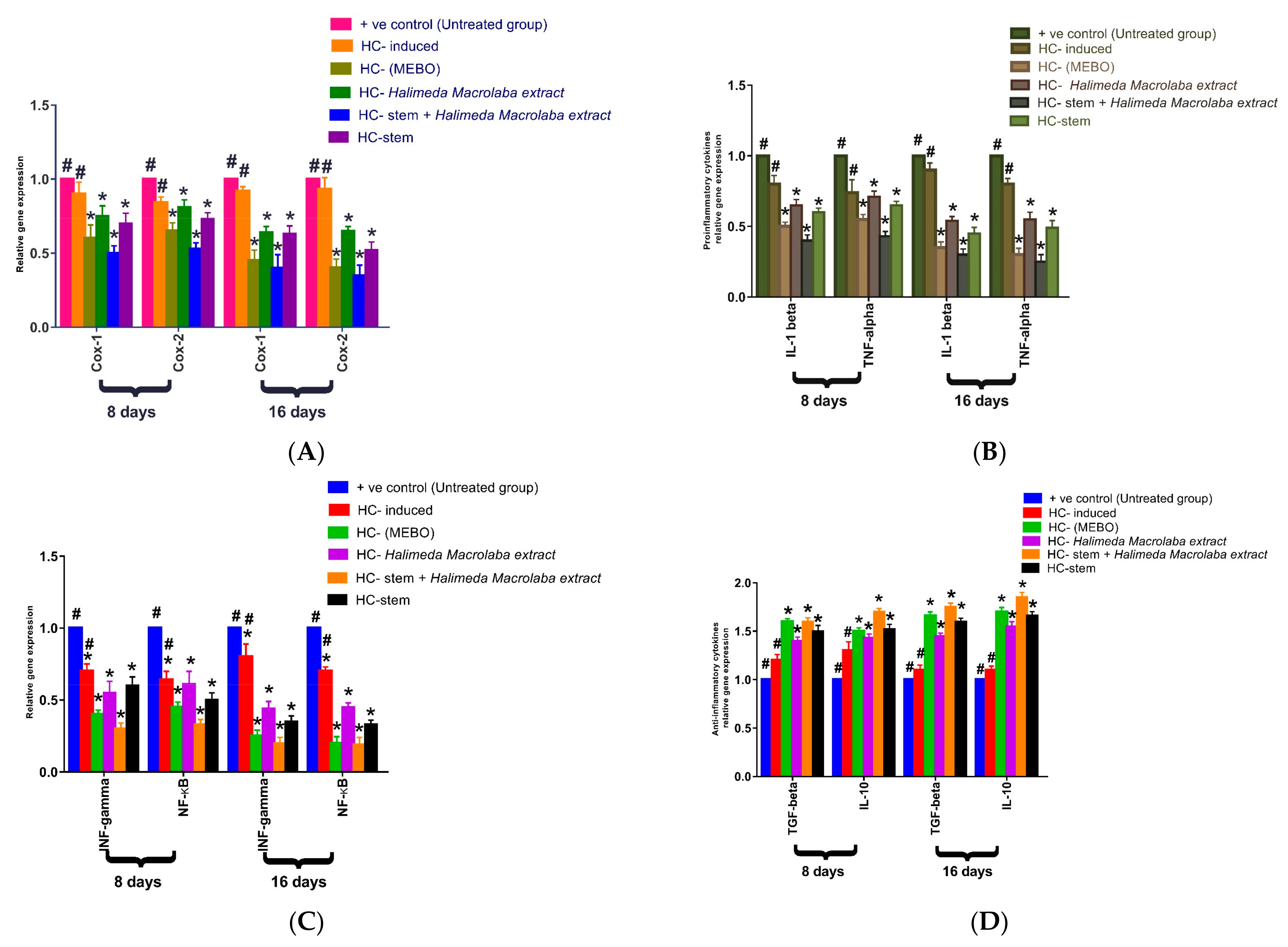
2.2.4. Gene Expression Results
3. Discussion
4. Materials and Methods
4.1. Metabolomic Analysis of Halimeda macroloba Extract
4.1.1. Algae Sample Collection and Identification
4.1.2. Extraction of Halimeda macroloba
4.1.3. Metabolomic Analysis
4.2. Biological Study
4.2.1. Incision Wound Model
4.2.2. Excisional Wound Creation
4.2.3. Induction of Immunocompromised Condition
4.2.4. Collection of Tissue Samples, Calculation of Percentage Wound Closure Rate
4.3. Isolation, Culture, Characterization, and Injection of Mesenchymal Stem Cells
4.3.1. Bone Marrow Isolation of BMMSCs
4.3.2. Culture of BMMSCs
4.3.3. Trypan Blue Exclusion Test of Cell Viability
4.3.4. Characterization of BM-MSCs by Flow Cytometric Phenotyping Analysis
4.3.5. Injection of MSC
4.4. Histopathological Study
4.5. Gene Expression Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S. Antioxidant and wound healing potential of vitis vinifera seeds supported by phytochemical characterization and docking studies. Antioxidants 2022, 11, 881. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Hamm, R.L. Factors that impair wound healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Concise review: Clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Domingues, C.C.; Kundu, N.; Kropotova, Y.; Ahmadi, N.; Sen, S. Antioxidant-upregulated mesenchymal stem cells reduce inflammation and improve fatty liver disease in diet-induced obesity. Stem Cells Transl. Med. 2019, 10, 280. [Google Scholar] [CrossRef]
- Ren, G.; Chen, X.; Dong, F.; Li, W.; Ren, X.; Zhang, Y.; Shi, Y. Concise review: Mesenchymal stem cells and translational medicine: Emerging issues. Stem Cells Transl. Med. 2012, 1, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal stromal cells induce peculiar alternatively activated macrophages capable of dampening both innate and adaptive immune responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Paus, R.; Tiede, S.; Day, P.; Bayat, A. Exploring the role of stem cells in cutaneous wound healing. Exp. Dermatol. 2009, 18, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Giesert, C.; Marxer, A.; Sutherland, D.R.; Schuh, A.C.; Kanz, L.; Bühring, H.J. Antibody W7C5 defines a CD109 epitope expressed on CD34+ and CD34− hematopoietic and mesenchymal stem cell subsets. Ann. N. Y. Acad. Sci. 2003, 996, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Sayed, A.M.; Abdelwahab, M.F.; Albohy, A.; Abdulrazik, B.S.; Ibrahim, A.M.; Bringmann, G.; Abdelmohsen, U.R. Identifying the specific-targeted marine cerebrosides against SARS-CoV-2: An integrated computational approach. RSC Adv. 2021, 11, 36042–36059. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.; Rafiquzzaman, S.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.; et al. Antioxidant and signal-modulating effects of brown seaweed-derived compounds against oxidative stress-associated pathology. Oxidative Med. Cell. Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef]
- Elmaidomy, A.H.; Zahran, E.M.; Soltane, R.; Alasiri, A.; Saber, H.; Ngwa, C.J.; Pradel, G.; Alsenani, F.; Sayed, A.M.; Abdelmohsen, U.R. New Halogenated Compounds from Halimeda macroloba Seaweed with Potential Inhibitory Activity against Malaria. Molecules 2022, 27, 5617. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Mohamad, S.A.; Yahia, R.; Badawi, A.M.; Sayed, A.M.; Abdelmohsen, U.R. Anti-otomycotic potential of nanoparticles of Moringa oleifera leaf extract: An integrated in vitro, in silico and phase 0 clinical study. Food Funct. 2022, 13, 11083–11096. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Sayed, A.M.; Alaaeldin, R.; Khattab, A.R.; Abdelmohsen, U.R. Bioactives and functional food ingredients with promising potential for management of cerebral and myocardial ischemia: A comprehensive mechanistic review. Food Funct. 2022, 13, 6859–6874. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Dunshea, F.R.; Suleria, H.A. Lc-esi-qtof/ms characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N.; Hashimoto, Y. Structures of Two Water Soluble Hemolysins Isolated from the Green Alga Ulva pertusa. Agric. Biol. Chem. 1975, 39, 2021–2025. [Google Scholar] [CrossRef]
- Koren-Goldshlager, G.; Aknin, M.; Kashman, Y.J. Cycloshermilamine D, a new pyridoacridine from the marine tunicate Cystodytes violatinctus. J. Nat. Prod. 2000, 63, 830–831. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhang, Q.; Wang, J. Integrated gas chromatograph-mass spectrometry (GC/MS) and MS/MS-based molecular networking reveals the analgesic and anti-inflammatory phenotypes of the sea slater Ligia exotica. Mar. Drugs 2019, 17, 395. [Google Scholar] [CrossRef]
- Wainai, T.; Truscott, B.; Idler, D.R. Isolation of 22-dehydrocholesterol from scallop. Can. J. Biochem. 1964, 42, 1331–1337. [Google Scholar]
- Rahman, A.-U.; Choudhary, M.I. Chapter 4—Chemistry and Biology of Steroidal Alkaloids from Marine Organisms. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: Cambridge, MA, USA, 1999; pp. 233–260. [Google Scholar]
- Dzeha, T.; Jaspars, M.; Tabudravu, J. Clionasterol, a triterpenoid from the Kenyan marine green macroalga Halimeda macroloba. West. Indian Ocean. J. Mar. Sci. 2003, 2, 157–161. [Google Scholar]
- Nazarudin, M.F.; Yasin, I.S.M.; Mazli, N.; Saadi, A.R.; Azizee, M.H.S.; Nooraini, M.A.; Saad, N.; Ferdous, U.T.; Fakhrulddin, I.M. Preliminary screening of antioxidant and cytotoxic potential of green seaweed, Halimeda opuntia (Linnaeus) Lamouroux. Saudi J. Biol. Sci. 2022, 29, 2698–2705. [Google Scholar] [CrossRef]
- Rink, B.E.; Amilon, K.R.; Esteves, C.L.; French, H.M.; Watson, E.; Aurich, C.; Donadeu, F.X. Isolation and characterization of equine endometrial mesenchymal stromal cells. Stem Cell Res. Ther. 2017, 8, 166. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Hussein, A.S.; Salem, M.A.; Khalil, H.E.; Yehia Desoukey, S.; Fouad, M.A.; Kamel, M.S. Antiulcer potential and molecular docking of flavonoids from Ocimum forskolei Benth., family Lamiaceae. Nat. Prod. Res. 2021, 35, 1933–1937. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdelmohsen, U.R.; Ayoub, A.T.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Kamel, M.S. Metabolic profiling, histopathological anti-ulcer study, molecular docking and molecular dynamics of ursolic acid isolated from Ocimum forskolei Benth.(family Lamiaceae). S. Afr. J. Bot. 2020, 131, 311–319. [Google Scholar] [CrossRef]
- Zahran, E.M.; Abdel-Maqsoud, N.M.R.; Tammam, O.Y.; Abdel-Rahman, I.M.; Elrehany, M.A.; Bakhsh, H.T.; Altemani, F.H.; Algehainy, N.A.; Alzubaidi, M.A.; Abdelmohsen, U.R. Scabicidal Potential of Coconut Seed Extract in Rabbits via Downregulating Inflammatory/Immune Cross Talk: A Comprehensive Phytochemical/GC-MS and In Silico Proof. Antibiotics 2022, 12, 43. [Google Scholar] [CrossRef]
- Pereira, L.d.P.; Mota, M.R.; Brizeno, L.A.; Nogueira, F.C.; Ferreira, E.G.; Pereira, M.G.; Assreuy, A.M. Modulator effect of a polysaccharide-rich extract from Caesalpinia ferrea stem barks in rat cutaneous wound healing: Role of TNF-α, IL-1β, NO, TGF-β. J. Ethnopharmacol. 2016, 187, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, R.M.; Simeonova, P.P.; Matheson, J.M.; Kommineni, C.; Guriel, J.L.; Sugawara, T.; Luster, M.I. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000, 14, 2525–2531. [Google Scholar] [CrossRef]
- Maccario, R.; Podestà, M.; Moretta, A.; Cometa, A.; Comoli, P.; Montagna, D.; Daudt, L.; Ibatici, A.; Piaggio, G.; Pozzi, S. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005, 90, 516–525. [Google Scholar] [PubMed]
- Zahran, E.M.; Abdelmohsen, U.R.; Shalash, M.M.; Salem, M.A.; Khalil, H.E.; Desoukey, S.Y.; Fouad, M.A.; Krischke, M.; Mueller, M.; Kamel, M.S. Local anaesthetic potential, metabolic profiling, molecular docking and in silico ADME studies of Ocimum forskolei, family Lamiaceae. Nat. Prod. Res. 2021, 35, 4757–4763. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.R. Guidelines for the use of animals in biomedical research. Thromb. Haemost. 1987, 58, 1078–1084. [Google Scholar] [CrossRef]
- Ang, E.; Lee, S.; Gan, C.; See, P.; Chan, Y.; Ng, L.; Machin, D. The role of alternative therapy in the management of partial thickness burns of the face--experience with the use of moist exposed burn ointment (MEBO) compared with silver sulphadiazine. Ann. Acad. Med. Singap. 2000, 29, 7–10. [Google Scholar] [PubMed]
- Keshri, G.K.; Gupta, A.; Yadav, A.; Sharma, S.K.; Singh, S.B. Photobiomodulation with pulsed and continuous wave near-infrared laser (810 nm, Al-Ga-As) augments dermal wound healing in immunosuppressed rats. PLoS ONE 2016, 11, e0166705. [Google Scholar] [CrossRef]
- Gupta, A.; Jain, G.; Raghubir, R. A time course study for the development of an immunocompromised wound model, using hydrocortisone. J. Pharmacol. Toxicol. Methods 1999, 41, 183–187. [Google Scholar] [CrossRef]
- Vidal, M.A.; Kilroy, G.E.; Lopez, M.J.; Johnson, J.R.; Moore, R.M.; Gimble, J.M. Characterization of equine adipose tissue-derived stromal cells: Adipogenic and osteogenic capacity and comparison with bone marrow-derived mesenchymal stromal cells. Vet. Surg. 2007, 36, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Adly, H.A.; Okby, A.E.-W.; Mahmoud, A.-A.Y.; El-Shamy, A.; Galhoom, R.A.-A.; Hashem, M.A.; Ahmed, M.F. Repopulation of multipotent stem cells derived from adult male rabbits on a polycaprolactone scaffold: An in vitro study. Al-Azhar Int. Med. J. 2021, 2, 37–42. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, 21, A-3B. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, J.; Xiao, X.-B.; Mai, H.-X.; Zheng, K.; Sun, W.-L.; Wang, L.; Liang, F.; Yang, Z.-L.; Liu, Y. Multiple injections of human umbilical cord-derived mesenchymal stromal cells through the tail vein improve microcirculation and the microenvironment in a rat model of radiation myelopathy. J. Transl. Med. 2014, 12, 246. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahran, E.M.; Mohyeldin, R.H.; El-Mordy, F.M.A.; Maher, S.A.; Tammam, O.Y.; Saber, E.A.; Altemani, F.H.; Algehainy, N.A.; Alanazi, M.A.; Jalal, M.M.; et al. Wound Restorative Power of Halimeda macroloba/ Mesenchymal Stem Cells in Immunocompromised Rats via Downregulating Inflammatory/Immune Cross Talk. Mar. Drugs 2023, 21, 336. https://doi.org/10.3390/md21060336
Zahran EM, Mohyeldin RH, El-Mordy FMA, Maher SA, Tammam OY, Saber EA, Altemani FH, Algehainy NA, Alanazi MA, Jalal MM, et al. Wound Restorative Power of Halimeda macroloba/ Mesenchymal Stem Cells in Immunocompromised Rats via Downregulating Inflammatory/Immune Cross Talk. Marine Drugs. 2023; 21(6):336. https://doi.org/10.3390/md21060336
Chicago/Turabian StyleZahran, Eman Maher, Reham H. Mohyeldin, Fatma Mohamed Abd El-Mordy, Sherif A. Maher, Omar Y. Tammam, Entesar Ali Saber, Faisal H. Altemani, Naseh A. Algehainy, Mohammad A. Alanazi, Mohammed M. Jalal, and et al. 2023. "Wound Restorative Power of Halimeda macroloba/ Mesenchymal Stem Cells in Immunocompromised Rats via Downregulating Inflammatory/Immune Cross Talk" Marine Drugs 21, no. 6: 336. https://doi.org/10.3390/md21060336
APA StyleZahran, E. M., Mohyeldin, R. H., El-Mordy, F. M. A., Maher, S. A., Tammam, O. Y., Saber, E. A., Altemani, F. H., Algehainy, N. A., Alanazi, M. A., Jalal, M. M., Elrehany, M. A., & Abdelmohsen, U. R. (2023). Wound Restorative Power of Halimeda macroloba/ Mesenchymal Stem Cells in Immunocompromised Rats via Downregulating Inflammatory/Immune Cross Talk. Marine Drugs, 21(6), 336. https://doi.org/10.3390/md21060336








