Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review
Abstract
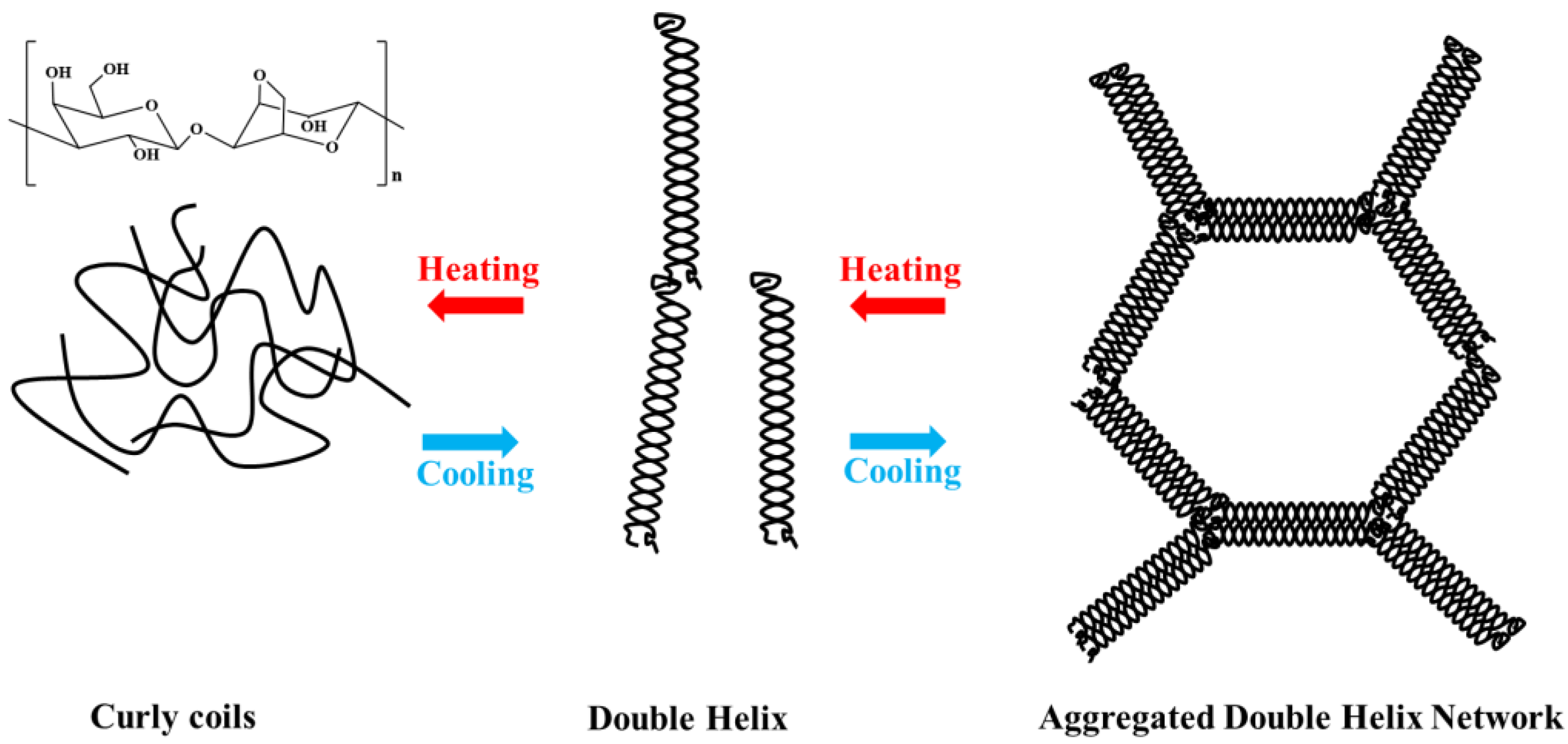
1. Introduction
2. Extraction and Modification of Agarose
2.1. Agarose Extraction
2.1.1. Agaropectin Precipitation Method
2.1.2. Agarose Precipitation Method
2.1.3. Ion Exchange Method
2.1.4. Ionic Liquid Method
| Method | Reagents | Seaweed Type | Highlights | Cite |
|---|---|---|---|---|
| Agaropectin precipitation | DMSO | Gelidium amansii | 1 wt% concentration gel strength was 1190 g/cm2; sulfate content was 0.28 wt% | Jeon et al. [23] |
| Quaternary ammonium compounds | Gracilaria cylindrica | 1 wt% concentration gel strength was 935 g/cm2; sulfate content was 0.17 wt% | Santos et al. [24] | |
| Gelidium amansii | 1 wt% concentration gel strength was 742 g/cm2; sulfate content was 0.63 wt% | Chew et al. [22] | ||
| Ion exchange | DEAE-Sephadex A-50 | Purified agar solution | sulfate content was 0.05 wt%; pyruvic acid content was below 0.01 wt% | Duckworth et al. [25] |
| DEAE-Cellulose suspension | Ahnfeltia plicata | 1 wt% concentration gel strength was 1417 g/cm2; sulfate content was 0.15 wt% | Zhang et al. [28] | |
| Ionic liquid | Choline-based bio-ionic liquids | Gracilaria dura | 1 wt% concentration gel strength was 1250 g/cm2; sulfate content was 0.21 wt% | Sharma et al. [30] |
| 1-ethyl-3-methylimidazolium acetate, choline acetate, 1-ethyl-3-methylimidazolium diethyl phosphate | Gracilaria dura | 1 wt% concentration gel strength was 600 g/cm2; sulfate content was 1.95 wt%; yield was 39 wt% | Trivedi et al. [29] | |
| Heat-compatible; strong-anion exchange; isopropanol | Gracilaria amansii | 1 wt% concentration gel strength was 853 g/cm2; sulfate content was 0.14 wt% | Wang et al. [31] |
2.1.5. Agarose Extraction by a Complex Method
2.2. Modification of Agarose
| Method | Type | Application | Modification Reagents | Results | Cite |
|---|---|---|---|---|---|
| Biomodification | Enzymatic modification | Edible packaging film | Galactose oxidase GAO-5F | Agarose is oxidized to polyaldehydes and can be cross-linked with gelatine for application in food packaging films. | Cao et al. [46] |
| Enzymatic modification | GH50 Agarosease Aga3420 | Efficient production of high purity neoagarobiose (NA2) in a low temperature environment. | Zhang et al. [47] | ||
| Physical modification | Electronic irradiation | Material properties of agarose hydrogels can be adjusted at a low doses of high energy electron irradiation. | Krömmelbein et al. [41] | ||
| Ultrasound | Drug delivery | 1-MHz Ultrasound | High frequency ultrasound sonication enhances the internal diffusivity of agarose gels and aid drug delivery. | Tsukamoto et al. [48] | |
| Compounding | Mucin | Crosslinking between mucin and agarose results in increased swelling, adhesion, hygroscopicity, and thermal properties. | Builders et al. [49] | ||
| Compounding | Drug delivery | Chitosan/γ-alumina | Hydrogel nanocomposites are efficient drug delivery systems for the chemotherapeutic agent 5-FU and simultaneously reduce its adverse effects. | Bayat et al. [50] | |
| Compounding | Drug delivery | Fe3O4/CS | Ability to release curcumin in response to pH | Pourmadadi et al. [51] | |
| Chemical modification | Esterification | Octenylsuccinic anhydride | Reduced gel strength, lower melting temperature and increased transparency | Xiao et al. [43] | |
| Esterification | Emulsifiers | Fatty acid derivatives | Improved emulsification properties | Xiao et al. [44] | |
| Sensor | Nucleobase guanine | Good fluorescent activity | Oza et al. [45] | ||
| Esterification | Drug delivery | Carbonyl diimidazole | Adsorbs more hydrophobic dyes and controls the release of hydrophobic dyes | Evans et al. [52] | |
| Esterification | Microcapsules | Dodecenylsuccinic anhydride | Prepared agarose microcapsules were used for the encapsulation of DHA and showed good oxidative stability and release properties. | Xiao et al. [53] | |
| Conjugated | Immunoaffinity chromatography columns | Avermectin polyclonal antibodies | Enables the rapid and sensitive simultaneous determination of avermectin, ivermectin, doramectin, and eprinomectin residues in the bovine liver and muscle in combination with LC-MA-MA | Hou et al. [54] | |
| Coupling | Hydrophobic interaction chromatography support | Phenyl ligand | Microspheres can be used to isolate lysozyme and bovine serum proteins and can tolerate higher flow rates. | Gustavsson et al. [55] |
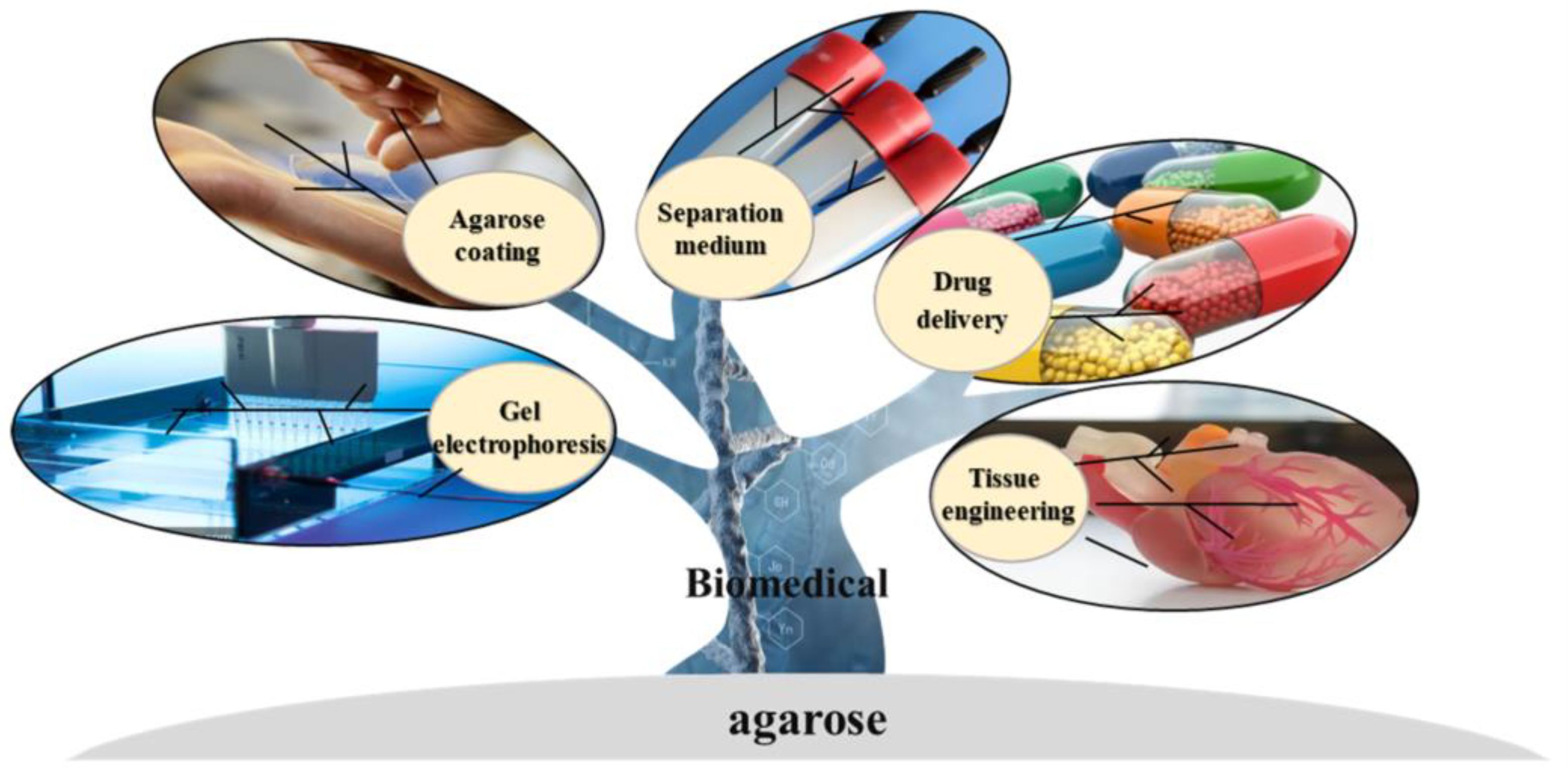
3. Biomedical Applications of Agarose and Its Derivatives
3.1. Agarose Gel Electrophoresis
3.2. Agarose Separation Medium
3.2.1. Affinity Chromatography
3.2.2. Size Exclusion Chromatography
3.2.3. Ion Exchange Chromatography
3.2.4. Hydrophobic Interaction Chromatography
3.3. Agarose Coating
3.4. Drug Delivery
3.5. Tissue Engineering
3.5.1. Agarose Hydrogel
3.5.2. 3D/4D Printed Brackets
4. Materials and Methods
5. Conclusions
6. Prospects and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ramkumar, V.S.; Prakash, S.; Ramasubburayan, R.; Pugazhendhi, A.; Gopalakrishnan, K.; Kannapiran, E.; Rajendran, R.B. Seaweeds: A resource for marine bionanotechnology. Enzyme Microb. Technol. 2016, 95, 45–57. [Google Scholar]
- El-Beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A.; Barqawi, A.A.; Mansour, A.T. Phytochemical and Potential Properties of Seaweeds and Their Recent Applications: A Review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Nan, F.R.; Feng, J.; Lv, J.P.; Liu, Q.; Fang, K.P.; Gong, C.Y.; Xie, S.L. Origin and evolutionary history of freshwater Rhodophyta: Further insights based on phylogenomic evidence. Sci. Rep. 2017, 7, 2934. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001, 13, 173–184. [Google Scholar] [CrossRef]
- MarketWatch Global Agarose Market Analysis and Business Growth Outlook [2023–2030]. Available online: https://www.marketwatch.com/ (accessed on 12 April 2023).
- Alba, K.; Kontogiorgos, V. Seaweed Polysaccharides (Agar, Alginate Carrageenan). In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Lee, Y.E.; Kim, H.; Seo, C.; Park, T.; Lee, K.B.; Yoo, S.Y.; Hong, S.C.; Kim, J.T.; Lee, J. Marine polysaccharides: Therapeutic efficacy and biomedical applications. Arch Pharm. Res. 2017, 40, 1006–1020. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, I.; Dheer, D.; Nagpal, M.; Kumar, P.; Venkatesh, D.N.; Puri, V.; Singh, I. A propitious role of marine sourced polysaccharides: Drug delivery and biomedical applications. Carbohydr. Polym. 2023, 308, 120448. [Google Scholar] [CrossRef]
- Xiao, Q.; Ma, M.Z.; Chen, J.; Zhang, Y.H.; Chen, F.Q.; Weng, H.F.; Xiao, A.F. Preparation of macroporous rigid agarose microspheres by pre-crosslinking with cyclic anhydride. Int. J. Biol. Macromol. 2022, 222, 41–54. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.S.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Gaharwar, A.K. Engineered Biomaterials for in Situ Tissue Regeneration. Tissue Eng. Part A 2022, 28, S590. [Google Scholar] [CrossRef]
- Ciancia, M.; Fernandez, P.V.; Leliaert, F. Diversity of Sulfated Polysaccharides From Cell Walls of Coenocytic Green Algae and Their Structural Relationships in View of Green Algal Evolution. Front. Plant Sci. 2020, 11, 554585. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Heredia, M.A.; Lapa, A.; Mendes, A.C.; Balcaen, L.; Samal, S.K.; Chai, F.; Van der Voort, P.; Stevens, C.V.; Parakhonskiy, B.V.; Chronakis, I.S.; et al. Bioinspired, biomimetic, double-enzymatic mineralization of hydrogels for bone regeneration with calcium carbonate. Mater. Lett. 2017, 190, 13–16. [Google Scholar] [CrossRef]
- Wu, F.; Xu, T.T.; Zhao, G.Y.; Meng, S.S.; Wan, M.M.; Chi, B.; Mao, C.; Shen, J. Mesoporous Silica Nanoparticles-Encapsulated Agarose and Heparin as Anticoagulant and Resisting Bacterial Adhesion Coating for Biomedical Silicone. Langmuir 2017, 33, 5245–5252. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.T.; Ma, Y.H.; Zhang, Y.N.; Cao, X.J.; Shi, Z.Y.; Liu, Y.; Ding, X.J. Significantly improved antifouling capability of silicone rubber surfaces by covalently bonded acrylated agarose towards biomedical applications. Colloids Surf. B-Biointerfaces 2023, 222, 112979. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of Flexible pH-Responsive Agarose/Succinoglycan Hydrogels for Controlled Drug Release. Polymers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Apte, G.; Lindenbauer, A.; Schemberg, J.; Rothe, H.; Nguyen, T.H. Controlling Surface-Induced Platelet Activation by Agarose and Gelatin-Based Hydrogel Films. ACS Omega 2021, 6, 10963–10974. [Google Scholar] [CrossRef]
- Krommelbein, C.; Xie, X.F.; Seifert, J.; Konieczny, R.; Friebe, S.; Kas, J.; Riedel, S.; Mayr, S.G. Electron beam treated injectable agarose/alginate beads prepared by electrospraying. Carbohydr. Polym. 2022, 298, 120024. [Google Scholar] [CrossRef]
- Kinoshita, K.; Iwase, M.; Yamada, M.; Yajima, Y.; Seki, M. Fabrication of multilayered vascular tissues using microfluidic agarose hydrogel platforms. Biotechnol. J. 2016, 11, 1415–1423. [Google Scholar] [CrossRef]
- Araki, C.; Arai, K. Studies on the chemical constitution of agar-agar. XXIV. Isolation of a new disaccharide as a reversion product from acidic hydrolysate. Bull. Chem. Soc. Jpn 1967, 40, 1452–1456. [Google Scholar] [CrossRef]
- Chew, K.W.; Show, P.L.; Yap, Y.J.; Juan, J.C.; Phang, S.M.; Ling, T.C.; Chang, J.S. Sonication and grinding pre-treatments on Gelidium amansii seaweed for the extraction and characterization of Agarose. Front. Environ. Sci. Eng. 2018, 12, 2. [Google Scholar] [CrossRef]
- Jeon, Y.-J.; Athukorala, Y.; Lee, J.-H. Characterization of Agarose Product from Agar Using DMSO. ALGAE 2005, 20, 61–67. [Google Scholar] [CrossRef]
- Santos, G.A.; Doty, M.S. Agarose from Gracilaria-Cylindrica. Bot. Mar. 1983, 26, 31–34. [Google Scholar] [CrossRef]
- Duckworth, M.; Yaphe, W. Preparation of agarose by fractionation from the spectrum of polysaccharides in agar. Anal. Biochem. 1971, 44, 636–641. [Google Scholar] [CrossRef]
- Ozturk, Y.; Ekmekci, Z. Removal of sulfate ions from process water by ion exchange resins kk. Miner. Eng. 2020, 159, 106613. [Google Scholar] [CrossRef]
- Cook, R.B.; Witt, H.J. Agarose composition, AQUEOUS Gel and Method of Making Same. U.S. Patent 4,290,911, 22 September 1981. [Google Scholar]
- Zhang, Y.; Fu, X.T.; Duan, D.L.; Xu, J.C.; Gao, X. Preparation and characterization of agar, agarose, agaropectin from the red alga Ahnfeltia plicata. J. Oceanol. Limnol. 2019, 37, 815–824. [Google Scholar] [CrossRef]
- Trivedi, T.; Kumar, A. Efficient Extraction of Agarose from Red Algae Using Ionic Liquids. Green Sustain. Chem. 2014, 4, 190–201. [Google Scholar] [CrossRef]
- Sharma, M.; Chaudhary, J.P.; Mondal, D.; Meena, R.; Prasad, K. A green and sustainable approach to utilize bio-ionic liquids for the selective precipitation of high purity agarose from an agarophyte extract. Green Chem. 2015, 17, 2867–2873. [Google Scholar] [CrossRef]
- Wang, T.P.; Chang, L.L.; Chang, S.N.; Wang, E.C.; Hwang, L.C.; Chen, Y.H.; Wang, Y.M. Successful preparation and characterization of biotechnological grade agarose from indigenous Gelidium amansii of Taiwan. Process Biochem. 2012, 47, 550–554. [Google Scholar] [CrossRef]
- Xiao, Q.; Yin, Q.; Ni, H.; Cai, H.N.; Wu, C.Z.; Xiao, A.F. Characterization and immobilization of arylsulfatase on modified magnetic nanoparticles for desulfation of agar. Int. J. Biol. Macromol. 2017, 94, 576–584. [Google Scholar] [CrossRef]
- Shukla, M.K.; Kumar, M.; Prasad, K.; Reddy, C.R.K.; Jha, B. Partial characterization of sulfohydrolase from Gracilaria dura and evaluation of its potential application in improvement of the agar quality. Carbohydr. Polym. 2011, 85, 157–163. [Google Scholar] [CrossRef]
- Wang, X.Y.; Duan, D.L.; Fu, X.T. Enzymatic desulfation of the red seaweeds agar by Marinomonas arylsulfatase. Int. J. Biol. Macromol. 2016, 93, 600–608. [Google Scholar] [CrossRef]
- Zhang, C.; An, D.; Xiao, Q.; Chen, F.Q.; Zhang, Y.H.; Weng, H.F.; Xiao, A.F. Convenient Agarose Preparation with Hydrogen Peroxide and Desulfation Process Analysis. Mar. Drugs 2021, 19, 297. [Google Scholar] [CrossRef]
- Zhang, C.; An, D.; Xiao, Q.; Weng, H.F.; Zhang, Y.H.; Yang, Q.M.; Xiao, A.F. Preparation, characterization, and modification mechanism of agar treated with hydrogen peroxide at different temperatures. Food Hydrocoll. 2020, 101, 105527. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Zhao, X.W.; Ye, F.Y.; Zhao, G.H. Hydrophobically modified polysaccharides and their self-assembled systems: A review on structures and food applications. Carbohydr. Polym. 2022, 284, 119182. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Song, X.N.; Lin, Y.; Xiao, Q.; Du, X.P.; Chen, Y.H.; Xiao, A.F. Antioxidant capacity and prebiotic effects of Gracilaria neoagaro oligosaccharides prepared by agarase hydrolysis. Int. J. Biol. Macromol. 2019, 137, 177–186. [Google Scholar] [CrossRef]
- Punia, S. Barley starch modifications: Physical, chemical and enzymatic—A review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef]
- Zhang, B.; Lan, W.; Xie, J. Chemical modifications in the structure of marine polysaccharide as serviceable food processing and preservation assistant: A review. Int. J. Biol. Macromol. 2022, 223, 1539–1555. [Google Scholar] [CrossRef]
- Krommelbein, C.; Mutze, M.; Konieczny, R.; Schonherr, N.; Griebel, J.; Gerdes, W.; Mayr, S.G.; Riedel, S. Impact of high-energy electron irradiation on mechanical, structural and chemical properties of agarose hydrogels. Carbohydr. Polym. 2021, 263, 117970. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.L.; Ye, J.; Zhao, P.; Xiao, M.T. Oxyalkylation modification as a promising method for preparing low-melting-point agarose. Int. J. Biol. Macromol. 2018, 117, 696–703. [Google Scholar] [CrossRef]
- Xiao, Q.; Weng, H.F.; Chen, G.; Xiao, A.F. Preparation and characterization of octenyl succinic anhydride modified agarose derivative. Food Chem. 2019, 279, 30–39. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, G.; Xiao, A.F. Preparation, characterization, and emulsification properties of agarose fatty acid derivatives with different hydrophobic chains. Int. J. Biol. Macromol. 2019, 141, 906–918. [Google Scholar] [CrossRef]
- Oza, M.D.; Meena, R.; Prasad, K.; Paul, P.; Siddhanta, A.K. Functional modification of agarose: A facile synthesis of a fluorescent agarose-guanine derivative. Carbohydr. Polym. 2010, 81, 878–884. [Google Scholar] [CrossRef]
- Cao, N.; Xia, G.; Sun, H.; Zhao, L.; Cao, R.; Jiang, H.; Mao, X.; Liu, Q. Characterization of a Galactose Oxidase from Fusarium odoratissimum and Its Application in the Modification of Agarose. Foods 2023, 12, 603. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Wang, J.X.; Zeng, R.Y.; Wang, D.Q.; Wang, W.X.; Tong, X.F.; Qu, W. Agarose-Degrading Characteristics of a Deep-Sea Bacterium Vibrio Natriegens WPAGA4 and Its Cold-Adapted GH50 Agarase Aga3420. Mar. Drugs 2022, 20, 692. [Google Scholar] [CrossRef]
- Tsukamoto, A.; Tanaka, K.; Kumata, T.; Yoshida, K.; Watanabe, Y.; Miyata, S.; Furukawa, K.S.; Ushida, T. 1-MHz ultrasound enhances internal diffusivity in agarose gels. Appl. Acoust. 2013, 74, 1117–1121. [Google Scholar] [CrossRef]
- Builders, P.F.; Kunle, O.O.; Adikwu, M.U. Preparation and characterization of mucinated agarose: A mucin-agarose physical crosslink. Int. J. Pharm. 2008, 356, 174–180. [Google Scholar] [CrossRef]
- Bayat, F.; Pourmadadi, M.; Eshaghi, M.M.; Yazdian, F.; Rashedi, H. Improving Release Profile and Anticancer Activity of 5-Fluorouracil for Breast Cancer Therapy Using a Double Drug Delivery System: Chitosan/Agarose/γ-Alumina Nanocomposite@Double Emulsion. J. Cluster Sci. 2023, 1–13. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ahmadi, M.; Yazdian, F. Synthesis of a novel pH-responsive Fe3O4/chitosan/agarose double nanoemulsion as a promising Nanocarrier with sustained release of curcumin to treat MCF-7 cell line. Int. J. Biol. Macromol. 2023, 235, 123786. [Google Scholar] [CrossRef]
- Evans, C.; Morimitsu, Y.; Nishi, R.; Yoshida, M.; Takei, T. Novel hydrophobically modified agarose cryogels fabricated using dimethyl sulfoxide. J. Biosci. Bioeng. 2022, 133, 390–395. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, G.; Zhang, Y.H.; Weng, H.F.; Cai, M.H.; Xiao, A.F. Evaluation of a novel self-emulsifiable dodecenyl succinylated agarose in microencapsulation of docosahexaenoic acid (DHA) through spray-chilling process. Int. J. Biol. Macromol. 2020, 163, 2314–2324. [Google Scholar] [CrossRef]
- Hou, X.L.; Li, X.W.; Ding, S.Y.; He, J.H.; Jiang, H.Y.; Shen, J.Z. Simultaneous analysis of avermectins in bovine tissues by LC-MS-MS with immunoaffinity chromatography cleanup. Chromatographia 2006, 63, 543–550. [Google Scholar] [CrossRef]
- Gustavsson, P.E.; Axelsson, A.; Larsson, P.O. Superporous agarose beads as a hydrophobic interaction chromatography support. J. Chromatogr. A 1999, 830, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Y.; Xiao, Q.; Xiao, Z.C.; Zhang, Y.H.; Weng, H.F.; Chen, F.Q.; Xiao, A.F. Hydrophobic modified agar: Structural characterization and application in encapsulation and release of curcumin. Carbohydr. Polym. 2023, 308, 120644. [Google Scholar] [CrossRef]
- Zhao, L.S.; Li, S.S.; Liang, C.; Qiao, L.Z.; Du, K.F. High-strength and low-crystallinity cellulose/agarose composite microspheres: Fabrication, characterization and protein adsorption. Biochem. Eng. J. 2021, 166, 107826. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.D.; Zhu, K.; Miao, Z.; Zhao, J.Z.; Che, X.J.; Hao, D.X.; Zhang, R.Y.; Ma, G.H. Manipulation of pore structure during manufacture of agarose microspheres for bioseparation. Eng. Life Sci. 2020, 20, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Tantray, J.A.; Mansoor, S.; Wani, R.F.C.; Nissa, N.U. (Eds.) Chapter 24—Agarose gel electrophoresis. In Basic Life Science Methods; Academic Press: Cambridge, MA, USA, 2023; pp. 103–106. [Google Scholar]
- Li, C.; Arakawa, T. Agarose native gel electrophoresis of proteins. Int. J. Biol. Macromol. 2019, 140, 668–671. [Google Scholar] [CrossRef]
- Tomioka, Y.; Arakawa, T.; Akuta, T.; Nakagawa, M.; Ishibashi, M. Analysis of proteins by agarose native gel electrophoresis in the presence of solvent additives. Int. J. Biol. Macromol. 2022, 198, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Akuta, T.; Nakagawa, M.; Sato, T.; Shibata, T.; Maruyama, T.; Okumura, C.J.; Kurosawa, Y.; Arakawa, T. Agarose native gel electrophoresis for characterization of antibodies. Int. J. Biol. Macromol. 2020, 151, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Abe, B.T.; Wesselhoeft, R.A.; Chen, R.; Anderson, D.G.; Chang, H.Y. Circular RNA migration in agarose gel electrophoresis. Mol. Cell 2022, 82, 1768–1777.e3. [Google Scholar] [CrossRef]
- Song, N.L.; He, X.; Zhao, Q.R.; Yan, T.D.; Wen, L. Cloning and expression of the tumstatin active peptides-T-7 and its derivant-T-7-NGR. Clin. Exp. Med. 2009, 9, 165–171. [Google Scholar]
- Green, M.R.; Sambrook, J. Recovery of DNA from Low-Melting-Temperature Agarose Gels: Organic Extraction. Cold Spring Harb. Protoc. 2020, 2020, 100461. [Google Scholar] [CrossRef] [PubMed]
- Tomioka, Y.; Nakagawa, M.; Sakuma, C.; Nagatoishi, S.; Tsumoto, K.; Arakawa, T.; Akuta, T. Ladder observation of bovine serum albumin by high resolution agarose native gel electrophoresis. Int. J. Biol. Macromol. 2022, 215, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, C.; Tomioka, Y.; Li, C.; Shibata, T.; Nakagawa, M.; Kurosawa, Y.; Arakawa, T.; Akuta, T. Analysis of protein denaturation, aggregation and post-translational modification by agarose native gel electrophoresis. Int. J. Biol. Macromol. 2021, 172, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Lira, R.B.; Steinkuhler, J.; Knorr, R.L.; Dimova, R.; Riske, K.A. Posing for a picture: Vesicle immobilization in agarose gel. Sci. Rep. 2016, 6, 25254. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Yazdian, F.; Koulivand, A.; Rahmani, E. Green synthesized polyvinylpyrrolidone/titanium dioxide hydrogel nanocomposite modified with agarose macromolecules for sustained and pH-responsive release of anticancer drug. Int. J. Biol. Macromol. 2023, 240, 124345. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, L.; Wu, J.; Huang, Y.D.; Zhao, L.; Wu, N.; Zhou, W.Q.; Hao, D.X.; Ma, G.H.; Su, Z.G. Fabrication of rigid and macroporous agarose microspheres by pre-cross-linking and surfactant micelles swelling method. Colloids Surf. B-Biointerfaces 2019, 182, 110377. [Google Scholar] [CrossRef]
- Iftekhar, S.; Ovbude, S.T.; Hage, D.S. Kinetic Analysis by Affinity Chromatography. Front. Chem. 2019, 7, 673. [Google Scholar] [CrossRef]
- Behar, G.; Renodon-Corniere, A.; Mouratou, B.; Pecorari, F. Affitins as robust tailored reagents for affinity chromatography purification of antibodies and non-immunoglobulin proteins. J. Chromatogr. A 2016, 1441, 44–51. [Google Scholar] [CrossRef]
- Yin, J.L.; Zheng, H.W.; Lin, H.; Sui, J.X.; Wang, B.C.; Pavase, T.R.; Cao, L.M. Boronic acid-functionalized agarose affinity chromatography for isolation of tropomyosin in fishes. J. Sci. Food Agric. 2019, 99, 6490–6499. [Google Scholar] [CrossRef]
- Fang, Y.M.; Lin, D.Q.; Yao, S.J. Review on biomimetic affinity chromatography with short peptide ligands and its application to protein purification. J. Chromatogr. A 2018, 1571, 1–15. [Google Scholar] [CrossRef]
- Yi, Y.; Shi, K.F.; Ding, S.W.; Hu, J.M.; Zhang, C.; Mei, J.F.; Ying, G.Q. A general strategy for protein affinity-ligand oriented-immobilization and screening for bioactive compounds. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2023, 1218, 123591. [Google Scholar] [CrossRef]
- Bai, Y.L.; Yang, S.T. Production and separation of formate dehydrogenase from Candida boidinii. Enzyme Microb. Technol. 2007, 40, 940–946. [Google Scholar] [CrossRef]
- Zheng, H.W.; Wang, C.Y.; Pavase, T.R.; Xue, C.H. Fabrication of copolymer brushes grafted superporous agarose gels: Towards the ultimate ideal particles for efficient affinity chromatography. Colloids Surf. B-Biointerfaces 2022, 217, 112705. [Google Scholar] [CrossRef] [PubMed]
- Barth, H.G. Size Exclusion Chromatography: A Teaching Aid for Physical Chemistry. J. Chem. Educ. 2018, 95, 1125–1131. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, L.; Huang, Y.D.; Zhu, K.; Che, X.J.; Du, Y.X.; Gao, J.W.; Hao, D.X.; Zhang, R.Y.; Wang, Q.B.; et al. Preparation and structural regulation of macroporous agarose microspheres for highly efficient adsorption of giant biomolecules. Colloid. Polym. Sci. 2022, 300, 691–705. [Google Scholar] [CrossRef]
- Zhao, L.; Che, X.J.; Huang, Y.D.; Zhu, K.; Du, Y.X.; Gao, J.W.; Zhang, R.Y.; Zhang, Y.Q.; Ma, G.H. Regulation on both pore structure and pressure-resistant property of uniform agarose microspheres for high-resolution chromatography. J. Chromatogr. A 2022, 1681, 463461. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, C.; Ma, W.D.; Xiong, Y.; Yao, J.; Yan, G.Q.; Chen, G.; Lu, H.J. A practical approach to enrich intact tryptic N-glycopeptides through size exclusion chromatography and hydrophilicity (SELIC) using an acrylamide-agarose composite gel system. Anal. Chim. Acta 2019, 1058, 107–116. [Google Scholar] [CrossRef]
- Stone, M.C.; Tao, Y.Y.; Carta, G. Protein adsorption and transport in agarose and dextran-grafted agarose media for ion exchange chromatography: Effect of ionic strength and protein characteristics. J. Chromatogr. A 2009, 1216, 4465–4474. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Zhou, S.Y.; Han, L.J.; Zhang, Q.Y.; Pritts, W.A. Impact of linker-drug on ion exchange chromatography separation of antibody-drug conjugates. Mabs 2019, 11, 1113–1121. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Williams, P.; Nigen, M.; Tamayo, V.M.; Doco, T.; Sanchez, C. Fractionation of Acacia seyal gum by ion exchange chromatography. Food Hydrocoll. 2020, 98, 105283. [Google Scholar] [CrossRef]
- Kristl, A.; Luksic, M.; Pompe, M.; Podgornik, A. Effect of Pressure Increase on Macromolecules’ Adsorption in Ion Exchange Chromatography. Anal. Chem. 2020, 92, 4527–4534. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, H.M.; Domanska, A.; Bamford, D.H. Monolithic ion exchange chromatographic methods for virus purification. Virology 2012, 434, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Ding, Y.Y.; Zhang, L.P.; Shi, G.; Sang, X.X.; Ni, C.H. Preparation of surface-modified, micrometer-sized carboxymethyl chitosan drug-loaded microspheres. J. Appl. Polym. Sci. 2018, 135, 45731. [Google Scholar] [CrossRef]
- Ljunglof, A.; Lacki, K.M.; Mueller, J.; Harinarayan, C.; van Reis, R.; Fahrner, R.; Van Alstine, J.M. Ion exchange chromatography of antibody fragments. Biotechnol. Bioeng. 2007, 96, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, A.R.; Paulo, P.M.R.; Prazeres, D.M.F. Scalable purification of single stranded DNA scaffolds for biomanufacturing DNA-origami nanostructures: Exploring anion-exchange and multimodal chromatography. Sep. Purif. Technol. 2022, 298, 121623. [Google Scholar] [CrossRef]
- Barroca-Ferreira, J.; Goncalves, A.M.; Santos, M.F.A.; Santos-Silva, T.; Maia, C.J.; Passarinha, L.A. A chromatographic network for the purification of detergent-solubilized six-transmembrane epithelial antigen of the prostate 1 from Komagataella pastoris mini-bioreactor lysates. J. Chromatogr. A 2022, 1685, 463576. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Sun, Y. Development of poly(methacrylate)-grafted Sepharose FF for cation-exchange chromatography of proteins. J. Chromatogr. A 2020, 1634, 461669. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Cui, Y.; Li, D.; Wang, H.; Ng, T.B. Isolation and Characterization of a Phaseolus vulgaris Trypsin Inhibitor with Antiproliferative Activity on Leukemia and Lymphoma Cells. Molecules 2017, 22, 187. [Google Scholar] [CrossRef]
- Lienqueo, M.E.; Salazar, O.; Henriquez, K.; Calado, C.R.C.; Fonseca, L.P.; Cabral, J.M.S. Prediction of retention time of cutinases tagged with hydrophobic peptides in hydrophobic interaction chromatography. J. Chromatogr. A 2007, 1154, 460–463. [Google Scholar] [CrossRef]
- Hall, T.; Kelly, G.M.; Emery, W.R. Use of mobile phase additives for the elution of bispecific and monoclonal antibodies from phenyl based hydrophobic interaction chromatography resins. J. Chromatogr. B-Anal. Technol. Biomed. Life Sci. 2018, 1096, 20–30. [Google Scholar] [CrossRef]
- Rodler, A.; Ueberbacher, R.; Beyer, B.; Jungbauer, A. Calorimetry for studying the adsorption of proteins in hydrophobic interaction chromatography. Prep. Biochem. Biotechnol. 2019, 49, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Murisier, A.; Verscheure, L.; Sandra, K.; Guillarme, D. Hydrophobic Interaction Chromatography (HIC) for the Characterization of Therapeutic Monoclonal Antibodies and Related Products, Part 2: Practical Considerations. LC GC Eur. 2021, 34, 139–148. [Google Scholar]
- Wang, L.H.; Fu, Q.X.; Yu, J.Y.; Liu, L.F.; Ding, B. Nanoparticle-doped polystyrene/polyacrylonitrile nanofiber membrane with hierarchical structure as promising protein hydrophobic interaction chromatography media. Compos. Commun. 2019, 16, 33–40. [Google Scholar] [CrossRef]
- Ren, K.; Li, Y.; Shi, F.; Wang, X.Y. Separation of lipopolysaccharides containing different fatty acid chains using hydrophobic interaction chromatography. Anal. Methods 2012, 4, 838–843. [Google Scholar] [CrossRef]
- Brandts, P.M.; Middelkoop, C.M.; Gelsema, W.J.; Deligny, C.L. Hydrophobic Interaction Chromatography of Simple Compounds on Alkyl-Agaroses with Different Alkyl Chain Lengths and Chain Densities—Mechanism and Thermodynamics. J. Chromatogr. 1986, 356, 247–259. [Google Scholar] [CrossRef]
- Mehta, A.; Grover, C.; Gupta, R. Purification of lipase from Aspergillus fumigatus using Octyl Sepharose column chromatography and its characterization. J. Basic Microbiol. 2018, 58, 857–866. [Google Scholar] [CrossRef]
- Holkova, I.; Rauova, D.; Mergova, M.; Bezakova, L.; Mikus, P. Purification and Product Characterization of Lipoxygenase from Opium Poppy Cultures (Papaver somniferum L.). Molecules 2019, 24, 4268. [Google Scholar] [CrossRef]
- Ghosh, S.; Saraswathi, A.; Indi, S.S.; Hoti, S.L.; Vasan, H.N. Ag@AgI, Core@Shell Structure in Agarose Matrix as Hybrid: Synthesis, Characterization, and Antimicrobial Activity. Langmuir 2012, 28, 8550–8561. [Google Scholar] [CrossRef]
- Li, M.; Neoh, K.G.; Kang, E.T.; Lau, T.; Chiong, E. Surface Modifi cation of Silicone with Covalently Immobilized and Crosslinked Agarose for Potential Application in the Inhibition of Infection and Omental Wrapping. Adv. Funct. Mater. 2014, 24, 1631–1643. [Google Scholar] [CrossRef]
- Chen, X.Y.; Li, H.J.; Qiao, X.N.; Jiang, T.Z.; Fu, X.; He, Y.; Zhao, X. Agarose oligosaccharide- silver nanoparticle- antimicrobial peptide-composite for wound dressing. Carbohydr. Polym. 2021, 269, 118258. [Google Scholar] [CrossRef]
- Stickler, D.J.; Lear, J.C.; Morris, N.S.; Macleod, S.M.; Downer, A.; Cadd, D.H.; Feast, W.J. Observations on the adherence of Proteus mirabilis onto polymer surfaces. J. Appl. Microbiol. 2006, 100, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Wers, E.; Lefeuvre, B. New hybrid agarose/Cu-Bioglass® biomaterials for antibacterial coatings. Korean J. Chem. Eng. 2017, 34, 2241–2247. [Google Scholar] [CrossRef]
- Li, W.T.; Huang, Z.X.; Cai, R.; Yang, W.; He, H.W.; Wang, Y.J. Rational Design of Ag/ZnO Hybrid Nanoparticles on Sericin/Agarose Composite Film for Enhanced Antimicrobial Applications. Int. J. Mol. Sci. 2021, 22, 105. [Google Scholar] [CrossRef]
- Li, M.; Mitra, D.; Kang, E.T.; Lau, T.; Chiong, E.; Neoh, K.G. Thiol-ol Chemistry for Grafting of Natural Polymers to Form Highly Stable and Efficacious Antibacterial Coatings. ACS Appl. Mater. Interfaces 2017, 9, 1847–1857. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, D.V.; Pozharitskaya, O.N.; Shikov, A.N.; Flisyuk, E.V.; Rusak, A.V.; Makarov, V.G. Rheological Study of Agar Hydrogels for Soft Capsule Shells. Pharm. Chem. J. 2014, 47, 556–558. [Google Scholar] [CrossRef]
- Felfel, R.M.; Gideon-Adeniyi, M.J.; Hossain, K.M.Z.; Roberts, G.A.F.; Grant, D.M. Structural, mechanical and swelling characteristics of 3D scaffolds from chitosan-agarose blends. Carbohyd. Polym. 2019, 204, 59–67. [Google Scholar] [CrossRef]
- Ribba, L.; Garcia, N.L.; D’Accorso, N.; Goyanes, S. Chapter 3—Disadvantages of Starch-Based Materials, Feasible Alternatives in Order to Overcome These Limitations. In Starch-Based Materials in Food Packaging; Villar, M.A., Barbosa, S.E., García, M.A., Castillo, L.A., López, O.V., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 37–76. [Google Scholar]
- Devi, L.S.; Das, A.J.; Das, A.B. Characterization of high amylose starch-microcrystalline cellulose based floatable gel for enhanced gastrointestinal retention and drug delivery. Carbohydr. Polym. Technol. Appl. 2022, 3, 100185. [Google Scholar] [CrossRef]
- Shikov, A.; Pozharitskaya, O.; Makarov, V.; Makarova, M. New Technology for Preparation of Herbal Extracts and Soft Halal Capsules on its Base. Am. -Eurasian J. Sustain. Agric. 2009, 3, 130–134. [Google Scholar]
- Awadhiya, A.; Tyeb, S.; Rathore, K.; Verma, V. Agarose bioplastic-based drug delivery system for surgical and wound dressings. Eng. Life Sci. 2017, 17, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, T.Y.; Wang, Q.B.; Zhang, G.F.; Huo, J.S.; Huang, J.; Wang, L.Y. Fabrication of uniform alginate-agarose microcapsules loading FeSO4 using water-oil-water-oil multiple emulsions system combined with premix membrane emulsification technique. Colloids Surf. A-Physicochem. Eng. Asp. 2016, 498, 128–138. [Google Scholar] [CrossRef]
- Yazdi, M.K.; Taghizadeh, A.; Taghizadeh, M.; Stadler, F.J.; Farokhi, M.; Mottaghitalab, F.; Zarrintaj, P.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; et al. Agarose-based biomaterials for advanced drug delivery. J. Control Release 2020, 326, 523–543. [Google Scholar] [CrossRef]
- Pozharitskaya, O.; Shikov, A.; Demchenko, D.; Flisyuk, E.; Makarov, V. Effect of Plasticizers on Moisture Absorption and Mechanical Properties of Agar Films. Farmatsiya 2017, 66, 18–23. [Google Scholar]
- Haglund, B.O.; Upadrashta, S.M.; Neau, S.H.; Cutrera, M.A. Dissolution Controlled Drug-Release from Agarose Beads. Drug Dev. Ind. Pharm. 1994, 20, 947–959. [Google Scholar] [CrossRef]
- Sakai, S.; Kawabata, K.; Tanaka, S.; Harimoto, N.; Hashimoto, I.; Mu, C.J.; Salmons, B.; Ijima, H.; Kawakami, K. Subsieve-size agarose capsules enclosing ifosfamide-activating cells: A strategy toward chemotherapeutic targeting to tumors. Mol. Cancer Ther. 2005, 4, 1786–1790. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Rossi, F.; Santoro, M.; Casalini, T.; Veglianese, P.; Masi, M.; Perale, G. Characterization and Degradation Behavior of Agar-Carbomer Based Hydrogels for Drug Delivery Applications: Solute Effect. Int. J. Mol. Sci. 2011, 12, 3394–3408. [Google Scholar] [CrossRef]
- Armenia, I.; Ayllon, C.C.; Herrero, B.T.; Bussolari, F.; Alfranca, G.; Grazu, V.; de la Fuente, J.M. Photonic and magnetic materials for on-demand local drug delivery. Adv. Drug Delivery Rev. 2022, 191, 114584. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Chen, H.; Jiang, W.; Zhu, C.; Toufouki, S.; Yao, S. A new deep eutectic solvent-agarose gel with hydroxylated fullerene as electrical “switch” system for drug release. Carbohydr. Polym. 2022, 296, 119939. [Google Scholar] [CrossRef]
- Rajabzadeh-Khosroshahi, M.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Rasekh, B. Chitosan/agarose/graphitic carbon nitride nanocomposite as an efficient pH-sensitive drug delivery system for anticancer curcumin releasing. J. Drug Delivery Sci. Technol. 2022, 74, 103443. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ahmadi, M.; Abdouss, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Hesari, Y. The synthesis and characterization of double nanoemulsion for targeted Co-Delivery of 5-fluorouracil and curcumin using pH-sensitive agarose/chitosan nanocarrier. J. Drug Delivery Sci. Technol. 2022, 70, 102849. [Google Scholar] [CrossRef]
- Samadi, A.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Eufrasio-da-silva, T. Ameliorating quercetin constraints in cancer therapy with pH-responsive agarose-polyvinylpyrrolidone -hydroxyapatite nanocomposite encapsulated in double nanoemulsion. Int. J. Biol. Macromol. 2021, 182, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.J.; Li, S.S.; Li, X.Y.; Wang, X.Y. Smart MXene/agarose hydrogel with photothermal property for controlled drug release. Int. J. Biol. Macromol. 2021, 190, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Wang, Y.M.; Zhang, L.L.; Xu, M.; Zhang, J.F.; Dong, W. Magnetic field-driven drug release from modified iron oxide-integrated polysaccharide hydrogel. Int. J. Biol. Macromol. 2018, 108, 558–567. [Google Scholar] [CrossRef]
- Alizadeh, R.; Zarrintaj, P.; Kamrava, S.K.; Bagher, Z.; Farhadi, M.; Heidari, F.; Komeili, A.; Gutierrez, T.J.; Saeb, M.R. Conductive hydrogels based on agarose/alginate/chitosan for neural disorder therapy. Carbohydr. Polym. 2019, 224, 115161. [Google Scholar] [CrossRef]
- Hasan, M.L.; Padalhin, A.R.; Kim, B.; Lee, B.T. Preparation and evaluation of BCP-CSD-agarose composite microsphere for bone tissue engineering. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2019, 107, 2263–2272. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef]
- Singh, Y.P.; Bhardwaj, N.; Mandal, B.B. Potential of Agarose/Silk Fibroin Blended Hydrogel for in Vitro Cartilage Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 21236–21249. [Google Scholar] [CrossRef]
- Garakani, S.S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2020, 143, 533–545. [Google Scholar] [CrossRef]
- Su, T.; Zhang, M.Y.; Zeng, Q.K.; Pan, W.H.; Huang, Y.J.; Qian, Y.N.; Dong, W.; Qi, X.L.; Shen, J.L. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact. Mater. 2021, 6, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Nagi, G.K.; Corcoran, A.A.; Agrawal, R.; Dubey, M.; Hunt, R.W. Algal polysaccharides for 3D printing: A review. Carbohydr. Polym. 2023, 300, 120267. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Lameirinhas, N.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. A Guide to Polysaccharide-Based Hydrogel Bioinks for 3D Bioprinting Applications. Int. J. Mol. Sci. 2022, 23, 6564. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Piou, M.; Darling, E.; Cormier, D.; Sun, J.; Wan, J.D. Bio-printing cell-laden Matrigel-agarose constructs. J. Biomater. Appl. 2016, 31, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef]
- Ding, A.; Lee, S.J.; Tang, R.; Gasvoda, K.L.; He, F.; Alsberg, E. 4D Cell-Condensate Bioprinting. Small 2022, 18, e2202196. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, R.; Zhang, L.; Cao, X. 4D Printing of Robust Hydrogels Consisted of Agarose Nanofibers and Polyacrylamide. ACS Macro Lett. 2018, 7, 442–446. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Ali, A.A.; El-Fiqi, A. Controlled delivery of therapeutic ions and antibiotic drug of novel alginate-agarose matrix incorporating selenium-modified borosilicate glass designed for chronic wound healing. J. Non-Cryst. Solids 2020, 534, 119889. [Google Scholar] [CrossRef]
- Veisi, H.; Varshosaz, J.; Rostami, M.; Mirian, M. Thermosensitive TMPO-oxidized lignocellulose/cationic agarose hydrogel loaded with deferasirox nanoparticles for photothermal therapy in melanoma. Int. J. Biol. Macromol. 2023, 238, 124126. [Google Scholar] [CrossRef]
- Nie, Z.; Peng, K.L.; Lin, L.Z.; Yang, J.Y.; Cheng, Z.K.; Gan, Q.; Chen, Y.; Feng, C.G. A conductive hydrogel based on nature polymer agar with self-healing ability and stretchability for flexible sensors. Chem. Eng. J. 2023, 454, 139843. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, F.; Xu, X.-W.; Chen, F.-Q.; Weng, H.-F.; Chen, J.; Ru, Y.; Xiao, Q.; Xiao, A.-F. Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review. Mar. Drugs 2023, 21, 299. https://doi.org/10.3390/md21050299
Jiang F, Xu X-W, Chen F-Q, Weng H-F, Chen J, Ru Y, Xiao Q, Xiao A-F. Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review. Marine Drugs. 2023; 21(5):299. https://doi.org/10.3390/md21050299
Chicago/Turabian StyleJiang, Feng, Xin-Wei Xu, Fu-Quan Chen, Hui-Fen Weng, Jun Chen, Yi Ru, Qiong Xiao, and An-Feng Xiao. 2023. "Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review" Marine Drugs 21, no. 5: 299. https://doi.org/10.3390/md21050299
APA StyleJiang, F., Xu, X.-W., Chen, F.-Q., Weng, H.-F., Chen, J., Ru, Y., Xiao, Q., & Xiao, A.-F. (2023). Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review. Marine Drugs, 21(5), 299. https://doi.org/10.3390/md21050299





