Abstract
Sargassum is one of the largest and most diverse genus of brown seaweeds, comprising of around 400 taxonomically accepted species. Many species of this genus have long been a part of human culture with applications as food, feed, and remedies in folk medicine. Apart from their high nutritional value, these seaweeds are also a well-known reservoir of natural antioxidant compounds of great interest, including polyphenols, carotenoids, meroterpenoids, phytosterols, and several others. Such compounds provide a valuable contribution to innovation that can translate, for instance, into the development of new ingredients for preventing product deterioration, particularly in food products, cosmetics or biostimulants to boost crops production and tolerance to abiotic stress. This manuscript revises the chemical composition of Sargassum seaweeds, highlighting their antioxidant secondary metabolites, their mechanism of action, and multiple applications in fields, including agriculture, food, and health.
1. Introduction
Sargassum (family Sargasseae, order Fucales) is a genus of brown algae spanning the ocean basins of the Atlantic, Pacific, and Indian Oceans, inhabiting mostly tropical and subtropical environments where it forms dense submarine forests [1]. In this genus, some species are even holopelagic, and give the name to the Sargasso Sea, bounded only by the currents around the North Atlantic gyre, where species such as Sargassum fluitans and Sargassum natans create extensive floating mats and a unique ecosystem providing habitats, breeding zones, migration pathways, and feeding areas to diverse marine species [2,3]. On the other hand, due to their invasive nature, Sargassum seaweeds may pose a threat to other marine ecosystems. Blooms of these holopelagic species are one of such cases that negatively affect the near-shore seagrass communities, coral colonies, and fauna, ultimately generating socioeconomical problems [2,4]. Sargassum muticum, introduced in the north-east Atlantic along the European coasts in the 1970s, is considered one of the most invasive seaweeds in Europe, capable of forming dense beds, replacing native seaweeds [5], and negatively affecting oyster farming and tourism in regions such as Normandy [6]. In turn, in some countries, such as Spain and Portugal, these seaweeds have been considered by some authors as an addition to the algal flora, rather than a threat [7,8], positively impacting the diversity of motile fauna and constituting a potential source of food for fish and cephalopod species [6].
The Sargassum genus was first discovered in 1820 and currently comprises near 400 taxonomically accepted species of varying morphologies [1,9]. Morphological plasticity is even observed within the same species in response to environmental conditions, seasons, age and reproductive stage [10]. In general, this genus is characterized by a thallus differentiated into a holdfast, and one to several main axis divided into branches containing leaf-like structures, vesicles, and receptacles (reproductive organ) [1] of diverse shapes and lengths. Excepting S. natans and S. fluitans, which reproduce only by fragmentation, Sargassum seaweeds reproduce sexually [11].
Sargassum spp. have been used over the centuries in folk applications [9,10]. For instance, the pharmacological properties of species, such as S. pallidum, S. confusum, S. fusiforme, S. fullvellum, S. henslowianum, S. thunbergii, S. horneri, S. siliquastrum, S. muticum, S. hemiphyllum, S. polycystum, and S. vachellianum are well-recognized in Traditional Chinese Medicine, and their use to treat diseases dates back to nearly 2000 years ago [10]. The most emblematic medicinal application of these seaweeds is, unquestionably, the treatment of thyroid-related disorders such as goiter, mostly due to their high content in iodine. However, Sargassum has been used to treat many other pathologies, including scrofula, oedema, arteriosclerosis, skin diseases, and chronic bronchitis, among others [10].
Owing to their composition in nutrients and bioactive compounds, Sargassum spp. have high potential for human consumption and animal feed, although some caution is necessary since they can accumulate heavy metals if grown in contaminated environments [2]. In China, S. fusiforme is one of the most cultivated and commonly consumed brown algae [12]. In tropical countries, such as the Philippines, Sargassum spp. is sometimes used as a cover for preserving fish freshness, and as a vegetable, fertilizer, or even an insect repellent [9]. Concerning animal feed, in the central Philippines, Sargassum is usually used for direct consumption or processed into animal feed for exportation [13]. S. polycystum and S. thunbergii are traditionally used to artificially feed sea cucumber grown in large-scale operations, and other Sargassum species, such as S. horneri, are being explored as substitutes [14]. Although the literature is still limited, reports on Sargassum benefits in animal feed can already be found, showing evidence that Sargassum wightii can be used to improve milk yield in Sahiwal cows [15] and Sargassum spp. to reduce cholesterol content of shrimp [16].
Similar to other seaweeds, Sargassum spp. have a long history of application in agriculture as well. In Portugal, “Sargaço” has a secular tradition of being used as a natural fertilizer; in Bermuda, these seaweeds are spread around banana trees as a mulch and fertilizer [17]; in the Caribbean, they are commercialized as mulch, and in Martinique and the Dominican Republic, production of Sargassum-based compost is a common practice.
On top of the above-mentioned applications, due to its richness in bioactive secondary compounds, Sargassum biomass is recognized as a valuable resource with high potential for the development of commercially viable products in distinct fields, including healthcare and pharmaceuticals [1]. Among them, phenolic compounds (including phlorotannins), terpenoids, and phytosterols are among the most recognized ones. In seaweeds, they perform vital roles usually working as herbivore deterrents, digestive inhibitors, as antibacterial and antifouling agents, UV protectors among other functions [18], but in the past years, they have been demonstrated to exert numerous bioactive and health promoting activities, specially due to their exceptional antioxidant properties. This work compiles relevant studies that focused on secondary antioxidant metabolites from Sargassum, from their basic chemistry to marketed products.
2. Antioxidant Secondary Metabolites
2.1. Phenolic Compounds
Phenolic compounds consist of monomeric, oligomeric, or polymeric compounds characterized by an aromatic ring linked directly to at least one hydroxyl group (–OH). In general, seaweeds are good sources of such compounds, albeit their phenolic profile is extremely variable according to intrinsic and extrinsic factors. In the particular case of this genus, the variations of the phenolic concentrations may range from 1.1% DW to 12.7% DW [19].
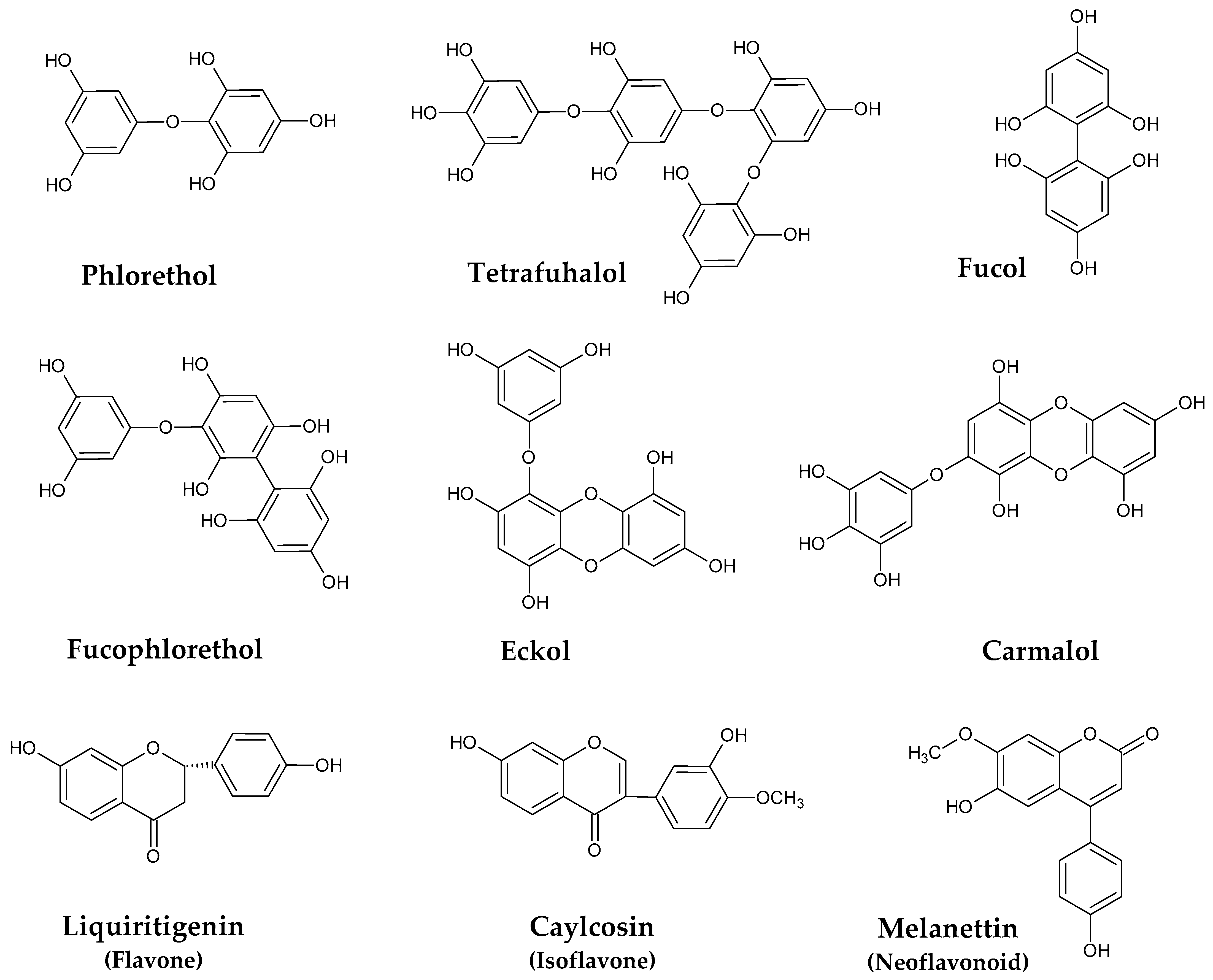
Phlorotannins are a characteristic and the most abundant class of polyphenols found in marine brown seaweeds. They are mainly stored in specialized membrane-bound vesicles named physodes [20], and their biosynthesis occurs via the acetate-malonate pathway. There is a large number of phlorotannins structures in nature, with sizes that can range from the simple monomer phloroglucinol, with 126 Da, to very large and complex polymers of 650 kDa [21]. Moreover, variation also occurs according to the type of interphloroglucinol linkage, and within these circumstances, four sub-classes can be pointed out (Figure 1), including phlorethols and fuhalols (ether linkage), fucols (C-C linkage), fucophlorethols (C-C and ether linkages), and eckols and carmalols (dibenzodoxine linkage) [22,23,24].
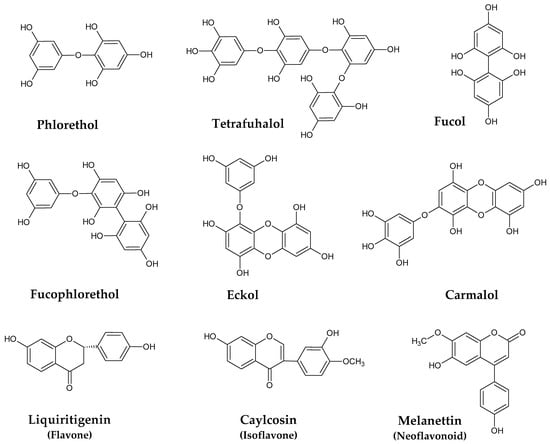
Figure 1.
Examples of phenolic compounds isolated from Sargassum spp.
Phlorotannins have gathered much attention during the last few years due to their numerous health properties, which include, among others, antioxidant, anti-inflammatory, and antitumor activities, and even gut microbiota modulatory effects [25,26,27,28,29]. However, the identification and characterization of these compounds is a very complex task due to the large number of similar features, their large size, the high susceptibility to oxidation, and the lack of commercially available standards [30]. Nevertheless, much effort has been made over the years to understand the chemistry of these compounds in distinct algae, including those belonging to the genus Sargassum. In general, the levels and the phlorotannins constituents found in seaweeds from this genus depend on factors, such as species, size, age and reproductive status, location, depth, nutrient enrichment, salinity, light exposure, ultraviolet radiation, harvest period, and several others [31]. According to Li et al. [23], fuhalol-type phlorotannins were predominant in a purified fraction of S. fusiforme (DP 2–10 monomers), although other relevant compounds ere detected, particularly phlorethols and fucophlorethols, with varying degree of polymerization (DP 2–11 monomers). The same authors also reported newly discovered eckols and carmalol derivatives. Some hydroxyfuhalols (fuhalols with more than one additional hydroxy group), such as hydroxytrifuhalol B, hydroxypentafuhalol A, hydroxyheptafuhalol B, and hydroxynonafuhalol A, were also detected and isolated from S. spinuligerum [32], while the most abundant phlorotannins in S. muticum collected in Norway were found to belong to fuhalols, hydroxyfuhalols, and phlorethols type [33].
Although less known than phlorotannins, other phenolic constituents in Sargassum species have been described as well (Figure 1). In this regard, the isoflavone caylcosin, the flavone liquiritigenin, and distinct neoflavonoids, including melanettin and stevenin, were identified in S. pallidum [34]. Other isoflavones, such as daidzin, genistein, sissotrin, formononetin, and biochanin A, were reported to occur in S. muticum and S. vulgare [35].
2.2. Terpenoids
Terpenoids represent a large and diverse group of natural occurring products derived from terpenes, i.e., organic compounds consisting of five-carbon isoprene units, containing oxygen molecules. Terpenes or terpenoids are grouped as hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), sesterterpenes (C25), triterpenes (C30), and tetraterpenes (C40).
Carotenoids are the most common tetraterpenes in nature. These have red, yellow, or orange colors, and they are essential for photosynthesis, participating also in photoprotection and in cell membrane stabilization in a wider context. Chemically, carotenoids are divided into carotenes, i.e., pure hydrocarbons containing no oxygen, and xanthophylls, i.e., oxygenated carotenes.
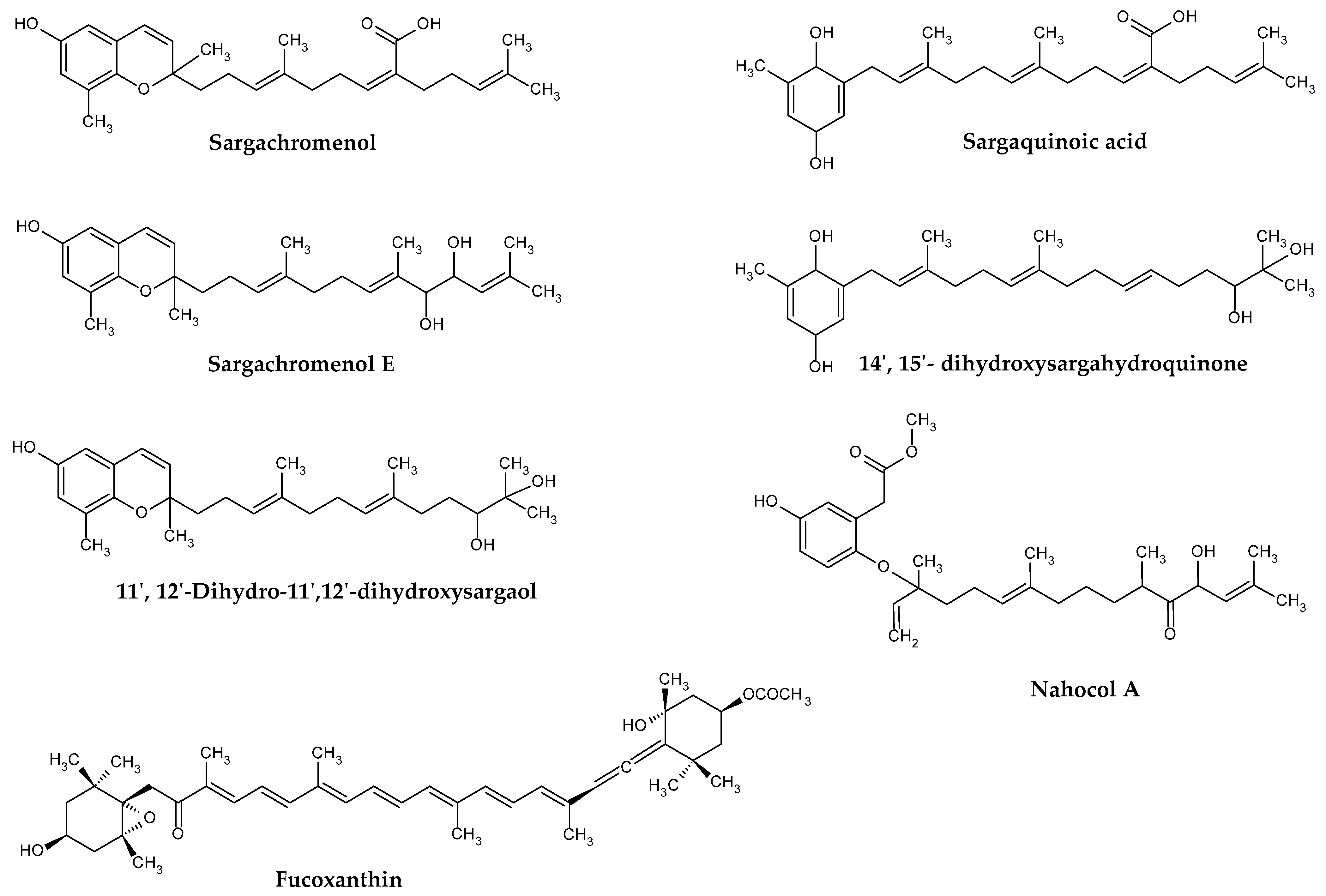
Fucoxanthin (Figure 2) is the major carotenoid produced by brown seaweeds. This orange-colored pigment has a unique chemical structure containing allene bonds, 5,6-monocyclic oxide, and acetylated groups. It occurs in two configurations, designated as trans or cis, although the former is thermodynamically more stable than the latter [36].
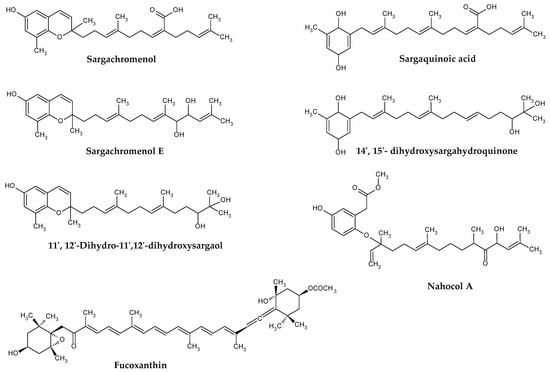
Figure 2.
Examples of terpenoids isolated from Sargassum spp.
The levels of this pigment are greatly variable between species and depend on the environmental conditions. For instance, in S. swartzii, fucoxanthin accounted for 0.17 mg/g dried weight (DW), while concentrations of 0.40 mg/g DW were described in S. vulgare and S. japonica, and of 0.70 mg/g DW in S. angustifolium and S. plagyophyllum [37]. These values are much lower than the concentrations of fucoxanthin described for S. horneri, S. fusiforme, S. thunbergii, and S. confusum (3.7; 1.1; 1.8; and 1.6 mg/g DW, respectively) from Hakodate, Japan [38]. Moreover, the pattern of seasonal variation is not the same in all species, and the content of fucoxanthin in Sargassum varies between species and may be affected by duration of sunshine and seawater temperature. Numura et al. [39] reported concentrations up to 4.49 mg/g DW in S. horneri collected in winter, while concentrations of 2.69 mg/g DW were observed in summer.
Brown algae, including those from Sargassum genus, also synthesize meroditerpenoids. These compounds have a mixed biosynthetic origin, comprising a polyprenyl chain attached to a p-benzoquinone or hydroquinone moiety [29]. The prefix “mero” stands for “part, partial, or fragment”, in Greek terminology, and thus, “meroditerpenoid” are partially derived from diterpenoids. Common precursors of such compounds in algae are geranylgeranyltoluquinol, γ-tocotrienol, and γ-dehydro-tocotrienol.
The first isolated meroterpenoids were γ-tocotrienol, and its 11′,12′-epoxide, from S. tortile in 1975 [40]. After that, many meroterpenoids including plastoquinone, chromanol, and chromene have been isolated from different Sargassum species (Table 1). Importantly, despite Sargassum meroterpenoids exhibit important biological and medicinal properties, no quantifications for these compounds have been reported in the literature so far.

Table 1.
Reported meroterpenoids in Sargassum spp.
2.3. Phytosterols
Phytosterols are fatty compounds produced by plants, and remarkably contribute as the major lipid constituents of biological membranes of plant cells. Sargassum species are considered good sources of phytosterols, such as fucosterol, β-sitosterol, and saringosterol, with low levels of cholesterol. Similar to brown algae in general, fucosterol is the most representative sterol in Sargassum seaweeds (see Table 2). Indeed, fucosterol alone was reported to account for 67% of the total phytosterol content in S. fusiforme [38]. Ergosterol was also found in relevant concentrations in different species, ranging from 4.0 µg/g DW in S. piluliferum to 42.8 µg/g DW in S. fusiforme. Moreover, according to Ito et al., seaweeds from the species S. horneri collected in different seasons and different locations were found to display significantly different concentrations of phytosterols (12.3–32.3 mg/g DW), demonstrating that these compounds are strongly affected by spatial and seasonal variations [65].

Table 2.
Examples of phytosterols identified from Sargassum spp.
3. Bioactive Potential of Sargassum Antioxidant Secondary Metabolites
Oxidation performs a fundamental role in our everyday lives, taking place, for instance, in cell metabolic processes and in food systems. The oxidative metabolism is essential for the survival of cells, but, on the downside, it is responsible of the production of free radicals and other reactive species which, in abnormal conditions, can cause destructive effects by oxidizing membrane lipids, cellular proteins, DNA and enzymes, and even cause disease [70]. In foods, oxidation is the major chemical deterioration, contributing for their rancidification, appearance of off-flavors, unpleasant texture or -color, loss of nutritional quality, and even compromise safety [71]. In this context, natural antioxidants have gathered much interest since they can be used as replacers of the synthetic ones that are used in the food industries and have been linked to multiple health benefits, offering protection against free radicals and oxidative stress in cells, radical-induced tissue injuries, and retardation of the onset and progress of chronic diseases.
3.1. In Chemico Studies
Distinct authors have proven that seaweeds from the genus Sargassum have high potential to serve as bio-source of antioxidant compounds. To measure their antioxidant potential, a primary screening is usually carried out using different chemical methods including, among others, radical scavenging activity, protection against lipid peroxidation, metal-ion chelating ability, and reducing capacity, which allow the evaluation of the compounds’ mechanistic intervention, concentration effectiveness, and synergistic effects. A joint summary of the antioxidant activity of Sargassum spp. extracts and isolated compounds is compiled in Table 3.
Radical scavenging is one of the most typical mechanisms of antioxidant activity and can be tested via a wide variety of in chemico methods. DPPH and ABTS+ radicals are the most commonly used due to their stability, reproducibility, and simplicity. However, although they are useful for an initial screening of the extracts’/compounds’ antioxidant activity, they have low biological relevance since they are synthetic and do not occur in biological systems [68]. For that, radicals, such as NO•, O2•− or HO•, are a more suitable approach since these are biologically produced during normal cellular activities [72].
Reducing power is another common method that has been used as a comparative tool among foods and algae. It essentially measures the antioxidant activity of a compound through its ability to stabilize radicals by donating electrons, usually involving the reduction potential of transition metals, such as iron (Ferric Ion Reducing Antioxidant Power, FRAP) or copper (Cupric Reducing Antioxidant Capacity, CUPRAC) [73].
Free radicals can also originate from heavy and transition metals, namely mercury, lead, arsenic, and iron, leading to diseases associated with oxidative stress. Therefore, another mechanism of estimating the antioxidant activity of Sargassum compounds is via their capacity to chelate transition metals, forming complex structures which decrease the metal reactivity and eases their excretion from the body.
Other systems, such as β-carotene bleaching or lipid peroxidation, have been reported on compounds extracted from Sargassum seaweeds, all of them employed for chemically screen their antioxidant activity of compounds at certain conditions.
Clearly, phenolic compounds, in particular phlorotannins, appear as the major and most well studied group of compounds contributing to the antioxidant properties of Sargassum seaweeds. Indeed, positive correlations between phenolic content and antioxidant activity have been reported in many studies using whole algae, their parts, extracts, or fractions [74,75,76,77]. Notably, studies on phlorotannins isolated from Sargassum have shown that compounds, such as triphlorethol B, tetraphlorethol C, and pentaphlorethol A, can be more effective than certain antioxidant references such as ascorbic acid or resveratrol at preventing lipid peroxidation or scavenging of O2•− radicals [78]. The phenolics group is not, however, the solo contributor for the antioxidant properties of Sargassum seaweeds.
Other molecules, such as pigments have been described for their strong radical scavenging and reducing power properties. This is the case of the pigment-rich extracts retrieved from S. cristaefolium, which were shown to exert promising dose-dependent antioxidant properties via DPPH and FRAP assays [79]. Likewise, a positive correlation between the antioxidant activity and the carotenoid content of methanol and ethanol extracts from S. siliquosum and S. polycystum was described on ABTS+•, DPPH• and FRAP [80]. More recently, a carotenoid-rich extract obtained from S. polycystum was found to be the most active on DPPH• and ORAC assays compared with those obtained from two other seaweeds, including Euchema denticulatum (red) and Caulerpa lentillifera (green), most likely due to its high content in fucoxanthin [81]. In fact, within the group of carotenoids, fucoxanthin clearly stands out as one of the most prominent compounds. Due to its structural features, specifically the presence of an allenic bond and a 5,6-monoepoxide motif, fucoxanthin has a strong proton donating capacity that translates into an exceptional antioxidant activity [82]. Indeed, several authors have described strong antioxidant activities in multiple chemical assays (e.g., DPPH•, ABTS+•, and FRAP) for different Sargassum spp.-derived extracts that contained significant concentrations of fucoxanthin [82,83,84]. Moreover, Raji et al. [85] reported that fucoxanthin isolated from S. wightii exhibited great radical scavenging properties with IC50 values if 79.55 µM and 75.99 µM in DPPH• and ABTS+• assays.
Apart from carotenoids, meroterpenoids have also been described as relevant contributors for the antioxidant properties of the Sargassum seaweeds. Among them, sargachromenol (SCM), sargaquinoic acid (SQA), and sargahydroquinoic acid (SHQA) stand out the most, with several authors reporting promising antioxidant activity measured via ABTS+•, DPPH•, FRAP, OH•, and O2•− assays for extracts containing these compounds [52,56]. In fact, after isolating SCM and SQA from S. micracanthum, Ham and coworkers observed that these two meroterpenoids displayed strong DPPH•− scavenging activities, with the latter being slightly better than the former (49.3 versus 100.2 µM) [86]. Concordantly, the scavenging effects of SCM, SQA, and SHQA isolated from S. serratifolium on DPPH• (IC50 = 8.0, 15.3 and 5.9 µg/mL, respectively) and OH• (IC50 = 0.26, 0.27 and 0.27 µg/mL, respectively) were found promising and even superior to that of the commercial antioxidant reference butyl hydroxytoluene (IC50 = 40.4 and 0.9 µg/mL, for DPPH• and OH•, respectively) [56]. Likewise, upon isolation from S. thunbergii, Seo et al. noticed that these three compounds were as good peroxynitrite (ONOO-) scavengers as L-ascorbic acid or penicillamine [87].
Derivatives of these compounds were shown to exert excellent antioxidant properties as well. According to Jang et al., sixteen different sargachromanols isolated from S. siliquastrum exhibited significant radical scavenging activity in the range of 87–91% at the concentration of 100 µg/mL [88]. Moreover, mojabanchromanol, isolated from the same species, showed equal or even better results than BHT, L-ascorbic acid, or α-tocopherol on TBARS and DPPH• assays [89]. Identical observations were described for thumbergol A and B, isolated from S. thunbergii, which were also as effective as BHT, L-ascorbic acid and α-tocopherol at scavenging DPPH•, ONOO−, and O2•− [60]. In turn, superior antioxidant activities comparing with L-ascorbic acid and α-tocopherol were described for other chromene derivatives retrieved from S. micracanthum, including 2-geranylgeranyl-6-methylbenzoquinone and its hydroquinone derivative, performing 28 to 300 times better inhibitory properties on lipid peroxidation than the referred standard compounds [50]. Two other derivatives from 2-geranylgeranyl-6-methyl-1,4-benzohydroquinone isolated from the same species were also found very active against lipid peroxidation, showing an inhibitory effect 40 times stronger than that of α-tocopherol [51].
Apart from these compounds, there are other less studied molecules that can still can contribute importantly for the antioxidant activity of Sargassum seaweeds. This is the case of (+)-epiloliolide, which was isolated from S. naozhouense and, despite not exerting as good DPPH• scavenging activity as ascorbic acid, it displayed a quite interesting IC50 value of 17 mM [54]. In turn, four terpenoids isolated from S. wightii, including 2α-hydroxy-(28,29)-frido-olean-12(13),21(22)-dien-20-propyl-21-hex-40′(Z)-enoate, 2α-hydroxy-(28,29)-frido-olean-12(13),21(22)-dien-20-prop-2(E)-en-21-butanoate, 2α-hydroxy-8(17),12E,14-labdatriene, and 3β,6β,13α-trihydroxy-8(17),12E,14-labdatriene exhibited DPPH• and ABTS+• scavenging effects comparable with that of BHT, and significantly better activities in the range of approximately 2–5 times comparing with α-tocopherol [64].
However, one should bear in mind that, for complex samples such as crude extracts, the antioxidant activities are a result of the interactions occurring between multiple compounds that can either lead to synergistic or antagonistic effects. Therefore, the presence of certain compounds individually recognized as antioxidants in a given sample may not translate into the expected antioxidant properties.

Table 3.
Selected in vitro antioxidant activity tests in non-cellular systems of extracts and purified compounds from Sargassum species reported during the last five years.
Table 3.
Selected in vitro antioxidant activity tests in non-cellular systems of extracts and purified compounds from Sargassum species reported during the last five years.
| Sargassum spp. Extracts and Compounds | ||||
|---|---|---|---|---|
| Sargassum spp. | Extraction Conditions | Bioactive Compounds | In Chemico Antioxidant Properties | Ref. |
| S. acinarium | 80% MeOH; 80% EtOH; 80% Act; H2O | Alkaloids; phenolics, steroids, terpenoids | TAA MeOH; EtOH; Act; H2O: 2.1; 2.8; 1.7; 0.7 mg AA/g DW; RP MeOH; EtOH; Act; H2O: 0.4; 1.3; 0.4; 0.5 mg AA/g DW | [76] |
| S. angustifolium | EtOH; 50% EtOH; H2O | Cardiac glycosides, saponins, steroids, flavonoids | DPPH● EtOH; 50% EtOH; H2O: 1/EC50 = 14.3; 10.0; 0.7 mg/mL; FICA EtOH; 50% EtOH; H2O (% at 1 mg/mL): 15.6; 19.7; 88.3%; RP EtOH; 50% EtOH; H2O (OD 700 nm at 5 mg/mL): 1.0; 0.4; 0.1; TBARS EtOH; 50% EtOH; H2O (% at 1 mg/mL): 68.9; 23.3; 4.9% | [90] |
| S. aquifolium | EtOH; 50% EtOH; H2O | Cardiac glycosides, saponins, steroids, flavonoids | DPPH● EtOH; 50% EtOH; H2O: 1/EC50 = 2.9; 20.0; 0.7 mg/mL; FICA EtOH; 50% EtOH; H2O (% at 1 mg/mL): 7.3; 20.1; 52.0%; RP EtOH; 50% EtOH; H2O (OD 700 nm at 5 mg/mL): 0.4; 0.5; 0.7; TBARS EtOH; 50% EtOH; H2O (% at 1 mg/mL): 29.3; 10.3; 8.9% | [90] |
| EtOH | Phenolics | DPPH●: IC50 = 828.2 µg/mL | [77] | |
| S. asperifolium | EtOH; 50% EtOH; H2O | Cardiac glycosides, flavonoids, saponins, steroids, condensed tannins | DPPH● EtOH; 50% EtOH; H2O: 1/EC50 = 13.3; 40.0; 0.7 mg/mL; FICA EtOH; 50% EtOH; H2O (% at 1 mg/mL): 4.3; 10.9; 42.9%; RP EtOH; 50% EtOH; H2O (OD 700 nm at 5 mg/mL): 1.0; 0.7; 0.2; TBARS EtOH; 50% EtOH; H2O (% at 1 mg/mL): 53.7; 11.3; 3.3% | [90] |
| S. boveanum | EtOH; 50% EtOH; H2O | Cardiac glycosides, alkaloids, saponins, steroids, flavonoids | DPPH● EtOH; 50% EtOH; H2O: 1/EC50 = 100.0; 20.0; 1.2 mg/mL; FICA EtOH; 50% EtOH; H2O (% at 1 mg/mL): 3.9; 23.4; 78.8%; RP EtOH; 50% EtOH; H2O (OD 700 nm at 5 mg/mL): 1.5; 1.1; 0.6; TBARS EtOH; 50% EtOH; H2O (% at 1 mg/mL): 58.9; 20.1; 1.3% | [90] |
| Ext: MeOH; Fract: DCM → EtOAc → BuOH → H2O | Phenolics | DPPH● MeOH; DCM; EtOAc; BuOH; H2O: EC50 = 1091.7; 245.9; 171.4; 779.9; 1987.1 ppm; ABTS●+ MeOH; DCM; EtOAc; BuOH; H2O: EC50 = 1204.6; 247.6; 219.5;487.9; 1556.4 ppm; FRAP MeOH; DCM; EtOAc; BuOH; H2O: EC50 = 535.8; 157.9; 129.2; 243.9; 1381.7 ppm | [91] | |
| S. cinctum | H2O; MeOH | Phenolics | TAA H2O; MeOH: 38.3; 21.9 mg AA/g DW; DPPH● H2O; MeOH: IC50 = 1.1; 1.2 mg/mL; FRAP H2O; MeOH: 12.3; 3.7 mg AA/g DW | [91] |
| S. coriifolium | MeOH; EtOH; H2O | Terpenoids; saponins; phlorotannins; cardiac glycosides; flavonoids; phenols | DPPH● MeOH; EtOH; H2O: IC50 = 1.0; 1.4; 2.7 mg/mL; ABTS●+ MeOH; EtOH; H2O: IC50 = 1.6; 2.2; 3.7 mg/mL; H2O2 MeOH; EtOH; H2O: IC50 = 2.0; 2.6; 4.7 mg/mL | [92] |
| S. crassifolium | EtOH | Phenolics | DPPH●: IC50 = 767.0 µg/mL | [75] |
| S. cristaefolium | EtOH | Phenolics | DPPH●: IC50 = 737.3 µg/mL | [77] |
| Ext: CHCl3:MeOH:H2O (1:2:0.8) Fract: DCM → Act → MeOH | Fucoxanthin, porphyrin derivatives, galactosyldiacylglycerols | TAA Ext; DCM; Act; MeOH: 39.2; 46.2; 66.1; 7.8 ìmol TE/g DPPH● Ext; DCM; Act; MeOH (at 0.5 mg/mL): 41.3; 48.6; 67.2; 11.9% FRAP Ext; DCM; Act; MeOH: 688.1; 368.1; 679.2; 132.1 ìmol FE/g | [79] | |
| S. duplicatum | EtOH; MeOH; EtOAc | Fucoxanthin | DPPH● EtOH; MeOH; EtOAc: IC50 = 93.8; 78.5; 112.3 ìg/mL | [83] |
| S. furcatum | DCM:MeOH (2:1) | Phenolics | DPPH●: EC50 = 0.5 mg/L; ABTS●+: EC50 = 0.3 mg/L | [93] |
| S. horneri | 70% MeOH | Phenolics | DPPH●: EC50 = 06 mg/mL; H2O2: EC50 = 83.9 mg/mL; O2●-: EC50 = 0.5 mg/mL; OH●: EC50 = 1.4 mg/mL; RP: EC50 = 0.2 mg/mL; FICA: 0.4 mg/mL | [94] |
| MeOH | Fucoxanthin, phenolics | DPPH● (at 2 mg/mL): 46.5%; RP (OD 700 nm at 2 mg/mL): 0.8 | [95] | |
| Ext: 80% MeOH Fract: CHCl3 | Phenolics, polysaccharides, sterols, Apo-9 fucoxanthinone | DPPH●Ext; CHCl3: IC50 = 1.1; 2.7 mg/mL; Alkyl | [96] | |
| S. linearifolium | 80% MeOH → hot H2O | Phenolics, alkaloids, terpenoids, sterols | DPPH● hot H2O: IC50 = 124.5 µg/mL; ABTS●+ hot H2O: IC50 = 257.1 µg/mL | [97] |
| 70% EtOH | Fucoxanthin, Phenolics | ABTS●+ (at 1 mg/mL): 34.5 mg TE/g extract; DPPH● (at 1 mg/mL): 15.6 mg TE/g DW; FRAP (at 1 mg/mL): 12.5 mg TE/g DW | [84] | |
| S. miyabei | 70% EtOH | Phenolics | DPPH● (% at 0.1 mg/mL): 42.8%; OH● (% at 0.1 mg/mL): 43.8%; LP (% at 0.1 mg/mL): 52.4% | [98] |
| 70% EtOH | SHQA, SCM, Phenolics | ABTS●+: 186.2 mg VCE/g; DPPH●: 193.7 mg VCE/g; FRAP: 1.0 mM FE/g | [52] | |
| S. muticum | 80% MeOH; 80% EtOH; 80% Act; H2O | Alkaloids; phenolics, steroids, terpenoids | TAA MeOH; EtOH; Act; H2O: 0.9; 3.1; 2.3; 0.7 mg AA/g DW; RP MeOH; EtOH; Act; H2O: 0.2; 1.4; 1.0; 0.7 mg AA/g DW | [76] |
| EtOH; Act; EtOAc; CHCl3; Hex | Phenolics | DPPH● EtOH; Act; EtOAc; CHCl3; Hex: 4.0; 5.4; 16.2; 6.1;4.7 mg TE/g DW; FRAP EtOH; Act; EtOAc; CHCl3; Hex: 4.2; 17.5; 18.3; 6.0; 6.2 mg AA/g DW | [99] | |
| CHCl3:MeOH (1:1); SC CO2 | Glycolipids | DPPH● CHCl3:MeOH; SC CO2: EC50 = 4.1; 0.9 mg/mL | [100] | |
| CHCl3:MeOH (1:1); SC CO2 | Phospholipids | DPPH● CHCl3:MeOH; SC CO2: EC50 = 4.8; 1.0 mg/mL | [100] | |
| 50% MeOH | Phenolics | DPPH●: IC50 = 1.64 mg/mL | [101] | |
| EAE-PLE 4h with alcalase or vicozyme | Phenolics; phlorotannins | TEAC alcalase; viscozyme: 0.5; 0.6 mmol TE/g | [102] | |
| S. naozhouense | 75% EtOH → Column chromatography | (+)-epiloliolide | DPPH●: IC50 = 17 mM | [54] |
| S. oligocystum | EtOH; 50% EtOH; H2O | Cardiac glycosides, alkaloids, saponins, steroids, flavonoids, condensed tannins | DPPH● EtOH; 50% EtOH; H2O: 1/EC50 = 12.5; 12.5; 5.0 mg/mL; FICA EtOH; 50% EtOH; H2O (% at 1 mg/mL): 7.3; 20.1; 52.0% RP EtOH; 50% EtOH; H2O (OD 700 nm at 5 mg/mL): 1.1; 0.6; 0.5 TBARS EtOH; 50% EtOH; H2O (% at 1bmg/mL): 59.3; 22.0; 2.7% | [54] |
| S. podocanthum | 70% EtOH | Fucoxanthin, Phenolics | ABTS●+ (at 1 mg/mL): 147.1 mg TE/g extract; DPPH● (at 1 mg/mL): 136.6 mg TE/g DW; FRAP (at 1 mg/mL): 29.6 mg TE/g DW | [84] |
| S. polycystum | CHCl3; Hex; MeOH; Act; 70% EtOH | Steroids, phenolics, saponins, terpenoids | TAA CHCl3; Hex; MeOH; Act; 70% EtOH (at 1.25 mg/mL): 68.0; 61.4; 121.0; 46.0; 120.0 mmol AA/g DW | [103] |
| EtOH | Phenolics | DPPH●: IC50 = 804.3 µg/mL | [77] | |
| H2O; MeOH | Phenolics | TAA H2O; MeOH: 43.3; 36.1 mg AA/g DW; DPPH● H2O; MeOH: IC50 = 1.0; 1.2 mg/mL; FRAP H2O; MeOH: 11.6; 3.0 mg AA/g DW | [104] | |
| EtOH | Carotenoids | DPPH● (at 1 mg/mL): 20.4%; ORAC: 42.1 mmol TE/100 g | [81] | |
| S. serrifolium | 70% EtOH | SHQA, SCM, Phenolics | ABTS●+: 99.5 mg VCE/g; DPPH●: 75.4 mg VCE/g; FRAP: 0.3 mM FE/g | [52] |
| 70% EtOH → HPLC purification | SHQA, SCM, SQA | ABTS●+ SHQA, SCM, SQA: IC50 = 13.8; 13.1; 47.3 µg/mL; DPPH● SHQA, SCM, SQA: IC50 = 5.9; 8.0; 15.3 µg/mL; O2●- SHQA, SCM, SQA: IC50 = 16.9; 14.5; 20.5 µg/mL; ROS: IC50 = 0.2; 0.2; 0.3 µg/mL | [56] | |
| S. tenerrium | H2O; MeOH | Phenolics | TAA H2O; MeOH: 52.0; 40.1 mg AA/g DW; DPPH● H2O; MeOH: IC50 = 0.8; 0.8 mg/mL; FRAP H2O; MeOH: 13.4; 4.0 mg AA/g DW | [104] |
| S. thunbergii | 70% EtOH | Phenolics | DPPH● (% at 0.1 mg/mL): 39.3 %; OH● (% at 0.1 mg/mL): 34.3 %; LP (% at 0.1 mg/mL): 31.0% | [98] |
| MeOH | Fucoxanthin, phenolics | DPPH● (at 2 mg/mL): 90.4%; RP (OD 700 nm at 2 mg/mL): 0.5 | [95] | |
| S. vestitum | 70% EtOH | Phenolics | ABTS●+: 40.3 mg TE/g DW; DPPH●: 111.8 mg TE/g DW; FRAP: 46.2 mg TE/g DW | [105] |
| MAE 70% EtOH | Phenolics | ABTS●+: 149.8 mg TE/g DW; DPPH●: 116.5 mg TE/g DW; FRAP: 68.0 mg TE/g DW | [105] | |
| UAE 70% EtOH | Phenolics | ABTS●+: 147.2 mg TE/g DW; DPPH●: 86.1 mg TE/g DW; FRAP: 60.1 mg TE/g DW | [105] | |
| 70% EtOH | Fucoxanthin, Phenolics | ABTS●+ (at 1 mg/mL): 183.4 mg TE/g extract; DPPH● (at 1 mg/mL): 209.5 mg TE/g DW; FRAP (at 1 mg/mL): 283.7 mg TE/g DW | [84] | |
| S. vulgare | Act | Phenolics | DPPH●: IC50 = 0.8 mg/mL; O2●-: IC50 = 1.0 mg/mL; RP (OD 700 nm at 1 mg/mL): 0.3 | [106] |
| MeOH; H2O; hot H2O | Phenolics | ABTS●+ MeOH; H2O; hot H2O: EC50 = 165.8; 73.3; 46.5 µg/mL; β-CBT MeOH; H2O; hot H2O: EC50 = 18.2; 142.7; 66.5 µg/mL; FRAP: 216.4; 102.1; 198.3µg TE/mL | [107] | |
| Ext: 70% Act Fract: Hex → EtOAc → H2O | Phlorotannins | ABTS●+Ext; Hex; EtOAc; H2O: IC50 = 72.9; 93.8; 25.1; 74.9 µg/mL; DPPH● Ext; Hex; EtOAc; H2O: IC50 = 97.4; 29.8; 25.8; 96.6 µg/mL O2●- Hex; EtOAc: IC50 = 37.1; 27.0 µg/mL; β-CBT Ext; Hex; EtOAc: IC50 = 65.2; 41.3; 72.1 µg/mL; | [108] | |
| S. wightii | SFE CO2:EtOH (94:6); EtOH; 60% EtOH; 40% EtOH; H2O | Phenolics, protein | TAA SFE; EtOH; 60% EtOH; 40% EtOH; H2O: 67.9; 21.6; 23.0; 31.1; 33.3 mg AA/g extract; FRAP SFE; EtOH; 60% EtOH; 40% EtOH; H2O: 57.3; 45.4; 55.7; 62.3; 68.7 mg AA/g extract | [109] |
| Hex → EtOAc → MeOH | Phlorotannins | DPPH● EtOAc: IC50 = 59.9 µg/mL; ABTS●+ EtOAc: IC50 = 51.0 µg/mL; FRAP EtOAc: 55.2 µg/mL | [110] | |
| EtOAc → Column chromatography → TLC | Fucoxanthin | DPPH●: IC50 = 79.6 µM; ABTS●+: IC50 = 76.0 µM | [85] | |
Act—Acetone; MeOH—Methanol; EtOH—Ethanol; EtOAc—Ethyl acetate; Hex—Hexane; DCM—Dichloromethane; SFE—Supercritical fluid extraction; MAE—Microwave assisted extraction; UAE—Ultrasound assisted extraction; β-CBT—β-Carotene bleaching test; FICA—Ferrous ion chelating activity; TAA—Total antioxidant activity; RP—Reducing power; LP—Lipid peroxidation; TE—Trolox equivalents; FE: Ferrous equivalent; DW—Dry weight; OD—Optical density, SHQA—Sargahydroquionic acid; SCM—Sargachromenol; SQA—Sargaquionicacid.
3.2. Cellular and In Vivo Studies
Following in chemico screening experiments, the use of biological models is essential to further corroborate the antioxidant activity of a compound and elucidate its mechanisms of action. The screening of the antioxidant activity of Sargassum spp. secondary metabolites in cell and animal models has thus been reported by a few authors. In general, oxidative stress inductors, such as H2O2, carbon tetrachloride (CCl4), tert-butyl hydroperoxide (t-BHP), and 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH), are applied, while the protective effects of secondary metabolites are estimated through monitorization of oxidative stress markers, such as the levels of intercellular reactive oxygen species (ROS), lipid peroxidation, enzymes, and transcription factors involved in redox homeostasis. Literature data on the antioxidant effects of secondary metabolites from Sargassum origin using cellular and in vivo models is summarized in Table 4 and Table 5, respectively.
3.2.1. Oxidative Stress Protective Effects
As in chemical studies, distinct authors have emphasized the oxidative stress-protective abilities of Sargassum spp. phenolic-rich extracts in biological systems., e.g., Pinteus et al. [111] demonstrated that two purified fractions obtained from the S. muticum extract (rich in phenolics, including phlorotannins), namely the MeOH fraction and 50:50 MeOH:DCM fraction, were able to reduce the H2O2—induced elevation of intracellular ROS in MCF-7 cells by 70% and 56%, respectively. Likewise, phenolic compounds together with sulfated polysaccharides were reported as the major contributors to the antioxidant activity of a S. polycystum Celluclast-assisted extract, which was demonstrated to significantly decrease the cell death brought on by H2O2-induced oxidative stress in zebrafish embryos to normal levels [112].
In a different in vivo model, a polyphenol-rich extract of S. pallidum containing mainly phlorotannins was reported to decrease lipid peroxidation by 37%, increase the levels of the antioxidant enzyme superoxide dismutase (SOD) levels by 47%, and restore GSH to the control levels in CCl4-induced oxidative stress in Wistar rats [113]. Following an identical behavior, a non-identified polyphenol-rich extract of Sargassum spp. led to an increase in GPx levels (8.14 ± 4.49 nmol/g) in the liver of seaweed-treated animals when compared to the non-treated CCl4-induced Wistar rats (9.88 ± 1.07 nmol/g) [113,114]. Furthermore, after administrating a phlorotannins-rich extract from S. hemiphyllum to CCl4-exposed Kunming mice, Zhao et al. [115] observed a statistically significant increase in SOD, catalase (CAT), glutathione peroxidase (GPx), and total antioxidant capacity (TAOC) in the serum, kidneys, liver, and brain (p < 0.05), which was accompanied by a significant decrease in lipid peroxidation in their livers (p < 0.05).
Terpenoids constitute another important group of compounds of Sargassum origin that have shown important antioxidant properties in cell and animal models. Among these, the carotenoid fucoxanthin, isolated from a methanolic extract of S. siliquastrum, was proven to significantly decrease ROS production in H2O2-stimulated Vero cells at 5 µM (p < 0.05), 50 µM and 100 µM (p < 0.01) [116]. Other terpenoids, such as meroterpenoids, extracted or isolated from several Sargassum spp., also demonstrated interesting protective effects on oxidative stress induction. Indeed, after treating t-BHP-stimulated HepG2 cells with a meroterpenoid-rich extract of S. serratifolium (mainly containing SHQA, SCM, and SQA), Lim et al. [57] showed a statistically significant ROS and lipid peroxidation reduction in a dose-dependent manner (p < 0.05). In addition, sargachromanols D, E, and K, and three other chromanols isolated from S. siliquastrum at 5 µg/mL, significantly restored GSH in H2O2-stimulated HT1080 cells [59].
A similar trend was described for (−)-loliolide, a different type of monoterpenoid, which was able to significantly reduce ROS levels (p < 0.01) on AAPH-stimulated Vero cells and zebrafish embryos at concentrations of 12.5 and 25 µg/mL. Interesting inhibition of lipid peroxidation was observed in a different study using zebrafish embryos as well [44]. Notably, an indole derivative, namely indole-6-carboxaldehyde, isolated from S. thunbergii, was found interfere strongly with the cellular antioxidant defenses of V79-4 cells, triggering a significant increase in the expression heme oxygenase-1 (HO-1), and most importantly, in nuclear factor-erythroid 2-related factor 2 (Nrf2), an important transcription factor that regulates the expression of intracellular antioxidant proteins [62].
3.2.2. Effects on Oxidative-Stress Related Disorders
Multiple diseases are frequently linked to oxidative stress. Considering this, many in vitro and in vivo experiments of Sargassum spp. revealed a variety of protective properties against diseases linked to the antioxidant activity associated with the secondary metabolites present in this brown seaweed.
UV irradiation leads to increased inflammation and skin damage through the induction of ROS. In UV-B irradiated HaCaT cells, i.e., immortalized aneuploid keratinocytes from adult human skin, extracts and/or compounds from different Sargassum species have been shown to decrease ROS production, lipid peroxidation, and increase antioxidant defense through induction of SOD and CAT activity. Accordingly, Piao et al. [117] and Han et al. [118] demonstrated that ethanolic and methanolic extracts from S. muticum and S. horneri, respectively, triggered a significant increase in SOD and CAT activity (p < 0.05), and a decrease in ROS and lipid peroxidation in UV-B-exposed HaCaT cells at all tested concentrations (S. muticum—12.5 µg/mL, 25 µg/mL, 50 µg/mL, 100 µg/mL, and S. horneri—31.6 µg/mL, 62.5 µg/mL, and 125 μg/mL, respectively). The authors also found a good correlation between their results observed and the high percentage of polyphenols commonly present in the extracts. S. horneri extract contained 7.45 ± 0.29% of total phenolics and 0.29 ± 0.07% of total flavonoids. In a different model using UV-irradiated zebrafish embryos, a polyphenol-rich ethanolic extract from S. thunbergii, demonstrated a decrease (at 0.8 µg/mL, 1.2 µg/mL, and 1.6 μg/mL concentrations, respectively) in ROS production by 71%, 71%, and 85%, respectively [119]. Fucoxanthin and the meroterpenoid tetraprenyltoluquinol chromane, isolated from S. siliquastrum and S. muticum, respectively, showed a similar trend in human dermal fibroblasts exposed to the same radiation [53,120]. Other terpenoids, such as (−)-loliolide and sargachromanol (SC) E, isolated from S. horneri, caused a reduction in ROS production induced by UV irradiation, in HaCaT cells and human dermal fibroblasts, respectively. Thus, (−)-loliolide only caused a significant inhibition at 12.5 µg/mL and 25 μg/mL, while SC E in all concentrations tested (5 µg/mL, 10 µg/mL, and 20 µmol/L) [45,47].
The effects of two S. virgatum extracts with high contents of polyphenols on spermatogenesis and infertility of male Wistar rats exposed to γ-irradiation were shown to increase the antioxidant enzymes SOD, CAT, GPx, and GSH at 100 mg/kg body weight (p < 0.05). At 400 mg/kg, the ethanolic extract displayed the best effect in the increased antioxidant enzymes: 86.38% for SOD, 80.87% for GPx, 90.04% for CAT, and 81.55% for GSH compared with the controls [121]. In a different approach, fucoxanthin, isolated from S. glaucescens, also stimulated these enzymes’ activity in plasma and testicles from Syrian hamsters, which were lowered by cisplatin exposure, a chemotherapeutic agent that is usually associated with infertility [122].
Accumulation of particulate matter in the lungs is another common cause that triggers oxidative stress and can lead to serious tissue damage and cardiopulmonary complications [123]. In this context, the treatment of particulate matter-exposed HaCaT and murine lung epithelial cells with polyphenol-rich S. horneri ethanol extracts exhibited promising effects towards counteracting ROS generation and lipid peroxidation, while notably improving SOD and CAT activities at 62.5 µg/mL and 125 µg/mL [123,124]. Similarly, in particulate matter exposed HaCaT cells and zebrafish embryos, a fucoxanthin-rich extract of S. fusiformis caused a reduction in several factors associated with inflammatory responses, including ROS production, reaching statistical significance at 25 µg/mL, 50 µg/mL, and 100 µg/mL [125]. Other compounds, such as (–)-loliolide, isolated from S. horneri, or fucosterol isolated from S. binderi, demonstrated interesting activities as well. S. horneri revealed a great capacity to boost cell viability through ROS reduction in fine dust-exposed HaCaT cells [46], while S. binderi not only caused a dose-dependent (12.5 µg/mL, 25 µg/mL, 50 µg/mL, and 100 µg/mL) increase in SOD and CAT activity, but also triggered a significant increase in HO-1 levels and Nrf2 nuclear translocation [126].
The synthetic drug methamphetamine (MA) has a neurodegenerative effect on the human brain and can increase the generation of ROS through dopamine oxidation. In fact, a hydroethanolic extract of S. angustifolium rich in polyphenols, including gallic acid, protocatechuic acid, gentisic acid, and hydroxybenzoic acid, led to a decrease in ROS generation on MA-exposed SH-SY5Y dopaminergic cells with doses of 80 and 160 μg/mL [127]. Similarly, fucoxanthin isolated from S. horneri prevented the effects of MA on ROS, significantly reducing their production (p < 0.05) and causing a significant increase in HO-1 expression in PC12 cells at 3 µM [128].
Researchers frequently induce inflammatory reactions in RAW 264.7 cells by exposing them to lipopolysaccharide (LPS). It can stimulate membrane-bound NADP oxidase and consequently, the generation of ROS. Ethanol, methanol, and ethyl acetate S. serratifolium extracts, whose major compounds identified were the meroterpenoids SHQA, SC, and SQA, were reported to dose-dependently (1.25–10 µg/mL) reduce LPS-induced ROS in LPS-stimulated macrophages [56]. The same trend was observed with SCM and SHQA isolated from S. horneri and S. macrocarpum, respectively [48,49]. In turn, an acetone:ethanol extract of S. glaucescens rich in fucoxanthin caused a decrease in LPS-induced ROS and O2•− production in the same cell line at concentrations ranging from 25 µg/mL to 100 µg/mL [122]. The anti-inflammatory properties of fucoxanthin are further evidenced by in vivo studies in rodents [129,130], including scenarios of induced asthma [131] and induced colon inflammation [132].
ROS are produced in high quantity in the mitochondria in scenarios of hyperglycemia and in the case of other metabolic disorders such as diabetes. In this sense, Lee et al. [133] treated glucose-stimulated INS-1 cells with an extract of S. sagamianum with the extract at 100 µg/mL and observed a reduction of ROS (by 122%) and lipid peroxidation, attributing these effects to the presence of secondary metabolites, such as phlorotannins and plastoquinones. A hydroethanolic extract of S. fusiformis rich in several secondary metabolites such as flavonoids, fucoxanthin, and fucosterol, also afforded a statistically significant increase in SOD and CAT activities accompanied by a notable decrease in lipid peroxidation in a high-fat diet and streptozotocin-induced ICR mice [134]. In fact, studies using diabetic mice showed that fucoxanthin lowers fasting glucose levels, increases plasma insulin levels and, when administered in tandem with metformin, induces the regeneration of pancreatic β-cells [135,136]. Usually diabetic mice have altered serum lipid profiles, with elevated values of triglycerides and cholesterol, which are reduced when treated with fucoxanthin [135]. These anti-diabetic and lipid profile regulating properties of fucoxanthin are the target of a clinical trial in patients with metabolic syndrome, currently under phase II [137].
Three phlorotannins isolated from S. carpophyllum were identified as anti-allergic agents. The compounds inhibited the activation of mast cells, the cells of the immune system responsible for the release of histamine, particularly when triggered by an allergen [138]. In addition to inhibiting the formation of ROS, phlorotannins are promising candidates for novel anti-allergic drugs. Choi et al. [54] showed that SHQA, isolated from S. serratifolium, prevented the activation of effector cells in allergic responses and caused a statistically significant reduction in ROS formation, once again demonstrating the antioxidant properties of meroterpenoids present in Sargassum spp.
In addition to the previously reported disease-counteracting effects, S. angustifolium hydroethanolic extracts rich in phenolic compounds were shown to dose-dependently (at 20 mg/kg, 40 mg/kg, and 80 mg/kg) improve defense against oxidative stress in rats, through the notable increase in the total antioxidant capacity in rats’ blood, and the decrease in lipid peroxidation brought on by hypertension and dyslipidemia, respectively [139,140].

Table 4.
Antioxidant effects of Sargassum spp. Secondary metabolites in cellular models.
Table 4.
Antioxidant effects of Sargassum spp. Secondary metabolites in cellular models.
| Sargassum spp. | Solvents | Secondary Antioxidants | Cells | Oxidative Stress Inductors | Effects | Ref. |
|---|---|---|---|---|---|---|
| S. angustifolium | 80% EtOH | PPS | SH-SY5Y | Methamphetamine | ↓ ROS | [127] |
| S. fusiformis | Ext: 80% MeOH Frac: Chl and water | fucoxanthin (↑%) | HaCaT | Particulate matter exposure | ↓ ROS | [125] |
| S. glaucescens | Ext: 70% Ace in EtOH Frac: 90% EtOH, EtOH:water:Hex (9:1:10) | fucoxanthin (↑%) | RAW 264.7 | LPS-stimulation | ↓ ROS/O2- | [122] |
| S. horneri | 80% MeOH | PPS (↑%) | HaCaT | UV-B | ↓ ROS | [118] |
| 95% EtOH | PPS (↑%) | MLE-12 | Particulate matter | ↓ ROS/LPO; ↑ SOD/CAT | [123] | |
| 70% EtOH | PPS (↑%), | HaCaT | Fine dust | ↓ ROS | [124] | |
| Ext: 70% EtOH Frac: Hex, DCM, EtOAc, BuOH | Fucosterol | BV2 HT22 | LPS (BV2 cells); Glu (HT22 cells) | BV2 cells: ↑ HO-1/Nrf2 (DCM fraction) HT22 cells: ↓ ROS (DCM fraction) | [141] | |
| S. muticum | Ext: MeOH:DCM Frac: MeOH:DCM, MeOH | PPS | MCF-7 | H2O2 | ↓ H2O2 | [111] |
| Ext: 80% EtOH Frac: Hex, DCM, EtOAc, BuOH, H2O | PPS | HaCaT | UV-B | ↓ ROS/LPO; ↑ SOD/CAT | [117] | |
| S. plagiophyllum | Distilled water | PPS | Human normal colon | H2O2 | ↓ ROS | [142] |
| S. polycystum and S. natans | Enzymes: Viscozyme, Celluclast, AMG, Termamyl, and Ultraflo | PPS | Chang | Extraction with different enzymes | ↑ ROS scavenging effects in Celluclast Ext | [112] |
| S. sagamanum | 80% EtOH | PPS | INS-1 | Glucose | ↓ ROS/LPO | [133] |
| S. serratiflium | 70% EtOH | SHQA, SC, SQA (↑%) | HepG2 | t-BHP | ↓ ROS/ LPO; Prevention of GSH oxidation; ↓ SOD/CAT; ↑ GST; ↑ Nrf2 Independent of t-BHP: ↑ HO-1/Nrf2 | [57] |
| Ext: EtOAc, MeOH, EtOH, Ace, Hex, Chl, H2O | SHQA, SC, SQA (↑%) | RAW 264.7 | LPS | ↑ TPC (EtOAc, MeOH and EtOH Ext) ↓ ROS (EtOAc, MeOH, and EtOH Ext) | [56] | |
| S. thunbergii | 40% EthOH | PPS (↑%) | L929 | UV-B | ↓ ROS/LPO; ↑ SOD and CAT | [119] |
| S. binderi | Ext: 70% EtOH Frac: Hex, Chl, EtOAc | Fucosterol | A549 | Fine dust | ↓ ROS; ↑ HO-1, SOD, CAT, and Nrf2 | [126] |
| S. carpophyllum | Ext: 80% MeOH Frac: Chl and H2O | 3 phlorotannins | RBL-2H3 | DNP-HAS | ↓ ROS | [138] |
| S. horneri | Ext: 80% MeOH Frac: Chl | (−)-Loliolide | Vero | AAPH | ↓ ROS | [44] |
| Ext: 80% MeOH Frac: Hex, EtOAc, MeOH, H2O | (−)-loliolide | HaCaT | UV-B | ↓ ROS | [45] | |
| Ext: MeOH Frac: Hex, 85% Aq MeOH, BuOH, H2O | SC E | HDF | UV-A | ↓ ROS, LPO and membrane protein oxidation | [47] | |
| Ext: 80% MeOH Frac: Hex, Chl, EtOAc | (−)-loliolide | HaCaT | Fine dust | ↓ ROS | [46] | |
| EtOH | Fucoxanthin | PC12 | Methamphetamine | ↓ ROS; ↑ SOD and CAT; ↑ HO-1 and Nrf2 | [128] | |
| Ext: 70% EtOH Frac: Hex, EtOAc | SCM | RAW 264.7 | LPS | ↓ ROS; ↑ HO-1/Nrf2 | [48] | |
| Ext: 70% EtOH Frac: Hex | Fucosterol | HDF | TNF-á/IFN-ã | ↓ ROS; ↑ HO-1/Nrf2 | [143] | |
| S. macrocarpum | 80% EtOH | SHQA | RAW 264.7 | LPS | ↓ ROS; ↑ HO-1 | [49] |
| S. muticum | Ext: MeOH Frac: Hex, DCM, EtOAc, BuOH | TPM | HDF | UV-A | ↓ ROS | [53] |
| S. serratifolium | Ext: 95% EtOH, H2O/ EtOH Frac: Hex, EtOAc, BuOH, H2O | SHQA | KU812F | PMACI | ↓ ROS | [144] |
| S. siliquastrum | Ext: 80% MeOH Frac: Chl | Fucoxanthin | Vero | H2O2 | ↓ ROS | [116] |
| Ext: MeOH Frac: Hex, 85% Aq MeOH, BuOH, H2O | SCM D, E, and K, 3 chromanols | HT1080 | H2O2 | ↓ ROS/LPO; ↑ GSH | [59] | |
| Ext: 80% MeOH Frac: Chl | Fucoxanthin | Human fibroblasts | UV-B | ↓ ROS | [120] | |
| S. thunbergii | Ext: 80% MeOH; Frac: Chl | I6CA | V79-4 | H2O2 | ↓ ROS; ↑ HO-1 and Nrf2 | [62,63] |
| Ext: MeOH Frac: Hex, 85% aq MeOH, BuOH, H2O | SC E, SC D, SHQA | HT1080 | Fe(II)/H2O2; AAPH | ↓ ROS; ↓ LPO | [61] |
↓: decreased; ↑: increased; AAPH: 2,2′-azobis(2-amidinopropane)dihydrochloride; Ace: acetone; Aq: aqueous; BuOH: butanol; CAT: catalase; Chl: chloroform; CHO: carbohydrates; CMF-DA: 5-chloromethylfluorescein diacetate; DCM: dichloromethane; DCFH-DA: 2′,7′-dichlorodihydrofluorescein diacetate; DPPP: diphenyl-1-pyrenylphosphin; DNP-HAS: dinitrophenyl-human serum albumin; EtOAc: ethyl acetate; EtOH: ethanol; Ext: extract; Frac: fractionation; Glu: glutamate; GSH: reduced glutathione; GST: glutathione S-transferase; H2O: water; H2O2: hydrogen peroxide; HDF: human dermal fibroblasts; Hex: Hexane; HO-1: heme oxygenase-1; HX: cyclohexane; I6CA: Indole-6-Carboxaldehyde; LPO: lipid peroxidation; LPS: lipopolysaccharide; mBrB: monobromobimane; MDA: malondialdehyde; MeOH: methanol; NBT: nitro blue tetrazolium; ND: non-defined; Nrf2: nuclear factor-erythroid 2-related factor 2; O2−: anion superoxide; PMACI: phorbol.

Table 5.
Antioxidant effects of Sargassum spp. Secondary metabolites in vivo models.
Table 5.
Antioxidant effects of Sargassum spp. Secondary metabolites in vivo models.
| Sargassum spp. | Solvents | Secondary Antioxidants | Animal Model | Oxidative Stress Inductors | Effects | Ref. |
|---|---|---|---|---|---|---|
| ND | H2O | PPS (↑%) | Wistar rats | CCl4 | ↑ GPx | [114] |
| S. angustifolium | 70% EtOH | PPS (↑%) | Wistar rats | Dexamethasone | ↓ LPO | [140] |
| 70% EtOH | PPS | Wistar rats | CdCl2 | ↑ TAOC | [139] | |
| S. fusiformis | Ext: 80% MeOH Frac: Chl, H2O | fucoxanthin (↑%) | Zebrafish embryos | Particulate matter | ↓ ROS | [125] |
| 95% EtOH | Flv, fucoxanthin, fucosterol | ICR mice | HFD/STZ | ↓ LPO; ↑ SOD and CAT | [134] | |
| S. glaucescens | Ext: 70% Ace in EtOH Frac: 90% EtOH, EtOH: H2O:Hex | Fucoxanthin (↑%) | Syrian hamsters | Cisplatin chemotherapy | ↑ SOD (plasma); ↑ GPx and CAT (testicles) | [122] |
| S. hemiphyllum | Ext: 70% EtOH Frac: EtOAc | Phlorotannins | Kunming mice | CCl4 | ↑ SOD (serum, kidney); ↑ TAOC (serum, kidney, and liver); ↑ CAT (kidney, brain, and liver); ↑ GPx (brain and liver); ↓ LPO (kidney) | [115] |
| S. longifollium | EtOH | Terpenoids, PPS, and Flv (encapsulated in Na-Caseinate matrix) | Fingerling Tilapia | A. salmonicida | SOD and LPO levels returned to normal | [145] |
| S. pallidum | 70% aq Ace | PPS (↑%) | Wistar rats | CCl4 | ↓ LPO; ↑ SOD and GSH | [113] |
| S. policystum | Enzymes: Celluclast | PPS | Zebrafish embryos | H2O2 | ↓ ROS | [112] |
| H2O | PPS (↑%) | Sprague–Dawley rats | HCF diet | ↓ LPO; ↑ SOD | [146] | |
| S. thunbergii | Ext: 40% EtOH Frac: EtOAc | PPS (↑%) | Zebrafish embryos | UV-B | ↓ ROS | [119] |
| S. virgatum | EtOH | PPS | Wistar rats | ã-irradiation | ↑ SOD, CAT, GPx, and GSH | [121] |
| S. vulgare | MeOH | ↑ Total phenolics and Flv | Wistar Rats | ASP | ↓ LPO; ↑ SOD and CAT | [147] |
| S. horneri | Ext: 80% MeOH Frac: Chl | (−)-Loliolide | Zebrafish | AAPH | ↓ LPO and ROS | [44] |
| Ext: 80% MeOH Frac: Hex, EtOAc, MeOH, H2O | (−)-Loliolide | Zebrafish | UV-B | ↓ LPO an ROS | [45] |
↓: decreased; ↑: increased; AAPH: 2,2′-azobis(2-amidinopropane)dihydrochloride; Aq: aqueous; CAR: carotene; CAT: catalase; CCl4: carbon tetrachloride; CdCl2: cadmium chloride; Chl: chloroform; CHO: carbohydrates; DCFH-DA: 2′,7′-dichlorodihydrofluorescein diacetate; DEN: diethylnitrosamine; DPPP: diphenyl-1-pyrenylphosphin; EtOH: ethanol; EtOAc: ethyl acetate; Ext: extract; Flv: flavonoids; Frac: fractionation; GPx: glutathione peroxidase; GSH: reduced glutathione; H2O: water; H2O2: hydrogen peroxide; HCF: high cholesterol/high fat; Hex: hexane; HLP: hyperlipidemia; HSD: high stocking density; LPO: lipid peroxidation; LPS: lipopolysaccharide; MDA: malondialdehyde; MeOH: methanol; MNNG: N-methyl-N’-nitro-N-nitrosoguanidine; NaCl: sodium chloride; ND: non defined; PFP: sodium pentafluoropropionate; PPS: polyphenols; RBC: red blood cells; ROS: reactive oxygen species; SA: S. aquifolium; SHE: S. horneri extract; SOD: superoxide dismutase; STZ: streptozotocin; TAOC: total antioxidant capacity; TCP: tocopherol; Vit C: vitamin C.
4. Applications in Non-Patented and in Patented Products
4.1. Food Applications
Apart from the well-known Asian cultural uses of Sargassum seaweeds as a food ingredient, these marine resources have recently raised much interest in applications in novel food compositions. Indeed, we have recently witnessed the appearance of products, such as a health-promoting tea, in which powdered Sargassum (3.3%) is combined with green tea, other seaweeds, and herbs [148], and a cocktail developed in the Caribbean called ‘pineapple gift’, which contains pineapple as a base, Sargassum extract, lemon juice, aquafaba, lavender, angostura bitters, and tequila [149]. Moreover, the incorporation of S. marginatum in pasta has been shown to improve its antioxidant properties and decrease pasta strands stickiness after cooking [150].
In South Korea, the development of innovative food formulations containing antioxidant-rich extracts of Sargassum is a growing practice, with focus on baked products, including bread and cakes. S. sagamianum antioxidant-rich extracts were incorporated into bread at doses of 0.25%, 0.50%, and 0.75%, and evaluated regarding antifungal and sensorial properties. The extracts, at a dose of 0.75%, were able to keep the bread free from mold for up to six days and significantly reduced the mold infestation in bread after nine days of storage when compared to the control (0% seaweed extract), thus translating into an increased shelf-life. The color of the enriched bread was darker compared to the control, with reddish-brown tones. The change in color, along with bitter taste and seaweed smell, made the enriched bread less accepted by panelists, following negative correlation between seaweed concentration and panelists’ acceptance [151]. Other novel seaweed-containing breads have been reported, including bread enriched with an extract of S. fulvellum [152] or an ethanol extract of S. siliquastrum [153]. The fortified breads, at doses of 1% and 2% in the case of S. fulvellum extract, and 0.1% and 1% in the case of S. siliquastrum extract, presented a slightly higher resistance against oxidation of the lipids in their composition and bacterial colonization with Bacillus subtillis. However, the extracts did not seem to reduce the ability of molds to grow. The higher doses of extracts caused breads to present unpleasant color, taste and aroma that made them less preferred by the sensorial evaluation panel. Nevertheless, the bread with 0.1% S. siliquastrum extract allowed combining increased resistance to microorganisms without a significant alteration in the overall sensorial properties.
S. fusiforme residue containing antioxidants and fiber was used as a fortifying ingredient in sponge cake. The residue was prepared by removing polysaccharides from S. fusiforme (with hot water), drying and superfine grinding to c.a. 10 μm particles. Sponge cake with S. fusiforme powder at a ratio of 10% presented a significant difference to control in color analysis, affording increased redness. It was, however, well rated on sensorial analysis, with slightly higher overall preference scores than the control sponge cake [154]. Combining an attractive orange color with antioxidant and nutraceutical properties, fucoxanthin is a useful ingredient for the food processing industry. In Japan, Sargassum is expected to become a most relevant source of food-grade fucoxanthin, as the preparation and subsequent incorporation of fucoxanthin-rich extracts from several species of Sargassum is the subject of a pending patent. The sources of fucoxanthin listed in this patent include S. fusiforme, S. sagamianum, S. yezoense, S. yendoi, S. horneri, S. thunbergii, S. patens, S. confusum, and algae from other genera [155]. In China, fucoxanthin-fortified meat products are patented for ‘improving DHA (docosahexaenoic acid) level in a human body’ [156]. Incorporation of fucoxanthin, obtained from Sargassum sp., into catfish sausages at 1%, 2%, 3%, or 4% mass percentage intensifies the color of the product and slightly increases its stability against microbial degradation [157]. The hedonic test of catfish sausages with the different concentrations of fucoxanthin shows significant differences (p < 0.05) for appearance, odor, flavor, texture, color, and overall quality score, with the catfish sausage with 1% fucoxanthin being the most preferred by the panelists.
4.2. Cosmetic Applications
Asian countries have also a long tradition of incorporating Sargassum, either as a whole plant or in the form of extracts, into cosmetic and skin-regenerating formulations [158]. Several aspects need to be considered for these applications to be feasible, which include the extraction yields of the target compounds, the amounts necessary to have a desirable effect, and the stability of the compounds in a certain formulation, among others. Nevertheless, there are currently various patented facial masks based on whole seaweeds of Sargassum aimed at skin hydration, nourishing and anti-aging, even though the active components responsible for the skin improvement effect are not identified [159,160,161,162,163,164]. Cosmetics based on extracts of S. muticum and S. serratifollium have also been patented for skin improvement, with anti-cellulite, skin-lightening, and/or anti-wrinkle benefits [165,166].
The broad range of cosmetic properties described for Sargassum in the abovementioned patents is related to the diversity of active components present in this seaweed, including phloroglucinol, phenolic compounds and/or phlorotannins, and fucoxanthin.
Phloroglucinol is used in a variety of cosmetics, mostly as a skin renewal and hair dyeing agent. In fact, it was patented by L’Oreal as an epidermal renewal-promoting agent under the claim of inducing keratinocyte proliferation [167]. With the expiry of the patent in 2018, it became available for any company to include in their compositions. Currently, phloroglucinol (in the trimethyl ether form) is found in various skincare formulations, including anti-wrinkle lotions, night creams, and lifting serums [168]. The application of phloroglucinol as a hair colorant is based on its ability to generate red color upon reaction with compounds, such as aldehydes or allyl groups [169]. Phloroglucinol can be found in several oxidative hair dye formulations, at concentrations ranging between 0.1% and 1% [170].
Polyphenolic extracts have properties that make them interesting ingredients for cosmetics formulation. These are typically dose-dependent, that is, they are more potent as the total phenolic content in the extract increases. For instance, the antimicrobial properties of the polyphenols from S. polycystum are dose-dependent, and they make this extract an effective preservative agent to replace chemical additives in lotions [171].
In France, antioxidant-rich extracts of S. muticum have been patented for use in cosmetics with the purpose of protecting against reactive oxygen radicals and maintaining a young skin appearance; the patent has expired in April 2022 [172]. In South Korea, polyphenol-rich extracts of a mixture of Sargassum horneri and Enteromorpha prolifera are patented as antioxidants for use in cosmetic formulations [173]. The polyphenols in S. plagyophyllum have, in addition to the antioxidant action, the ability to inhibit collagenase, making them useful for the preparation of natural anti-wrinkle products [174].
The ability to inhibit tyrosinases is another characteristic with cosmetic interest observed in Sargassum phlorotannins [175]. This class of enzymes is involved in the production of the skin pigment, melanin, and compounds able to inhibit them are much sought after for their skin whitening effect. Moreover, phlorotannins are trending ingredients in the industry of natural cosmetics because they can combine anti-aging and UV-protecting activities. A phlorotannin-rich fermented extract of S. vulgare, registered under the tradename DermalRx® FSE, is available as a natural ingredient for cosmetics from the biotechnological company Biocogent. The product is claimed to provide ‘strong anti-pollution activity’ while inhibiting oxidation and inflammation, being indicated by the manufacturer as most suited for incorporation into cosmetic products with protective and repairing properties [176].
Fucoxanthin, a carotenoid with important radical-scavenging and anti-UV properties, is employed in a variety of cosmetics, from sun lotions and firming serums to more specialized formulas that treat acne and help accelerate scar regeneration [177]. Moreover, its ability to interfere with the metabolism of lipids is making this compound an attractive ingredient for slimming cosmetic formulations. An anti-cellulite cosmetic composition based on fucoxanthin (e.g., from S. fulvellum) was patented under the claim of inducing apoptosis of the fat cells, thereby reducing cellulite [178].
4.3. Applications as Biostimulants
Although there is a long tradition of applications of Sargassum as fertilizers in agriculture, the effects of this genus as biostimulants are still not well explored. Nevertheless, some there are already some reports describing promising applications of these seaweeds as biostimulants. Notably, Sembera et al. [179] reported a compost based on Sargassum with equal or superior quality to current compost standards. Moreover, over the last years, Sargassum has been explored regarding its potential to be used as crop biostimulants and/or novel products for crop protection. In this regard, [180] demonstrated the positive effect of S. angustifolium extracts on the growth of Lens esculenta. Additionally, a Sargassum spp. compost was described to improve mangrove seedlings development in dry nurseries, a fact that can represent a promising strategy in mangrove restauration [31]. S. vulgare extracts were also reported to increase the germination of Triticum durum [181] and Phaseolus vulgaris [130] under salt stress. Regarding crop protection, Shahriari and coworkers [129] recently highlighted the potential of S. angustifolium extracts to the improvement of drought tolerance in Brassica napus L., while Han et al. [131] remarked the positive effect of S. horneri extracts on the thermal tolerance of Neopyropia yezoensis algae. In a different approach, the treatment of Solanum lycopersicum with S. tenerrimum and S. fusiforme extracts was found to enhance its resistance to the fungi Macrophomina phaseolina, [132] and to reduce the late blight and gray mold diseases [182], respectively.
5. Concluding Remarks
A variety of antioxidant compounds found in Sargassum seaweeds have been proven capable of improving shelf-life, nutritional, and public health status as food, food adjuvants, and pharmaceutical or phytopharmaceutical/biostimulant products. Currently, there are new potential markets for such seaweeds and growing interest on the exploitation of their products as natural sources with high antioxidant activity. This trend is clearly expressed by the increasing amount of research and innovation that has been taken in this field during the recent years with great prominence in the food and cosmetic sectors which are putting strong efforts in the development of new products containing extracts and/or antioxidant compounds from this genus. Nevertheless, joint efforts of research and industries are still necessary in order to raise awareness to the full potential of these natural marine resources and build an economical value chain.
Author Contributions
Conceptualization—S.M.C.; literature review and writing of the original draft—M.D.C., R.S.-R., S.S., S.S.B., A.M.S.S. and S.M.C. All authors contributed to writing—reviewing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through the projects UIDB/50006/2020 and UIDP/50006/2020. S.S. thanks FCT for funding through program DL 57/2016–Norma transitória (Ref. SFRH/BPD/74299/2010). M.D.C. and S.M.C thank LAQV-REQUIMTE for research contract. R.S.-R thanks FCT/MCTES and ESF (European Social Fund) through NORTE 2020 (Programa Operacional Região Norte) for her PhD grant ref. 2022.14518.BD.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mattio, L.; Payri, C.E. 190 Years of Sargassum Taxonomy, Facing the Advent of DNA Phylogenies. Bot Rev. 2011, 77, 31–70. [Google Scholar] [CrossRef]
- Amador-Castro, F.; Garcia-Cayuela, T.; Alper, H.S.; Rodriguez-Martinez, V.; Carrillo-Nieves, D. Valorization of pelagic sargassum biomass into sustainable applications: Current trends and challenges. J. Environ. Manag. 2021, 283, 112013. [Google Scholar] [CrossRef]
- Sargasso Sea Commission About the Sargasso Sea. Available online: http://www.sargassoseacommission.org/sargasso-sea/about-the-sargasso-sea (accessed on 5 January 2023).
- Rodriguez-Martinez, R.E.; Jordan-Dahlgren, E.; Hu, C.M. Spatio-temporal variability of pelagic Sargassum landings on the northern Mexican Caribbean. Remote Sens. Appl. Soc. Environ. 2022, 27, 100767. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Engelen, A.H.; Santos, R. Habitat-related differences in recruitment and survival of early recruits of the invasive Sargassum muticum (Phaeophyta, Sargassaceae) in northern Portugal. Hydrobiologia 2012, 683, 287–296. [Google Scholar] [CrossRef]
- Raoux, A.; Pezy, J.-P.; Sporniak, T.; Dauvin, J.-C. Does the invasive macro-algae Sargassum muticum (Yendo) Fensholt, 1955 offer an appropriate temporary habitat for mobile fauna including non indigenous species? Ecological. Indic. 2021, 126, 107624. [Google Scholar] [CrossRef]
- Fernandez, C. Boom-bust of Sargassum muticum in northern Spain: 30 years of invasion. Eur. J. Phycol. 2020, 55, 285–295. [Google Scholar] [CrossRef]
- Engelen, A.H.; Primo, A.L.; Cruz, T.; Santos, R. Faunal differences between the invasive brown macroalga Sargassum muticum and competing native macroalgae. Biol. Invasions 2013, 15, 171–183. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Hassan, H.M.; Elmaidomy, A.H.; Abdelmohsen, U.R. Pharmacological and natural products diversity of the brown algae genus Sargassum. RSC Adv. 2020, 10, 24951–24972. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Chapter 3—Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Fleurence, J., Levine, I., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 41–106. [Google Scholar]
- Desrochers, A.; Cox, S.-A.; Oxenford, H.A.; Tussenbroek, B. Sargassum Uses Guide: A Resource for Caribbean Researchers, Entrepreneurs and Policy Makers; FAO: Rome, Italy; University of the West Indies, Cave Hill Campus: Bridgetown, Barbados, 2020; p. 172. [Google Scholar]
- Aaron-Amper, J.; Largo, D.B.; Handugan, E.R.B.; Nini, J.L.; Alingasa, K.M.A.; Gulayan, S.J. Culture of the tropical brown seaweed Sargassum aquifolium: From hatchery to field out-planting. Aquacult. Rep. 2020, 16, 102591. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Jiang, X.D.; Li, H.Y.; Liu, T.H.; Ji, L.; Sun, Y.Q. Utilization of different seaweeds (Sargassum polycystum, Sargassum thunbergii, Sargassum horneri, Enteromorpha prolifera, Macrocystis pyrifera, and the residue of M. pyrifera) in the diets of sea cucumber Apostichopus japonicus (Selenka, 1867). Algal. Res. 2022, 61, 102591. [Google Scholar] [CrossRef]
- Singh, B.K.; Chopra, R.C.; Rai, S.N.; Verma, M.P.; Mohanta, R.K. Nutritional Evaluation of Seaweed on Nutrient Digestibility, Nitrogen Balance, Milk Production and Composition in Sahiwal Cows. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 437–443. [Google Scholar] [CrossRef]
- Casas-Valdez, M.; Portillo-Clark, G.; Aguila-Ramírez, N.; Rodríguez-Astudillo, S.; Sánchez-Rodríguez, I.; Carrillo-Domínguez, S. Efecto del alga marina Sargassum spp. sobre las variables productivas y la concentración de colesterol en el camarón café, Farfantepenaeus californiensis (Holmes, 1900). Rev. Biol. Mar. Oceanogr. 2006, 41, 97–105. [Google Scholar] [CrossRef]
- Resources, Government of Bermuda—Department of Environmental and Natural Resources Sargassum spp. Seaweeds. Available online: https://environment.bm/sargassum-seaweed (accessed on 5 January 2023).
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.C.; Pages, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed Secondary Metabolites with Beneficial Health Effects: An Overview of Successes in In Vivo Studies and Clinical Trials. Mar. Drugs 2020, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Pires, S.M.G.; Silva, S.; Costa, F.; Braga, S.S.; Pinto, D.C.G.A.; Silva, A.M.S.; Cardoso, S.M. Overview of Phlorotannins' Constituents in Fucales. Mar. Drugs 2022, 20, 754. [Google Scholar] [CrossRef]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Chapter Twelve—Bioactive Components from Seaweeds: Cosmetic Applications and Future Development. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 345–378. [Google Scholar]
- Li, Y.X.; Wijesekara, I.; Li, Y.; Kim, S.K. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Catarino, M.D.; Amarante, S.J.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Brown Algae Phlorotannins: A Marine Alternative to Break the Oxidative Stress, Inflammation and Cancer Network. Foods 2021, 10, 1478. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.; Cruz, M.T.; Mateus, N.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins from Fucus vesiculosus: Modulation of Inflammatory Response by Blocking NF-κB Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 6897. [Google Scholar] [CrossRef]
- Catarino, M.D.; Fernandes, I.; Oliveira, H.; Carrascal, M.; Ferreira, R.; Silva, A.M.S.; Cruz, M.T.; Mateus, N.; Cardoso, S.M. Antitumor Activity of Fucus vesiculosus-Derived Phlorotannins through Activation of Apoptotic Signals in Gastric and Colorectal Tumor Cell Lines. Int. J. Mol. Sci. 2021, 22, 7604. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Circuncisao, A.R.; Neves, B.; Marcal, C.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Impact of Gastrointestinal Digestion on the Anti-Inflammatory Properties of Phlorotannins from Himanthalia elongata. Antioxidants 2022, 11, 1518. [Google Scholar] [CrossRef]
- Catarino, M.D.; Marcal, C.; Bonifacio-Lopes, T.; Campos, D.; Mateus, N.; Silva, A.M.S.; Pintado, M.M.; Cardoso, S.M. Impact of Phlorotannin Extracts from Fucus vesiculosus on Human Gut Microbiota. Mar. Drugs 2021, 19, 375. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of Phlorotannins Extraction from Fucus vesiculosus and Evaluation of Their Potential to Prevent Metabolic Disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [PubMed]
- Trench, C.; Thomas, S.L.; Thorney, D.; Maddix, G.M.; Francis, P.; Small, H.; Machado, C.B.; Webber, D.; Tonon, T.; Webber, M. Application of Stranded Pelagic Sargassum Biomass as Compost for Seedling Production in the Context of Mangrove Restoration. Front Environ. Sci. 2022, 10, 932293. [Google Scholar] [CrossRef]
- Martinez, J.H.I.; Castaneda, H.G.T. Preparation and Chromatographic Analysis of Phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef]
- Montero, L.; Sanchez-Camargo, A.P.; Garcia-Canas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibanez, E. Anti-proliferative activity and chemical characterization by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry of phlorotannins from the brown macroalga Sargassum muticum collected on North-Atlantic coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.Y.; Shao, C.L.; Wei, Y.X.; Wang, B.G.; Sun, L.L.; Zheng, C.J.; Guan, H.S. Chemical constituents from Sargassum pallidum (Turn.) C. Agardh. Biochem. Syst. Ecol. 2009, 37, 127–129. [Google Scholar] [CrossRef]
- Klejdus, B.; Lojkova, L.; Plaza, M.; Snoblova, M.; Sterbova, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Analysis of Fucoxanthin Content and Purification of All-Trans-Fucoxanthin from Turbinaria turbinata and Sargassum plagyophyllum by SiO2 Open Column Chromatography and Reversed Phase-HPLC. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1340–1354. [Google Scholar] [CrossRef]
- Terasaki, M.; Hirose, A.; Narayan, B.; Baba, Y.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Evaluation of Recoverable Functional Lipid Components of Several Brown Seaweeds (Phaeophyta) from Japan with Special Reference to Fucoxanthin and Fucosterol Contents. J. Phycol. 2009, 45, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Kamogawa, H.; Susanto, E.; Kawagoe, C.; Yasui, H.; Saga, N.; Hosokawa, M.; Miyashita, K. Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J. Appl. Phycol. 2013, 25, 1159–1169. [Google Scholar] [CrossRef]
- Kato, T.; Kumanireng, A.S.; Ichinose, I.; Kitahara, Y.; Kakinuma, Y.; Kato, Y. Structure and synthesis of active component from a marine alga, Sargassum tortile, which induces the settling of swimming larvae of Coryne uchidai. Chem. Lett. 1975, 4, 335–338. [Google Scholar] [CrossRef]
- Tsuchiya, N.; Sato, A.; Haruyama, H.; Watanabe, T.; Iijima, Y. Nahocols and isonahocols, endothelin antagonists from the brown alga, Sargassum autumnale. Phytochemistry 1998, 48, 1003–1011. [Google Scholar] [CrossRef]
- Reddy, P.; Urban, S. Meroditerpenoids from the southern Australian marine brown alga Sargassum fallax. Phytochemistry 2009, 70, 250–255. [Google Scholar] [CrossRef]
- Herath, K.H.I.N.M.; Kim, H.J.; Jang, J.H.; Kim, H.S.; Kim, H.J.; Jeon, Y.J.; Jee, Y. Mojabanchromanol Isolated from Sargassum horneri Attenuates Particulate Matter Induced Inflammatory Responses via Suppressing TLR2/4/7-MAPK Signaling in MLE-12 Cells. Mar. Drugs 2020, 18, 355. [Google Scholar] [CrossRef]
- Kim, H.S.; Wang, L.; Fernando, I.P.S.; Je, J.G.; Ko, S.C.; Kang, M.C.; Lee, J.M.; Yim, M.J.; Jeon, Y.J.; Lee, D.S. Antioxidant efficacy of (-)-loliolide isolated from Sargassum horneri against AAPH-induced oxidative damage in Vero cells and zebrafish models in vivo. J. Appl. Phycol. 2020, 32, 3341–3348. [Google Scholar] [CrossRef]
- Wang, L.; Kim, H.S.; Je, J.G.; Fu, X.T.; Huang, C.X.; Ahn, G.; Oh, J.Y.; Sanjeewa, K.K.A.; Xu, J.C.; Gao, X.; et al. In Vitro and In Vivo Photoprotective Effects of (-)-Loliode Isolated from the Brown Seaweed, Sargassum horneri. Molecules 2021, 26, 6898. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Jeon, Y.J.; Kim, H.S.; Fernando, I.P.S.; Ahn, G. (-)-Loliolide Isolated fromSargassum horneriProtects against Fine Dust-Induced Oxidative Stress in Human Keratinocytes. Antioxidants 2020, 9, 474. [Google Scholar] [CrossRef]
- Kim, J.A.; Ahn, B.N.; Kong, C.S.; Kim, S.K. The chromene sargachromanol E inhibits ultraviolet A-induced ageing of skin in human dermal fibroblasts. Brit. J. Dermatol. 2013, 168, 968–976. [Google Scholar] [CrossRef]
- Han, E.J.; Jayawardena, T.U.; Jang, J.H.; Fernando, I.P.S.; Jee, Y.; Jeon, Y.J.; Lee, D.S.; Lee, J.M.; Yim, M.J.; Wang, L.; et al. Sargachromenol Purified from Sargassum horneri Inhibits Inflammatory Responses via Activation of Nrf2/HO-1 Signaling in LPS-Stimulated Macrophages. Mar. Drugs 2021, 19, 497. [Google Scholar] [CrossRef] [PubMed]
- Joung, E.J.; Cao, L.; Lee, B.; Gwon, W.G.; Park, S.H.; Kim, H.R. Sargahydroquinoic Acid, a Cyclooxygenase-2 Inhibitor, Attenuates Inflammatory Responses by Regulating NF-κB Inactivation and Nrf2 Activation in Lipopolysaccharide-Stimulated Cells. Inflammation 2021, 44, 2120–2131. [Google Scholar] [CrossRef]
- Iwashima, M.; Mori, J.; Ting, X.; Matsunaga, T.; Hayashi, K.; Shinoda, D.; Saito, H.; Sankawa, U.; Hayashi, T. Antioxidant and antiviral activities of plastoquinones from the brown alga Sargassum micracanthum, and a new chromene derivative converted from the plastoquinones. Biol. Pharm. Bull. 2005, 28, 374–377. [Google Scholar] [CrossRef]
- Mori, J.; Iwashima, M.; Wakasugi, H.; Saito, H.; Matsunaga, T.; Ogasawara, M.; Takahashi, S.; Suzuki, H.; Hayashi, T. New plastoquinones isolated from the brown alga, Sargassum micracanthum. Chem. Pharm. Bull. 2005, 53, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Cao, L.; Jeong, S.J.; Kim, H.R.; Nam, T.J.; Lee, S.G. The Comparison of Total Phenolics, Total Antioxidant, and Anti-Tyrosinase Activities of Korean Sargassum Species. J. Food Qual. 2021, 2021, 6640789. [Google Scholar] [CrossRef]
- Balboa, E.M.; Li, Y.X.; Ahn, B.N.; Eom, S.H.; Dominguez, H.; Jimenez, C.; Rodriguez, J. Photodamage attenuation effect by a tetraprenyltoluquinol chromane meroterpenoid isolated from Sargassum muticum. J. Photoch. Photobiol. B 2015, 148, 51–58. [Google Scholar] [CrossRef]
- Peng, Y.; Huang, R.M.; Lin, X.P.; Liu, Y.H. Norisoprenoids from the Brown Alga Sargassum naozhouense Tseng et Lu. Molecules 2018, 23, 348. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Tsutsumi, S.; Takada, Y.; Kimura, J. Antibacterial Quinone Metabolites from the Brown Alga, Sargassum sagamianum. B Chem. Soc. Jpn. 2008, 81, 1125–1130. [Google Scholar] [CrossRef]
- Lim, S.; Choi, A.H.; Kwon, M.; Joung, E.J.; Shin, T.; Lee, S.G.; Kim, N.G.; Kim, H.R. Evaluation of antioxidant activities of various solvent extract from Sargassum serratifolium and its major antioxidant components. Food Chem. 2019, 278, 178–184. [Google Scholar] [CrossRef]
- Lim, S.; Kwon, M.; Joung, E.J.; Shin, T.; Oh, C.W.; Choi, J.S.; Kim, H.R. Meroterpenoid-Rich Fraction of the Ethanolic Extract from Sargassum serratifolium Suppressed Oxidative Stress Induced by Tert-Butyl Hydroperoxide in HepG2 Cells. Mar. Drugs 2018, 16, 374. [Google Scholar] [CrossRef]
- Jung, M.; Jang, K.H.; Kim, B.; Lee, B.H.; Choi, B.W.; Oh, K.B.; Shin, J. Meroditerpenoids from the Brown Alga Sargassum siliquastrum. J. Nat. Prod. 2008, 71, 1714–1719. [Google Scholar] [CrossRef]
- Lee, J.I.; Seo, Y. Chromanols from Sargassum siliquastrum and Their Antioxidant Activity in HT 1080 Cells. Chem. Pharm. Bull. 2011, 59, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Park, K.E.; Kim, Y.A.; Lee, H.J.; Yoo, J.S.; Ahn, J.W.; Lee, B.J. Isolation of tetraprenyltoluquinols from the brown alga Sargassum thunbergii. Chem. Pharm. Bull. 2006, 54, 1730–1733. [Google Scholar] [CrossRef]
- Kim, J.A.; Kong, C.S.; Seo, Y.W.; Kim, S.K. Sargassum thunbergii extract inhibits MMP-2 and-9 expressions related with ROS scavenging in HT1080 cells. Food Chem. 2010, 120, 418–425. [Google Scholar] [CrossRef]
- Kim, S.O.; Choi, Y.H. Indole-6-Carboxaldehyde Isolated from Sargassum thunbergii (Mertens) Kuntze Prevents Oxidative Stress-Induced Cellular Damage in V79-4 Chinese Hamster Lung Fibroblasts through the Activation of the Nrf2/HO-1 Signaling Pathway. Cell Physiol. Biochem. 2020, 54, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Heo, S.J.; Ko, S.C.; Park, W.S.; Choi, I.W.; Yi, M.; Jung, W.K. Indole-6-carboxaldehyde isolated from Sargassum thunbergii inhibits the expression and secretion of matrix metalloproteinase-9. Int. J. Mol. Med. 2019, 44, 1979–1987. [Google Scholar] [CrossRef]
- Maneesh, A.; Chakraborty, K. Previously undescribed fridooleanenes and oxygenated labdanes from the brown seaweed Sargassum wightii and their protein tyrosine phosphatase-1B inhibitory activity. Phytochemistry 2017, 144, 19–32. [Google Scholar] [CrossRef]
- Terasaki, M.; Kawagoe, C.; Ito, A.; Kumon, H.; Narayan, B.; Hosokawa, M.; Miyashita, K. Spatial and seasonal variations in the biofunctional lipid substances (fucoxanthin and fucosterol) of the laboratory-grown edible Japanese seaweed (Sargassum horneri Turner) cultured in the open sea. Saudi J. Biol. Sci. 2017, 24, 1475–1482. [Google Scholar] [CrossRef]
- Magura, J.; Moodley, R.; Jonnalagadda, S.B. Toxic metals (As and Pb) in Sargassum elegans Suhr (1840) and its bioactive compounds. Int. J. Environ. Health Res. 2019, 29, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Mulder, M.; Bogie, J.; Hoeks, C.; Schepers, M.; Tuabe, A.; Leijten, F.; Chintapakorn, Y.; Struik, D.; Liu, H.B.; Hellings, N.; et al. Dietary Sargassum fusiforme Improves Memory and Reduces Amyloid Plaque Load in an Alzheimer's Disease Mouse Model. Atherosclerosis 2019, 287, E57. [Google Scholar] [CrossRef]
- Ito, M.; Koba, K.; Hikihara, R.; Ishimaru, M.; Shibata, T.; Hatate, H.; Tanaka, R. Analysis of functional components and radical scavenging activity of 21 algae species collected from the Japanese coast. Food Chem. 2018, 255, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.L.; Liu, C.P.; Gao, L.; Lu, Y.B. One-Step Preparative Separation of Phytosterols from Edible Brown Seaweed Sargassum horneri by High-Speed Countercurrent Chromatography. Mar. Drugs 2019, 17, 691. [Google Scholar] [CrossRef]
- Malinin, N.L.; West, X.Z.; Byzova, T.V. Oxidation as "The Stress of Life". Aging-Us 2011, 3, 906–910. [Google Scholar] [CrossRef]
- Decker, E.A.; Elias, R.J.; McClements, D.J. Oxidation in Foods and Beverages and Antioxidant Applications: Management in Different Industry Sectors; Elsevier: Amsterdam, The Netherlands, 2010; Volume 2. [Google Scholar]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef]
- Chakraborty, K.; Maneesh, A.; Makkar, F. Antioxidant Activity of Brown Seaweeds. J. Aquat. Food Prod. Technol. 2017, 26, 406–419. [Google Scholar] [CrossRef]
- Wu, Y.J.; Gao, H.Q.; Wang, Y.X.; Peng, Z.T.; Guo, Z.Q.; Ma, Y.X.; Zhang, R.F.; Zhang, M.W.; Wu, Q.; Xiao, J.; et al. Effects of different extraction methods on contents, profiles, and antioxidant abilities of free and bound phenolics of Sargassum polycystum from the South China Sea. J. Food Sci. 2022, 87, 968–981. [Google Scholar] [CrossRef]
- Ismail, G.A.; Gheda, S.F.; Abo-shady, A.M.; Abdel-karim, O.H. In vitro potential activity of some seaweeds as antioxidants and inhibitors of diabetic enzymes. Food Sci. Technol. 2020, 40, 681–691. [Google Scholar] [CrossRef]
- Prasedya, E.S.; Martyasari, N.W.R.; Abidin, A.S.; Ilhami, B.T.K.; Padmi, H.; Widyastuti, S.; Sunarwidhi, A.L.; Sunarpi, H. Antioxidant activity of brown macroalgae Sargassum ethanol extract from Lombok coast, Indonesia. IOP Conf. Series Earth Environ. Sci. 2021, 712, 012038. [Google Scholar] [CrossRef]
- Taniguchi, R.; Ito, C.; Keitoku, S.; Miyake, Y.; Itoigawa, M.; Matsui, T.; Shibata, T. Analysis on the Structure of Phlorethols Isolated From the Warm-Temperate Brown Seaweed Sargassum carpophyllum and Their Antioxidant Properties. Nat. Prod. Commun. 2022, 17. [Google Scholar] [CrossRef]
- Saraswati; Giriwono, P.E.; Iskandriati, D.; Tan, C.P.; Andarwulan, N. In-vitro anti-inflammatory activity, free radical (DPPH) scavenging, and ferric reducing ability (FRAP) of Sargassum cristaefolium lipid-soluble fraction and putative identification of bioactive compounds using UHPLC-ESI-ORBITRAP-MS/MS. Food Res. Int. 2020, 137, 109702. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.W.S.; Tan, K.M.; Chew, L.Y.; Kong, K.W.; Yan, S.W. Application of Two-Level Full Factorial Design for the Extraction of Fucoxanthin and Antioxidant Activities from Sargassum siliquosum and Sargassum polycystum. J. Aquat. Food Prod. Technol. 2018, 27, 446–463. [Google Scholar] [CrossRef]
- Balasubramaniam, V.; Chelyn, L.J.; Vimala, S.; Fairulnizal, M.N.M.; Brownlee, I.A.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Yan, X.J.; Chuda, Y.; Suzuki, M.; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem. 1999, 63, 605–607. [Google Scholar] [CrossRef]
- Savira, A.D.R.; Amin, M.N.G.; Alamsjah, M.A. The effect of different type of solvents on the antioxidant activity of fucoxanthin extract from brown seaweed Sargassum duplicatum. IOP Conf. Series Earth Environ. Sci. 2021, 718, 012010. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Comparison of chemical profile and antioxidant properties of the brown algae. Int. J. Food Sci. Technol. 2018, 53, 174–181. [Google Scholar] [CrossRef]
- Raji, V.; Loganathan, C.; Sadhasivam, G.; Kandasamy, S.; Poomani, K.; Thayumanavan, P. Purification of fucoxanthin from Sargassum wightii Greville and understanding the inhibition of angiotensin 1-converting enzyme: An in vitro and in silico studies. Int. J. Biol. Macromol. 2020, 148, 696–703. [Google Scholar] [CrossRef]
- Ham, Y.M.; Kim, K.N.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Chemical Constituents from Sargassum micracanthum and Antioxidant Activity. Int. J. Pharmacol. 2010, 6, 147–151. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, H.J.; Park, K.E.; Kim, Y.A.; Ahn, J.W.; Jong, S.Y.; Lee, B.J. Peroxynitrite-scavenging constituents from the brown alga Sargassum thunbergii. Biotechnol. Bioprocess Eng. 2004, 9, 212–216. [Google Scholar] [CrossRef]
- Jang, K.H.; Lee, B.H.; Choi, B.W.; Lee, H.S.; Shin, J. Chromenes from the brown alga Sargassum siliquastrum. J. Nat. Prod. 2005, 68, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Cho, J.Y.; Kang, S.E.; Hong, Y.K.; Ahn, D.H. Antioxidant activity of mojabanchromanol, a novel chromene, isolated from brown alga Sargassum siliquastrum. J. Environ. Biol. 2008, 29, 479–484. [Google Scholar] [PubMed]
- Farvin, K.H.S.; Surendraraj, A.; Al-Ghunaim, A.; Al-Yamani, F. Chemical profile and antioxidant activities of 26 selected species of seaweeds from Kuwait coast. J. Appl. Phycol. 2019, 31, 2653–2668. [Google Scholar] [CrossRef]
- Savaghebi, D.; Barzegar, M.; Mozafari, M.R. Manufacturing of nanoliposomal extract from Sargassum boveanum algae and investigating its release behavior and antioxidant activity. Food Sci. Nutr. 2020, 8, 299–310. [Google Scholar] [CrossRef]
- Sobuj, M.K.A.; Islam, M.A.; Haque, M.A.; Islam, M.M.; Alam, M.J.; Rafiquzzaman, S.M. Evaluation of bioactive chemical composition, phenolic, and antioxidant profiling of different crude extracts of Sargassum coriifolium and Hypnea pannosa seaweeds. J. Food Meas. Charact. 2021, 15, 1653–1665. [Google Scholar] [CrossRef]
- Vasconcelos, J.B.; de Vasconcelos, E.R.T.P.P.; Urrea-Victoria, V.; Bezerra, P.S.; Reis, T.N.V.; Cocentino, A.L.M.; Navarro, D.M.A.F.; Chow, F.; Areces, A.J.; Fujii, M.T. Antioxidant activity of three seaweeds from tropical reefs of Brazil: Potential sources for bioprospecting. J. Appl. Phycol. 2019, 31, 835–846. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.J.; Jee, Y.; Jeon, Y.J.; Kim, H.J. Antioxidant potential of Sargassum horneri extract against urban particulate matter-induced oxidation. Food Sci. Biotechnol. 2020, 29, 855–865. [Google Scholar] [CrossRef]
- Chen, C.J.; Han, L.S.; Yao, M.K.; Shi, L.; Zhang, M.S.; Shi, Y.P.; Liu, C.H.; Bai, X.F.; Liu, X.; Liu, X.; et al. Comparative Studies on Antioxidant, Angiotensin-Converting Enzyme Inhibitory and Anticoagulant Activities of the Methanol Extracts from Two Brown Algae (Sargassum horneri and Sargassum thunbergii). Russ. J. Mar. Biol. 2021, 47, 380–387. [Google Scholar] [CrossRef]
- Kim, H.S.; Sanjeewa, K.K.A.; Fernando, I.P.S.; Ryu, B.; Yang, H.W.; Ahn, G.; Kang, M.C.; Heo, S.T.; Je, J.G.; Jeon, Y.J. A comparative study of Sargassum horneri Korea and China strains collected along the coast of Jeju Island South Korea: Its components and bioactive properties. Algae 2018, 33, 341–349. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, Antioxidant, and Anti-Tumor Activities of Sargassum linearifolium and Cystoseira crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, Y.; Yuan, Y.; Farooq, M.A.; Fayyaz, M.S.; Su, D.X.; Zeng, Q.Z.; Rahaman, A. Process optimization and antioxidative activity of polyphenols derived from different seaweed species Sargassum miyabei, Undaria pinnatifida Suringar, and Sargassum thunbergii. Food Sci. Nutr. 2022, 10, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenco-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of Bioactive Properties in Brown Algae from the Northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef]
- Terme, N.; Boulho, R.; Kucma, J.P.; Bourgougnon, N.; Bedoux, G. Radical scavenging activity of lipids from seaweeds isolated by solid-liquid extraction and supercritical fluids. Ocl 2018, 25, D505. [Google Scholar] [CrossRef]
- Le Lann, K.; Jegou, C.; Stiger-Pouvreau, V. Effect of different conditioning treatments on total phenolic content and antioxidant activities in two Sargassacean species: Comparison of the frondose Sargassum muticum (Yendo) Fensholt and the cylindrical Bifurcaria bifurcata R. Ross. Phycol. Res. 2008, 56, 238–245. [Google Scholar] [CrossRef]
- Sanchez-Camargo, A.D.; Montero, L.; Stiger-Pouvreau, V.; Tanniou, A.; Cifuentes, A.; Herrero, M.; Ibanez, E. Considerations on the use of enzyme-assisted extraction in combination with pressurized liquids to recover bioactive compounds from algae. Food Chem. 2016, 192, 67–74. [Google Scholar] [CrossRef]
- Nazarudin, M.F.; Paramisparam, A.; Khalid, N.A.; Albaz, M.N.; Shahidan, M.S.; Yasin, I.S.M.; Isha, A.; Abu Zarin, M.; Aliyu-Paiko, M. Metabolic variations in seaweed, Sargassum polycystum samples subjected to different drying methods via 1H NMR-based metabolomics and their bioactivity in diverse solvent extracts. Arab. J. Chem. 2020, 13, 7652–7664. [Google Scholar] [CrossRef]
- Vasanthi, C.; Rao, V.A.; Babu, R.N.; Sriram, P.; Karunakaran, R. In-vitro antioxidant activities of aqueous and alcoholic extracts of Sargassum species-Indian brown seaweed. J. Food Process. Preserv. 2020, 44, e14877. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimum conditions of microwave-assisted extraction for phenolic compounds and antioxidant capacity of the brown alga Sargassum vestitum. Sep. Sci. Technol. 2018, 53, 1711–1723. [Google Scholar] [CrossRef]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T. Brown macroalgae from the Adriatic Sea as a promising source of bioactive nutrients. J. Food Meas. Charact. 2019, 13, 330–338. [Google Scholar] [CrossRef]
- Santos, J.P.; Torres, P.B.; dos Santos, D.Y.A.C.; Motta, L.B.; Chow, F. Seasonal effects on antioxidant and anti-HIV activities of Brazilian seaweeds. J. Appl. Phycol. 2019, 31, 1333–1341. [Google Scholar] [CrossRef]
- Chouh, A.; Nouadri, T.; Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phlorotannins of the Brown Algae Sargassum vulgare from the Mediterranean Sea Coast. Antioxidants 2022, 11, 1055. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Geethalakshmi, V.; Anandan, R.; Jayarani, R.; Mathew, S. Screening of effective solvents for obtaining antioxidant-rich seaweed extracts using principal component analysis. J. Food Process. Preserv. 2020, 44, e14716. [Google Scholar] [CrossRef]
- Vijayan, R.; Chitra, L.; Penislusshiyan, S.; Palvannan, T. Exploring bioactive fraction of Sargassum wightii: In vitro elucidation of angiotensin-I-converting enzyme inhibition and antioxidant potential. Int. J. Food Prop. 2018, 21, 674–684. [Google Scholar] [CrossRef]
- Pinteus, S.; Lemos, M.F.L.; Silva, J.; Alves, C.; Neugebauer, A.; Freitas, R.; Duarte, A.; Pedrosa, R. An Insight into Sargassum muticum Cytoprotective Mechanisms against Oxidative Stress on a Human Cell In Vitro Model. Mar. Drugs 2017, 15, 353. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Ranasinghe, P.; Gunasekara, U.K.D.S.S.; Jeon, Y.J. Antioxidant and anti-inflammatory functionality of ten Sri Lankan seaweed extracts obtained by carbohydrase assisted extraction. Food Sci. Biotechnol. 2018, 27, 1761–1769. [Google Scholar] [CrossRef]
- Sprygin, V.G.; Kushnerova, N.F.; Fomenko, S.E.; Drugova, E.S.; Lesnikova, L.N.; Merzlyakov, V.Y.; Momot, T.V. The Influence of an Extract from the Marine Brown Alga Sargassum pallidum on the Metabolic Reactions in the Liver under Experimental Toxic Hepatitis. Russ. J. Mar. Biol. 2017, 43, 479–484. [Google Scholar] [CrossRef]
- Altinok-Yipel, F.; Tekeli, I.O.; Ozsoy, S.Y.; Guvenc, M.; Sayin, S.; Yipel, M. Investigation of hepatoprotective effect of some algae species on carbon tetrachloride-induced liver injury in rats. Arch. Physiol. Biochem. 2020, 126, 463–467. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Yang, X.Q.; Gong, Z.Q.; Pan, M.Z.; Han, Y.L.; Liu, Y. Antioxidant activities of crude phlorotannins from Sargassum hemiphyllum. J. Huazhong Univ. Sci. Technol. 2016, 36, 449–455. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoon, W.J.; Kang, H.K.; Yoo, E.S.; Koh, Y.S.; Kim, D.S.; Lee, N.H.; Hyun, J.W. Protective Effect of the Ethyl Acetate Fraction of Sargassum muticum Against Ultraviolet B-Irradiated Damage in Human Keratinocytes. Int. J. Mol. Sci. 2011, 12, 8146–8160. [Google Scholar] [CrossRef]
- Han, E.J.; Kim, S.Y.; Han, H.J.; Kim, H.S.; Kim, K.N.; Fernando, I.P.S.; Madusanka, D.M.D.; Dias, M.K.H.M.; Cheong, S.H.; Park, S.R.; et al. UVB protective effects of Sargassum horneri through the regulation of Nrf2 mediated antioxidant mechanism. Sci. Rep. 2021, 11, 9963. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chen, H.H.; Qu, H.D.; Qiao, K.; Xu, M.; Wu, J.N.; Su, Y.C.; Shi, Y.; Liu, Z.Y.; Wang, Q. Photoprotective effects of Sargassum thunbergii on ultraviolet B-induced mouse L929 fibroblasts and zebrafish. BMC Complement. Med. 2022, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Jeon, Y.J. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B Biol. 2009, 95, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Semaida, A.I.; El-Khashab, M.A.; Saber, A.A.; Hassan, A.I.; Elfouly, S.A. Effects of Sargassum virgatum extracts on the testicular measurements, genomic DNA and antioxidant enzymes in irradiated rats. Int. J. Radiat. Biol. 2022, 98, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.T.; Sudirman, S.; Hsieh, M.C.; Hu, J.Y.; Kong, Z.L. Oral supplementation of fucoxanthin-rich brown algae extract ameliorates cisplatin-induced testicular damage in hamsters. Biomed. Pharmacother. 2020, 125, 109992. [Google Scholar] [CrossRef]
- Kim, H.J.; Herath, K.H.I.N.M.; Dinh, D.T.T.; Kim, H.S.; Jeon, Y.J.; Kim, H.J.; Jee, Y. Sargassum horneri ethanol extract containing polyphenols attenuates PM-induced oxidative stress via ROS scavenging and transition metal chelation. J. Funct. Foods 2021, 79, 104401. [Google Scholar] [CrossRef]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.S.; Jeon, Y.J.; Jee, Y.; Kim, K.N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Dai, Y.L.; Jiang, Y.F.; Lu, Y.A.; Yu, J.B.; Kang, M.C.; Jeon, Y.J. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo. Toxicol. Rep. 2021, 8, 349–358. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef]
- Salari, Z.; Abbasnejad, M.; Hesni, M.A.; Esmaeili-Mahani, S. Effect of the Sargassum angustifolium Extract on Methamphetamine-Induced Cytotoxicity in SH-SY5Y Cells. Altern. Med. 2022, 2022, 9978235. [Google Scholar] [CrossRef]
- Wei, J.X.; Mou, C.Y.; Bao, Y.J.; Xie, Y.F.; Jin, H.X.; Shen, H.W.; Zhou, W.H.; Zhang, J.R.; He, S.; Chen, B.J.; et al. Fucoxanthin alleviates methamphetamine-induced neurotoxicity possibly via the inhibition of interaction between Keap1 and Nrf2. J. Funct. Foods 2021, 86, 104713. [Google Scholar] [CrossRef]
- Shahriari, A.G.; Mohkami, A.; Niazi, A.; Parizipour, M.H.G.; Habibi-Pirkoohi, M. Application of Brown Algae (Sargassum angustifolium) Extract for Improvement of Drought Tolerance in Canola (Brassica napus L.). Iran. J. Biotechnol. 2021, 19, 22–29. [Google Scholar] [CrossRef]
- Latique, S.; Elouaer, M.; Souguir, M.; Aloui, H.; Hannachi, C.; Chernane, H.; Mansori, M.; Elkaoua, M. Effect of seaweed extract of Sargassum vulgare on germination behavior of two bean cultivars (Phaseolus vulgaris L) under salt stress. IOSR J. Agric. Vet. Sci. 2014, 7, 116–120. [Google Scholar] [CrossRef]
- Han, S.; Park, J.S.; Umanzor, S.; Yarish, C.; Kim, J.K. Effects of extraction methods for a new source of biostimulant from Sargassum horneri on the growth of economically important red algae, Neopyropia yezoensis. Sci. Rep. 2022, 12, 11878. [Google Scholar] [CrossRef] [PubMed]
- Khedia, J.; Dangariya, M.; Nakum, A.K.; Agarwal, P.; Panda, A.; Parida, A.K.; Gangapur, D.R.; Meena, R.; Agarwal, P.K. Sargassum seaweed extract enhances Macrophomina phaseolina resistance in tomato by regulating phytohormones and antioxidative activity. J. Appl. Phycol. 2020, 32, 4373–4384. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, H.A.; Han, J.S. Sargassum sagamianum extract protects INS-1 pancreatic β cells against high glucose-induced apoptosis. Cytotechnology 2019, 71, 389–399. [Google Scholar] [CrossRef]
- Wu, S.Y.; Zuo, J.H.; Cheng, Y.; Zhang, Y.; Zhang, Z.S.; Wu, M.J.; Yang, Y.; Tong, H.B. Ethanol extract of Sargarsum fusiforme alleviates HFD/STZ-induced hyperglycemia in association with modulation of gut microbiota and intestinal metabolites in type 2 diabetic mice. Food Res. Int. 2021, 147, 110550. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Tanideh, N. Antidiabetic effect of fucoxanthin extracted from Sargassum angustifolium on streptozotocin-nicotinamide-induced type 2 diabetic mice. Food Sci. Nutr. 2021, 9, 3521–3529. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef]
- Guadalajara, U.O. Effect of Fucoxanthin on the Metabolic Syndrome, Insulin Sensitivity and Insulin Secretion. Available online: https://ClinicalTrials.gov/show/NCT03613740 (accessed on 30 September 2022).
- Matsui, T.; Ito, C.; Itoigawa, M.; Shibata, T. Three phlorotannins from Sargassum carpophyllum are effective against the secretion of allergic mediators from antigen-stimulated rat basophilic leukemia cells. Food Chem. 2022, 377, 131992. [Google Scholar] [CrossRef] [PubMed]
- Safaeian, L.; Yegdaneh, A.; Mobasherian, M. The Effect of Sargassum angustifolium Ethanol Extract on Cadmium Chloride-Induced Hypertension in Rat. Res. J. Pharmacogn. 2021, 8, 81–89. [Google Scholar] [CrossRef]
- Yegdaneh, A.; Safaeian, L.; Halvaei-Varnousfaderani, M.; Bazvand, S. Antihyperlipidemic and Antioxidant Effects of Ethanol Fraction of Sargassum angustifolium in Dexamethasone-Induced Dyslipidemic Rats. Res. J. Pharmacogn. 2022, 9, 39–49. [Google Scholar] [CrossRef]
- Ko, W.; Lee, H.; Kim, N.; Jo, H.G.; Woo, E.R.; Lee, K.; Han, Y.S.; Park, S.R.; Ahn, G.; Cheong, S.H.; et al. The Anti-Oxidative and Anti-Neuroinflammatory Effects of Sargassum horneri by Heme Oxygenase-1 Induction in BV2 and HT22 Cells. Antioxidants 2021, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Sengkhim, R.; Peerakietkhajorn, S.; Jeanmard, N.; Pongparadon, S.; Khuituan, P.; Thitiphatphuvanon, T.; Surinlert, P.; Tipbunjong, C. Effects of Sargassum plagiophyllum extract pretreatment on tissue histology of constipated mice. Trop. J. Pharm. Res. 2021, 20, 2339–2346. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.J.; Jee, Y.; Kim, H.J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-κB/MAPK Pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef]
- Choi, K.S.; Shin, T.S.; Chun, J.; Ahn, G.; Han, E.J.; Kim, M.J.; Kim, J.B.; Kim, S.H.; Kho, K.H.; Kim, D.H.; et al. Sargahydroquinoic acid isolated from Sargassum serratifolium as inhibitor of cellular basophils activation and passive cutaneous anaphylaxis in mice. Int. Immunopharmacol. 2022, 105, 108567. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. Protective efficacy of microencapsulated seaweed extracts for preventing Aeromonas infections in Oreochromis mossambicus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 218, 36–45. [Google Scholar] [CrossRef]
- Dousip, A.; Matanjun, P.; Sulaiman, M.R.; Tan, T.S.; Ooi, Y.B.H.; Lim, T.P. Effect of seaweed mixture intake on plasma lipid and antioxidant profile of hyperholesterolaemic rats. J. Appl. Phycol. 2014, 26, 999–1008. [Google Scholar] [CrossRef]
- Ibrahim, R.Y.M.; Hammad, H.B.I.; Gaafar, A.A.; Saber, A.A. The possible role of the seaweed Sargassum vulgare as a promising functional food ingredient minimizing aspartame-associated toxicity in rats. Int. J. Environ. Health Res. 2022, 32, 752–771. [Google Scholar] [CrossRef]
- He, W.; Sun, D. A Kind of Health Care Complex Tea and Preparation Method Thereof. China Patent CN104222378B, 17.08.2016, 17 August 2016. [Google Scholar]
- Practical and Creative Uses of Sargassum Seaweed in the Caribbean. Available online: https://www.villapalmarcancun.com/blog/destination/practical-and-creative-uses-of-sargassum-seaweed-in-the-caribbean (accessed on 5 January 2023).
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N. Influence of Indian Brown Seaweed (Sargassum marginatum) as an Ingredient on Quality, Biofunctional, and Microstructure Characteristics of Pasta. Food Sci. Technol. Int. 2009, 15, 471–479. [Google Scholar] [CrossRef]
- Kim, M.-J.; Kim, K.-B.-W.-R.; Lee, C.-J.; Kwak, J.-H.; Kim, D.-H.; SunWoo, C.; Jung, S.-A.; Kang, J.-Y.; Kim, H.-J.; Choi, J.-S. Effect of Sargassum sagamianum extract on shelf-life and improved quality of morning bread. Korean J. Food Sci. Technol. 2011, 43, 723–728. [Google Scholar] [CrossRef]
- Kim, M.-J.; Song, E.-J.; Kim, K.-B.-W.-R.; Lee, C.-J.; Jung, J.-Y.; Kwak, J.-H.; Choi, M.-K.; Kim, D.-H.; SunWoo, C.; Choi, J.-S. Effect of Sargassum fulvellum extracts on shelf-life and quality improvement of bread. J. Korean Soc. Food Sci. Nutr. 2011, 40, 867–874. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, K.-B.-W.-R.; Song, E.-J.; Kim, J.-H.; Kim, A.-R.; Kim, M.-J.; Moon, J.-H.; Kang, H.-M.; Lee, H.-D.; Hong, Y.-K. Effect of extracts from Sargassum siliquastrum on shelf-life and quality of bread. J. Korean Soc. Food Sci. Nutr. 2008, 37, 490–496. [Google Scholar] [CrossRef]
- Gan, L.J.; You, Q.; Luo, Y.M.; Ye, Y.T.; Lei, L.; Deng, Z.Y.; Rong, H. Effect of superfine grinding Sargassum fusiforme residue powder on sponge cakes properties. LWT Food Sci. Technol. 2022, 165, 113735. [Google Scholar] [CrossRef]
- Miyashita, K.; Abe, M. Fucoxanthin-Enriched Algae Product and Method for Producing the Same. Japan Patent JP2013031369A, 14 February 2013. [Google Scholar]
- Zhang, T.; Fu, S.; Wang, Y.; Xue, C.; Mao, X.; Xue, Y.; Wang, J.; Chang, Y.; Tang, Q.; Jiang, X. Application of Fucoxanthin and/or Fucoxanthin in Preparation for Improving DHA Level of Human Body. China Patent CN112674337A, 20 April 2021. [Google Scholar]
- Aditya, N.W.; Amin, M.N.G.; Alamsjah, M.A. The effect of fucoxanthin as coloring agent on the quality of catfish sausage. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012080. [Google Scholar] [CrossRef]
- Redmond, S.; Kim, J.K.; Yarish, C.; Pietrak, M.; Bricknell, I. Culture of Sargassum in Korea: Techniques and Potential for Culture in the US; The University of Maine: Orono, ME, USA, 2014. [Google Scholar]
- Lee, G.S.; Lee, D.H.; Ko, H.J.; Kim, K.B.; Kwon, S.W.; Pyo, H.B.; Lee, W.J.; Hyun, C.G. Sargassum sp. Extract that Have Low Photo-Induced Cytotoxicity, Preparation Method Thereof and Cosmetic Composition Containing the Same. Republic of Korea Patent KR1020110006444B1, 19 May 2011. [Google Scholar]
- He, M. Face Mask made of Seawead. China Patent CN1147374A, 16 April 1997. [Google Scholar]
- Cai, C.; Yang, Y.; Zheng, Y.; Hu, Y.; He, P.; Jia, R.; Wu, W.; Wu, H.; Zhou, L.; Zhang, Z. Sargassum horneri Boiled Alga Clay Mask and Preparation Method Thereof. China Patent CN103720619B, 16 April 2014. [Google Scholar]
- Li, X. Seaweed Mud Non-Woven Mask and Preparation Method Thereof. China Patent CN103876980A, 25 June 2014. [Google Scholar]
- Yang, L. Nano-Collagen Moistening Skin-Care Facial Mask. China Patent CN104323975A, 4 February 2015. [Google Scholar]
- Ltd., Q.A.M.D.C. Seaweed Paste Mask. China Patent CN103690464A, 2 April 2014. [Google Scholar]
- Mei, B.C.; Lyga, J.W. Sargassum muticum Extracts and Methods of Use. WO2014158854A1, 2 October 2014. [Google Scholar]
- Kim, H.R.; Im, K.T.; Im, S.J. Methods for the Preparation of Nano-emulsion Containing Skin-Whitening, Anti-Wrinkle and Anti-Inflammatory Cosmetic Composition and Use Thereof. Republic of Korea Patent KR1020210073174B1, 18 January 2022. [Google Scholar]
- Breton, L.; Girerd, F.; Renault, B. Use of Phloroglucinol in a Composition. Canada Patent CA2255222C, 16 December 2018. [Google Scholar]
- Decoder, I. Phloroglucinol Trimethyl Ether. Available online: https://incidecoder.com/ingredients/phloroglucinol-trimethyl-ether (accessed on 6 October 2022).
- Ismay, D. Some Colour Reactions of Phloroglucinol. J. Soc. Chem. Ind. 1950, 69, 58–60. [Google Scholar] [CrossRef]
- Andersen, F.A. Final report on the safety assessment of phloroglucinol. J. Am. Coll. Toxicol. 1995, 14, 468–475. [Google Scholar]
- Sipahutar, Y.H.; Albaar, N.; Purnamasari, H.B.; Kristiany, M.G.; Prabowo, D.H.G. Seaweed extract (Sargassum polycystum) as a preservative on sunscreen cream with the addition of seaweed porridge. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012072. [Google Scholar] [CrossRef]
- Pellegrini, M.; Andre, G.; Pellegrini, L. Cosmetic or Dermatological Compositions, e.g. for Combating Skin Aging, Containing Extract of Sargassum Muticum Brown Algae Having e.g. Antiradical and DNA Protecting Action. France Patent FR2838342A1, 17 June 2005. [Google Scholar]
- Shin, Y.-S.; Shin, C.-W.; Shin, Y.-W.; Park, Y.-H. Cosmetic Composition Including Extracts of Sargassum Horneri and Enteromorpha Prolifera. Republic of Korea Patent KR20190087199A, 24 July 2019. [Google Scholar]
- Mansauda Karlah Lifie, R.; Effionora, A.; Tati, N. Antioxidant and Anti-Collagenase Activity of Sargassum Plagyophyllum Extract as an Anti-Wrinkle Cosmetic Ingredient. Pharmacogn. J. 2018, 10, 932–936. [Google Scholar] [CrossRef]
- Gazali, M. Aktivitas Inhibitor Tirosinase Pada Ekstrak Alga Cokelat Sargassum Sp. Agardh Asal Pesisir Lhok Bubon, Kabu-paten Aceh Barat. J. Perikan. Terpadu 2018, 1, 26–40. [Google Scholar] [CrossRef]
- Biocogent. DermalRx. Available online: https://www.biocogent.com/dermalrx (accessed on 5 January 2023).
- Decoder, I. Fucoxanthin. Available online: https://incidecoder.com/ingredients/fucoxanthin (accessed on 6 October 2022).
- Hwang, Y. Anti-Obesity Cream Composition Containing Fucoxanthin. WO2011152692A2, 8 December 2011. [Google Scholar]
- Sembera, J.A.; Meier, E.J.; Waliczek, T.M. Composting as an Alternative Management Strategy for Sargassum Drifts on Coastlines. Horttechnology 2018, 28, 80–84. [Google Scholar] [CrossRef]
- Mendoza-Morales, L.T.; Mendoza-González, A.C.; Mateo-Cid, L.E.; Rodríguez-Dorantes, A. Analysis of the effect as biostimulants of Sargassum vulgare and Ulva fasciata extracts on Lens esculenta growth. Mex. J. Biotechnol. 2019, 4, 15–28. [Google Scholar] [CrossRef]
- Latique, S.; Elouaer, M.A.; Chernane, H.; Hannachi, C.; Elkaoua, M. Effect of seaweed liquid extract of Sargassum vulgare on growth of durum wheat seedlings (Triticum durum L.) under salt stress. Int. J. Innov. Appl. Stud. 2014, 7, 1430. [Google Scholar]
- Sbaihat, L.; Takeyama, K.; Koga, T.; Takemoto, D.; Kawakita, K. Induced Resistance in Solanum lycopersicum by Algal Elicitor Extracted from Sargassum fusiforme. Sci. World J. 2015, 2015, 870520. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).