Possible Role of Docosahexaenoic Acid in Response to Diarrhetic Shellfish Toxins in the Mussel Perna viridis
Abstract
1. Introduction
2. Results
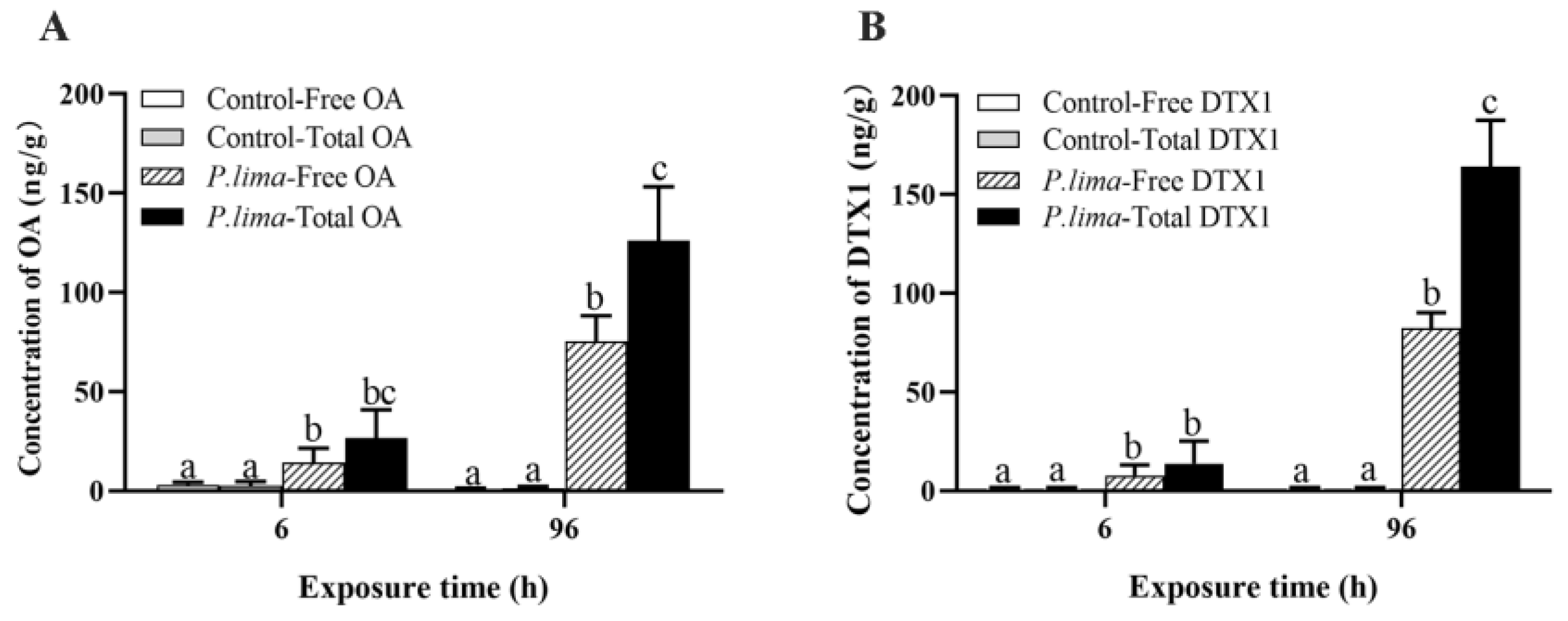
2.1. Changes in Accumulation and Esterification of DTSs after Exposure to P. lima
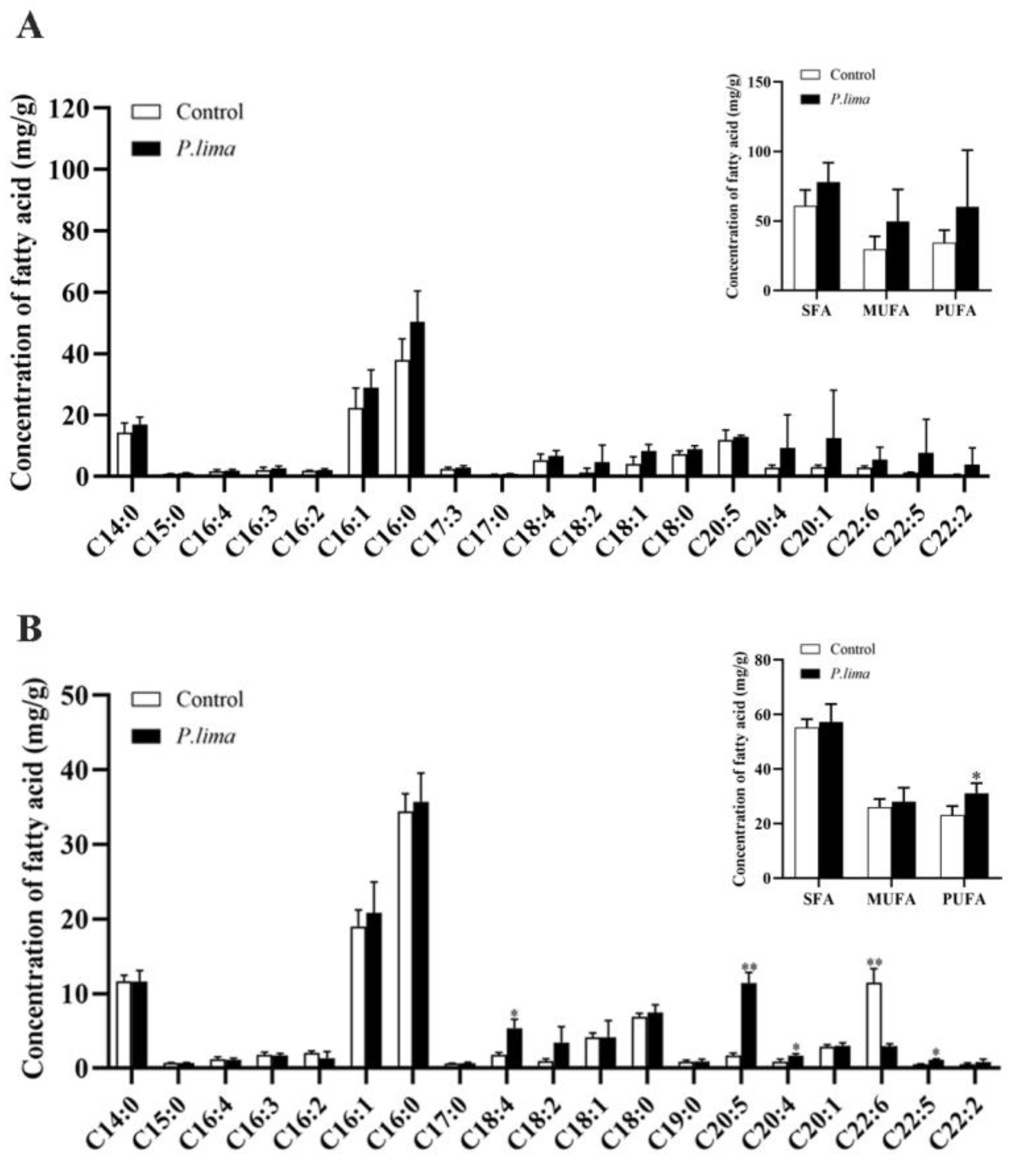
2.2. Changes in Fatty Acid Levels in the Digestive Gland after Exposure to P. lima
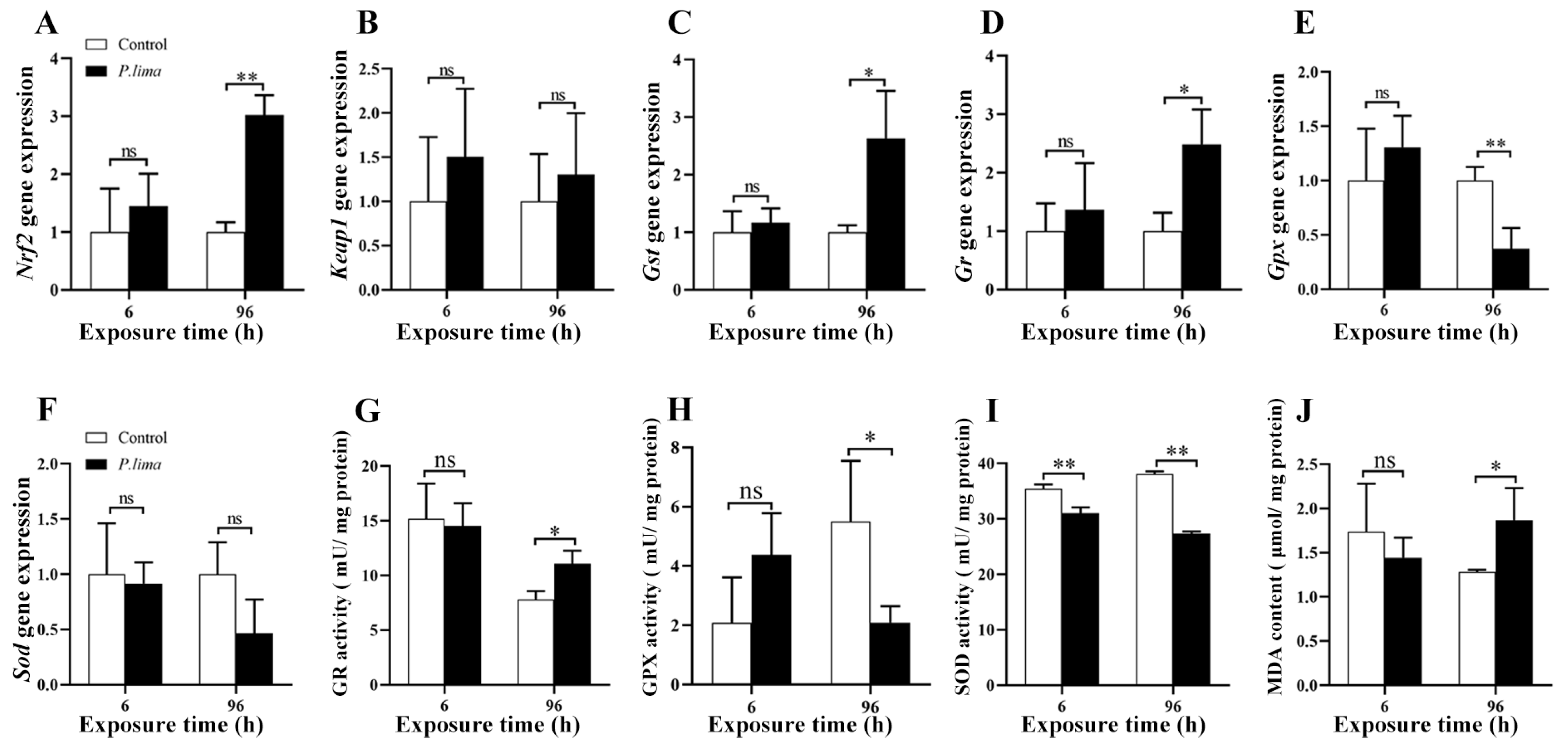
2.3. Changes in Nrf2/ARE Signaling Pathway in the Digestive Gland after Exposure to P. lima
2.4. Changes in the Esterification Level of DSTs after Addition of DHA
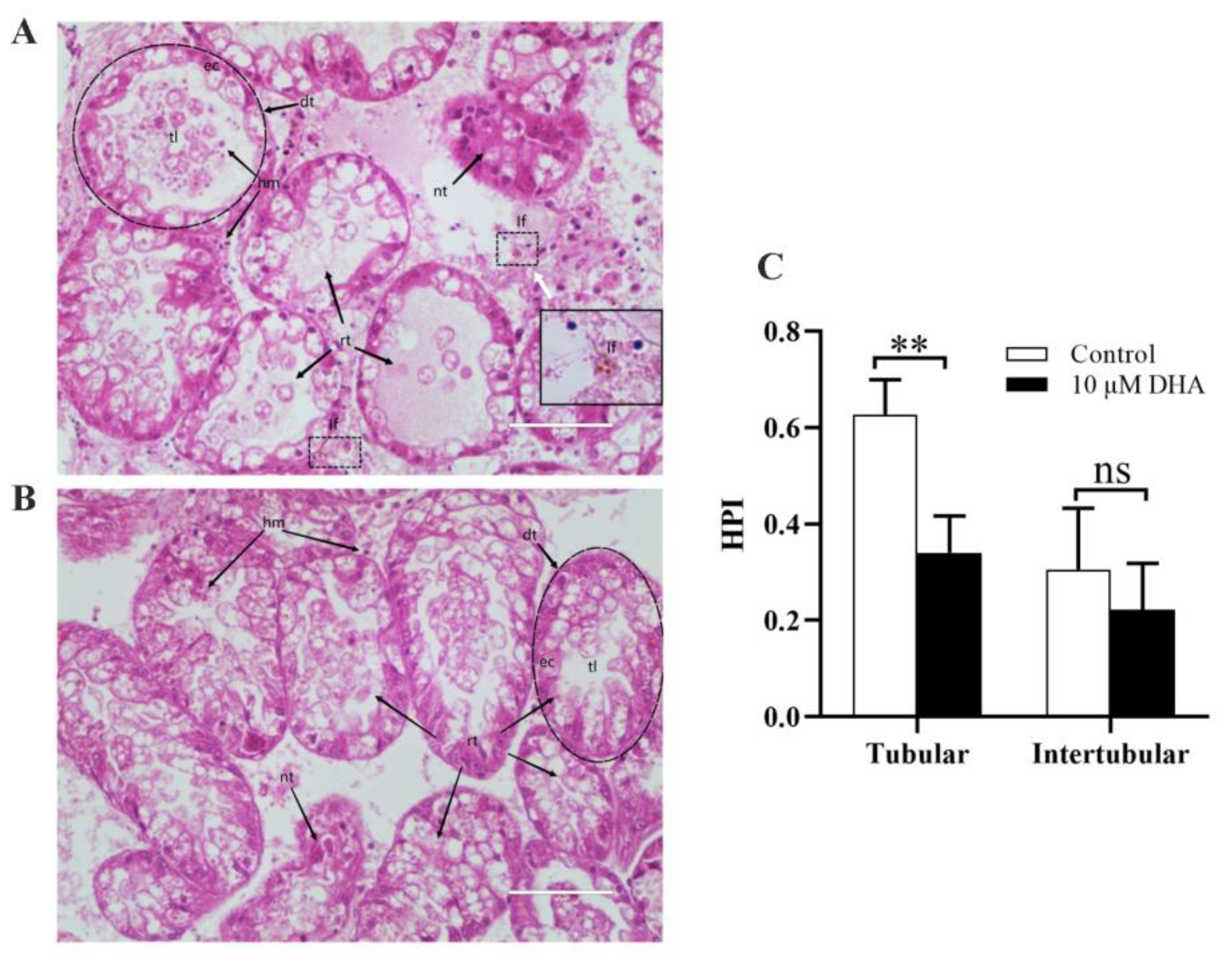
2.5. DHA Reduced Damage Caused by DSTs in the Digestive Gland
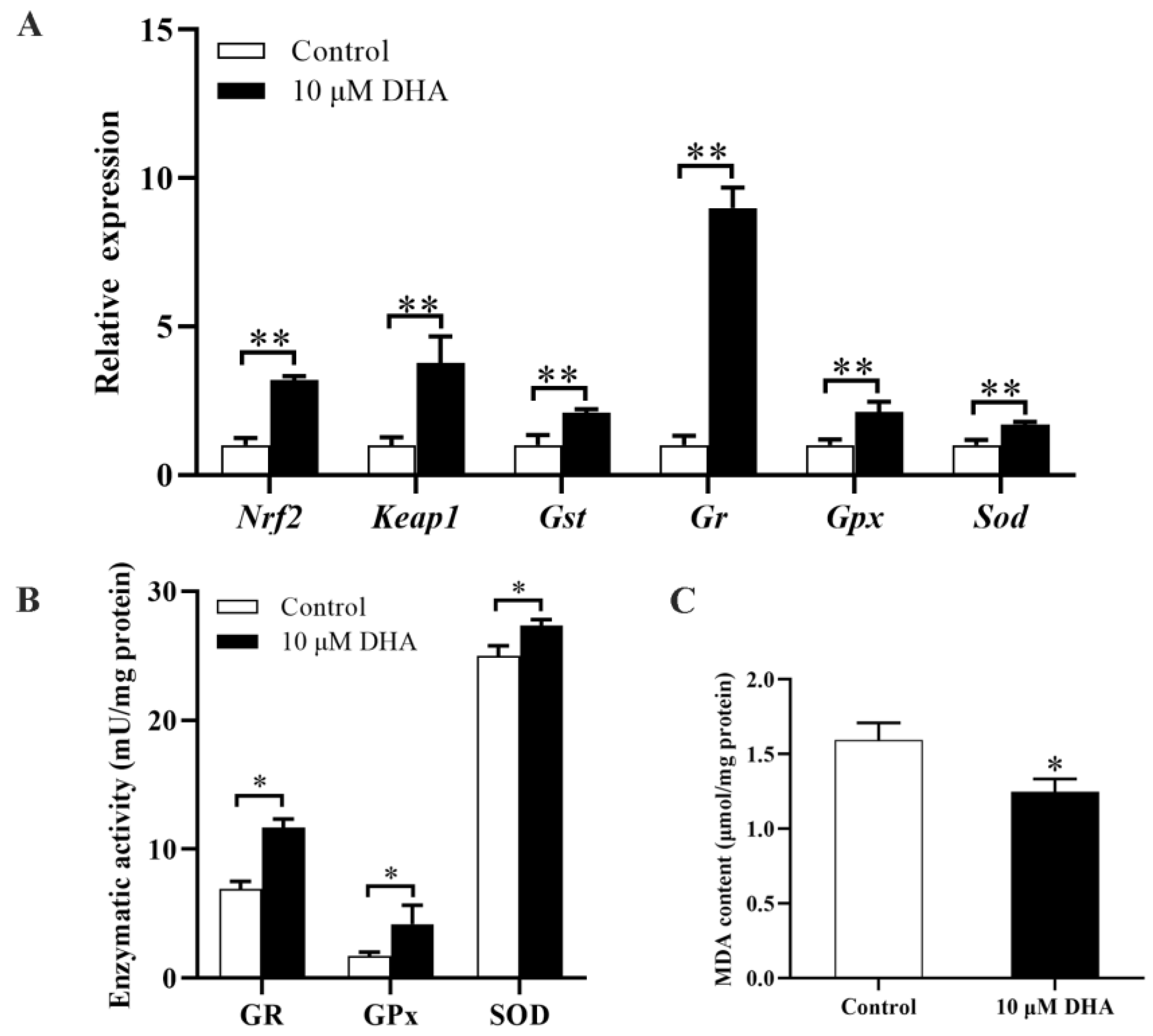
2.6. DHA Activated the Nrf2/ARE Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Animal Maintenance and Algae
4.2. Experimental Design
4.3. Toxin Analysis
4.4. Analysis of Fatty Acids
4.5. RNA Extraction and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.6. Detection of Oxidative Stress Biomarkers
4.7. Histological Examination
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Reguera, B.; Velo-Suárez, L.; Raine, R.; Park, M.G. Harmful Dinophysis species: A review. Harmful Algae 2012, 14, 87–106. [Google Scholar] [CrossRef]
- Chen, T.; Xu, X.; Wei, J.; Chen, J.; Miu, R.; Huang, L.; Zhou, X.; Fu, Y.; Yan, R.; Wang, Z.; et al. Food-borne disease outbreak of diarrhetic shellfish poisoning due to toxic mussel consumption: The first recorded outbreak in China. PLoS ONE 2013, 8, e65049. [Google Scholar] [CrossRef]
- Taylor, M.; McIntyre, L.; Ritson, M.; Stone, J.; Bronson, R.; Bitzikos, O.; Rourke, W.; Galanis, E.; Team, O. Outbreak of diarrhetic shellfish poisoning associated with mussels, British Columbia, Canada. Mar. Drugs 2013, 11, 1669–1676. [Google Scholar] [CrossRef]
- Sellner, K.G.; Doucette, G.J.; Kirkpatrick, G.J. H Harmful algal blooms: Causes, impacts and detection. J. Ind. Microbiol. Biotechnol. 2003, 30, 383–406. [Google Scholar] [CrossRef]
- Buratti, S.; Franzellitti, S.; Poletti, R.; Ceredi, A.; Montanari, G.; Capuzzo, A.; Fabbri, E. Bioaccumulation of algal toxins and changes in physiological parameters in Mediterranean mussels from the North Adriatic Sea (Italy). Environ. Toxicol. 2013, 28, 451–470. [Google Scholar] [CrossRef]
- Reguera, B.; Riobo, P.; Rodriguez, F.; Diaz, P.A.; Pizarro, G.; Paz, B.; Franco, J.M.; Blanco, J. Dinophysis toxins: Causative organisms, distribution and fate in shellfish. Mar. Drugs 2014, 12, 394–461. [Google Scholar] [CrossRef]
- Takai, A.; Murata, M.; Torigoe, K.; Isobe, M.; Mieskes, G.; Yasumoto, T. Inhibitory effect of okadaic acid derivatives on protein phosphatases. A study on structure-affinity relationship. Biochem. J. 1992, 284, 539–544. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Opinion of the scientific panel on contaminants in the food chain on a request from the European Commission on marine biotoxins in shellfish–okadaic acid and analoguesn. EFSA J. 2008, 589, 1–62. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Pasaro, E.; Mendez, J.; Laffon, B. Okadaic acid: More than a diarrheic toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.K.; Rai, S.; Nath, C. Okadaic acid induced neurotoxicity: An emerging tool to study Alzheimer’s disease pathology. Neurotoxicology 2013, 37, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, J.; Hao, Y.; Nie, Y.; Li, Z.; Qian, M.; Liang, Q.; Yu, J.; Zeng, M.; Wu, K. Differentiated tumor immune microenvironment of Epstein–Barr virus-associated and negative gastric cancer: Implication in prognosis and immunotherapy. Oncotarget 2017, 8, 67094–67103. [Google Scholar] [CrossRef] [PubMed]
- Svensson, S.; Särngren, A.; Förlin, L. Mussel blood cells, resistant to the cytotoxic effects of okadaic acid, do not express cell membrane p-glycoprotein activity (multixenobiotic resistance). Aquat. Toxicol. 2003, 65, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Prado-Alvarez, M.; Florez-Barros, F.; Sexto-Iglesias, A.; Mendez, J.; Fernandez-Tajes, J. Effects of okadaic acid on haemocytes from Mytilus galloprovincialis: A comparison between field and laboratory studies. Mar. Environ. Res. 2012, 81, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Prego-Faraldo, M.V.; Valdiglesias, V.; Laffon, B.; Mendez, J.; Eirin-Lopez, J.M. Early genotoxic and cytotoxic effects of the toxic dinoflagellate Prorocentrum lima in the mussel Mytilus galloprovincialis. Toxins 2016, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Prado-Alvarez, M.; Florez-Barros, F.; Mendez, J.; Fernandez-Tajes, J. Effect of okadaic acid on carpet shell clam (Ruditapes decussatus) haemocytes by in vitro exposure and harmful algal bloom simulation assays. Cell Biol. Toxicol. 2013, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J. Accumulation of dinophysis toxins in bivalve molluscs. Toxins 2018, 10, 453. [Google Scholar] [CrossRef]
- Yanagi, T.; Murata, M.; Torigoe, K.; Yasumoto, T. Biological activities of semisynthetic analogs of dinophysistoxin-3, the major diarrhetic shellfish toxin. Agric. Bioi. Chem. 1989, 53, 525–529. [Google Scholar] [CrossRef]
- Suzuki, T.; Ota, H.; Yamasaki, M. Direct evidence of transformation of dinophysistoxin-1 to 7-O-acyl-dinophysistoxin-1 (dinophysistoxin-3) in the scallop Patinopecten yessoensis. Toxicon 1999, 37, 187–198. [Google Scholar] [CrossRef]
- Rossignoli, A.E.; Fernandez, D.; Regueiro, J.; Marino, C.; Blanco, J. Esterification of okadaic acid in the mussel Mytilus galloprovincialis. Toxicon 2011, 57, 712–720. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Chen, W.-C.; Li, H.-Y.; Liu, J.-S.; Jiang, T.; Yang, W.-D. P-glycoprotein expression in Perna viridis after exposure to Prorocentrum lima, a dinoflagellate producing DSP toxins. Fish Shellfish Immunol. 2014, 39, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Vidal, A.; Ruiz, Y.; Suárez, P.; Martinez, A.A.; Rossignoli, A.; Blanco, J.; Garcia, O.; San Juan, F. Accumulation of Okadaic Acid and Detoxifying Enzymes in the Digestive Gland of Mytilus galloprovincialis during exposure to DSP. In Molluscan Shellfish Safety; Springer: Berlin/Heidelberg, Germany, 2014; pp. 217–225. [Google Scholar] [CrossRef]
- Zou, Y.; Wei, X.M.; Weng, H.W.; Li, H.Y.; Liu, J.S.; Yang, W.D. Expression profile of eight glutathione S-transferase genes in Crassostrea ariakensis after exposure to DSP toxins producing dinoflagellate Prorocentrum lima. Toxicon 2015, 105, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, S.W.; Kim, H.J.; Kang, J.W.; Park, S.C. Detoxification- and immune-related transcriptomic analysis of gills from bay scallops (Argopectenirradians) in response to algal toxin okadaic acid. Toxins 2018, 10, 308. [Google Scholar] [CrossRef]
- Lv, J.J.; Yuan, K.K.; Lu, M.Y.; He, Z.B.; Li, H.Y.; Yang, W.D. Responses of JNK signaling pathway to the toxic dinoflagellate Prorocentrum lima in the mussel Perna viridis. Ecotoxicol. Environ. Saf. 2021, 227, 112905. [Google Scholar] [CrossRef]
- Tan, K.; Ma, H.; Li, S.; Zheng, H. Bivalves as future source of sustainable natural omega-3 polyunsaturated fatty acids. Food Chem. 2020, 311, 125907. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zheng, H. Endogenous LC-PUFA biosynthesis capability in commercially important mollusks. Crit. Rev. Food Sci. Nutr. 2020, 62, 2836–2844. [Google Scholar] [CrossRef]
- Gao, L.; Wang, J.; Sekhar, K.R.; Yin, H.; Yared, N.F.; Schneider, S.N.; Sasi, S.; Dalton, T.P.; Anderson, M.E.; Chan, J.Y.; et al. Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J. Biol. Chem. 2007, 282, 2529–2537. [Google Scholar] [CrossRef]
- Hendriks, I.E.; van Duren, L.A.; Herman, P.M.J. Effect of dietary polyunsaturated fatty acids on reproductive output and larval growth of bivalves. J. Exp. Mar. Biol. Ecol. 2003, 296, 199–213. [Google Scholar] [CrossRef]
- Nevejan, N.; Saez, I.; Gajardo, G.; Sorgeloos, P. Supplementation of EPA and DHA emulsions to a Dunaliella tertiolecta diet: Effect on growth and lipid composition of scallop larvae, Argopecten purpuratus (Lamarck, 1819). Aquaculture 2003, 217, 613–632. [Google Scholar] [CrossRef]
- Sühnel, S.; Lagreze, F.; Zanette, G.; Magalhães, A.R.M.; Ferreira, J.F. Effect of the fatty acid EPA and DHA in the conditioning of the scallop Nodipecten nodosus (Linné, 1758). Aquaculture 2012, 330–333, 167–171. [Google Scholar] [CrossRef]
- Shahbazi, A.; Zakaria, M.P.; Yap, C.K.; Tan, S.G.; Surif, S.; Mohamed, C.A.R.; Sakari, M.; Bakhtiari, A.R.; Bahry, P.S.; Chandru, K.; et al. Use of different tissues of Perna viridis as biomonitors of polycyclic aromatic hydrocarbons (PAHs) in the coastal waters of Peninsular Malaysia. Environ. Forensics 2010, 11, 248–263. [Google Scholar] [CrossRef]
- Yap, C.K.; Shahbazi, A.; Zakaria, M.P. Concentrations of heavy metals (Cu, Cd, Zn and Ni) and PAHs in Perna viridis collected from seaport and non-seaport waters in the Straits of Johore. Bull. Environ. Contam. Toxicol. 2012, 89, 1205–1210. [Google Scholar] [CrossRef] [PubMed]
- Prego-Faraldo, M.V.; Martinez, L.; Mendez, J. RNA-Seq analysis for assessing the early response to DSP toxins in Mytilus galloprovincialis digestive gland and gill. Toxins 2018, 10, 417. [Google Scholar] [CrossRef] [PubMed]
- He, Z.B.; Duan, G.F.; Liang, C.Y.; Li, H.Y.; Liu, J.S.; Yang, W.D. Up-regulation of Nrf2-dependent antioxidant defenses in Perna viridis after exposed to Prorocentrum lima. Fish Shellfish Immunol. 2019, 90, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.M.; Lu, M.Y.; Duan, G.F.; Li, H.Y.; Liu, J.S.; Yang, W.D. Responses of CYP450 in the mussel Perna viridis after short-term exposure to the DSP toxins-producing dinoflagellate Prorocentrum lima. Ecotoxicol. Environ. Saf. 2019, 176, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Dou, M.; Jiao, Y.H.; Zheng, J.W.; Zhang, G.; Li, H.Y.; Liu, J.S.; Yang, W.D. De novo transcriptome analysis of the mussel Perna viridis after exposure to the toxic dinoflagellate Prorocentrum lima. Ecotoxicol. Environ. Saf. 2020, 192, 110265. [Google Scholar] [CrossRef] [PubMed]
- Konoki, K.; Onoda, T.; Watanabe, R.; Cho, Y.; Kaga, S.; Suzuki, T.; Yotsu-Yamashita, M. In vitro acylation of okadaic acid in the presence of various bivalves’ extracts. Mar. Drugs 2013, 11, 300–315. [Google Scholar] [CrossRef]
- Qiu, J.; Ji, Y.; Fang, Y.; Zhao, M.; Wang, S.; Ai, Q.; Li, A. Response of fatty acids and lipid metabolism enzymes during accumulation, depuration and esterification of diarrhetic shellfish toxins in mussels (Mytilus galloprovincialis). Ecotoxicol. Environ. Saf. 2020, 206, 111223. [Google Scholar] [CrossRef]
- Blanco, J.; Arevalo, F.; Correa, J.; Morono, A. Lipophilic toxins in Galicia (NW Spain) between 2014 and 2017: Incidence on the main molluscan species and analysis of the monitoring efficiency. Toxins 2019, 11, 612. [Google Scholar] [CrossRef]
- Vale, P. Detailed profiles of 7-O-acyl esters in plankton and shellfish from the Portuguese coast. J. Chromatogr. A 2006, 1128, 181–188. [Google Scholar] [CrossRef]
- Lv, J.J.; Yuan, K.K.; Lu, G.X.; Li, H.Y.; Kwok, H.F.; Yang, W.D. Responses of ABCB and ABCC transporters to the toxic dinoflagellate Prorocentrum lima in the mussel Perna viridis. Aquat. Toxicol. 2022, 254, 106368. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Zhang, H.; Li, S.; Ma, H.; Zheng, H. Lipid nutritional quality of marine and freshwater bivalves and their aquaculture potential. Crit. Rev. Food Sci. Nutr. 2021, 62, 6990–7014. [Google Scholar] [CrossRef] [PubMed]
- Knauer, J.; Southgate, P.C. Growth and fatty acid composition of Pacific oyster (Crassostrea gigas) spat fed a microalga and microcapsules containing varying amounts of eicosapentaenoic and docosahexaenoic acid. J. Shellfish Res. 1997, 16, 447–454. [Google Scholar]
- Prasad, P.; Anjali, P.; Sreedhar, R.V. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit. Rev. Food Sci. Nutr. 2021, 61, 1725–1737. [Google Scholar] [CrossRef]
- Pes, K.; Friese, A.; Cox, C.J.; Laizé, V.; Fernández, I. Biochemical and molecular responses of the Mediterranean mussel (Mytilus galloprovincialis) to short-term exposure to three commonly prescribed drugs. Mar. Environ. Res. 2021, 168, 105309. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Xiao, B.; Cui, D.; Lin, Y.; Zeng, J.; Li, J.; Cao, M.-J.; Liu, J. Antioxidant activity of docosahexaenoic acid (DHA) and its regulatory roles in mitochondria. J. Agric. Food Chem. 2021, 69, 1647–1655. [Google Scholar] [CrossRef]
- Magalhães, R.; Martins, N.; Fontinha, F.; Couto, A.; Serra, C.R.; Santos, R.A.; Olsen, R.E.; Peres, H.; Oliva-Teles, A. Effects of dietary ARA, DHA, and carbohydrates levels on gilthead sea bream liver and intestine oxidative stress, tissue histomorphology, and gut microbiota. Aquaculture 2022, 552, 738014. [Google Scholar] [CrossRef]
- Costa, P.M.; Carreira, S.; Costa, M.H.; Caeiro, S. Development of histopathological indices in a commercial marine bivalve (Ruditapes decussatus) to determine environmental quality. Aquat. Toxicol. 2013, 126, 442–454. [Google Scholar] [CrossRef]
- De Jesús Romero-Geraldo, R.; Garcia-Lagunas, N.; Hernandez-Saavedra, N.Y. Crassostrea gigas exposure to the dinoflagellate Prorocentrum lima: Histological and gene expression effects on the digestive gland. Mar. Environ. Res. 2016, 120, 93–102. [Google Scholar] [CrossRef]
- Neves, R.A.F.; Santiago, T.C.; Carvalho, W.F.; Silva, E.D.S.; da Silva, P.M.; Nascimento, S.M. Impacts of the toxic benthic dinoflagellate Prorocentrum lima on the brown mussel Perna perna: Shell-valve closure response, immunology, and histopathology. Mar. Environ. Res. 2019, 146, 35–45. [Google Scholar] [CrossRef]
- Ye, M.H.; Li, D.W.; Cai, Q.D.; Jiao, Y.H.; Liu, Y.; Li, H.Y.; Yang, W.D. Toxic responses of different shellfish species after exposure to Prorocentrum lima, a DSP toxins producing dinoflagellate. Toxins 2022, 14, 461. [Google Scholar] [CrossRef]
- Li, D.W.; Cen, S.Y.; Liu, Y.H.; Balamurugan, S.; Zheng, X.Y.; Alimujiang, A.; Yang, W.D.; Liu, J.S.; Li, H.Y. A type 2 diacylglycerol acyltransferase accelerates the triacylglycerol biosynthesis in heterokont oleaginous microalga Nannochloropsis oceanica. J. Biotechnol. 2016, 229, 65–71. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Holm, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Joshy, A.; Sharma, S.R.K.; Mini, K.G.; Gangadharan, S.; Pranav, P. Histopathological evaluation of bivalves from the southwest coast of India as an indicator of environmental quality. Aquat. Toxicol. 2022, 243, 106076. [Google Scholar] [CrossRef]


| Gene Name | Primer Sequence (5′-3′) | Amplicon Size (bp) | |
|---|---|---|---|
| Ef1α | F: | CACTCCGTCTTCCACTCCA | 131 |
| R: | CCTCTGGCATTGACTCGTG | ||
| Rpl3 | F: | GGTGGCACTATCTCCCAGAA | 98 |
| R: | GCCATCTGGACGTTACACCT | ||
| Uba52 | F: | TTACATTTGGTCCTGCGTCTC | 135 |
| R: | CAGTTGGTAGCCCTTTGATGA | ||
| Rpl13 | F: | TAAAGACTGGCAACGCTATGT | 155 |
| R: | TCACAACTGGTCGGAGAAG | ||
| Rpl37 | F: | GTCGCAATAAGACGCACACGTTG | 179 |
| R: | GTGCCTCATTCGACCAGTTCCG | ||
| Nrf2 | F: | TCAACCTGGACAGGAACCCA | 90 |
| R: | TATCGCGACAGTGTGGACCT | ||
| Keap1 | F: | TATCGCTCCAATGAACACGG | 173 |
| R: | AAGCACTTCTGGGGCTACGC | ||
| Gst | F: | GTTGGCTCGAAATTAAGTATGGC | 108 |
| R: | AAACTCCTCCAGTATTTTCTGGTCT | ||
| Gr | F: | TTACTCCAGTTGCCATAGCAGCAG | 113 |
| R: | TGGATGTGAGAACACCACAGTAGC | ||
| Gpx | F: | CAACGACCCCCAGATTCAGA | 80 |
| R: | TCTAGAGTCGGTAGGAGCCAT | ||
| Sod | F: | GCAACATTCCTTCAGCACCT | 154 |
| R: | CCTTGTTCCAAAAGCCTAATTG | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, K.-K.; Chen, Z.-M.; Liu, Y.-X.; Li, H.-Y.; Yang, W.-D. Possible Role of Docosahexaenoic Acid in Response to Diarrhetic Shellfish Toxins in the Mussel Perna viridis. Mar. Drugs 2023, 21, 155. https://doi.org/10.3390/md21030155
Yuan K-K, Chen Z-M, Liu Y-X, Li H-Y, Yang W-D. Possible Role of Docosahexaenoic Acid in Response to Diarrhetic Shellfish Toxins in the Mussel Perna viridis. Marine Drugs. 2023; 21(3):155. https://doi.org/10.3390/md21030155
Chicago/Turabian StyleYuan, Kuan-Kuan, Zi-Min Chen, Ya-Xin Liu, Hong-Ye Li, and Wei-Dong Yang. 2023. "Possible Role of Docosahexaenoic Acid in Response to Diarrhetic Shellfish Toxins in the Mussel Perna viridis" Marine Drugs 21, no. 3: 155. https://doi.org/10.3390/md21030155
APA StyleYuan, K.-K., Chen, Z.-M., Liu, Y.-X., Li, H.-Y., & Yang, W.-D. (2023). Possible Role of Docosahexaenoic Acid in Response to Diarrhetic Shellfish Toxins in the Mussel Perna viridis. Marine Drugs, 21(3), 155. https://doi.org/10.3390/md21030155







