Identification of Antioxidative Peptides Derived from Arthrospira maxima in the Biorefinery Process after Extraction of C-Phycocyanin and Lipids
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening Promising Enzymes for Producing Antioxidative Peptides from Spent Biomass of A. maxima after the Recovery of C-PC and Lipids

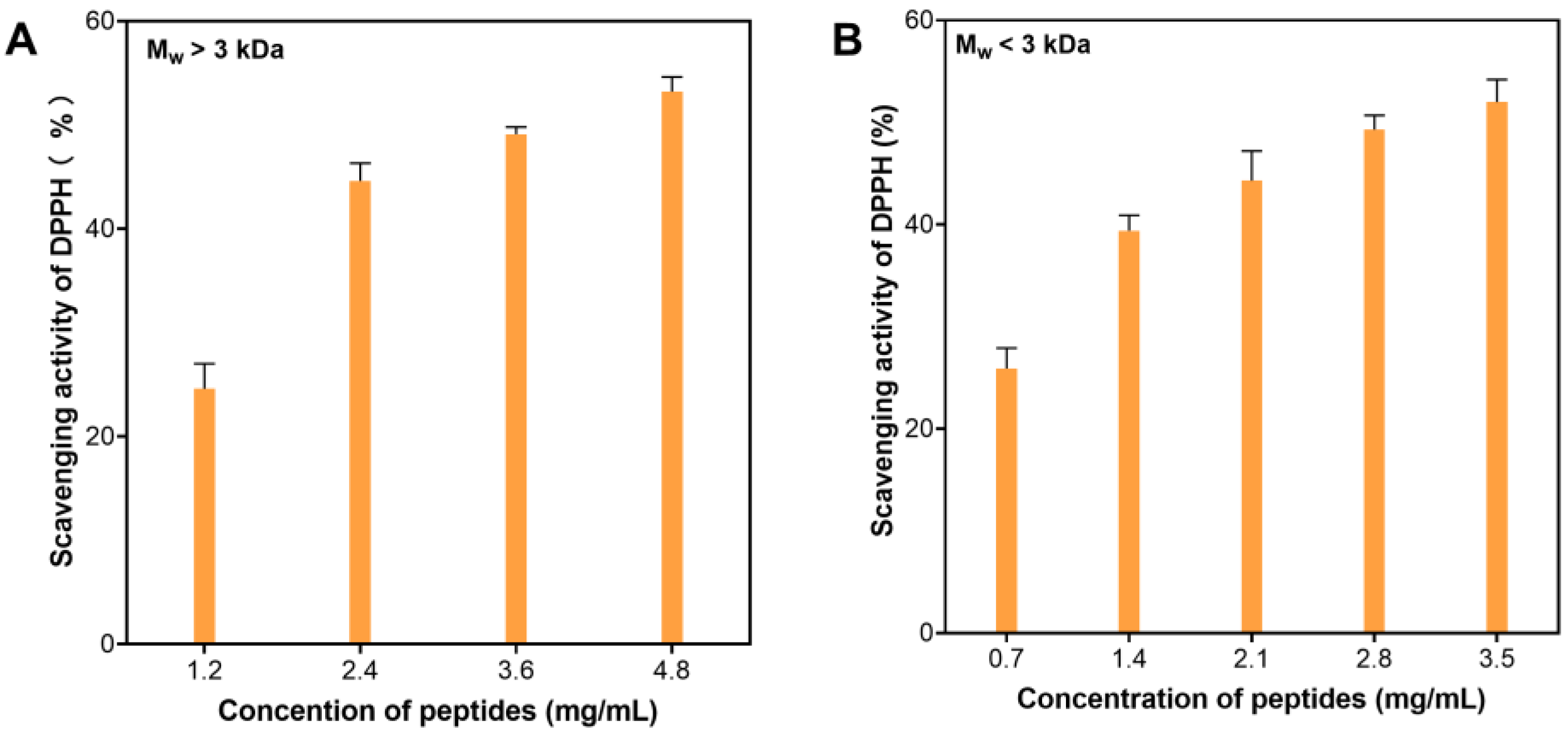
2.2. Antioxidative Activities of Peptide Fractions by 3-kDa Ultrafiltration
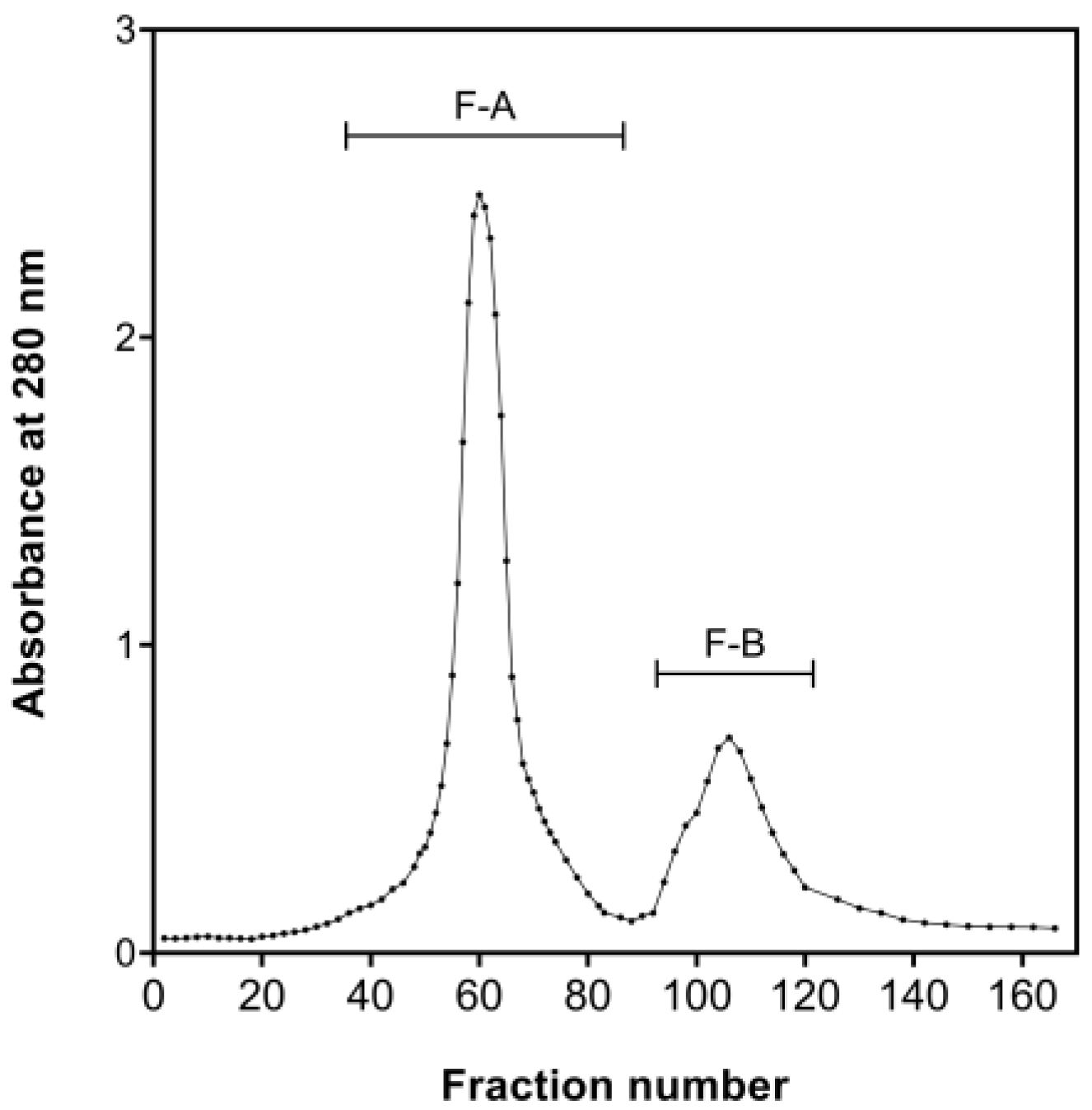
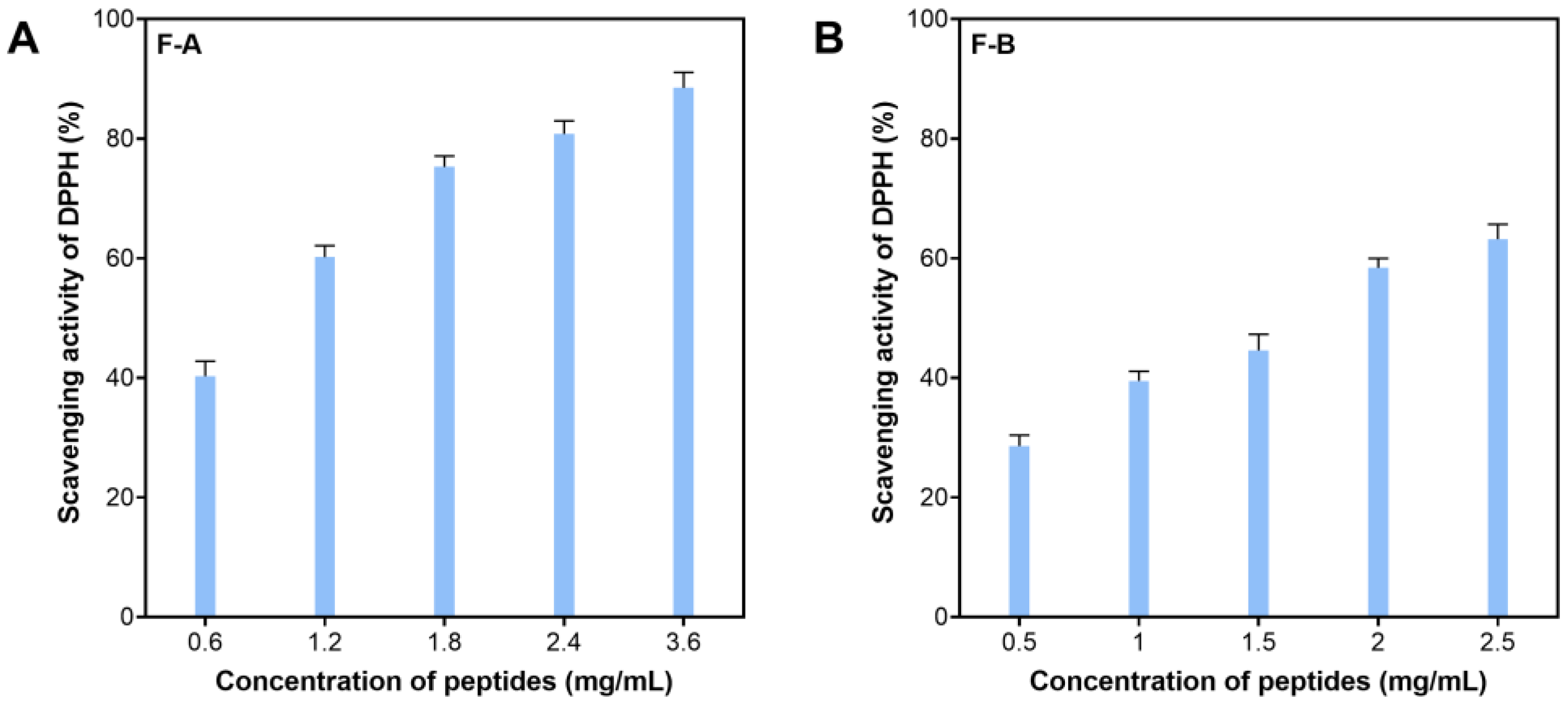
2.3. Antioxidative Peptides Purified by Chromatography Gel Filtration
2.4. Prediction of Bioactive Potential of Peptides Identified from LC-MS/MS Results
| Peptide Sequence | PepRank | Molecular Weight (Da) | Potential Bioactivities | Protein Group |
|---|---|---|---|---|
| KNAMPAFNGRL | 0.86 | 1218.4 | ACE inhibitor, DPP IV inhibitor | Cytochrome C6 |
| RALGFDFRR | 0.83 | 1137.3 | ACE inhibitor, Antioxidative, DPP IV inhibitor, DPP III inhibitor, Activating ubiquitin-mediated proteolysis | Chlorophyll a/b binding light-harvesting protein |
| KAPGFGDRR | 0.78 | 1003.1 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Antiamnestic, Antithrombotic, Regulating | 60 kDa chaperonin |
| RHTPFFKG | 0.77 | 989.1 | ACE inhibitor, DPP IV inhibitor, Antioxidative | Elongation factor |
| RNPAIFRG | 0.75 | 930.1 | ACE inhibitor, DPP IV inhibitor | Carbohydrate-selective porin OprB |
| KFFYPNFQTRV | 0.75 | 1446.7 | ACE inhibitor, DPP IV inhibitor, Alpha-glucosidase inhibitor, Renin inhibitor, CaMPDE inhibitor | Phycobilisome linker polypeptide |
| RGQWTVGFNRM | 0.71 | 1351.6 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Renin inhibitor, Neuropeptide | Phycobilisome linker polypeptide |
| KFFYGNSQVRF | 0.68 | 1392.6 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, CaMPDE inhibitor, Renin inhibitor, Immunomodulating | Phycobilisome linker polypeptide |
| KAGYLFPEIARR | 0.67 | 1420.7 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Renin inhibitor, Neuropeptide, Alpha-glucosidase inhibitor | LL-diaminopimelate aminotransferase |
| RDNVLRF | 0.63 | 919 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Renin inhibitor, Stimulating | Orange carotenoid protein |
| RIPPYRN | 0.63 | 915.1 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Alpha-amylase inhibitor, Anti-inflammatory, Alpha-glucosidase inhibitor | Polypeptide-transport-associated domain protein ShlB-type |
| RNLGAGSQFNLPRN | 0.62 | 1543.7 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Renin inhibitor | Extracellular solute-binding protein family 3 |
| RSIPTLMIFKG | 0.60 | 1262.6 | ACE inhibitor, DPP IV inhibitor | Thioredoxin |
| RQMSLLLRR | 0.60 | 1172.4 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Renin inhibitor, Stimulating, Regulating, Antioxidative | Hypothetical protein |
| RLQLLARF | 0.58 | 1016.2 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Renin inhibitor, Stimulating, Antioxidative, Activating ubiquitin-mediated proteolysis | Methyltransferase type 11 |
| RFGIISVRF | 0.58 | 1094.3 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Stimulating | Uncharacterized protein |
| KFVVGGPQGDSGLTGRK | 0.58 | 1702.9 | ACE inhibitor, DPP IV inhibitor, Regulating, Antiamnestic, Antithrombotic, Renin inhibitor, CaMPDE inhibitor | S-adenosylmethionine synthase |
| KVAINGFGRI | 0.57 | 1074.3 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor | Glyceraldehyde-3-phosphate dehydrogenase |
| KADSLISGAAQAVYNKF | 0.54 | 1783.0 | ACE inhibitor, DPP IV inhibitor, DPP III inhibitor, Stimulating, Regulating, Alpha-glucosidase inhibitor, CaMPDE inhibitor, Renin inhibitor, Hypotensive, Antioxidative | C-phycocyanin alpha subunit |
| KIGLFGGAGVGKT | 0.54 | 1204.4 | ACE inhibitor, DPP IV inhibitor, Regulating, Immunomodulating | ATP synthase subunit beta |
| RAGGYTRL | 0.52 | 893.0 | ACE inhibitor, DPP IV inhibitor, Activating ubiquitin-mediated proteolysis | Dihydroorotase |
| KRPDFIAPGGNAAGQRE | 0.52 | 1783.9 | ACE inhibitor, DPP IV inhibitor, Regulating, Antiamnestic, Antithrombotic, Immunomodulating, Hypotensive, Neuropeptide | Phycobilisome protein |
2.5. Antioxidative Peptides Identified Based on In Silico Analysis
| Peptide Sequences | Activity | Bioactive Sequences | Fragmentation Locations |
|---|---|---|---|
| RALGFDFRR | Antioxidative | LGF | (3-5) |
| RHTPFFKG | Antioxidative | RHT | (1-3) |
| RQMSLLLRR | Antioxidative | LLR | (6-8) |
| RLQLLARF | Antioxidative | LQL | (2-4) |
| KADSLISGAAQAVYNKF | Antioxidative | GAA | (9-11) |
| KADSLISGAAQAVYNKF | Antioxidative | VY | (14-15) |
2.6. In Silico Simulated Gastrointestinal Digestion of the Antioxidative Peptides
| Peptides | Results of Enzyme Action | Locations of Released Peptides | Active Fragment Sequence | Location | Bioactivities of Identified Peptide |
|---|---|---|---|---|---|
| RALGFDFRR | RAL-GF-DF-RR | (1-3) (4-5) (6-7) (8-9) | GF RR DF | (4-5) (8-9) (6-7) | ACE inhibitor, Dipeptidyl peptidase IV inhibitor |
| RHTPFFKG | RH-TPF-F-K-G | (1-2) (3-5) (6-6) (7-7) (8-8) | RH | (1-2) | Dipeptidyl peptidase IV inhibitor |
| RQMSLLLRR | RQM-SL-L-L-RR | (1-3) (4-5) (6-6) (7-7) (8-9) | RR SL | (8-9) (4-5) | ACE inhibitor, Dipeptidyl peptidase IV inhibitor |
| RLQLLARF | RL-QL-L-ARF | (1-2) (3-4) (5-5) (6-8) | RL QL | (1-2) (3-4) | ACE inhibitor, Dipeptidyl peptidase IV inhibitor |
| KADSLISGAAQAVYNKF | KADSL- ISGAAQAVY-N-KF | (1-5) (6-14) (15-15) (16-17) | KF | (16-17) | ACE inhibitor Dipeptidyl peptidase IV inhibitor |
| Peptide | Active Fragment Sequence | Location | DH (%) | AE | W |
|---|---|---|---|---|---|
| RALGFDFRR | GF RR DF | (4-5) (8-9) (6-7) | 37.50 | 0.33 | 0.50 |
| RHTPFFKG | RH | (1-2) | 57.14 | 0.13 | 0.17 |
| RQMSLLLRR | RR SL | (8-9) (4-5) | 50.00 | 0.11 | 0.50 |
| RLQLLARF | RL QL | (1-2) (3-4) | 42.86 | 0.13 | 0.17 |
| KADSLISGAAQAVYNKF | KF | (16-17) | 18.75 | 0.06 | 0.10 |
2.7. Prediction of Toxicity and Physicochemical Properties of Released Bioactive Peptide Fractions after In Silico Digestion
| Peptide | Prediction | Hydrophobicity | Hydrophilicity | Charge | pI | Molecular Weight (Da) |
|---|---|---|---|---|---|---|
| GF | Non-Toxic | 0.39 | −1.25 | 0.00 | 5.88 | 222.26 |
| RR | Non-Toxic | −1.76 | 3.00 | 2.00 | 12.01 | 330.40 |
| DF | Non-Toxic | −0.05 | 0.25 | −1.00 | 3.8 | 280.29 |
| RH | Non-Toxic | −1.08 | 1.25 | 1.50 | 10.11 | 311.36 |
| SL | Non-Toxic | 0.14 | −0.75 | 0.00 | 5.88 | 218.27 |
| RL | Non-Toxic | −0.61 | 0.60 | 1.00 | 10.11 | 287.38 |
| QL | Non-Toxic | −0.08 | −0.80 | 0.00 | 5.88 | 259.33 |
| KF | Non-Toxic | −0.25 | 0.25 | 1.00 | 9.11 | 293.38 |
3. Methods
3.1. Materials
3.2. C-PC and Lipids Extracted to Generate the Spent Biomass
3.3. Enzymatic Hydrolysis
3.4. Antioxidation Assays
3.4.1. Scavenging Activities of DPPH
3.4.2. Scavenging Activities of Hydroxyl Free Radicals
3.4.3. Scavenging Activities of Superoxide Anion Free Radicals
3.4.4. Measurement of the Total Antioxidative Capacity
3.5. Ultrafiltration to Fractionate Peptides into Two Fractions with Different Molecular Weights and Antioxidative Activity
3.6. Chromatographical Gel Filtration to Purify Bioactive Peptides
3.7. LC-MS/MS Analysis of Peptides
3.8. Prediction of Bioactive Potential of Identified Peptides from the LC-MS/MS Results
3.9. Toxicity and Physicochemical Properties of Bioactive Peptides Released after In Silico Proteolysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, F.; Li, M.; Wang, Q.; Yan, J.; Han, S.; Ma, C.; Ma, P.; Liu, X.; McClements, D.J. Future foods: Alternative proteins, food architecture, sustainable packaging, and precision nutrition. Crit. Rev. Food Sci. Nutr. 2022, 1–22. [Google Scholar] [CrossRef]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a sustainable source of edible proteins and bioactive peptides-current trends and future prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Su, P.; Zhen, G.; Nguyen, T.T.; Diao, Y.; Heimann, K.; Zhang, W. An efficient protein isolation process for use in Limnospira maxima: A biorefinery approach. J. Food Compos. Anal. 2021, 104, 104173. [Google Scholar] [CrossRef]
- Chapman, J.; Power, A.; Netzel, M.E.; Sultanbawa, Y.; Smyth, H.E.; Truong, V.K.; Cozzolino, D. Challenges and opportunities of the fourth revolution: A brief insight into the future of food. Crit. Rev. Food Sci. Nutr. Food Sci. 2022, 62, 2845–2853. [Google Scholar] [CrossRef]
- Kell, S. Editorial foreword for “environment, development and sustainability” journal. Environ. Dev. Sustain. 2022, 24, 2983–2985. [Google Scholar] [CrossRef]
- Aiking, H.; de Boer, J. The next protein transition. Trends Food Sci. Technol. 2020, 105, 515–522. [Google Scholar] [CrossRef]
- Xu, X.; Sharma, P.; Shu, S.; Lin, T.-S.; Ciais, P.; Tubiello, F.; Smith, P.; Campbell, N.; Jain, A. Global greenhouse gas emissions from animal-based foods are twice those of plant-based foods. Nat. Food 2021, 2, 724–732. [Google Scholar] [CrossRef]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and supply of high-quality food protein for human consumption: Sustainability, challenges, and innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef]
- Lamsal, B.; Wang, H.; Pinsirodom, P.; Dossey, A.T. Applications of Insect-Derived Protein Ingredients in Food and Feed Industry. J. Am. Oil Chem. Soc. 2019, 96, 105–123. [Google Scholar] [CrossRef]
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae based production of single-cell protein. Curr. Opin. Biotechnol. 2022, 75, 102705. [Google Scholar] [CrossRef]
- Rawiwan, P.; Peng, Y.; Paramayuda, I.G.P.B.; Quek, S.Y. Red seaweed: A promising alternative protein source for global food sustainability. Trends Food Sci. Technol. 2022, 123, 37–56. [Google Scholar] [CrossRef]
- van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of Novel Protein Sources (Insects, Microalgae, Seaweed, Duckweed, and Rapeseed) and Legislative Aspects for Their Application in Food and Feed Production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Tian, J.; Bryksa, B.C.; Yada, R.Y. Feeding the world into the future–food and nutrition security: The role of food science and technology. Front. Life Sci. 2016, 9, 155–166. [Google Scholar] [CrossRef]
- Park, H.; Lee, C.G. Theoretical calculations on the feasibility of microalgal biofuels: Utilization of marine resources could help realizing the potential of microalgae. Biotechnol. J. 2016, 11, 1461–1470. [Google Scholar] [CrossRef]
- Herrera, A.; Boussiba, S.; Napoleone, V.; Hohlberg, A. Recovery of c-phycocyanin from the cyanobacterium Spirulina maxima. J. Appl. Phycol. 1989, 1, 325–331. [Google Scholar] [CrossRef]
- Kathiravan, A.; Udayan, E.; Ranjith Kumar, R. Bioprospecting of Spirulina biomass using novel extraction method for the production of C-Phycocyanin as effective food colourant. Vegetos 2022, 35, 484–492. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Y.; Xue, M.; Dun, Y.; Li, S.; Peng, N.; Liang, Y.; Zhao, S. Purification and identification of antioxidant peptides from enzymatic hydrolysate of Spirulina platensis. J. Microbiol. Biotechnol. 2016, 26, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Fan, X.; Zheng, Q.; Wang, J.; Zhang, X. Anti-oxidant, hemolysis inhibition, and collagen-stimulating activities of a new hexapeptide derived from Arthrospira (Spirulina) platensis. J. Appl. Phycol. 2018, 30, 1655–1665. [Google Scholar] [CrossRef]
- Carrizzo, A.; Conte, G.M.; Sommella, E.; Damato, A.; Ambrosio, M.; Sala, M.; Scala, M.C.; Aquino, R.P.; De Lucia, M.; Madonna, M.; et al. Novel potent decameric peptide of Spirulina platensis reduces blood pressure levels through a PI3K/AKT/eNOS-dependent mechanism. Hypertension 2019, 73, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ren, D.F.; Xue, Y.L.; Sawano, Y.; Miyakawa, T.; Tanokura, M. Isolation of an antihypertensive peptide from alcalase digest of Spirulina platensis. J. Agric. Food Chem. 2010, 58, 7166–7171. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X. Inhibitory effects of small molecular peptides from Spirulina (Arthrospira) platensis on cancer cell growth. Food Funct. 2016, 7, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Aiello, G.; Bollati, C.; Bartolomei, M.; Arnoldi, A.; Lammi, C. Phycobiliproteins from Arthrospira platensis (spirulina): A new source of peptides with dipeptidyl peptidase-iv inhibitory activity. Nutrients 2020, 12, 794. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Song, P.; Zou, M.H. Redox regulation of endothelial cell fate. Cell. Mol. Life Sci. 2014, 71, 3219–3239. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Suetsuna, K.; Chen, J.R. Identification of antihypertensive peptides from peptic digest of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef]
- Anekthanakul, K.; Senachak, J.; Hongsthong, A.; Charoonratana, T.; Ruengjitchatchawalya, M. Natural ACE inhibitory peptides discovery from Spirulina (Arthrospira platensis) strain C1. Peptides 2019, 118, 170107. [Google Scholar] [CrossRef]
- Bai, R. Advanced Process Development toward Biorefinery of Spirulina Biomass for the Production of Functional Proteins and Peptides. Ph.D. Thesis, Flinders University, Adelaide, Australia, 2022. [Google Scholar]
- Nguyen, T.T.; Heimann, K.; Zhang, W. Protein recovery from underutilised marine bioresources for product development with nutraceutical and pharmaceutical bioactivities. Mar. Drugs 2020, 18, 391. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Yang, X.R.; Zhao, Y.Q.; Qiu, Y.T.; Chi, C.F.; Wang, B. Preparation and Characterization of Gelatin and Antioxidant Peptides from Gelatin Hydrolysate of Skipjack Tuna (Katsuwonus pelamis) Bone Stimulated by in vitro Gastrointestinal Digestion. Mar. Drugs 2019, 17, 78. [Google Scholar] [CrossRef]
- Xia, E.; Zhai, L.; Huang, Z.; Liang, H.; Yang, H.; Song, G.; Li, W.; Tang, H. Optimization and Identification of Antioxidant Peptide from Underutilized Dunaliella salina Protein: Extraction, In Vitro Gastrointestinal Digestion, and Fractionation. BioMed Res. Int. 2019, 2019, 6424651. [Google Scholar] [CrossRef]
- Sathya, R.; MubarakAli, D.; MohamedSaalis, J.; Kim, J.-W. A Systemic Review on Microalgal Peptides: Bioprocess and Sustainable Applications. Sustainability 2021, 13, 3262. [Google Scholar] [CrossRef]
- Ovando, C.A.; Carvalho, J.C.D.; de Vinícius Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional properties and health benefits of bioactive peptides derived from Spirulina: A review. Food Rev. Int. 2018, 34, 34–51. [Google Scholar] [CrossRef]
- Cai, W.W.; Hu, X.M.; Wang, Y.M.; Chi, C.F.; Wang, B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs 2022, 20, 626. [Google Scholar] [CrossRef]
- Olsen, T.H.; Yesiltas, B.; Marin, F.I.; Pertseva, M.; García-Moreno, P.J.; Gregersen, S.; Overgaard, M.T.; Jacobsen, C.; Lund, O.; Hansen, E.B.; et al. AnOxPePred: Using deep learning for the prediction of antioxidative properties of peptides. Sci. Rep. 2020, 10, 21471. [Google Scholar] [CrossRef] [PubMed]
- Janairo, J.I.B. Machine Learning for the Cleaner Production of Antioxidant Peptides. Int. J. Pept. Res. Ther. 2021, 27, 2051–2056. [Google Scholar] [CrossRef]
- Grønning, A.G.B.; Kacprowski, T.; Schéele, C. MultiPep: A hierarchical deep learning approach for multi-label classification of peptide bioactivities. Biol. Methods Protoc. 2021, 6, bpab021. [Google Scholar] [PubMed]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Novel functional food ingredients from marine sources. Curr. Opin. Food Sci. 2015, 2, 123–129. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Bioactive peptides from shrimp shell processing discards: Antioxidant and biological activities. J. Funct. Foods 2017, 34, 7–17. [Google Scholar] [CrossRef]
- Acquah, C.; Di Stefano, E.; Udenigwe, C.C. Role of hydrophobicity in food peptide functionality and bioactivity. J. Food Bioact. 2018, 4, 88–98. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Chai, T.-T.; Law, Y.-C.; Wong, F.-C.; Kim, S.-K. Enzyme-assisted discovery of antioxidant peptides from edible marine invertebrates: A review. Mar. Drugs 2017, 15, 42. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Jiang, A. Antioxidant peptides isolated from sea cucumber Stichopus Japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Wong, F.-C.; Xiao, J.; Ong, M.G.L.; Pang, M.-J.; Wong, S.-J.; Teh, L.-K.; Chai, T.-T. Identification and characterization of antioxidant peptides from hydrolysate of blue-spotted stingray and their stability against thermal, pH and simulated gastrointestinal digestion treatments. Food Chem. 2019, 271, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C. Technology Bioinformatics approaches, prospects and challenges of food bioactive peptide research. Trends Food Sci. Technol. 2014, 36, 137–143. [Google Scholar] [CrossRef]
- Senadheera, T.R.; Hossain, A.; Dave, D.; Shahidi, F. In Silico Analysis of Bioactive Peptides Produced from Underutilized Sea Cucumber By-Products—A Bioinformatics Approach. Mar. Drugs 2022, 20, 610. [Google Scholar] [CrossRef]
- Ji, D.; Udenigwe, C.C.; Agyei, D. Antioxidant peptides encrypted in flaxseed proteome: An in silico assessment. Food Sci. Hum. Wellness 2019, 8, 306–314. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Pliszka, M.; Mogut, D.; Darewicz, M. Characteristics of biopeptides released in silico from collagens using quantitative parameters. Foods 2020, 9, 965. [Google Scholar] [CrossRef]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.S.; Chang, Y.W. Bioactivities of enzymatic protein hydrolysates derived from Chlorella sorokiniana. Food Sci. Nutr. 2019, 7, 2381–2390. [Google Scholar] [CrossRef]
- Gao, S.; Zhao, B.X.; Long, C.; Heng, N.; Guo, Y.; Sheng, X.H.; Wang, X.G.; Xing, K.; Xiao, L.F.; Ni, H.M.; et al. Natural Astaxanthin Improves Testosterone Synthesis and Sperm Mitochondrial Function in Aging Roosters. Antioxidants 2022, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, C.; Yan, Y.; Wang, H.; Wang, L.; Jiang, J.; Chen, S.; Chen, F. The transcriptional coactivator CmMBF1c is required for waterlogging tolerance in Chrysanthemum morifolium. Hortic. Res. 2022, 9, uhac215. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Zhou, X.; Wang, Y.; Chen, D.; Chen, G.; Li, Y.; He, J. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. 2018, 17, 139. [Google Scholar] [CrossRef]
- Wiśniewski, J.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Xu, M.; Udenigwe, C.C.; Agyei, D. Physicochemical characterisation, molecular docking, and drug-likeness evaluation of hypotensive peptides encrypted in flaxseed proteome. Curr. Res. Food Sci. 2020, 3, 41–50. [Google Scholar] [CrossRef]
- Gupta, S.; Kapoor, P.; Chaudhary, K.; Gautam, A.; Kumar, R.; Consortium, O.S.D.D.; Raghava, G.P. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE 2013, 8, e73957. [Google Scholar] [CrossRef]
| Protease | Temperature/°C | pH | Dosage of Enzyme (U/g-Sample) | Unit of Activity (U/g-Enzyme) |
|---|---|---|---|---|
| Papain | 45 | 7.5 | 6600 | 1.1 × 105 |
| Alcalase 2.4 L | 60 | 9.0 | 2340 | 3.9 × 104 |
| Protamex 1.6 | 55 | 7.5 | 9000 | 1.5 × 105 |
| Trypsin | 50 | 9.0 | 10,200 | 1.7 × 105 |
| Alcalase | 55 | 8.5 | 14,400 | 2.4 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, R.; Nguyen, T.T.; Zhou, Y.; Diao, Y.; Zhang, W. Identification of Antioxidative Peptides Derived from Arthrospira maxima in the Biorefinery Process after Extraction of C-Phycocyanin and Lipids. Mar. Drugs 2023, 21, 146. https://doi.org/10.3390/md21030146
Bai R, Nguyen TT, Zhou Y, Diao Y, Zhang W. Identification of Antioxidative Peptides Derived from Arthrospira maxima in the Biorefinery Process after Extraction of C-Phycocyanin and Lipids. Marine Drugs. 2023; 21(3):146. https://doi.org/10.3390/md21030146
Chicago/Turabian StyleBai, Renao, Trung T. Nguyen, Yali Zhou, Yong Diao, and Wei Zhang. 2023. "Identification of Antioxidative Peptides Derived from Arthrospira maxima in the Biorefinery Process after Extraction of C-Phycocyanin and Lipids" Marine Drugs 21, no. 3: 146. https://doi.org/10.3390/md21030146
APA StyleBai, R., Nguyen, T. T., Zhou, Y., Diao, Y., & Zhang, W. (2023). Identification of Antioxidative Peptides Derived from Arthrospira maxima in the Biorefinery Process after Extraction of C-Phycocyanin and Lipids. Marine Drugs, 21(3), 146. https://doi.org/10.3390/md21030146









