Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum
Abstract
1. Introduction
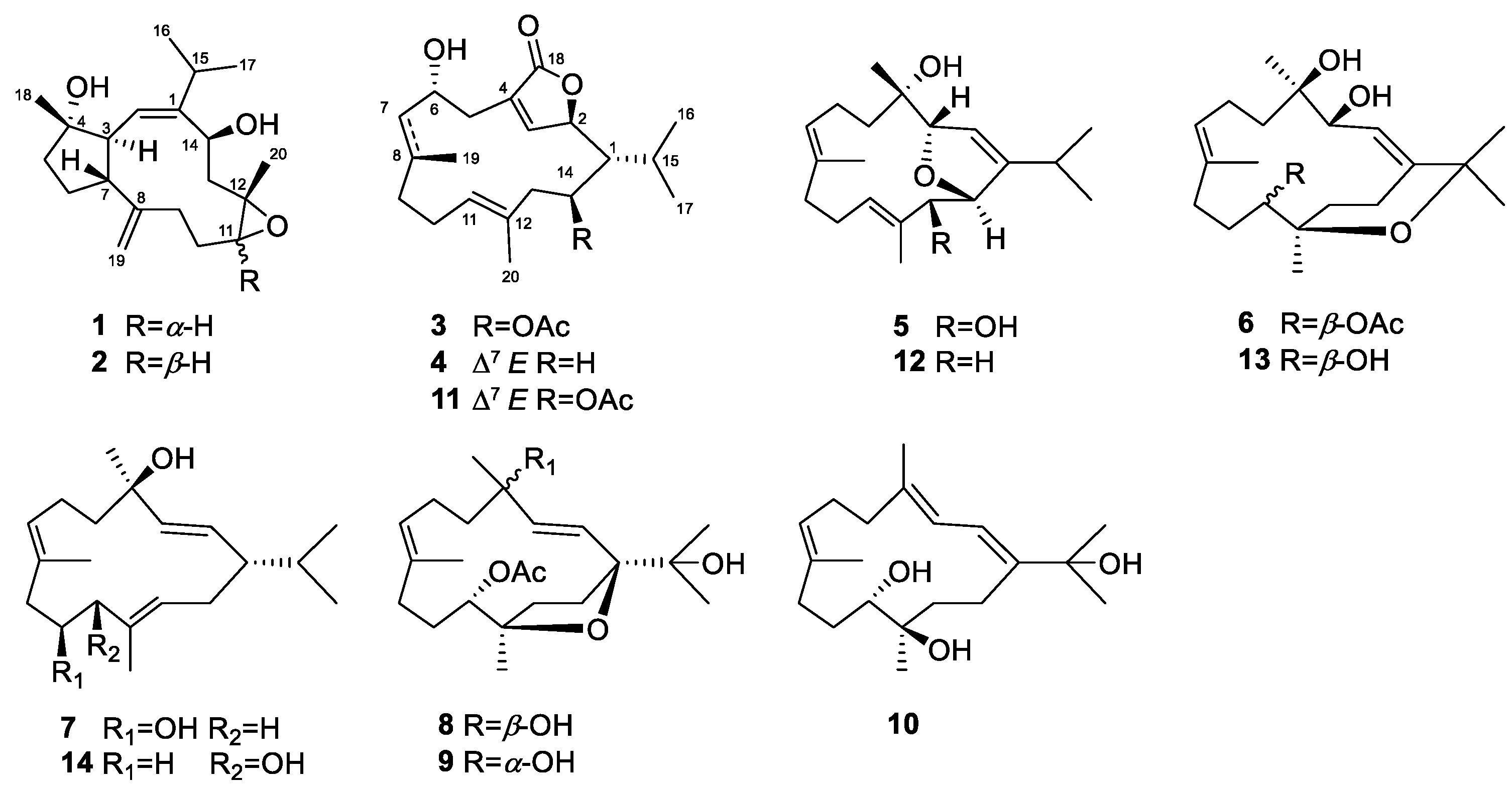
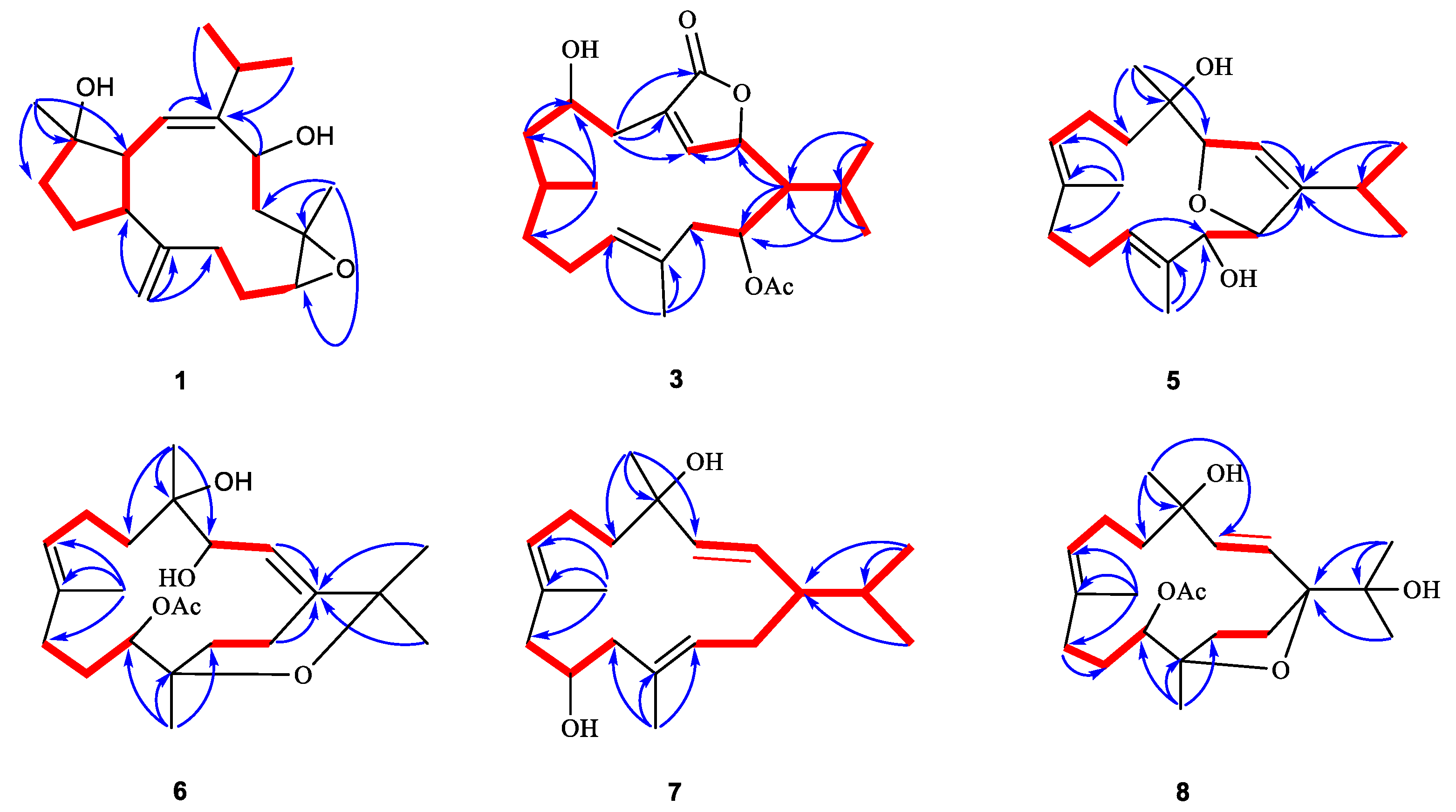
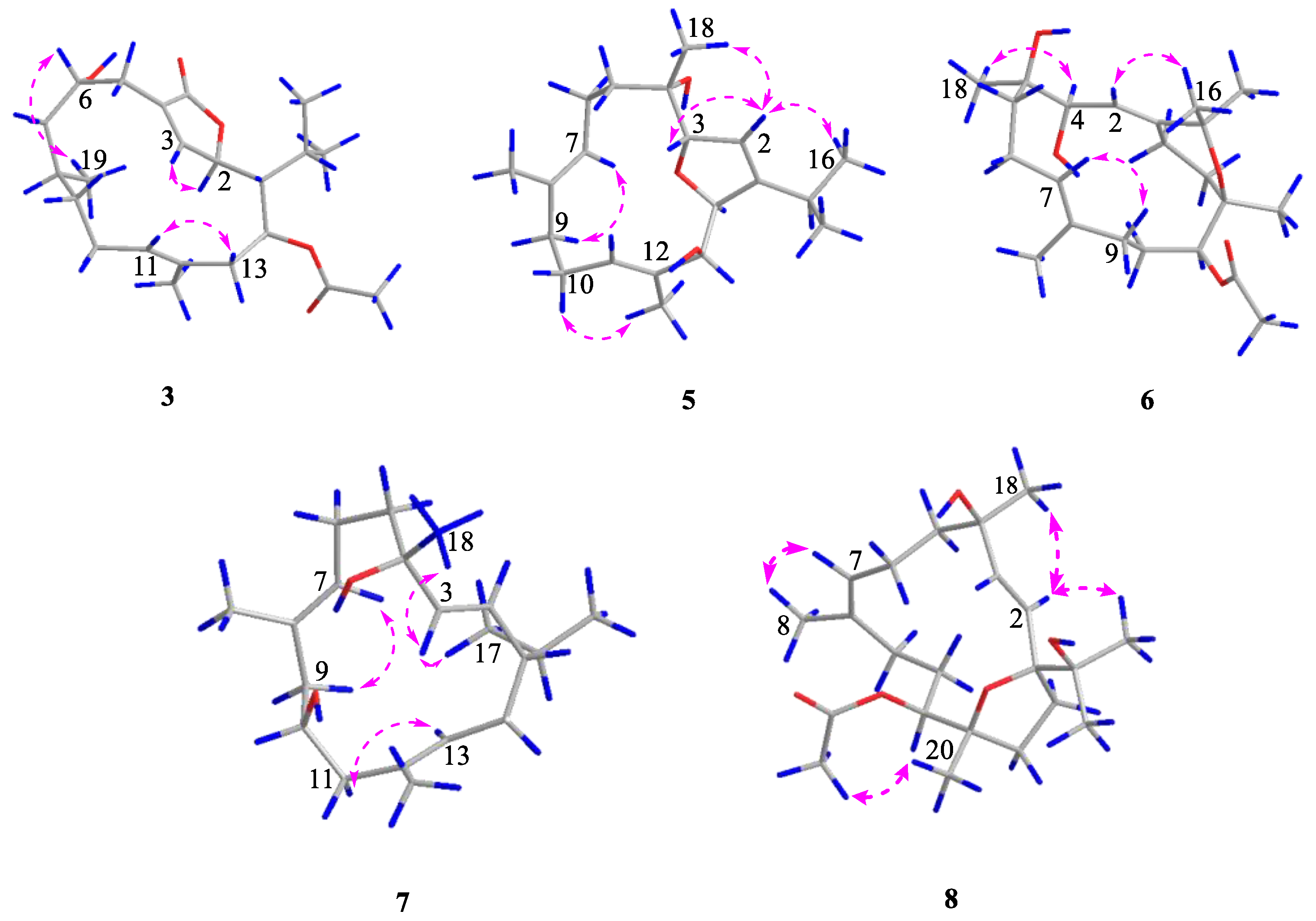
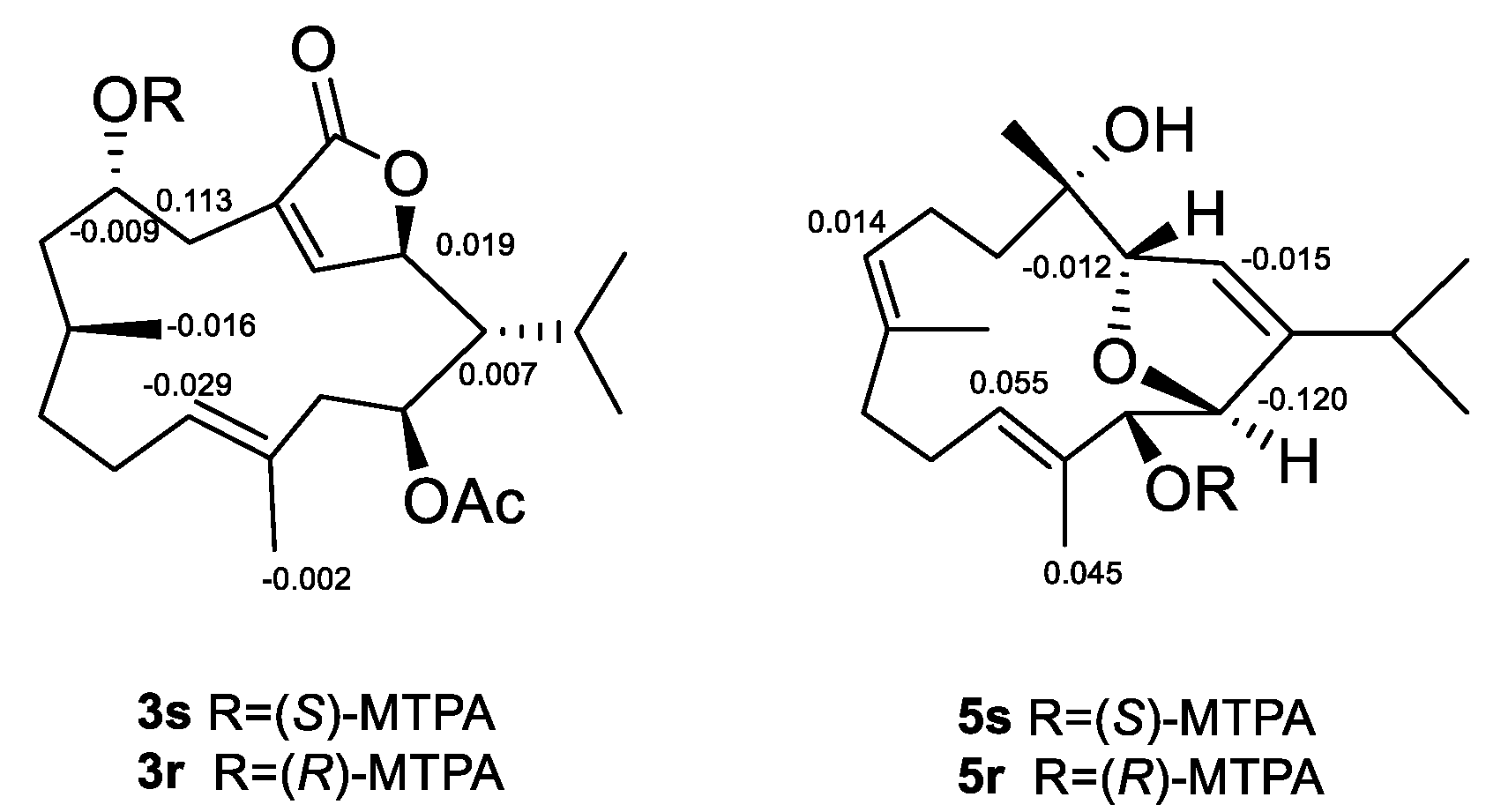
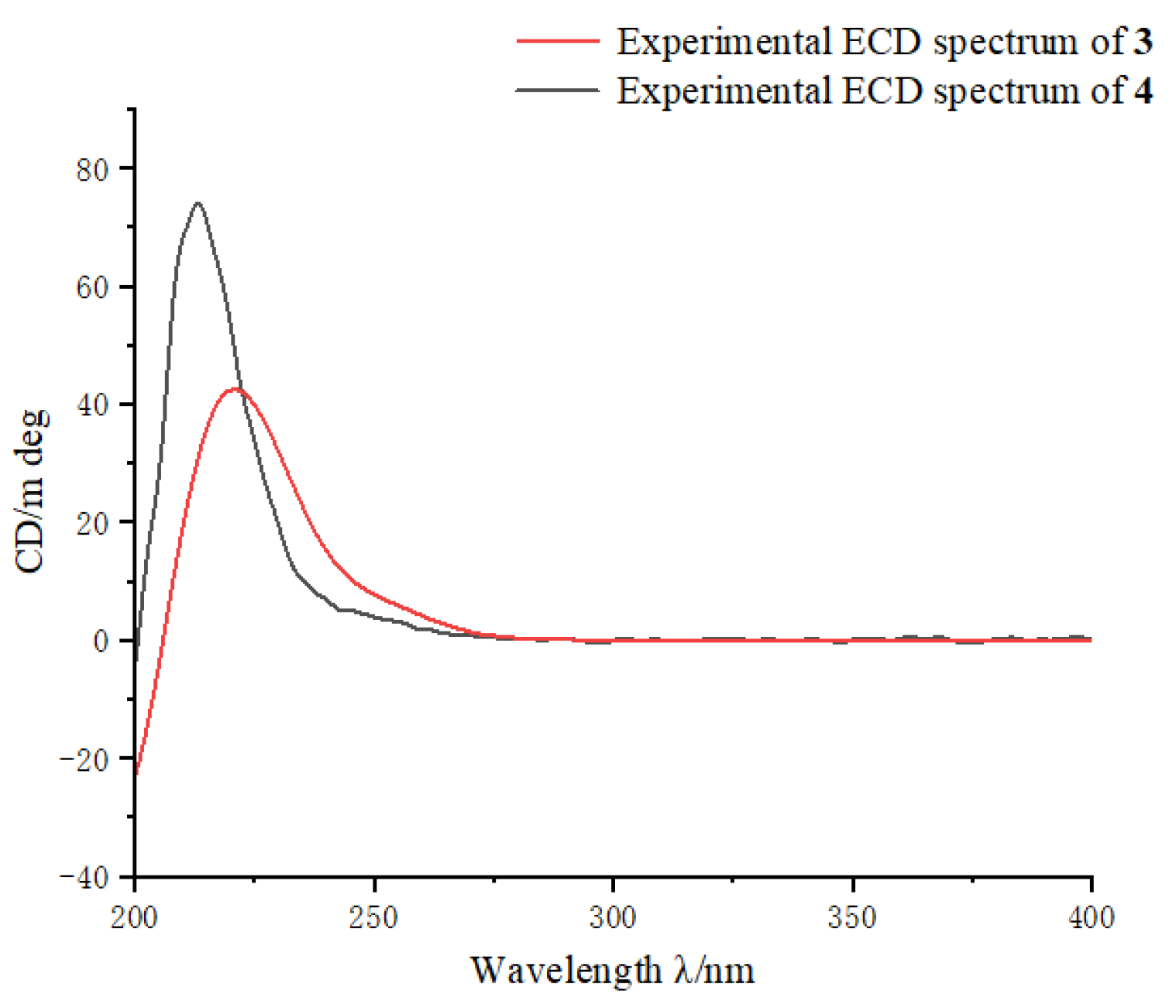
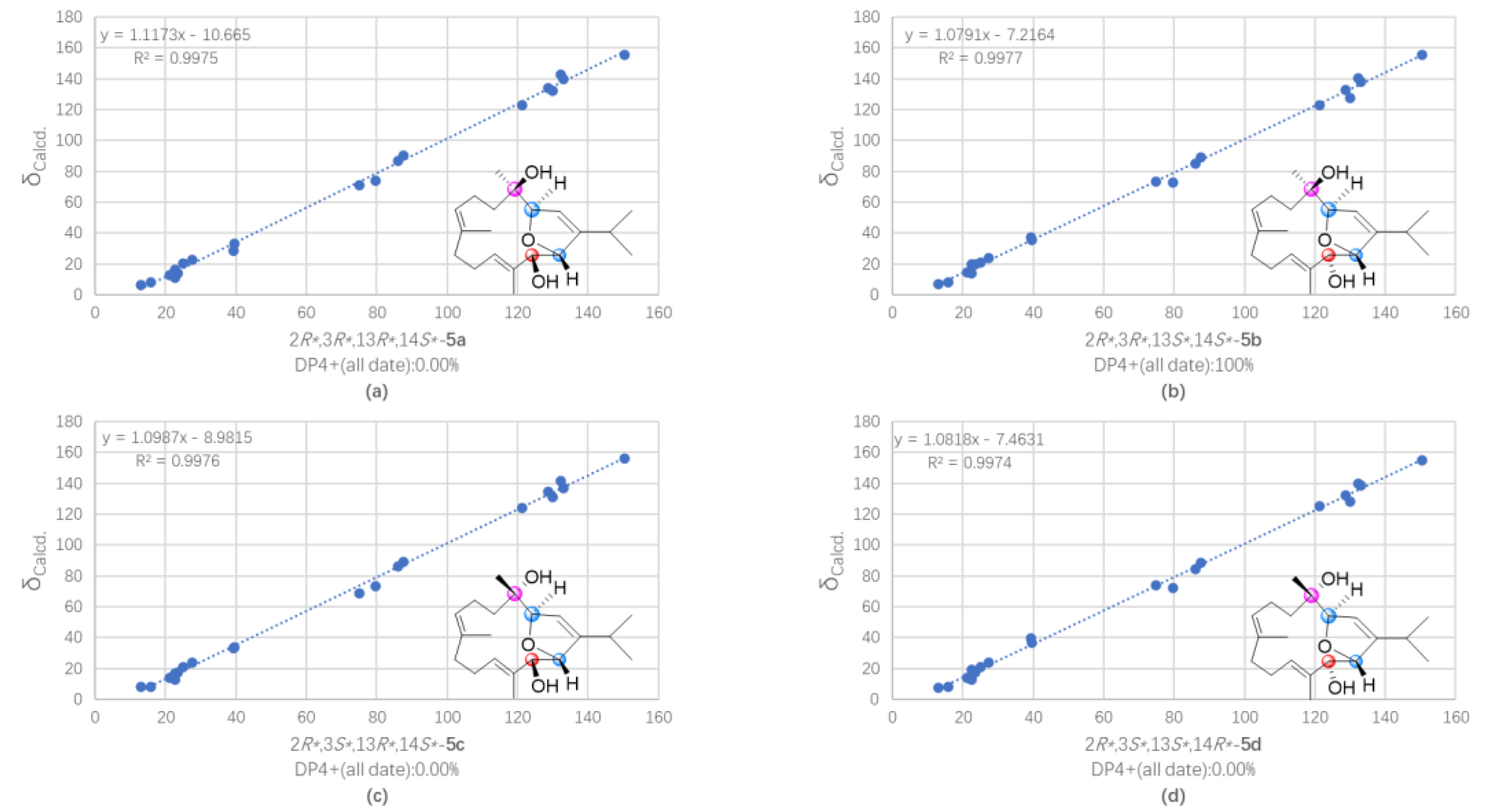
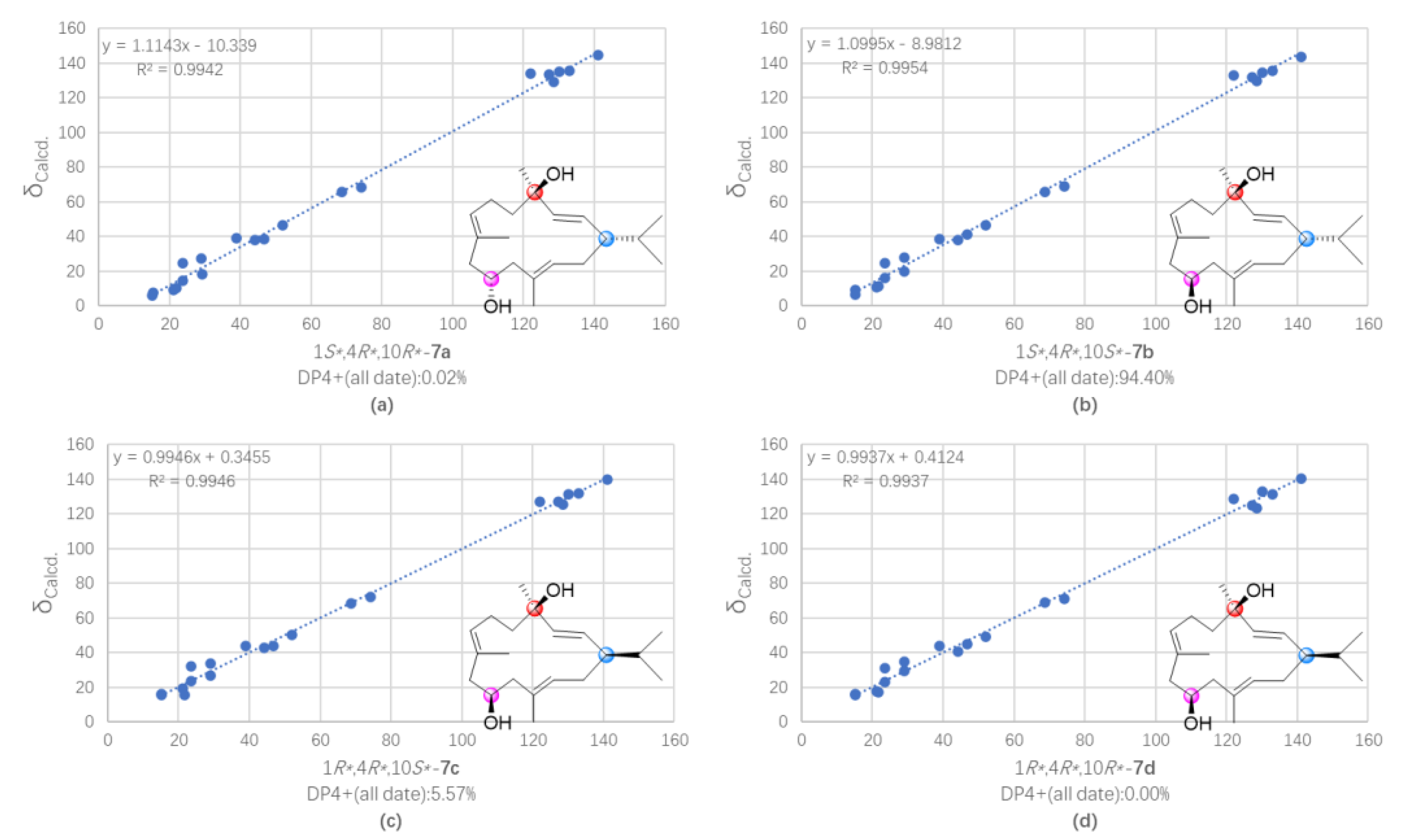
2. Results and Discussion
3. Materials and Methods
3.1. Subsection
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data of Compounds
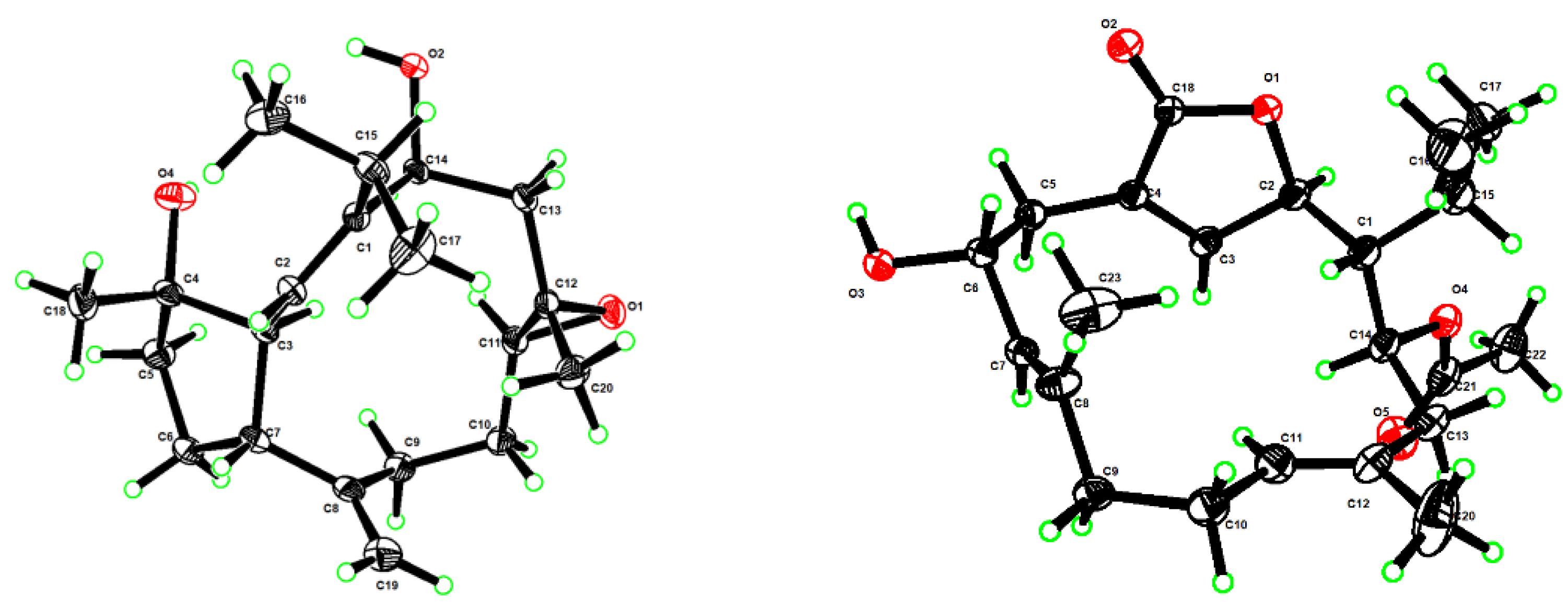
3.5. X-ray Crystallographic Analysis for Compounds 1 and 4
3.6. Esterification of Compounds 3 and 5 with MTPA Chlorides
3.7. QM-NMR Calculation of Compounds 5 and 7
3.8. Cytotoxic Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anjaneyulu, A.S.R.; Rao, G.V. Chemical constituents of the soft coral species of Sarcophyton genus: A review. J. Indian Chem. Soc. 1997, 74, 272–278. [Google Scholar]
- Liang, L.-F.; Guo, Y.-W. Terpenes from the soft corals of the genus Sarcophyton: Chemistry and biological activities. Chem. Biodivers. 2013, 10, 2161–2196. [Google Scholar] [CrossRef]
- Zubair, M.S.; Al-Footy, K.O.; Ayyad, S.-E.N.; Al-Lihaibi, S.S.; Alarif, W.M. A review of steroids from Sarcophyton species. Nat. Prod. Res. 2016, 30, 869–879. [Google Scholar] [CrossRef]
- Elkhawas, Y.A.; Elissawy, A.M.; Elnaggar, M.S.; Mostafa, N.M.; Al-Sayed, E.; Bishr, M.M.; Singab, A.N.B.; Salama, O.M. Chemical diversity in species belonging to soft coral genus Sacrophyton and its impact on biological activity: A review. Mar. Drugs 2020, 18, 41. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, H.; Wang, P.; Gong, W.; Xue, M.; Zhang, H.; Liu, T.; Liu, B.; Yi, Y.; Zhang, W. Bioactive polyoxygenated steroids from the South China Sea soft coral, Sarcophyton sp. Mar. Drugs 2013, 11, 775–787. [Google Scholar] [CrossRef]
- Chen, W.-T.; Liu, H.-L.; Yao, L.-G.; Guo, Y.-W. 9,11-Secosteroids and polyhydroxylated steroids from two South China Sea soft corals Sarcophyton trocheliophorum and Sinularia flexibilis. Steroids 2014, 92, 56–61. [Google Scholar] [CrossRef]
- Ngoc, N.T.; Hanh, T.T.H.; Quang, T.H.; Cuong, N.X.; Nam, N.H.; Thao, D.T.; Thung, D.C.; Kiem, P.V.; Minh, C.V. Polyhydroxylated steroids from the Vietnamese soft coral Sarcophyton ehrenbergi. Steroids 2021, 176, 108932. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, A.-N.; Shao, C.-L.; Li, L.; Xu, Y.; Qian, P.-Y. Chemical constituents of soft coral Sarcophyton infundibuliforme from the South China Sea. Biochem. Syst. Ecol. 2011, 39, 853–856. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Huang, C.-Y.; Chen, S.-R.; Weng, J.-R.; Tu, T.-H.; Cheng, Y.-B.; Wu, S.-H.; Sheu, J.-H. New hydroquinone monoterpenoid and cembranoid-related metabolites from the soft coral Sarcophyton tenuispiculatum. Mar. Drugs 2021, 19, 8. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Murthy, M.V.R.K.; Gowri, P.M.; Venugopal, M.; Laatsch, H. A rare prostaglandin from the soft coral Sarcophyton crassocaule of the Indian Ocean. J. Nat. Prod. 2000, 63, 1425–1426. [Google Scholar] [CrossRef]
- Cheng, Z.-B.; Deng, Y.-L.; Fan, C.-Q.; Han, Q.-H.; Lin, S.-L.; Tang, G.-H.; Luo, H.-B.; Yin, S. Prostaglandin derivatives: Nonaromatic phosphodiesterase-4 inhibitors from the soft coral Sarcophyton ehrenbergi. J. Nat. Prod. 2014, 77, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-Y.; Wen, Z.-H.; Chiou, S.-F.; Tsai, C.-W.; Wang, S.-K.; Hsu, C.-H.; Dai, C.-F.; Chiang, M.Y.; Wang, W.-H.; Duh, C.-Y. Ceramide and cerebrosides from the octocoral Sarcophyton ehrenbergi. J. Nat. Prod. 2009, 72, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, N.A.; Ibrahim, A.K.; Radwan, M.M.; Zaitone, S.A.; Gomaa, M.; ElSohly, M.A.; Hassanean, H.A.; Ahmed, S.A. Mechanism of action of antiepileptic ceramide from Red Sea soft coral Sarcophyton auritum. Bioorg. Med. Chem. Lett. 2015, 25, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, T.; Dembitsky, V.M. γ-Lactones from the soft corals Sarcophyton trocheliophorum and Lithophyton arboreum. Tetrahedron 2001, 57, 8743–8749. [Google Scholar] [CrossRef]
- Shaaban, M.; Ghani, M.A.; Issa, M.Y. New naturally occurring compounds from Sarcophyton trocheliophorum. Biointerface Res. App. Chem. 2022, 12, 2285–2331. [Google Scholar] [CrossRef]
- Rodrigues, I.G.; Miguel, M.G.; Mnif, W. A brief review on new naturally occurring cembranoid diterpene derivatives from the soft corals of the genera Sarcophyton, Sinularia, and Lobophytum since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef]
- Shaaban, M.; Yassin, F.Y.; Soltan, M.M. Calamusins J-K: New anti-angiogenic sesquiterpenes from Sarcophyton glaucum. Nat. Prod. Res. 2021, 35, 5720–5731. [Google Scholar] [CrossRef]
- Sawant, S.S.; Youssef, D.T.A.; Sylvester, P.W.; Wali, V.; Sayed, K.A.E. Antiproliferative sesquiterpenes from the Red Sea soft coral Sarcophyton glaucum. Nat. Prod. Commun. 2007, 2, 117–119. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.R.; Rao, V.L.; Sastry, V.G.; Rao, D.V. Trocheliophorin: A novel rearranged sesquiterpenoid from the Indian Ocean soft coral Sarcophyton trocheliophorum. J. Asian Nat. Prod. Res. 2008, 10, 597–601. [Google Scholar] [CrossRef]
- Ye, F.; Li, J.; Wu, Y.; Zhu, Z.-D.; Mollo, E.; Gavagnin, M.; Gu, Y.-C.; Zhu, W.-L.; Li, X.-W.; Guo, Y.-W. Sarinfacetamides A and B, nitrogenous diterpenoids with tricyclo [6.3.1.01,5] dodecane scaffold from the South China Sea soft coral Sarcophyton infundibuliforme. Org. Lett. 2018, 20, 2637–2640. [Google Scholar] [CrossRef]
- Lin, K.-H.; Lin, Y.-C.; Huang, C.-Y.; Tseng, Y.-J.; Chen, S.-R.; Cheng, Y.-B.; Hwang, T.-L.; Wang, S.-Y.; Chen, H.-Y.; Dai, C.-F.; et al. Cembranoid-related diterpenes, novel secoditerpenes, and an unusual bisditerpene from a Formosan soft coral Sarcophyton tortuosum. Bull. Chem. Soc. Jpn. 2021, 94, 2774–2783. [Google Scholar] [CrossRef]
- Bu, Q.; Yang, M.; Yan, X.-Y.; Yao, L.-G.; Guo, Y.-W.; Liang, L.-F. New flexible cembrane-type macrocyclic diterpenes as TNF-α inhibitors from the South China Sea soft coral Sarcophyton mililatensis. Int. J. Biol. Macromol. 2022, 222, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, J.; Shi, X.; Li, K.; Li, F.; Tang, X.; Li, G.; Li, P. Sarcoeleganolides C–G, five new cembranes from the South China Sea soft coral Sarcophyton elegans. Mar. Drugs 2022, 20, 574. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.A.; Elshamy, A.I.; Abd El-Razek, M.H.; Abdel-Tawab, A.M.; Ali, S.K.; Aboelmagd, M.; Suenaga, M.; Pare, P.W.; Umeyama, A.; Hegazy, M.-E.F. Sarcoconvolutums F and G: Polyoxygenated cembrane-type diterpenoids from Sarcophyton convolutum, a Red Sea soft coral. Molecules 2022, 27, 5835. [Google Scholar] [CrossRef]
- Sun, P.; Cai, F.-Y.; Lauro, G.; Tang, H.; Su, L.; Wang, H.-L.; Li, H.H.; Mándi, A.; Kurtán, T.; Riccio, R.; et al. Immunomodulatory biscembranoids and assignment of their relative and absolute configurations: Data set modulation in the density functional theory/nuclear magnetic resonance approach. J. Nat. Prod. 2019, 82, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Cuadrado, C.; Gao, C.; Wu, Q.; Li, X.; Pang, T.; Daranas, A.H.; Guo, Y.; Li, X. Polyoxygenated anti-inflammatory biscembranoids from the soft coral Sarcophyton tortuosum and their stereochemistry. Chin. Chem. Lett. 2021, 32, 271–276. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Huang, J.; Wang, Y.; Leng, X.; Li, T.; Ouyang, H.; Yan, X.; He, S. Bistrochelides H−L: Biscembranoids from the South China Sea soft coral Sarcophyton serenei. Phytochemistry 2022, 204, 113438. [Google Scholar] [CrossRef]
- Nguyen, N.B.; Chen, L.-Y.; Chen, P.-J.; El-Shazly, M.; Hwang, T.-L.; Su, J.-H.; Su, C.-H.; Yen, P.-T.; Peng, B.-R.; Lai, K.-H. MS/MS molecular networking unveils the chemical diversity of biscembranoid derivatives, neutrophilic inflammatory mediators from the cultured soft coral Sarcophyton trocheliophorum. Int. J. Mol. Sci. 2022, 23, 15464. [Google Scholar] [CrossRef]
- Al-Footy, K.O.; Alarif, W.M.; Asiri, F.; Aly, M.M.; Ayyad, S.-E.N. Rare pyrane-based cembranoids from the Red Sea soft coral Sarcophyton trocheliophorum as potential antimicrobial–antitumor agents. Med. Chem. Res. 2015, 24, 505–512. [Google Scholar] [CrossRef]
- Mohamed, T.A.; Elshamy, A.I.; Abdel-Tawab, A.M.; AbdelMohsen, M.M.; Ohta, S.; Pare, P.W.; Hegazy, M.-E.F. Oxygenated cembrene diterpenes from Sarcophyton convolutum: Cytotoxic sarcoconvolutum A–E. Mar. Drugs 2021, 19, 519. [Google Scholar] [CrossRef]
- Li, J.-F.; Zeng, Y.-B.; Li, W.-S.; Luo, H.; Zhang, H.-Y.; Guo, Y.-W. Xishaglaucumins A—J, new cembranoids with anti-inflammatory activities from the South China Sea soft coral Sarcophyton glaucum. Chin. J. Chem. 2022, 40, 79–90. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, X.; Han, X.; Feng, D.; Luo, X.; Ofwegen, L.v.; Li, P.; Li, G. Sarcoglaucins A-I, new antifouling cembrane-type diterpenes from the South China Sea soft coral Sarcophyton glaucum. Org. Chem. Front. 2019, 6, 2004–2013. [Google Scholar] [CrossRef]
- Badria, F.A.; Guirguis, A.N.; Perovic, S.; Steffen, R.; Müller, W.E.G.; Schröder, H.C. Sarcophytolide: A new neuroprotective compound from the soft coral Sarcophyton glaucum. Toxicology 1998, 131, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-Q.; Chen, J.; Wu, M.-J.; Zhang, H.-Y.; Liang, L.-F.; Guo, Y.-W. Uncommon capnosane diterpenes with neuroprotective potential from South China Sea soft coral Sarcophyton boettgeri. Mar. Drugs 2022, 20, 602. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2022, 39, 1122–1171. [Google Scholar] [CrossRef]
- Liu, J.; Gu, Y.-c.; Su, M.-z.; Guo, Y.-w. Chemistry and bioactivity of secondary metabolites from South China Sea marine fauna and flora: Recent research advances and perspective. Acta Pharmacol. Sin. 2022, 43, 3062–3079. [Google Scholar] [CrossRef]
- Liang, L.-F.; Kurtán, T.; Mándi, A.; Yao, L.-G.; Li, J.; Zhang, W.; Guo, Y.-W. Unprecedented diterpenoids as a PTP1B inhibitor from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller. Org. Lett. 2013, 15, 274–277. [Google Scholar] [CrossRef]
- Yang, M.; Li, X.-L.; Wang, J.-R.; Lei, X.; Tang, W.; Li, X.-W.; Sun, H.; Guo, Y.-W. Sarcomililate A, an unusual diterpenoid with tricyclo [11.3.0.02,16] hexadecane carbon skeleton, and its potential biogenetic precursors from the Hainan soft coral Sarcophyton mililatensis. J. Org. Chem. 2019, 84, 2568–2576. [Google Scholar] [CrossRef]
- Yang, Q.-B.; Wu, Q.; Chen, J.-K.; Liang, L.-F. The soft coral Sarcophyton trocheliophorum: A warehouse of terpenoids with structural and pharmacological diversity. Mar. Drugs 2023, 21, 30. [Google Scholar] [CrossRef]
- Yao, L.-G.; Zhang, H.-Y.; Liang, L.-F.; Guo, X.-J.; Mao, S.-C.; Guo, Y.-W. Yalongenes A and B, two new cembranoids with cytoprotective effects from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller. Helv. Chim. Acta 2012, 95, 235–239. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Gao, T.-R.; Yang, M.; Yao, L.-G.; Guo, Y.-W. Further new cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Fitoterapia 2021, 151, 104902. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Bie, W.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Sarcophyolides B–E, new cembranoids from the soft coral Sarcophyton elegans. Mar. Drugs 2013, 11, 3186–3196. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Guo, Y.-W.; Mollo, E.; Gavagnin, M.; Cimino, G. Sarcophytonolides E−H, cembranolides from the Hainan soft coral Sarcophyton latum. J. Nat. Prod. 2006, 69, 819–822. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.-F.; Chen, W.-T.; Li, X.-W.; Wang, H.-Y.; Guo, Y.-W. New bicyclic cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum. Sci. Rep. 2017, 7, 46584. [Google Scholar] [CrossRef]
- Wen, T.; Ding, Y.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Sinulaflexiolides A-K, cembrane-type diterpenoids from the Chinese soft coral Sinularia flexibilis. J. Nat. Prod. 2008, 71, 1133–1140. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kondo, K.; Osabe, K.; Mitsuhashi, H. Marine terpenes and terpenoids. V. Oxidation of sarcophytol A, a potent anti-tumor-promoter from the soft coral Sarcophyton glaucum. Chem. Pharm. Bull. 1988, 36, 2331–2341. [Google Scholar] [CrossRef]
- Li, S.-W.; Cuadrado, C.; Yao, L.-G.; Daranas, A.H.; Guo, Y.-W. Quantum mechanical–NMR-aided configuration and conformation of two unreported macrocycles isolated from the soft coral Lobophytum sp.: Energy calculations versus coupling constants. Org. Lett. 2020, 22, 4093–4096. [Google Scholar] [CrossRef]
- Raldugin, V.A.; Pleshkov, I.G.; Gatilov, Y.V.; Yaroshenko, N.I.; Salenko, V.L.; Shevtsov, S.A.; Pentegova, V.A. Photosensitized oxidation of isocembrol. VII. Products of reaction at the C11 double bond. Chem. Nat. Compd. 1984, 20, 45–52. [Google Scholar] [CrossRef]
| No. | 1 a | 3 b | 5 b | 6 b | 7 b | 8 a |
|---|---|---|---|---|---|---|
| 1 | 1.55 (d, 10.7) | 1.54 (br s) | ||||
| 2 | 5.14 (d, 10.6) | 4.95 (d, 10.7) | 5.51 (d, 1.4) | 5.42 (m) | 5.51 (dd, 15.6, 9.6) | 5.56 (d, 15.6) |
| 3 | 2.84 (t, 10.6) | 7.44 (t, 1.8) | 4.66 (dq, 5.5, 1.4) | 4.40 (br s) | 5.63 (d, 15.6) | 6.13 (d, 15.9) |
| 4 | ||||||
| 5 | 1.84 (m) | 2.75 (dt, 13.2, 1.2) | 1.83 (m) | 1.94 (m) | 1.89 (ddd, 13.4, 8.6, 1.9) | 1.87 (m) |
| 1.78 (m) | 2.45 (dd, 13.3, 10.5) | 1.53 (m) | 1.76 (m) | 1.58 (m) | 1.69 (m) | |
| 6 | 1.66 (m) | 4.22 (t, 9.8) | 2.34 (m) | 2.06 (m) | 2.24 (m) | 2.64 (m) |
| 1.92 (m) | ||||||
| 7 | 2.48 (t, 7.1) | 1.63 (m) | 5.22 (m) | 5.24 (t, 6.3) | 5.11 (d, 7.8) | 5.43 (dd, 10.6, 4.0) |
| 1.35 (m) | ||||||
| 8 | 1.29 (m) | |||||
| 9 | 2.33 (m) | 1.36 (m) | 2.10 (m) | 1.96 (m) | 2.25 (m) | 2.00 (m) |
| 2.00 (t, 13.5) | 1.26 (m) | 1.67 (m) | 2.12 (m) | |||
| 10 | 2.21 (m) | 2.08 (m) | 2.26 (m) | 1.86 (m) | 4.07 (d, 10,9) | 1.90 (m) |
| 1.46 (m) | 2.11 (m) | 1.43 (m) | 1.55 (m) | |||
| 11 | 2.89 (dd, 10.6, 2.9) | 5.05 (t, 7.7) | 5.32 (m) | 5.04 (d, 10.3) | 2.09 (m) | 5.08 (d, 9.4) |
| 12 | ||||||
| 13 | 2.25 (m) | 2.26 (dd, 13.5, 3.0) | 4.29 (t, 4.3) | 1.94 (m) | 5.24 (t, 7.7) | 1.78 (m) |
| 1.65 (m) | 2.20 (dd, 13.5, 10.9) | 1.61 (m) | 1.59 (m) | |||
| 14 | 4.88 (dd, 11.2, 5.1) | 5.02 (ddd, 10.8, 4.2, 1.1) | 4.84 (td, 5.0, 1.6) | 3.04 (m) | 2.07 (m) | 2.33 (td, 11.8, 7.4) |
| 2.38 (m) | 1.68 (m) | |||||
| 15 | 2.48 (m) | 2.22 (m) | 2.65 (m) | 1.70 (m) | ||
| 16 | 1.12 (d, 6.5) | 1.11 (d, 6.6) | 1.16 (d, 6.8) | 1.37 (s) | 0.96 (d, 6.6) | 1.11 (s) |
| 17 | 1.16 (d, 6.5) | 1.12 (d, 6.6) | 1.08 (d, 6.9) | 1.30 (s) | 0.84 (d, 6.7) | 1.13 (s) |
| 18 | 1.16 (s) | 1.00 (s) | 1.20 (s) | 1.30 (s) | 1.35 (s) | |
| 19 | 4.88 (s) | 0.77 (d, 6.0) | 1.56 (s) | 1.56 (s) | 1.54 (s) | 1.71 (s) |
| 4.69 (s) | ||||||
| 20 | 1.12 (s) | 1.67 (s) | 1.56 (s) | 1.10 (s) | 1.57 (m) | 1.17 (s) |
| OAc | 2.09 (s) | 2.08 (s) | 2.05 (s) |
| No. | 1 | 3 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|
| 1 | 149.90, qC | 49.67, CH | 150.34, qC | 146.95, qC | 51.96, CH | 91.52, qC |
| 2 | 125.85, CH | 81.19, CH | 121.20, CH | 121.34, CH | 122.10, CH | 129.84, CH |
| 3 | 51.08, CH | 151.06, CH | 87.63, CH | 73.16, CH | 141.04, CH | 137.41, CH |
| 4 | 81.64, qC | 131.39, qC | 74.93, qC | 75.86, qC | 74.20, qC | 72.47, qC |
| 5 | 41.04, CH2 | 37.42, CH2 | 39.37, CH2 | 39.18, CH2 | 44.21, CH2 | 41.64, CH2 |
| 6 | 25.90, CH2 | 66.40, CH | 22.57, CH2 | 21.75, CH2 | 23.66, CH2 | 22.90, CH2 |
| 7 | 54.09, CH | 47.19, CH2 | 128.74, CH | 127.40, CH | 127.31, CH | 130.06, CH |
| 8 | 148.54, qC | 28.31, CH | 133.16, qC | 134.03, qC | 130.05, qC | 132.11, qC |
| 9 | 24.65, CH2 | 37.25, CH2 | 39.64, CH2 | 33.90, CH2 | 46.88, CH2 | 34.86, CH2 |
| 10 | 28.07, CH2 | 24.31, CH2 | 25.00, CH2 | 25.03, CH2 | 68.82, CH | 27.37, CH2 |
| 11 | 62.28, CH | 129.20, CH | 130.08, CH | 72.70, CH | 38.96, CH2 | 77.36, CH |
| 12 | 59.06, qC | 130.80, qC | 132.19, qC | 74.15, qC | 132.94, qC | 84.58, qC |
| 13 | 45.46, CH2 | 41.43, CH2 | 79.67, CH | 29.33, CH2 | 128.47, CH | 35.80, CH2 |
| 14 | 69.34, CH | 73.92, CH | 86.05, CH | 20.95, CH2 | 23.68, CH2 | 30.76, CH2 |
| 15 | 27.09, CH | 25.81, CH | 27.43, CH | 74.60, qC | 29.13, CH | 72.84, qC |
| 16 | 21.23, CH3 | 24.93, CH3 | 21.11, CH3 | 30.42, CH3 | 21.15, CH3 | 26.01, CH3 |
| 17 | 23.55, CH3 | 18.74, CH3 | 22.57, CH3 | 28.85, CH3 | 21.85, CH3 | 24.62, CH3 |
| 18 | 27.70, CH3 | 172.97, qC | 23.36, CH3 | 21.72, CH3 | 29.17, CH3 | 29.00, CH3 |
| 19 | 112.26, CH2 | 18.25, CH3 | 15.84, CH3 | 17.41, CH3 | 15.52, CH3 | 16.33, CH3 |
| 20 | 23.05, CH3 | 18.47, CH3 | 12.89, CH3 | 23.63, CH3 | 15.09, CH3 | 20.69, CH3 |
| OAc | 171.13, qC | 170.47, qC | 170.17, qC | |||
| 21.35, CH3 | 21.75, CH3 | 21.42, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-T.; Yu, D.-D.; Su, M.-Z.; Luo, H.; Cao, J.-G.; Liang, L.-F.; Yang, F.; Guo, Y.-W. Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum. Mar. Drugs 2023, 21, 69. https://doi.org/10.3390/md21020069
Song Y-T, Yu D-D, Su M-Z, Luo H, Cao J-G, Liang L-F, Yang F, Guo Y-W. Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum. Marine Drugs. 2023; 21(2):69. https://doi.org/10.3390/md21020069
Chicago/Turabian StyleSong, Yu-Ting, Dan-Dan Yu, Ming-Zhi Su, Hui Luo, Jian-Guo Cao, Lin-Fu Liang, Fan Yang, and Yue-Wei Guo. 2023. "Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum" Marine Drugs 21, no. 2: 69. https://doi.org/10.3390/md21020069
APA StyleSong, Y.-T., Yu, D.-D., Su, M.-Z., Luo, H., Cao, J.-G., Liang, L.-F., Yang, F., & Guo, Y.-W. (2023). Structurally Diverse Diterpenes from the South China Sea Soft Coral Sarcophyton trocheliophorum. Marine Drugs, 21(2), 69. https://doi.org/10.3390/md21020069











