Abstract
Parasitic diseases, especially those caused by protozoans and helminths, such as malaria, trypanosomiasis, leishmaniasis, Chagas disease, schistosomiasis, onchocerciasis, and lymphatic filariasis, are the cause of millions of morbidities and deaths every year, mainly in tropical regions. Nature has always provided valuable antiparasitic agents, and efforts targeting the identification of antiparasitic drugs from plants have mainly focused on glycophytes. However, salt-tolerant plants (halophytes) have lately attracted the interest of the scientific community due to their medicinal assets, which include antiparasitic properties. This review paper gathers the most relevant information on antiparasitic properties of halophyte plants, targeting human uses. It includes an introduction section containing a summary of some of the most pertinent characteristics of halophytes, followed by information regarding the ethnomedicinal uses of several species towards human parasitic diseases. Then, information is provided related to the antiprotozoal and anthelmintic properties of halophytes, determined by in vitro and in vivo methods, and with the bioactive metabolites that may be related to such properties. Finally, a conclusion section is presented, addressing perspectives for the sustainable exploitation of selected species.
1. Introduction
Human parasitic diseases are predominantly linked to tropical or subtropical areas. However, climate change and the increased mobility of humans and animals trigger vector migration and the upsurge of parasitic infections in developed countries. Parasitic diseases continue to play a major role in human health, particularly infections caused by protozoans and helminths, such as malaria, trypanosomiasis, leishmaniasis, Chagas disease, schistosomiasis, onchocerciasis, lymphatic filariasis, and helminthiases, which are common in tropical regions and cause millions of morbidities and deaths every year [1,2].
For centuries, nature has been a source of medicines for the treatment of a vast array of diseases, with the first records of the ethnomedicinal uses of plants dating back to 2600 BC in Mesopotamia [3]. The easy access to terrestrial plants helps to explain their popularity as a source of bioactive products and innovative drug leads used in the pharmaceutical industry [4]. The importance of plant-based molecules as antiparasitic agents was reinforced by the Nobel Prize laureates in physiology or medicine in 2015, where Youyou Tu was honored for her discovery of artemisinin, a novel drug for the therapy against malaria, which was derived from Artemisia annua L., a plant used in the Chinese traditional medicine [5].
Most research targeting the identification of antiparasitic agents has focused on glycophytes, but salt-tolerant plants (halophytes) have lately aroused the interest of the scientific community due to their multiple medicinal properties, including antiparasitic activities. The harsh habitats where halophytes thrive, such as salt marshes, maritime dunes, and marine cliffs, expose them to extremely variable abiotic conditions, including salinity, light intensity, drought, and temperature [6,7]. This stressful environment contributes to the synthesis and accumulation of bioactive metabolites, including phenolics, alkaloids, and terpenes, conferring to halophyte species important medicinal properties, such as antioxidant, anti-inflammatory, antimicrobial, anti-tumoral, anti-infective, and antiparasitic activities [8,9,10,11,12,13,14,15]. In fact, several halophyte species are used as medicinal (e.g., Mesembryanthemum edule L. (syn. Carpobrotus edulis L.), and/or dietary plants (e.g., Chenopodium quinoa Willd, Salicornia spp.), mainly in rural areas where traditional medicine is the only source of health treatments. Moreover, as the problems associated with the salinization of soils and water bodies and the increasing competition for scarce freshwater resources increase [16,17], recruiting wild halophytes with economic potential is one of the suggested strategies to reduce the damage caused by the salinization of soil and water [18]. While most of the crop species used in traditional agriculture are salt sensitive (glycophytes), having a 10% yield decrease as the soil salinity increases over the 4–8 dS/m range, the growth of several halophytes is stimulated within a salinity range of 15–25 dS/m [18]. Halophytes are, therefore, a real strategy as alternative highly salt-tolerant crops that can cope with adverse saline conditions, to be used in the exploitation of degraded agricultural lands with the irrigation with brackish water for sustainable water management and soil conservation when establishing cost-efficient and environmental-friendly agro-ecosystems.
Reports on the traditional medicinal uses of halophytes comprise 43 families and more than 180 species in the Mediterranean, the Arabian Sea, and Syrian regions [12,19,20]. Some of these halophytes, such as Chenopodium album and Artemisia ramosissima have ethnomedicinal uses for parasitic diseases, including protozoal and helminthic infections [12,18,19], and several scientific reports confirmed their activities by in vitro and in vivo methods and identified their main bioactive constituents. There are several review papers detailing the biological properties of halophytic plant species [12,13,14,15], but information related to the properties of such plants is still dispersed in the literature. Aiming to fulfill this gap, this review provides a comprehensive outline of the ethnomedicinal uses of several species against human parasitic illnesses and the in vitro and in vivo antiprotozoal and anthelmintic properties of such plants, along with the bioactive metabolites that may be responsible for such assets. Finally, a conclusion section is presented, addressing perspectives for the sustainable exploitation of selected species in the context of sustainability and climate change.
2. Methodology
This review provides a focused overview of the potential use of halophytes as sources of bioactive molecules and/or natural products to tackle human parasitic diseases. A systematic search was performed to find all English articles with available full text related to the subject from 1993 until December 2022. Searches were performed by consulting several databases, including PubMed, Web of Science, Embase, and Google Scholar (as a search engine). The keywords “ethnomedicinal”, “traditional use”, “halophyte” and/or “salt tolerant plant” were used, alone or in combination with, for example, “antiparasitic”, “antiprotozoal“, and “anthelminthic” and its hyphenated variations. The classification of the plant species as halophytes was confirmed by a search in the eHALOPH database, and/or the description of their occurrence in coastal areas.
3. Ethnomedicinal Uses of Halophyte Plants as Antiparasitic Agents
Studies focusing on the ethnomedicinal uses of halophytes are limited [12,19,20,21]. Table 1 summarizes several halophytes species and their ethnopharmacological uses related to the treatment of human parasitic diseases. In brief, species such as Chenopodium album, Artemisia ramosissima, Helichrysum italicum, Portulaca oleracea, and Limoniastrum monopetalum are used to treat intestinal helminthic infections in different regions, such as Portugal, Nepal, Pakistan, Libya, Tunisia, the North Sea, India, Spain, and Italy [22,23,24,25,26,27,28,29]. Others, like Dysphania ambrosioides and Portulaca oleracea are used for their antiprotozoal properties in Albania, Cyprus, Iran, Egypt, and Brazil [28,29]. Several species (e.g., Limonium vulgare, Portulaca olearacea, Rumex crispus, Elaeagnus ramosíssima, Salsola kali, Dysphania ambrosioides, Chenopodium album) have been described as anti-diarrheic, which could be related to both helminthic and protozoal parasitic infections [21,22,28,29,30,31,32,33]. Such traditional uses demonstrate the importance and extensive uses of halophyte plants in folk medicine, especially in the Mediterranean region.

Table 1.
Examples of halophytes used in ethnomedicine towards parasitic diseases.
Herbal medicine has always had a fundamental role in human welfare, and it is still of utmost importance in many cultures today. In this sense, the ethnopharmacological knowledge of populations can serve as a base to search for novel bioactive compounds. Considering this, scientific research has explored and produced numerous studies about medicinal plants’ biological properties and chemical constituents not only to advance plant drug discovery but also to validate the plant’s traditional uses. However, for halophytes with folk uses, research on their bioactivities and subsequent evidence are still scarce for many of those plants. The next sections review some of the most relevant published work related to antiprotozoal (Section 4) and anthelmintic (Section 5) properties of halophyte species.
4. Halophyte Plants as Sources of Antiprotozoal Agents
Aligned to their traditional uses as antiparasitic agents, halophytes have proven by in vitro and in vivo research approaches their potential as sources of molecules with activity towards different protozoa species. Most antiprotozoal studies on natural products focus particularly on neglected tropical diseases (NTDs), a group of twenty infectious illnesses that include, for example, leishmaniasis, human African trypanosomiasis (HAT), Chagas disease, and schistosomiasis. NTDs affect more than 1 billion people worldwide, particularly very poor populations in tropical and subtropical areas in 149 countries [38,39,40]). Leishmaniasis is caused by more than 20 Leishmania species, while trypanosomiasis is ascribed to Trypanosoma, either the Trypanosoma brucei complex (sleeping sickness, human African trypanosomiasis) or T. cruzi (Chagas disease, American trypanosomiasis) [40]. Malaria, referred to as a “disease of poverty”, is no longer recognized as an NTD [41] and is caused by protozoa of the genus Plasmodium, namely P. falciparum, P. vivax, P. malariae, and P. ovale, which are specific for humans [42].
4.1. In Vitro Activities and Bioactive Constituents
Most of the reports on the antiparasitic activity of halophyte species include an in vitro screening, followed by the determination of the chemical composition of raw extracts, and less frequently of purified fractions or pure compounds. Sixteen species belonging to 14 different families have been described with in vitro antiprotozoal activity, and those reports are summarized in Table 2.
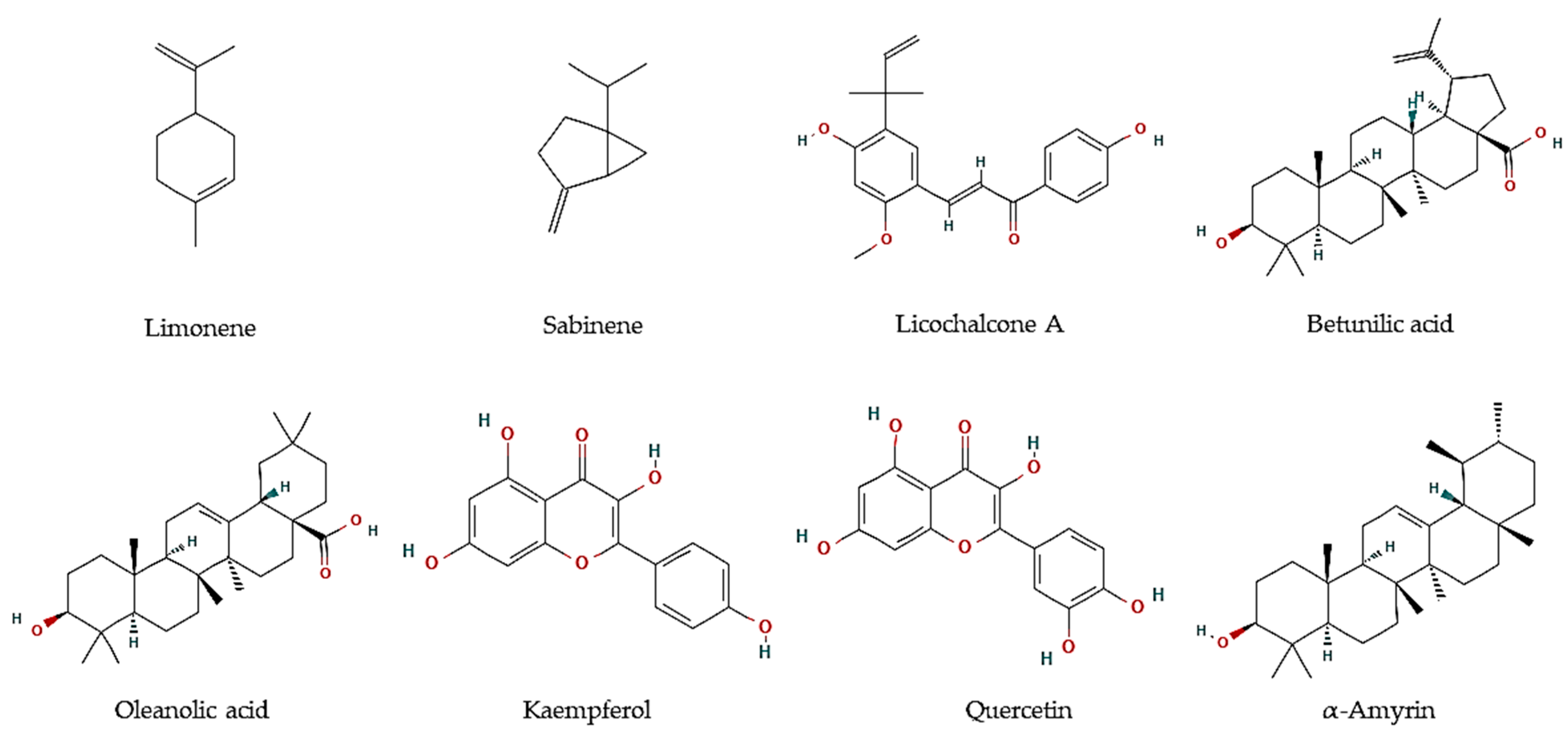
Essential oils are described as a composite mixture of volatile molecules obtained from aromatic plants, mostly by hydrodistillation, and display highly relevant biological properties, including antiparasitic activities. Essential oils were the main target to evaluate the potential antiprotozoal properties of halophyte species, mostly against Leishmania and Trypanosoma parasites. For example, the essential oil of flowering aerial parts of Crithmum maritimum was highly effective towards T. brucei parasites (EC50 = 5.0 µg/mL), which was linked to its mono-terpene hydrocarbon content, such as in limonene (EC50 = 5.6 µM; Figure 1) and sabinene (EC50 = 6.0 µM; Figure 1) [43]. However, they were less effective against L. infantum promastigotes (IC50 = 122 and 205 µg/mL, respectively) [44]. The antiparasitic features of major compounds, including monoterpene hydrocarbons, sesquiterpene hydrocarbons, oxygen-containing sesquiterpenoids, and diterpenoids, are well described [44]. In turn, the essential oil of leaves and fruits of Pistacia lentiscus exerted high inhibitory effects against promastigotes of Leishmania major, L. tropica, and L. infantum, with IC50 values varying between 8 and 26.2 µg/mL [45]. The major volatile components were myrcene and α-pinene in leaves, and α-pinene and limonene, in fruits, all reported with antileishmanial activities [46,47]. In another work, essential oils from leaves of P. lentiscus collected from two areas in Tunisia were tested against L. major intramacrophage and axenic amastigote forms [48], displaying moderate activities against intracellular amastigote (IC50 = 12.5–35.6 µg/mL), and high activity against L. major axenic amastigote forms (IC50 = 0.5 µg/mL) [48]. The main compounds were identified as pinene, β-myrcene, d-limonene, O-cymene, terpinen-4-ol, β-pinene, and α-phellandrene, which may disrupt parasite intracellular metabolic pathways [48].
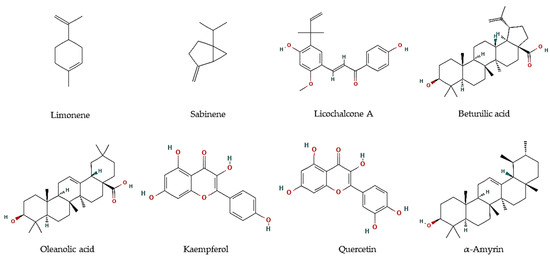
Figure 1.
Chemical structures of pure compounds reported with antiprotozoal properties.

Table 2.
In vitro antiprotozoal activity of halophyte species.
Table 2.
In vitro antiprotozoal activity of halophyte species.
| Family/Species | Plant Organ | Extract/Fraction/Compound | Chemical Components | Protozoal Species | Results * | References |
|---|---|---|---|---|---|---|
| Amaranthaceae | ||||||
| Dysphania ambrosioides (L.) Mosyakin & Clemants (syn. Chenopodium ambrosioides L.) | Aerial organs | Essential oil | Terpinolene | L. amazonensis, L. donovani | Epimastigotes (IC50 = 21.3 µg/mL), and trypomastigotes (IC50 = 28.1 µg/mL) | [49] |
| T. cruzi | Epimastigotes (IC50 = 21.3 µg/mL), trypomastigotes (IC50 = 28.1 µg/mL), and amastigotes (IC50 = 50.2 µg/mL) | [49] | ||||
| Aerial parts containing immature seeds | Ethanol ethylacetate extract | Ascaridole [1]; (−)-(2S,4S)-p-mentha-1(7),8-dien-2-hydroperoxide [2]; (−)-(2R,4S)-p-mentha-1(7),8-dien-2-hydroperoxide [3] (−)-(1R,4S)-p-mentha-2,8-dien-1-hydroperoxide [4] (−)-(1S,4S)-p-mentha-2,8-dien-1-hydroperoxide [5]. | T. cruzi (epimastigotes) | MLC [1] = 23 μM; MLC [2] = 1.2 μM; MLC [3] = 1.6 μM; MLC [4]= 3.1 μM; and MLC [5]= 0.8 μM | [50] | |
| Leaves | Hydroalchoholic extract | ND | Giardia lamblia (trophozoites) | IC50 = 198 µg/mL | [33] | |
| Leaves | 70 % Ethanol extract | ND | Plasmodium falciparum | IC50 = 25.4 μg/mL | [51] | |
| Leaves | Essential oil | Ascaridole | Entamoeba histolytica (trophozoites) | IC50 = 700 µg/mL | [52] | |
| Anacardiaceae | ||||||
| Pistacia lentiscus L. | Leaves and fruits | Essential oil | Leaves: Myrcene and α-pinene; Fruits: α-pinene and limonene | Leishmania major, L. tropica, L. infantum (clinical isolates) | IC50 = 8–26.2 µg/mL | [45] |
| Leaves | Essential oil | α-pinene, β-myrcene, D-limonene, o-cymene, terpinen-4- ol, β-pinene, α-phellandrene | Leishmania major | Intramacrophage amastigote: IC50 = 12.5–35.6 µg/mL; Axenic amastigote: IC50 = 0.5–56.1 µg/mL | [48] | |
| Apiaceae | ||||||
| Crithmum maritimum L. | Aerial organs | Essential oil | Limonene, γ-terpinene and sabinene | Trypanossoma brucei | IC50 = 5.0 µg/mL | [43] |
| Limonene, sabinene | Limonene: EC50 = 5.6 µM Sabinene: EC50 = 6.0 µM | |||||
| Aerial organs | Essential oil | α-pinene, p-cymene β-phellandrene, Z-β -ocimene, γ-terpinene, thymyl-methyl oxide, dillapiole | L. infantum (promastigotes) | IC50 = 122 µg/mL | [44] | |
| Flowers | Decoction | Falcarindiol | Trypanosoma cruzi | Extract: EC50 = 17.7 µg/mL, SI > 5.65 Fraction: EC50 = 0.47 µg/mL, SI = 59.6 | [53] | |
| Eryngium maritimum L. | Aerial organs | Essential oil | α-pinene, germacrene D, bicyclogermacrene, germacrene, δ-cadinene | L. infantum (promastigotes) | IC50 = 205 µg/mL | [44] |
| Foeniculum vulgare Mill. | Seeds | Essential oil, n-hexane, methanol, and water extracts | E-anethole | Trichomonas vaginalis | Methanol and hexane extracts: MLC = 360 µg/mL Essential oil and anethole: MLC = 1600 µg/ml | [54] |
| Seeds | Water extract | Hesperidin, ferulic acid, chlorogenic acid | Blastocystis spp. | 48h: IC50 = 224 µg/mL; 72h: IC50 = 175 µg/mL | [55] | |
| Asteraceae | ||||||
| Inula crithmoides L. | Aerial organs | Dichloromethane extract | Gallic, syringic, salicylic caffeic, coumaric, and rosmarinic acids; epicatechin, epigalocatechin gallate, catechin hydrate, quercetin, and apigenin | Leishmania infantum | Intracellular amastigotes: 70% at 125 µg/mL; Promastigotes: 26.5% at 125 µg/mL | [56] |
| Caryophyllaceae | ||||||
| Spergularia rubra (L.) J.Presl & C.Presl and | Aerial organs | Dichloromethane extract | Catechin hydrate | Leishmania infantum | Intracellular amastigotes: 25% at 125 µg/mL; promastigotes: 16.7% at 125 µg/mL | [56] |
| Cyperaceae | ||||||
| Cyperus rotundus L. | Tuber of root | Ethyl acetate extract | ND | Plasmodium falciparum | Sensitive strain 3D7: IC50 = 5.1 µg/mL; resistant strain INDO: IC50 = 4 µg/mL | [57] |
| Combretaceae | ||||||
| Laguncularia racemosa (L.) C.F. Gaertn. | Leaves | Chloroform:methanol (1:1) extract | Triterpenoids, phenols | P. falciparum | 60.1 % at 6.25 μg/mL | [58] |
| Fabaceae | ||||||
| Glycyrrhiza glabra L. | Roots | Water extract | Licochalcone A | Leishmania major (promastigotes) | Extract: > 90% death at 1:100 and 1:200 dilutions | [59] |
| L. donovani (promastigotes) | > 90% death at 1:100 dilution | [59] | ||||
| Licochalcone A | L. major | Amastigotes: 0 % infection at 5 and 10 μg/mL Promastigotes: 0.4 % at 1:100 | [59] | |||
| Juncaceae | ||||||
| Juncus acutus L. | Roots | Dichloromethane extract and Fraction 8 | Phenanthrenes, dihydrophenanthrenes, and benzocoumarins | Trypanosoma cruzi (trypomastigotes) | Extract: IC50 < 20 µg/mL; Fraction 8: IC50 = 4.1 µg/mL, SI: 1.5 | [60] |
| Nitrariaceae | ||||||
| Peganum harmala L. | Seeds | Water extract | ND | L. major (Promastigotes, amastigotes) | Promastigotes: IC50 = 40 µg/mL; Amastigotes: 50% reduction of infection at 10 and 40 µg/mL at 48h | [61] |
| Seeds | Hydroalchoholic extract | Harmaline, harmine, and beta-carboline | L. major (promastigotes) | IC50 = 59.4 µg/mL | [62] | |
| Seeds | Water extract | ND | L. donovani (promastigotes, axenic amastigotes) | Promastigotes: ED50 = 458,000 µg/mL at 72 h; Axenic amastigotes: ED50 = 6000 µg/mL at 72 h | [63] | |
| Seeds, Roots | Methanol extract | ND | L. tropica | Seeds: IC50 = 18.6 µg/mL; Roots: IC50 = 16.4 µg/mL | [64] | |
| Plantaginaceae | ||||||
| Plantago major | Seeds | 80% Ethanol | ND | P. falciparum | IC50 = 40.0 µg/mL | [65] |
| Polygonaceae | ||||||
| Rumex crispus L. | Leaves, roots | Methanol and ethanol extract | ND | Trypanosoma brucei brucei | Etanol root: IC50: 9.7 μg/mL | [66] |
| Plasmodium falciparum 3D7 strain | Methanol leaves: IC50 = 15 μg/mL | [66] | ||||
| Portulacaceae | ||||||
| Portulaca oleraceae | Leaves, stems | Essential oils | Phytol, squalene, palmitic acid, ethyllinoleate, ferulic acid, linolenic acid, scopoletin, linoleic acid, rhein, apigenin, bergapten | L. major (promastigotes) | Leaves: IC50 = 360 µg/mL; Stems: IC50 = 680 µg/mL | [67] |
| Tetrameristaceae | ||||||
| Pelliciera rhizophorae Planch. & Triana | Leaves | Methanol:Chloroform (1:1) fraction | α-amyrin, β-amyrine, ursolic acid, oleanolic acid, betulinic acid, brugierol, iso-brugierol, kaempferol, quercetin, and quercetin | Leishmania donovani | Oleanolic acid: IC50 = 5.3 μM Kaempferol: IC50 = 22.9 μM Quercetin:IC50 = 3.4 μM | [68] |
| Trypanosoma cruzi | α-Amyrin: IC50 = 19.0 μM | [68] | ||||
| Plasmodium falciparum | Betulinic acid: IC50 = 18.0 μM | [68] |
ND: not determined. IC50: half-maximal inhibitory concentration; EC50: half-maximal effective concentration; LC50: median lethal concentration; MLC: minimum lethal concentration; SI = Selectivity index. *: IC50 < 15 µg/mL and SI > 3 are considered promising for development as drug leads [69].
Some essential oils from other species have been described with antiparasitic activities, but with higher IC50 values, such as those from P. oleracea leaves and stems (IC50 = 360 and 680 µg/mL) on L. major promastigotes [67], or from F. vulgare seeds against T. vaginalis (MLC = 1600 µg/mL) [54]. The essential oil of D. ambrosioides aerial organs have been investigated for its in vitro activity against L. amazonensis and L. donovani, being highly active towards their epimastigotes (IC50 = 21.3 µg/mL) and trypomastigotes (IC50 = 28.1 µg/mL), as well as towards T. cruzi amastigotes (IC50 = 50.2 µg/mL) [49]. Terpinolene was the major active component [49]. In addition, ascaridole, identified as the main component of D. ambrosioides leaves’ essential oil, also exhibited in vitro activity against E. histolytica parasites, which are responsible for amebiasis, a parasitic disease considered a public health problem in developing countries [52].
Extraction with organic solvents has less expression than the extraction of essential oils, but even so, it also proves to be very effective in the extraction of compounds with antiprotozoal activity from salt-tolerant species. In this context, Oliveira and colleagues [60] performed an in vitro screening of T. cruzi trypomastigotes on 94 samples belonging to 31 halophytes species from Southern Portugal. From those, the dichloromethane extract of Juncus acutus roots was the most active (IC50 < 20 µg/mL), which was further fractionated, affording one active fraction with an IC50 of 4.1 µg/mL and selectivity index (SI) of 1.5. The active constituents were identified as phenanthrenes, dihydrophenanthrenes, and benzocoumarins [60]. C. maritimum flower decoction also presented anti-T. cruzi activity with an EC50 value of 17.7 µg/mL and SI of 5.65, and a fraction rich in falcarindiol has an increased activity (EC50 = 0.47 µg/mL) and selectivity (SI = 59.6) [53].
The same research group also screened 25 salt-tolerant plant species from southern Portugal for promastigotes and intracellular amastigotes of L. infantum, and the highest activity was obtained with the dichloromethane extract of S. rubra and I. crithmoides aerial organs [56]. The active extracts from I. crithmoides were rich in phenolic acids (gallic, syringic, salicylic caffeic, coumaric, and rosmarinic acids) and flavonoids (epicatechin, epigallocatechin gallate, catechin hydrate, quercetin, and apigenin), while catechin hydrate was detected in S. rubra [56].
In addition, hexane and methanol extracts of F. vulgare seeds were highly active against T. vaginalis (MLC = 360 µg/mL) [54], whereas aqueous extracts exhibit anti-Blastocystis activity with IC50 values ranging between 223.8 and 174.9 µg/mL [55]. The predominant compounds in F. vulgare were hesperidin, ferulic, and chlorogenic acid [55]. Several authors reported the in vitro antimalarial properties of G. glabra, particularly of root and aerial part extracts [65,70,71]. Furthermore, Licochalcone A (Figure 1) was isolated from its root water extract, with activity against L. major amastigotes (0% infected cells at 5 and 10 µg/mL) and promastigotes (0.4% infected cells at 1:100). Moreover, major components of a methanol:chloroform fraction obtained from the leaves of the tea mangrove Pelliciera rhizophorae, showed high antiprotozoal activity against L. donovani (Oleanolic acid: IC50 = 5.3 μM; Kaempferol: IC50 = 22.9 μM; Quercetin: IC50 = 3.4 μM), T. cruzi (α-amyrin: IC50 = 19.0 μM), as well as P. falciparum (Betulinic acid: IC50 = 18.0 μM) (Figure 1) [68].
Other authors reported the in vitro anti-Plasmodium efficacy of ethanolic extracts of Plantago major seeds (P. falciparum: IC50 = 40.0 µg/mL) [65] and C. rotundus tuber root ethyl acetate extract (P. falciparum IC50 = 5.1 µg/mL and 4 µg/mL for sensitive and resistant strains, respectively) [57]. Peganum harmala seeds and aerial parts extracts have been extensively studied for their antileishmanial properties against L. major, L. donovani, and L. tropica as sustained by different authors [61,62,63,64].
Overall, some authors defined that extracts with IC50 values below 15 µg/mL and selectivity above 3 can be considered promising for further development as drug leads [69]. Following this guideline, the fraction of flower decoction of C. maritimum can be considered the most promising sample with anti-T. cruzi activity by coupling both criteria (EC50 = 0.47 µg/mL; SI = 59.6) [53].
4.2. In Vivo Studies
Only a few authors evaluated the in vivo antiprotozoal potential of halophytes, including only three species and using mainly rodents as models. These reports are summarized in Table 3.
The most studied species was D. ambrosioides, investigated by five different authors. For instance, essential oil from its aerial parts was more effective against experimental cutaneous leishmaniasis by L. amazonensis in BALB/c mice than its pure main components, namely ascaridole, carvacol, and caryophyllene oxide [72]. In turn, hydroalcoholic extracts of its leaves displayed in vivo antimalarial properties against BALB/c mice infected with P. berghei intraperitoneally [51], and in vivo effects on C3H/HePas mice infected with L. amazonensis promastigotes [73]. Moreover, ascaridole was the main component in D. ambrosioides leaves’ essential oil and exhibited in vitro and in vivo activity against E. histolytica parasites [52]. Besides, other authors reported the anti-Plasmodium efficacy of ethanolic extracts of A. officinalis flowers (P. falciparum IC50 = 62.7 µg/mL; P. berghei suppression of parasitemia in vivo [400 mg/kg] = 62.86 %), and of P. major seeds (P. falciparum: IC50 = 40.0 µg/mL; P. berghei suppression of parasitemia in vivo [400 mg/kg] = 22.5%) [65].

Table 3.
In vivo antiprotozoal activity of halophyte species.
Table 3.
In vivo antiprotozoal activity of halophyte species.
| Family/Species | Plant Organ | Extract/Fraction/Compound | Chemical Components | Assay | Results | References |
|---|---|---|---|---|---|---|
| Amaranthaceae | ||||||
| Dysphania ambrosioides (L.) Mosyakin & Clemants (syn. Chenopodium ambrosioides L.) | Aerial organs | Essential oils | Ascaridole, carvacrol, caryophyllene oxide | Cutaneous leishmaniasis-L. amazonensis in BALB/c mice | Prevented lesion development compared with untreated animals | [72] |
| Mix of ascaridole, carvacrol, caryophyllene oxide | Cutaneous leishmaniasis-L. amazonensis in BALB/c mice | Cause death of animals after 3 days of treatment | [72] | |||
| Leaves | Essential oil | Ascaridole | Entamoeba histolytica HM-1 in IMSS strain Golden hamsters infected with trophozoites | 8 mg/kg and 80 mg/kg reverted the infection | [52] | |
| Leaves | 70% Ethanol | ND | BALB/c mice infected with P. berghei | Increased survival and decreased parasitaemia | [51] | |
| Leaves | 70% Ethanol | ND | C3H/HePas mice infected with Leishmania amazonensis promastigotes | Reduced nitric oxide production and the parasite load | [73] | |
| Malvaceae | ||||||
| Althaea officinalis L. | Flowers | 80% Ethanol | ND | P. berghei infected female Swiss albino mice | Suppression of parasitemia = 62.86 %, at a dose of 400 mg/kg | [65] |
| Plantaginaceae | ||||||
| Plantago major L. | Seeds | 80% Ethanol | ND | P. berghei infected female Swiss albino mice | Suppression of parasitemia = 22.46 %, at a dose of 400 mg/kg | [65] |
ND: not determined.
5. Halophyte Plants as Sources of Anthelmintic Agents
Supported by their traditional uses, some halophyte species have also demonstrated in vitro and in vivo potential as sources of molecules with anthelmintic activity. Most anthelmintic studies on natural products focus mainly on the model nematode Caenorhabditis elegansi, or gastrointestinal nematodes (GINs), namely Haemonchus contortus and Trichostrongylus colubriformis, a leading cause of production loss in agricultural animal systems worldwide [74,75]. Since different Trichostrongylus species, as for example, T. colubriformis, can infect humans in different areas of the world (e.g., Iran, Laos, Australia) [76], that species was included in this review.
5.1. In Vitro Activities and Bioactive Constituents
Most of the reports on the anthelmintic activity of halophytes were performed only in vitro, and only less than half included a chemical characterization of its major constituents. Twelve species from 9 families have been described with in vitro anthelmintic properties, and those reports are included in Table 4.
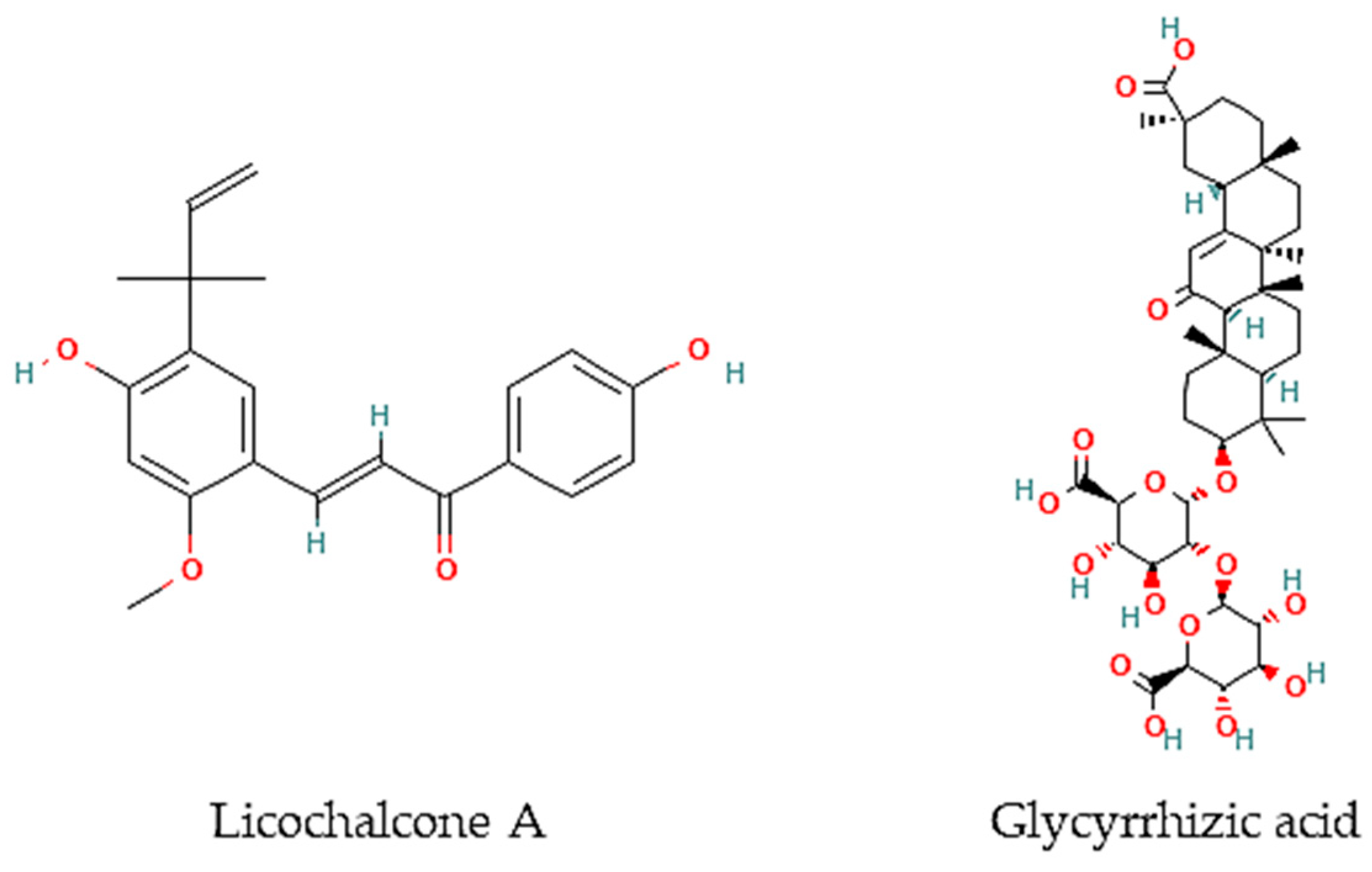
Oliveira and colleagues [77] have screened 80% acetone extracts from 8 halophyte species for their anthelmintic capacity using the Larval Ensheathment Inhibition Assay (LEIA) and Egg Hatching Inhibition Assay (EHIA) for T. colubriformis. P. lentiscus, L. monopetalum, C. mariscus, and H. italicum picardi were the most active in both GINs and life stages of the nematodes [77]. In particular, C. mariscus aerial parts collected during summer were more active against T. colubriformis (EC50 = 77.8 µg/mL) [78]. Moreover, inflorescences presented the highest anthelmintic activity against T. colubriformis [EC50 (LEIA) = 78.6 µg/mL; IC50 (EHIA) = 848.2 µg/mL]. Flavan-3-ols, proanthocyanidins, luteolin, and glycosylated flavonoids are the main active components [78]. Some authors have also tested some halophyte extracts against S. mansoni, which is a helminth species and the causal agent of schistosomiasis in humans. Methanol extracts of C. ambrosioides reduced S. mansoni cercariae infectivity [79], as well as essential oils from F. vulgare that showed moderate in vitro activity against S. mansoni worms, but with more remarkable effects in egg development, possibly attributable to the two main constituents detected in the essential oil, I-anethole and limonene [80]. A compound isolated from G. inflata, licochalcone A (Figure 2), presented an LC50 of 9 μM towards S. mansoni female and male adult worms [81].
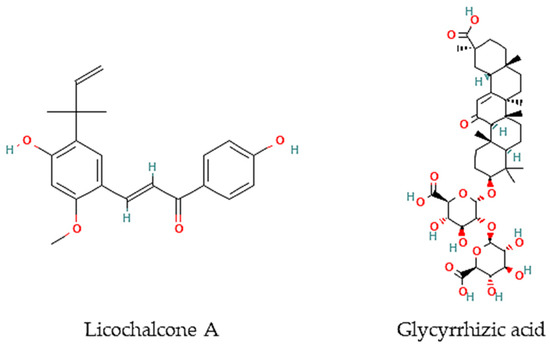
Figure 2.
Chemical structures of pure compounds reported with anthelmintic properties.
Besides, other authors have focused on other less frequently tested parasite species. For instance, the roots of G. glabra contain glycyrrhizic acid (Figure 2) that is very active in vitro against B. malayi microfilarae (IC50 = 1.20 μM), one of the most important causative agents of human lymphatic filariasis [82].

Table 4.
In vitro anthelmintic activity of halophyte species.
Table 4.
In vitro anthelmintic activity of halophyte species.
| Family/Species | Plant Organ | Extract/Fraction/Compound | Chemical Components | Assay | Results | References |
|---|---|---|---|---|---|---|
| Apiaceae | ||||||
| Foeniculum vulgare Mill. | Fresh leaves | Essential oil | I-anethole and limonene | Schistosoma mansoni adult worms (pairs) and eggs | 50% activity at 100,000 µg/mL (24 and 120 h) | [80] |
| Asteraceae | ||||||
| Helichrysum italicum (Roth) G. Don subsp. picardi (Boiss. & Reut.) Franco | Aerial parts | 80% acetone extract | Caffeoylquinic and dicaffeoylquinic acids and quercetin glycosides | Trichostrongylus colubriformis | IC50 (LEIA) = 132 µg/mL; IC50 (EHIA) = 3707 µg/mL | [77] |
| Inula crithmoides L. | Aerial parts | 80% acetone extract | ND | Trichostrongylus colubriformis | IC50 (LEIA) = 1031 µg/mL | [77] |
| Cyperaceae | ||||||
| Cladium mariscus L. Pohl | Aerial parts | 80% acetone extract | Proanthocyanins, phenolic acids, and luteolin | Trichostrongylus colubriformis | IC50 (LEIA) = 77.8 µg/mL; IC50 (EHIA) = 2575 µg/mL | [77] |
| Aerial parts, leaves, and inflorescences collected during spring, summer, autumn, and winter | 80% acetone extract | Flavan-3-ols, proanthocyanidins, luteolin, and glycosylated flavonoids | Trichostrongylus colubriformis | Summer: EC50 (LEIA) = 77.8 µg/mL; Spring: IC50 (EHIA) = 2275 µg/mL; Leaves: EC50 (LEIA) = 81.1 µg/mL; IC50 (EHIA) = 2289 µg/mL; Inflorescences: EC50 (LEIA) = 78.6 µg/mL; IC50 (EHIA) = 848 µg/mL | [78] | |
| Convolvulaceae | ||||||
| Calystegia soldanela (L.) R. Br. | Aerial parts | 80% acetone extract | ND | Trichostrongylus colubriformis | IC50 (LEIA) = 2711 µg/mL | [77] |
| Fabaceae | ||||||
| Glycyrrhiza glabra L. | Roots | Glycyrrhizic acid | Brugia malayi microfilarae in vitro | IC50 = 1.20 μM | [82] | |
| Glycyrrhiza inflata Batalin | ND | Licochalcone A | S. mansoni (female and male adult worms) | LC50 = 9 μM | [81] | |
| Medicago marina L. | Aerial parts | 80% acetone extract | ND | Trichostrongylus colubriformis | IC50 (LEIA) = 211 µg/mL | [77] |
| Plantaginaceae | ||||||
| Plantago coronopus L. | Aerial parts | 80% acetone extract | ND | Trichostrongylus colubriformis | IC50 (LEIA) = 212 µg/mL | [77] |
| Plumbaginaceae | ||||||
| Limoniuastrum monopetalum (L.) Boiss. | Aerial parts | 80% acetone extract | Sulphated and/or methylated flavonoids | Trichostrongylus colubriformis | IC50 (LEIA) = 1024 µg/mL; IC50 (EHIA) = 2102 µg/mL | [77] |
| Poaceae | ||||||
| Cynodon dactylon (L.) Pers. | ND | Methanol extract | ND | Hymenolepis diminuta | 40,000 µg/mL: paralysis and mortality at 4.12 h and 5.16 h, respectively | [83] |
| Rubiaceae | ||||||
| Crucianella marítima L. | Aerial parts | 80% acetone extract | ND | Trichostrongylus colubriformis | IC50 (LEIA) = 1024 µg/mL | [83] |
ND: not determined.
5.2. In Vivo Studies
There is a reduced number of studies focusing on in vivo anthelmintic properties of halophytic species. In fact, to our best knowledge, only three species were tested, mostly using rodents and sheep as models (Table 5).
Pistacia lentiscus is the most studied halophyte species due to its high level of tannins, known to exhibit high anthelmintic activity, as confirmed by in vivo studies against T. colubriformis. Infected goats fed with aerials parts of P. lentiscus presented a 16% reduction in fecal oocyst counts, whereas, in lambs, the reduction ranged between 55.2 and 61.3% [84,85]. In turn, S. mansoni infected mice treated orally with a methanol extract of C. ambrosioides (1250 mg/kg/day) exhibited a 53.7% total worm burden decrease and a 60.3% ova/g tissue in liver reduction, 9 weeks post-infection [86]. Hymenolepis diminuta infected Wistar rats were treated with C. dactylon methanol extract (800 mg/kg) enabling a reduction of 77.6% in eggs and 79% in adult worms counts, respectively [83].

Table 5.
In vivo anthelmintic activity of halophyte species.
Table 5.
In vivo anthelmintic activity of halophyte species.
| Family/Species | Plant Organ | Extract/Fraction/Compound | Chemical Components | Assay | Results | References |
|---|---|---|---|---|---|---|
| Amaranthaceae | ||||||
| Dysphania ambrosioides (L.) Mosyakin & Clemants (syn. Chenopodium ambrosioides L.) | ND | Methanol | ND | S. mansoni infected mice | 1250 mg/kg/day exhibited a 53.7% total worm burden decrease and a 60.3% ova/g tissue in liver reduction | [79,86] |
| Anacardiaceae | ||||||
| Pistacia lentiscus L. | Aerial parts | ND | Tannins | Teladorsagia circumcincta, Trichostrongylus colubriformis, and Chabertia ovina infected goats | Reduced fecal oocyst counts in approx. 16% | [85] |
| ND | Tannins | T. colubriformis infected lambs | Reduction of 55.2–61.3% on faecal egg counts | [84] | ||
| Poaceae | ||||||
| Cynodon dactylon (L.) Pers. | ND | Methanol extract | ND | Hymenolepis diminuta infected Wistar rats | 800 mg/kg: 77.6 and 79% reduction in egg and worms’ reduction, respectively | [83] |
ND: Not determined.
6. Conclusions
The importance of halophytes as sources of food and medicinal commodities is rising. Their role is of particular importance in the context of soil and water salinization since they can be commercially cultivated in underutilized saline areas and under sustainable saline agricultural systems. This review collected for the first time data on halophytes’ ethnomedical properties towards human parasitic infections, as well as the existing scientific reports on its in vitro and in vivo experiments on antiprotozoal and anthelmintic properties. Sixteen species from 14 different families have reported antiprotozoal properties, with most studies focusing on different strains of Leishmania, Trypanosoma and Plasmodium parasites. In this context, eight pure compounds occurring in halophyte species were identified with antiprotozoal properties, namely limonene and sabinene against T. brucei, licochalcone A against L. major, oleanolic acid, kaempferol and quercetin anti-L. donovani, and betulinic acid and α-amyrin towards T. cruzi and P. falciparum, respectively.
In turn, 15 species from 12 families were studied for their anthelmintic capacity, mainly against Trichostrongylus gastrointestinal nematodes. Essential oils were the most represented type of extract, followed by extracts made with different combinations of water and organic solvents. From the in vivo studies, the most promising species is D. ambrosioides, including essential oil from aerial organs towards cutaneous leishmaniasis and hydroalcoholic extracts of its leaves, with antimalarial properties. Ascaridole, the major metabolite detected in D. ambrosioides leaves essential oil, exhibited in vitro and in vivo activity against E. histolytica parasites, responsible for amebiasis. Regarding anthelmintic properties, the most promising species is P. lentiscus, as confirmed by in vivo studies of T. circumcincta, and T. colubriformis nematodes. Furthermore, a few compounds were isolated from halophyte species with anthelmintic properties, such as licochalcone A from G. inflata with anti-S. mansoni activity, and glycyrrhizic acid obtained from G. glabra roots with in vitro activity against B. malayi microfilariae. Therefore, halophytes have shown to be promising sources of compounds with potential application as antiparasitic commodities for both humans and animals, including neglected tropical diseases related to protozoan, and gastrointestinal nematodes infections. The main gap in the search for halophytic products with antiparasitic activity is the isolation and identification of novel molecules. Thus, efforts must be made in this regard.
Author Contributions
Formal analysis, L.C., M.J.R., M.O., C.G.P., and G.Z.; Funding acquisition, L.C. and G.Z.; Project administration, L.C.; Writing—original draft, L.C., M.J.R., G.Z., M.O., and C.G.P.; Writing—review & editing, L.C. and M.J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Science and Technology (FCT) and the Portuguese National Budget through projects UIDB/04326/2020 and UID/DTP/04138/2020. It also received funding through the HaloFarMs project (PRIMA/0002/2019), which is part of the Partnership on Research and Innovation in the Mediterranean Area (PRIMA) program supported by the European Union. M.J.R. was supported through the FCT program contract (UIDP/04326/2020) and L.C. by the FCT Scientific Employment Stimulus (CEECIND/00425/2017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wink, M. Medicinal plants: A source of anti-parasitic secondary metabolites. Molecules 2012, 17, 12771–12791. [Google Scholar] [CrossRef] [PubMed]
- Pisarski, K. The Global Burden of Disease of Zoonotic Parasitic Diseases: Top 5 Contenders for Priority Consideration. Trop. Med. Infect. Dis. 2019, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef]
- The Nobel Prize. Available online: https://www.nobelprize.org/prizes/medicine/2015/tu/facts/ (accessed on 28 December 2022).
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. N. Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Grigore, M.N.; Villanueva, M.; Boscaiu, M.; Vicente, O. Do halophytes really require salts for their growth and development? An experimental approach. Not. Sci. Biol. 2012, 4, 23–29. [Google Scholar] [CrossRef]
- Menezes-Benavente, L.; Teixeira, F.K.; Kamei, C.L.A.; Margis-Pinheiro, M. Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.). Plant Sci. 2004, 166, 323–331. [Google Scholar] [CrossRef]
- Amor, N.B.; Jimé Nez, A.B.; Megdiche, W.; Lundqvist, M.; Sevilla, F.; Abdelly, C. Response of antioxidant systems to NaCl stress in the halophyte Cakile maritima. Physiol. Plant. 2006, 126, 446–457. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Whid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of halophytes: Multiple origins of salt tolerance in land plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Halophytic herbs of the Mediterranean basin: An alternative approach to health. Food Chem. Toxicol. 2018, 114, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.; Moulaert, I.; Asselman, J.; van der Biest, K.; van de Pol, L.; Drouillon, M.; Everaert, G. Prioritizing ecosystem services for marine management through stakeholder engagement. Ocean Coast. Manag. 2022, 225, 106228. [Google Scholar] [CrossRef]
- Ferreira, M.J.; Pinto, D.C.G.A.; Cunha, Â.; Silva, H. Halophytes as medicinal plants against human infectious diseases. Appl. Sci. 2022, 12, 7493. [Google Scholar] [CrossRef]
- Panta, S.; Flowers, T.; Lane, P.; Doyle, R.; Haros, G.; Shabala, S. Halophyte agriculture: Success stories. Environ. Exp. Bot. 2014, 107, 71–83. [Google Scholar] [CrossRef]
- Loconsole, D.; Murillo-Amador, B.; Cristiano, G.; De Lucia, B. Halophyte Common Ice Plants: A future solution to arable land salinization. Sustainability 2019, 11, 6076. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Al-Oudat, M.; Qadir, M. The Halophytic Flora of Syria; International Center for Agricultural Research in the Dry Areas (ICARDA): Beirut, Lebanon, 2011. [Google Scholar]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant properties, phenolic composition, bioactive compounds and nutritive value of medicinal halophytes commonly used as herbal teas. South Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Liebezeit, G.; Künnemann, T.D.; Gad, G. Biotechnological potential of North Sea salt marsh plants—A review of traditional knowledge. Prog. Ind. Microbiol. 1999, 35, 77–84. [Google Scholar]
- Cornara, L.; La Rocca, A.; Marsili, S.; Mariotti, M.G. Traditional uses of plants in the Eastern Riviera (Liguria, Italy). J. Ethnopharmacol. 2009, 125, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, D.; Mengoni, A.; Aouani, M.E.; Bazzicalupo, M.; Mhamdi, R. Genetic diversity and salt tolerance of Sinorhizobium populations from two Tunisian soils. Ann. Microbiol. 2010, 60, 541–547. [Google Scholar] [CrossRef]
- Viegas, A.D.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Y.; Maibam, B.C.; Biswas, D.; Laisharm, S.; Deb, L.; Talukdar, N.C.; Borah, J.C. Anti-diabetic potential of selected ethno-medicinal plants of north east India. J. Ethnopharmacol. 2015, 171, 37–41. [Google Scholar] [CrossRef]
- Biodiversidade a Seus Pés. Available online: http://almargem.org/biodiv/ (accessed on 28 December 2022).
- Chamkhi, I.; Hnini, M.; Aurag, J. Conventional medicinal uses, phytoconstituents, and biological activities of Euphorbia officinarum L.: A systematic review. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 9971085. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, K.S.; Choudhary, A.K.; Bana, R.S.; Sharma, V.K.; Gupta, G.; Rajpoot, S.K.; Bhupenchandra, I.; Choudhary, M.; Jakhar, P.; et al. Sole- or dual-crop basis residue-mulching and Zn-fertilization lead to improved productivity, rhizo-modulation and soil health in zero-tilled pigeonpea–wheat cropping system. J. Soil Sci. Plant Nutr. 2022, 22, 1193–1214. [Google Scholar] [CrossRef]
- Novais, H.M.; Santos, I.; Mendes, S.; Pinto-Gomes, C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park. J. Ethnopharmacol. 2004, 93, 183–195. [Google Scholar] [CrossRef] [PubMed]
- González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Ghasemian, A.; Eslami, M.; Hasanvand, F.; Bozorgi, H.; Al-abodi, H.R. Eucalyptus camaldulensis properties for use in the eradication of infections. Comp. Immunol. Microbiol. Infect. Dis. 2019, 65, 234–237. [Google Scholar] [CrossRef]
- Neiva, V.A.; Ribeiro, M.N.S.; Nascimento, F.R.F.; Cartágenes, M.S.S.; Coutinho-Moraes, D.F.; Amaral, F.M.M. Plant species used in giardiasis treatment: Ethnopharmacology and In Vitro evaluation of anti-Giardia activity. Rev. Bras. Farmacogn. 2014, 24, 215–224. [Google Scholar] [CrossRef]
- França, F.; Lago, E.L.; Marsden, P.D. Plants used in the treatment of leishmanial ulcers due to Leishmania (Viannia) braziliensis in an endemic area of Bahia, Brazil. Rev. Soc. Bras. Med. Trop. 1996, 29, 229–232. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; El-Mleeh, A.; Abdel-Daim, M.M.; Prasad Devkota, H. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Abbasi, N.; Hosseini, M.; Rafieian-Kopaei, M. Concise review: Medicinal plants are effective against leishmaniasis. Biomed. Res. Ter. 2017, 4, 1733–1748. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Mohamed, A.A. Survey on medicinal plants and spices used in Beni-Sueif, Upper Egypt. J. Ethnobiol. Ethnomed. 2011, 7, 18. [Google Scholar] [CrossRef]
- Ferreira, F.N.A.; Ferreira, W.M.; da Silva Inácio, D.F.; Speridião Silva Neta, C.; das Neves Mota, K.C.; da Costa Júnior, M.B.; da Rocha, L.F.; Caicedo, W.O. In Vitro digestion and fermentation characteristics of tropical ingredients, co-products and by-products with potential use in diets for rabbits. Anim. Feed Sci. Technol. 2019, 252, 1–10. [Google Scholar] [CrossRef]
- Hotez, P.J. NTDs in the 2020s: An epic struggle of effective control tools versus the Anthropocene. PLoS Negl. Trop. Dis. 2020, 19, e0007872. [Google Scholar] [CrossRef]
- Nweze, J.A.; Mbaoji, F.N.; Li, Y.M.; Yang, L.Y.; Huang, S.S.; Chigor, V.N.; Eze, E.A.; Pan, L.X.; Zhang, T.; Yang, D.F. Potentials of marine natural products against malaria, leishmaniasis, and trypanosomiasis parasites: A review of recent articles. Infect. Dis. Poverty 2021, 10, 9. [Google Scholar] [CrossRef]
- Tempone, A.G.; Pieper, P.; Borborema, S.E.T.; Thevenard, F.; Lago, J.H.G.; Croft, S.L.; Anderson, E.A. Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria. Nat. Prod. Rep. 2021, 38, 2214–2235. [Google Scholar] [CrossRef]
- Sato, S. Plasmodium—A brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthropol. 2021, 40, 1. [Google Scholar] [CrossRef]
- Kamte, S.L.N.; Ranjbarian, F.; Cianfaglione, K.; Sut, S.; Dall’Acqua, S.; Bruno, M.; Afshar, F.H.; Iannarelli, R.; Benelli, G.; Cappellacci, L.; et al. Identification of highly effective antitrypanosomal compounds in essential oils from the Apiaceae family. Ecotoxicol. Environ. Saf. 2018, 156, 154–165. [Google Scholar] [CrossRef]
- Machado, M.; Santoro, G.; Sousa, M.C.; Salgueiro, L.; Cavaleiro, C. Activity of essential oils on the growth of Leishmania infantum promastigotes. Flavour Fragr. J. 2010, 25, 6–9. [Google Scholar] [CrossRef]
- Bouyahya, A.; Assemian, I.C.C.; Mouzount, H.; Bourais, I.; Et-Touys, A.; Fellah, H.; Benjouad, A.; Dakka, N.; Bakri, Y. Could volatile compounds from leaves and fruits of Pistacia lentiscus L. constitute a novel source of anticancer, antioxidant, antiparasitic and antibacterial drugs? Ind. Crop Prod. 2019, 128, 62–69. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crop Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Essid, R.; Rahali, F.Z.; Msaada, K.; Sghair, I.; Hammami, M.; Bouratbine, A.; Aoun, K.; Limam, F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northern Tunisia. Ind. Crop Prod. 2015, 77, 795–802. [Google Scholar] [CrossRef]
- Maaroufi, Z.; Cojean, S.; Loiseau, P.M.; Yahyaoui, M.; Agnely, F.; Abderraba, M.; Mekhloufi, G. In Vitro antileishmanial potentialities of essential oils from Citrus limon and Pistacia lentiscus harvested in Tunisia. Parasitol. Res. 2021, 120, 1455–1469. [Google Scholar] [CrossRef]
- Borges, A.R.; Aires, J.R.; Higino, T.M.; de Medeiros, M.D.; Citó, A.M.; Lopes, J.A.; de Figueiredo, R.C. Trypanocidal and cytotoxic activities of essential oils from medicinal plants of Northeast of Brazil. Exp. Parasitol. 2012, 132, 123–128. [Google Scholar] [CrossRef]
- Kiuchi, F.; Itano, Y.; Uchiyama, N.; Honda, G.; Tsubouchi, A.; Nakajima-Shimada, J.; Aoki, T. Monoterpene hydroperoxides with trypanocidal activity from Chenopodium ambrosioides. J. Nat. Prod. 2002, 65, 509–512. [Google Scholar] [CrossRef]
- Cysne, D.N.; Fortes, T.S.; Reis, A.S.; de Paulo Ribeiro, B.; Dos Santos Ferreira, A.; do Amaral, F.M.; Guerra, R.N.; Marinho, C.R.; Nicolete, R.; Nascimento, F.R. Antimalarial potential of leaves of Chenopodium ambrosioides L. Parasitol. Res. 2016, 115, 4327–4334. [Google Scholar] [CrossRef]
- Avila-Blanco, M.E.; Rodríguez, M.G.; Moreno Duque, J.L.; Muñoz-Ortega, M.; Ventura-Juárez, J. Amoebicidal activity of essential oil of Dysphania ambrosioides (L.) Mosyakin & Clemants in an amoebic liver abscess hamster model. Evid. Based Complement. Alternat. Med. 2014, 2014, 930208. [Google Scholar]
- Pereira, C.G.; Moraes, C.B.; Franco, C.H.; Feltrin, C.; Grougnet, R.; Barbosa, E.G.; Panciera, M.; Correia, C.R.D.; Rodrigues, M.J.; Custódio, L. In Vitro anti-Trypanosoma cruzi activity of halophytes from southern Portugal reloaded: A special focus on sea fennel (Crithmum maritimum L.). Plants 2021, 10, 2235. [Google Scholar] [CrossRef]
- Karami, F.; Dastan, D.; Fallah, M.; Matini, M. In Vitro ctivity of Foeniculum vulgare and its main essential oil component trans-anethole on Trichomonas vaginalis. Iran. J. Parasitol. 2019, 14, 631–638. [Google Scholar] [PubMed]
- Méabed, E.; El-Sayed, N.M.; Abou-Sreea, A.; Roby, M. Chemical analysis of aqueous extracts of Origanum majorana and Foeniculum vulgare and their efficacy on Blastocystis spp. cysts. Phytomedicine 2018, 43, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Rodrigues, M.J.; Pereira, C.; Lima do Monte Neto, R.; Junior, P.A.S.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; Barreira, L.; Custódio, L. First report of the in Vitro antileishmanial properties of extremophile plants from the Algarve Coast. Nat. Prod. Res. 2018, 32, 600–604. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Bagavan, A.; Rahuman, A.A.; Mohanakrishnan, D.; Kamaraj, C.; Elango, G.; Zahir, A.A.; Sahal, D. Antiplasmodial potential of selected medicinal plants from eastern Ghats of South India. Exp. Parasitol. 2013, 134, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Cherigo, L.; de Sedas, A.; Spadafora, C.; Martinez-Luis, S. Evaluation of antiparasitic, anticancer, antimicrobial and hypoglycemic properties of organic extracts from Panamanian mangrove plants. Asian Pac. J. Trop. Med. 2018, 11, 32–39. [Google Scholar] [CrossRef]
- Chen, M.; Christensen, B.; Blom, J.; Lemmich, E.; Nadelmann, L.; Fich, K.; Theander, T.G.; Kharazmi1, A. licochalcone A, a novel antiparasitic agent with potent activity against human pathogenic protozoan species of Leishmania. Antimicrob. Agents Chemother. 1993, 37, 2550–2556. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Junior, P.A.S.; Rodrigues, M.J.; DellaGreca, M.; Barreira, L.; Murta, S.M.F.; Romanha, A.J.; Custódio, L. Unlocking the In Vitro anti-Trypanosoma cruzi activity of halophyte plants from the southern Portugal. Asian Pac. J. Trop. Dis. 2016, 9, 735–741. [Google Scholar] [CrossRef]
- Yousefi, R.; Ghaffarifar, F.; Dalim Asl, A. The effect of Alkanna tincturia and Peganum harmala extracts on Leishmania major (MRHO/IR/75/ER) In Vitro. Iran. J. Parasitol. 2009, 4, 40–47. [Google Scholar]
- Rahimi-Moghaddam, P.; Ebrahimi, S.A.; Ourmazdi, H.; Selseleh, M.; Karjalian, M.; Haj-Hassani, G.; Alimohammadian, M.H.; Mahmoudian, M.; Shafiei, M. In Vitro and In Vivo activities of Peganum harmala extract against Leishmania major. J. Res. Med. Sci. 2011, 16, 1032–1039. [Google Scholar]
- Al-Tae, A.A.; Al-Bashir, N.M.; Al-Dulimy, S.K. The efficiency of Mytrus comminis and Peganum harmala plant extracts on pro and axenic amastigote stages of Leishmania donovani. Tikrit Med. J. 2009, 15, 93–99. [Google Scholar]
- Madah, M.; Haddad, S.; Khazem, M. Evaluation of the effect of Peganum harmala extracts on the In Vitro viability of Leishmania tropica promastigotes in comparison to Glucantime. J. Parasit. Dis. 2020, 44, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Sangian, H.; Faramarzi, H.; Yazdinezhad, A.; Mousavi, S.J.; Zamani, Z.; Noubarani, M.; Ramazani, A. Antiplasmodial activity of ethanolic extracts of some selected medicinal plants from the northwest of Iran. Parasitol. Res. 2013, 112, 3697–3701. [Google Scholar] [CrossRef] [PubMed]
- Idris, O.A.; Wintola, O.A.; Afolayan, A.J.; Uzor, P.F. Evaluation of the bioactivities of Rumex crispus L. leaves and root extracts using toxicity, antimicrobial, and antiparasitic Assays. Evid. Based Complement. Alternat. Med. 2019, 2019, 6825297. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.G.; Doudi, M.; Abedi, S. An In Vitro study of antileishmanial effect of Portulaca oleracea extract. J. Vector Borne Dis. 2016, 53, 362–369. [Google Scholar]
- López, D.; Cherigo, L.; Spadafora, C.; Loza-Mejía, M.A.; Martínez-Luis, S. Phytochemical composition, antiparasitic and α-glucosidase inhibition activities from Pelliciera rhizophorae. Chem. Cent. J. 2015, 9, 53. [Google Scholar] [CrossRef]
- do Carmo, M.A.V.; Fidelis, M.; Sanchez, C.A.; Castro, A.P.; Camps, I.; Colombo, F.A.; Marques, M.J.; Myoda, T.; Granato, D.; Azevedo, L. Camu-camu (Myrciaria dubia) seeds as a novel source of bioactive compounds with promising antimalarial and antischistosomicidal properties. Food Res. Int. 2020, 136, 109334. [Google Scholar] [CrossRef]
- Esmaeili, S.; Naghibi, F.; Mosaddegh, M.; Sahranavard, S.; Ghafari, S.; Abdullah, N.R. Screening of antiplasmodial properties among some traditionally used Iranian plants. J. Ethnopharmacol. 2009, 121, 400–404. [Google Scholar] [CrossRef]
- Ramazani, A.; Tavakolizadeh, M.; Ramazani, S.; Kheiri-Manjili, H.; Eskandari, M. Antiplasmodial property of Glycyrrhiza glabra traditionally used for malaria in Iran: Promising activity with high selectivity index for malaria. J. Arthropod-Borne Dis. 2018, 12, 135–140. [Google Scholar] [CrossRef]
- Monzote, L.; Pastor, J.; Scull, R.; Gille, L. Antileishmanial activity of essential oil from Chenopodium ambrosioides and its main components against experimental cutaneous leishmaniasis in BALB/c mice. Phytomedicine 2014, 21, 1048–1052. [Google Scholar] [CrossRef]
- Patrício, F.J.; Costa, G.C.; Pereira, P.V.; Aragão-Filho, W.C.; Sousa, S.M.; Frazão, J.B.; Pereira, W.S.; Maciel, M.C.; Silva, L.A.; Amaral, F.M.; et al. Efficacy of the intralesional treatment with Chenopodium ambrosioides in the murine infection by Leishmania amazonensis. J. Ethnopharmacol. 2008, 115, 313–319. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, J.F.; Sleebs, B.E.; Gasser, R.B. An appraisal of natural products active against parasitic nematodes of animals. Parasit. Vectors 2019, 12, 306. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Yoonuan, T.; Sanguankiat, S.; Nuamtanong, S.; Pongvongsa, T.; Phimmayoi, I.; Phanhanan, V.; Boupha, B.; Moji, K.; Waikagul, J. Short report: Human Trichostrongylus colubriformis infection in a rural village in Laos. Am. J. Trop. Med. Hyg. 2011, 84, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Lima, C.S.; Ketavong, S.; Llorent-Martínez, E.J.; Hoste, H.; Custódio, L. Disclosing the bioactive metabolites involved in the In Vitro anthelmintic effects of salt-tolerant plants through a combined approach using PVPP and HPLC-ESI-MSn. Sci. Rep. 2021, 11, 24303. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Lima, C.S.; Llorent-Martínez, E.J.; Hoste, H.; Custódio, L. Impact of seasonal and organ-related fluctuations on anthelmintic properties and chemical profile of Cladium mariscus (L.) Pohl extracts. Front. Plant Sci. 2022, 13, 934644. [Google Scholar] [CrossRef]
- Kamel, E.G.; El-Emam, M.A.; Mahmoud, S.S.M.; Fouda, F.M.; Bayaumy, F.E. Attenuation of Schistosoma mansoni cercarial infectivity to albino mice by methanol extract of some plant species. Pestic. Biochem. Physiol. 2010, 98, 342–348. [Google Scholar] [CrossRef]
- Wakabayashi, K.A.; de Melo, N.I.; Aguiar, D.P.; de Oliveira, P.F.; Groppo, M.; da Silva Filho, A.A.; Rodrigues, V.; Cunha, W.R.; Tavares, D.C.; Magalhães, L.G.; et al. Anthelmintic effects of the essential oil of fennel (Foeniculum vulgare Mill., Apiaceae) against Schistosoma mansoni. Chem. Biodivers. 2015, 12, 1105–1114. [Google Scholar] [CrossRef]
- Souza, R.L.; Gonçalves, U.O.; Badoco, F.R.; de Souza Galvão, L.; Santos, R.; de Carvalho, P.; de Carvalho, L.; da Silva Filho, A.A.; Veneziani, R.; Rodrigues, V.; et al. Licochalcone A induces morphological and biochemical alterations in Schistosoma mansoni adult worms. Biomed. Pharmacother. 2017, 96, 64–71. [Google Scholar] [CrossRef]
- Kalani, K.; Kushwaha, V.; Verma, R.; Murthy, P.K.; Srivastava, S.K. Glycyrrhetinic acid and its analogs: A new class of antifilarial agents. Bioorg. Med. Chem. Lett. 2013, 23, 2566–2570. [Google Scholar] [CrossRef]
- Yadav, A.K.; Nath, P. Anthelmintic effects and toxicity of Cynodon dactylon (L.) Pers. in rodent models. J. Intercult. Ethnopharmacol. 2017, 6, 407–413. [Google Scholar] [CrossRef]
- Manolaraki, F.; Sotiraki, S.; Stefanakis, A.; Skampardonis, V.; Volanis, M.; Hoste, H. Anthelmintic activity of some Mediterranean browse plants against parasitic nematodes. Parasitology 2010, 137, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Markovics, A.; Cohen, I.; Muklada, H.; Glasser, T.A.; Dvash, L.; Ungar, E.D.; Azaizeh, H.; Landau, S.Y. Consumption of Pistacia lentiscus foliage alleviates coccidiosis in young goats. Vet. Parasitol. 2012, 186, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kamel, E.G.; El-Emam, M.A.; Mahmoud, S.S.M.; Fouda, F.M.; Bayaumy, F.E. Parasitological and biochemical parameters in Schistosoma mansoni-infected mice treated with methanol extract from the plants Chenopodium ambrosioides, Conyza dioscorides and Sesbania sesban. Parasitol. Int. 2011, 60, 388–392. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).