Abstract
Culex pipiens mosquitoes are transmitters of many viruses and are associated with the transmission of many diseases, such as filariasis and avian malaria, that have a high rate of mortality. The current study draws attention to the larvicidal efficacy of three methanolic algal extracts, Cystoseira myrica, C. trinodis, and C. tamariscifolia, against the third larval instar of Cx. pipiens. The UPLC-ESI-MS analysis of three methanol fractions of algal samples led to the tentative characterization of twelve compounds with different percentages among the three samples belonging to phenolics and terpenoids. Probit analysis was used to calculate the lethal concentrations (LC50 and LC90). The highest level of toxicity was attained after treatment with C. myrica extract using a lethal concentration 50 (LC50) of 105.06 ppm, followed by C. trinodis (135.08 ppm), and the lowest level of toxicity was achieved by C. tamariscifolia (138.71 ppm) after 24 h. The elevation of glutathione-S-transferase (GST) and reduction of acetylcholine esterase (AChE) enzymes confirm the larvicidal activity of the three algal extracts. When compared to untreated larvae, all evaluated extracts revealed a significant reduction in protein, lipid, and carbohydrate contents, verifying their larvicidal effectiveness. To further support the observed activity, an in silico study for the identified compounds was carried out on the two tested enzymes. Results showed that the identified compounds and the tested enzymes had excellent binding affinities for each other. Overall, the current work suggests that the three algal extractions are a prospective source for the development of innovative, environmentally friendly larvicides.
1. Introduction
Human health is one of the most alarming effects of global warming in a variety of intricate ways. The population is significantly impacted by the changed spatial distribution of various infectious disease vectors, such as mosquitoes [1]. Changes in temperature may increase the possibility of the transmission of numerous mosquito-borne diseases, including the West Nile virus, dengue fever, and avian malaria. This is due to the fact that temperature affects both the pathogenic organisms’ and vector species’ life-cycle dynamics. Warmer temperatures increase mosquito reproduction and accelerate the development of the microorganisms they distribute [2]. In urban areas, female Culex pipiens feed on a range of vertebrate hosts, which may help to promote the spread of West Nile virus among birds and occasionally to populations of humans and other mammals [3]. Despite being extensively dispersed now, Cx. pipiens are native to Africa, Asia, the Middle East, and Europe [4]. Frequent vector management is the primary strategy for preventing and controlling the spread of mosquito-borne diseases [5]. In the past decades, conventional chemical insecticides have been created to combat diseases spread by mosquitoes [6]. Chemical pesticides are hazardous to humans and have a harmful impact on the environment and other living things; moreover, the unwise use of synthetic insecticides leads to resistance in mosquito species [7,8]. Researchers are searching for pest control alternatives that have fewer negative side effects. As a result, attention has been drawn to research on natural products that tries to identify the active components of natural insecticide agents that are secure and target-specific. Also, developing larvicides from marine sources might be another technique of mosquito control. Success stories in the search for pesticides with a natural origin have been documented [9,10,11,12].
Algae are organisms that resemble plants and have photosynthetic pigments in their cells. They are most commonly found in freshwater, marine, and wastewater ecosystems and range in size from microalgae to macroalgae. Algae are regarded as a rich source of a variety of biologically active molecules [13]. They also create a number of secondary metabolites with a wide range of chemical compositions, some of which are known insecticides. Secondary metabolites extracted from algae have been demonstrated to have a substantial effect on mosquito larvae [14].
Marine macroalgae, generally known as seaweed, presented a new tactic in the field of pest control [15]. Seaweeds are distributed in shallow coastal waters, estuaries, and intertidal and deep-sea regions [16]. They are typically categorized into three large pigmentation-based groups: Chlorophyta (green algae), Phaeophyta (brown algae), and Rhodophyta (red algae) [17]. Seaweeds, with their great chemical diversity, are utilized as a foodstuff for human and animal food, fertilizers, cosmetics, and medicinal products [18,19]. Seaweed extracts have proven competence for controlling and repelling various insect pests such as cereal aphids, Schizaphis graminum [20], the cotton stainer bug, Dysdercus cingulatus [21], mosquitoes [22,23], the tomato moth, Tuta absoluta [24], the termite, Microtermes obesi [25], maize weevil, Sitophilus zeamais [26] and the Asian citrus psyllid, Diaphorina citri [27]. Some studies documented the insecticidal effects of seaweeds of Egyptian origin as Caulerpa prolifera, Caulerpa serrulata, Jania rubens, Nitophyllum punctatum Padina pavonica, Chara vulgaris, Parachlorella kessleri, Ulva intestinalis and Cladophora glomerata [28,29].
Cystoseira is a widespread brown algae genus found throughout the Mediterranean region. Numerous substances have been identified from various species of the Mediterranean brown algae of the genus Cystoseira, including terpenoids, alkaloids, polysaccharides, and steroids; however, very few investigations on the pharmacological characteristics of these substances and different Cystoseira species have been published [30,31,32,33,34]. It is crucial to research the bioactive ingredients of seaweeds and assess their efficacy for mosquito control. Information on the structure-activity relationships of active chemicals that are responsible for the killing action can be obtained by understanding the chemical components of seaweed [14]. Recently, the dependence on secondary metabolites from natural sources has been in great demand to control various ailments and defense against several conditions [35,36,37,38,39,40].
Based on the prospective findings, the objective of this study is to investigate the chemical composition of the methanol fraction of three Cystoseira species, C. myrica, C. trinodis, and C. tamariscifolia, using UPLC/MS analysis along with a screening of the three species to generate eco-friendly larvicide algal extracts and assess their bioactivity against Cx. pipiens larvae. This is besides the correlation of their activity through molecular docking of the major constituents.
2. Results and Discussion
2.1. UPLC-ESI-MS Analysis for Characterization of Cystoseira myrica, C. trinodis, and C. tamariscifolia Methanol Fraction
Metabolic profiling of three algal species, C. myrica, C. trinodis, and C. tamariscifolia methanol fraction, was achieved using UPLC/ESI/MS analysis (Supplementary Figures S1–S3). Twelve compounds with different concentrations were annotated in the methanol fraction of the three Cystoseira species. Most of them belonged to the class of terpenoids and flavonoids (Table 1). Compounds were tentatively identified based on the mass of the molecular ion peaks and comparison with bibliographic references, as shown in (Table 1). On the one hand, a major meroditerpenoid with molecular formula C18H26O2 (1) and with a mass ion peak at m/z 273 was identified in C. myrica and C. tamariscifolia, and it was previously reported from C. baccata [41]. Acyclic diterpene (2E, l0E)-1-hydroxy-6,13-diketo-7- methylene-3,11,15-trimethylhexadeca-2,l0,l4-triene (3) with mass ion peak at m/z 317 identified in C. tamariscifolia and previously reported from C. crinite [42]. A phlorotannin with a mass ion peak at m/z 517 and molecular formula C24H22O13 was identified as bifuhalol hexacetate (5) in C. myrica. Oxocrinol (9) was identified as a linear terpenoid with a mass ion peak at m/z 223 and molecular formula C14H24O2 in C. tamariscifolia, and it was previously reported from C. crinite [43]. Hydroazulene diterpene cystoseirol monoacetate (10) with mass ion peak at m/z 397 and molecular formula C22H36O5 identified in the three algal extracts, and it was previously isolated from C. myrica [44]. Moreover, α-linolenic acid (12) with a mass ion peak at m/z 277 was identified in C. tamariscifolia. Linolenic acid was previously identified in C. compressa and C. indica extracts [31,45].
It is worth noting that Phenolic compounds are also identified in the tested methanol fractions as compound (2) myricetin and compound (6) quercetin, which had been identified previously using the HPLC technique from family Sargassaceae in Sargassum latifolium and S. wightii [46,47]. On the other hand, some tentatively identified constituents, including luteolin (4), cyanidin-3-O-glucoside (7), along with 7,8-methylenedioxycoumarin (8), and apigenin (11).
Our results revealed the presence of variable secondary metabolites with different percentages of the methanol fraction of the three species under investigation. The chemical structures of the major tentatively-identified compounds are presented in (Figure 1).


Table 1.
Metabolite profiling of Cystoseira myrica, C. trinodis, and C. tamariscifolia Methanol fraction via UPLC-ESI-MS in the negative ion mode.
Table 1.
Metabolite profiling of Cystoseira myrica, C. trinodis, and C. tamariscifolia Methanol fraction via UPLC-ESI-MS in the negative ion mode.
| No. | Rt (min) | Compound Name | [M − H]− (m/z) | Molecular Formula | Relative Amount (%) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| C. myrica | C. trinodis | C. tamariscifolia | ||||||
| 1 | 1.29 | Compound 7 | 273.00 | C18H26O2 | 8.10 | - | 7.65 | [41] |
| 2 | 1.56 | Myricetin | 317.05 | C15H10O8 | - | - | 9.99 | [48] |
| 3 | 1.75 | (2E, l0E)-1-hydroxy-6,13-diketo-7- methylene-3,11,15-trimethylhexadeca-2, l0, l4-triene | 317.00 | C20H30O3 | - | - | 3.45 | [42] |
| 4 | 2.04 | Luteolin-7- glucoside | 447.05 | C21H20O11 | - | - | 2.36 | [49] |
| 5 | 3.67 | Bifuhalol hexacetate | 517.00 | C24H22O13 | 5.83 | - | - | [50] |
| 6 | 4.60 | Quercetin | 301.00 | C15H10O7 | 0.36 | 0.18 | - | [51] |
| 7 | 5.05 | Cyanidin-3-O- glucoside | 485.05 | C21H21O11+ | 48.27 | 10.06 | - | [52] |
| 8 | 5.58 | 7,8-Methylenedioxycoumarin | 189.10 | C10H6O4 | - | - | 13.87 | [53] |
| 9 | 7.32 | Oxocrinol | 223.10 | C14H24O2 | - | - | 6.50 | [43] |
| 10 | 7.86 | Cystoseirol monoacetate | 397.05 | C22H36O5 | 1.29 | 0.49 | 1.21 | [44] |
| 11 | 8.73 | Apigenin | 269.20 | C15H10O5 | - | - | 1.40 | [54] |
| 12 | 23.68 | α-Linolenic acid | 277.00 | C18H30O2 | - | - | 4.79 | [55] |
Rt: Retention time recorded for each compound.
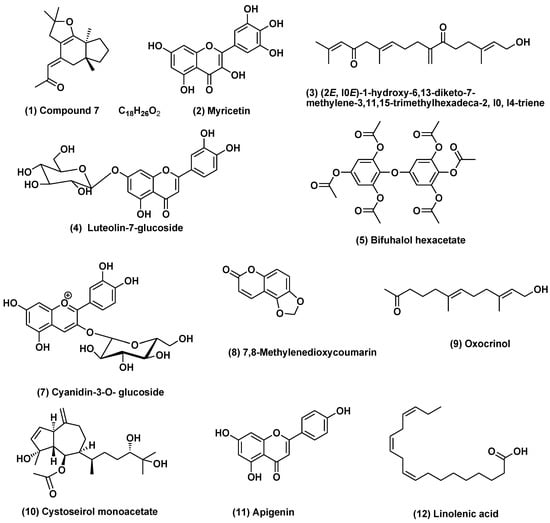
Figure 1.
Structures of compounds identified in Cystoseira myrica, C. trinodis, and C. tamariscifolia methanol fraction using UPLC-ESI-MS in the negative ion mode. Compounds (1, 2, 3, 5, 9, 10, and 12) identified in different Cystoseira species and the family Sargassaceae.
2.2. Larvicidal Bioassay
The bioassay test of the methanol fraction of C. myrica, C. trinodis, and C. tamariscifolia was conducted on the third instar larvae of Cx. pipiens. The mortality of Cx. pipiens larvae was calculated at 24 h, 48 h, and 72 h post-treatment (Table 2). The results demonstrated that the toxicity of the tested extracts significantly increased with time, extract concentration, and length of exposure time. With higher concentrations and longer exposure times, there was a higher percentage of larval mortality. The mortality of Cx. pipiens larvae started on the first day of exposure and continued until the third day. The highest level of toxicity was attained by C. myrica, followed by C. trinodis at LC50 105.06 and 135.08 ppm, respectively. The lowest level of toxicity was achieved by C. tamariscifolia at LC50 (138.71 ppm) at 24 h post-treatment (Table 2).

Table 2.
Susceptibility of Culex pipiens to C. myrica, C. trinodis, and C. tamariscifolia 24 h, 48 h, and 72 h post-treatment.
The activity of the tested extracts was arranged as follows: C. myrica > C. trinodis > C. tamariscifolia (Table 2). In general, C. tamariscifolia showed low convergent toxicity, while C. myrica and C. trinodis displayed adequate action against Cx. pipiens larvae. The low slope values suggested that the tested population of Cx. pipiens larvae are uniform.
Similar larvicidal activities were reported from different brown algae extracts against Cx. pipiens larvae as methanol extract of Sargassum dentifolium, Dictyota dichotoma, and Padina boryana exhibited LC50 values of 306.86, 266.85, and 295.52 ppm, respectively, after 24 h post-exposure [9]. Another study reported the larvicidal activity of the ethanolic extract of Cystoseira barbata against larvae of Aedes albopictus [56]. The MgO-NPs of Cystoseira crinita extract were tested against the house fly Musca domestica at different concentrations. It showed the highest mortality percentages, 99.0%, 95.0%, 92.2%, and 81.0% for 1st, 2nd, and 3rd instars’ larvae and pupa of M. domestica, respectively, at 10 μg/mL MgO-NPs [57]. The three tested extracts induced abnormalities in the treated larvae and also in the pupae that resulted from treated larvae (Supplementary Figure S4).
2.3. Biochemical Analysis
Insects synthesize many detoxifying enzymes, including esterases, oxidases, and reductases, to react with and detoxify a variety of invasive pesticides [58]. We investigated the activity of two different enzymes, glutathione-S-transferase (GST) and acetylcholinesterase (AChE), to gain an insight into the mechanisms involved in the 3rd larval instar of Culex pipiens. Through inhibition of AChE, the essential enzyme in regulating the level of acetylcholine in cholinergic synapses of insects will result in permanent neural excitation/stimulation, paralysis, ataxia, and eventually death [59,60]. The obtained results showed the activity of the GST enzyme significantly increased due to treatment with C. myrica, C. trinodis, and C. tamariscifolia. While the activity of AChE was decreased 24 h and 48 h post-treatment with the tested extracts as compared to the untreated group, as shown in (Table 3). Our results were in accordance with the previous reports that confirmed the role of GST and AChE in the detoxification process of the extract. Abdel Haleem et al. (2022) reported an increase in the intracellular glutathione content when the larvae were treated with different algal extracts [9]. Also, our findings are consistent with those of Huang et al. (2013), which discovered an increase in intracellular glutathione concentration after treating larvae with a polyphenolic-rich extract [54,61].

Table 3.
Quantitative analysis of glutathione-S-transferase and acetylcholinesterase activity at different time intervals.
2.4. Determination of Total Proteins, Total Lipids, and Total Carbohydrates
As proteins, carbohydrates, and lipids are the three most important components required for larval growth and development, the decline in these three components led to impaired larval development, which eventually led to larval death.
The biochemical changes in the whole-body tissue of Cx. pipiens larvae (carbohydrate, protein, and lipid) are shown in (Figure 2). All tested extracts showed a significant reduction in protein, lipids, and carbohydrate contents of treated larvae compared to untreated larvae, which may impair the survival and development of the larvae. Our results were in accordance with previous reports that reported that larvicides have a deleterious impact on larval growth and development, causing alterations in their metabolic and biochemical processes [62].
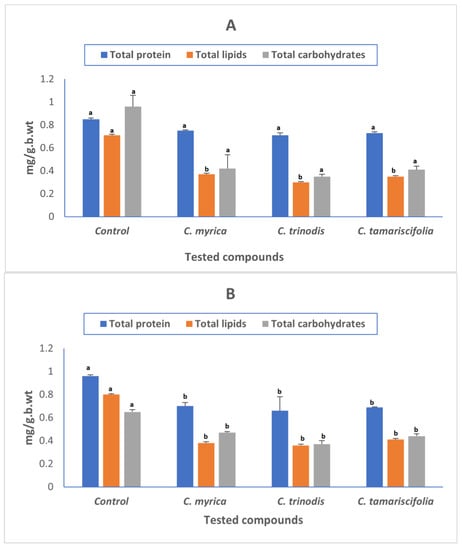
Figure 2.
Effect of Cystoseira myrica, C. trinodis, and C. tamariscifolia extracts on biomolecules availability in Culex pipiens 3rd instar larvae 24 h (A), 48 h (B) post-treatment. (a,b) Different letters indicate that the mean is significantly different from control at p < 0.05.
2.5. In Silico Studies
2.5.1. Binding Mode with AChE Enzyme
The main compounds identified in the methanol fraction of C. myrica, C. trinodis and C. tamariscifolia, and λ-cyhalothrin (control) were docked separately into the active pocket of AChE (PDB ID: 6XYS), to investigate the nature of binding with the crucial enzymes and discover some insights about the mode of action.
The meroditerpenoid with molecular formula C18H26O2 (1) and compound (2) myricetin showed good binding affinities to AChE without forming any hydrogen bonds (Supplementary Figure S5); only exposure to the receptor controls their binding effectiveness. Moreover, compound (3), with its long carbon chain, is bound strongly to Trp321 via H-arene interactions (Figure 3). While compounds (5) and (10) showed a high binding affinity with a binding energy of −7.26 and 7.28 Kcal/mol, respectively, but without forming any hydrogen bonds. Also, compound (9) showed high binding energy equal to −7.28 Kcal/mol (Table 4). In addition, α-linolenic acid (12) (BE = −7.97 Kcal/mol) adapted to the pocket through the formation of ion-H (Figure 4B) and H-arene (Figure 4E) interactions with Trp83 and Trp321 residues, respectively. The reference insecticide, λ-cyhalothrin, exhibited a very close binding behavior to (2) and (10) (Figure 3 and Figure 4).
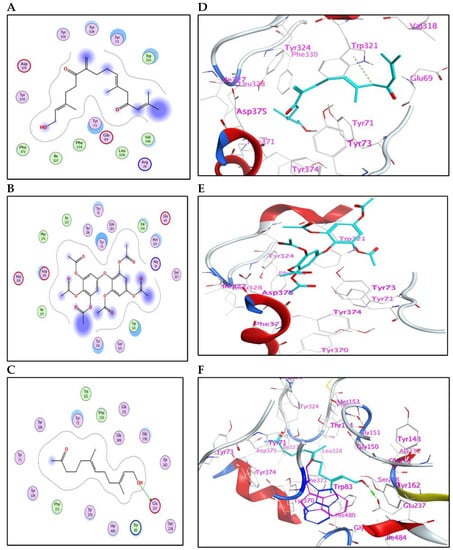
Figure 3.
(A–C) 2D-binding interaction profile for compounds 3, 5, and 9, respectively, with the active pocket of the AChE enzyme. (D–F) In-depth 3D ligand-AChE interaction mode for compounds 3, 5, and 9, respectively.

Table 4.
The binding data for major compounds identified in C. myrica, C. trinodis, and C. tamariscifolia methanol fraction with AChE and GST active sites.
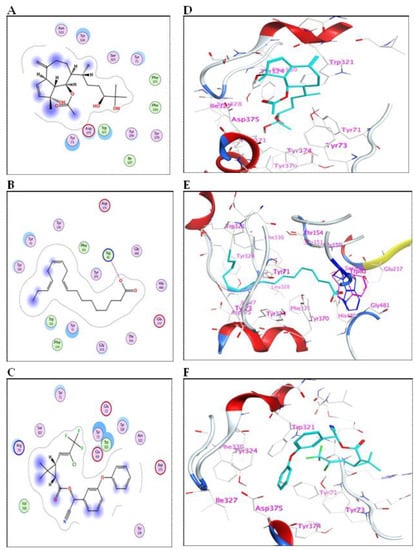
Figure 4.
(A–C) 2D-binding interaction profile for compounds 10, 12, and λ-cyhalothrin, respectively, with the active pocket of AChE enzyme. (D–F) In-depth 3D ligand-AChE interaction mode for compounds 10, 12, and λ-cyhalothrin, respectively.
The 2D-binding interaction profile for compounds 4, 7, 8, and 11 with the active pocket of the AChE enzyme are represented in (Supplementary Figure S6).
Collectively, the activity of C. myrica fraction toward Cx. pipiens could be mainly attributed to the binding of compounds (1 and 5) with the AChE enzyme. For the C. trinodis fraction, compound (10), although its small concentration, shows high binding affinity. Regarding the C. tamariscifolia fraction, compounds (3, 9, and 12) furnish the activity, and maybe in all extracts, each compound possesses a partial effect.
2.5.2. Binding Mode with GST Enzyme
The compounds identified in the methanol fraction of C. myrica, C. trinodis, C. tamariscifolia, and λ-cyhalothrin were subjected to docking into the pocket of GST protein (PDB ID: 1M0U). This study may help in predicting the mode of action of these derivatives as insecticides and discovering their abilities to bind with crucial enzymes.
The results for docking and the GST-binding parameters are depicted in (Figure 5 and Figure 6) and (Table 4). Compound (3) showed a comparable binding effect with the reference (BE = −5.75 Kcal/mol), although it could not form any type of interaction with the residues of the GST pocket (Figure 5A,D). This may be due to its long carbon chain that accurately fits the size of the GST pocket. Also, compound (9) has a similar behavior to compound (3) because of structural similarity; it could form one weak hydrogen bond with Ser110 (Figure 5F). Compound (5) binds with the GST pocket by forming one hydrogen bond (Figure 5E). Besides, compound (10) possesses two hydrogen bonds with two crucial amino acid residues and a value of binding energy (−6.06 Kcal/mol). Three hydrogen bonds control the binding of compound (20) with the pocket of GST (Figure 6B,E). It shares the formation of H-bonds with two crucial residues, Arg145 and Tyr208. The reference insecticide, λ-cyhalothrin exhibited only pi-pi interaction with Phe55 (Figure 6F). It can be inferred from the docking data that Phe55, Gln96, Ser110, Arg145, and Tyr208 represent the crucial amino acid residues in the active pocket of GST.
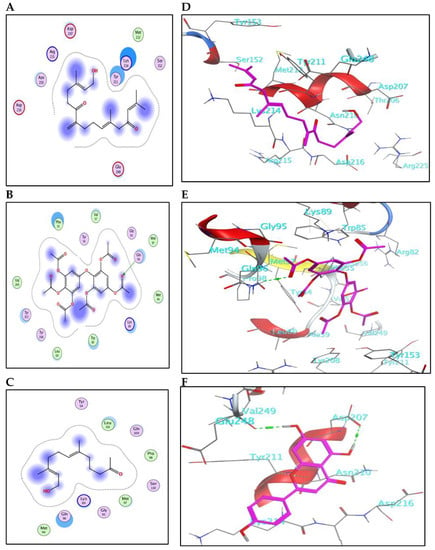
Figure 5.
(A–C) The 2D-binding interaction profile for compounds 3, 5, and 9, respectively, with the pocket of GST protein. (D–F) In-depth 3D ligand-GST interaction mode for compounds 3, 5, and 9, respectively.
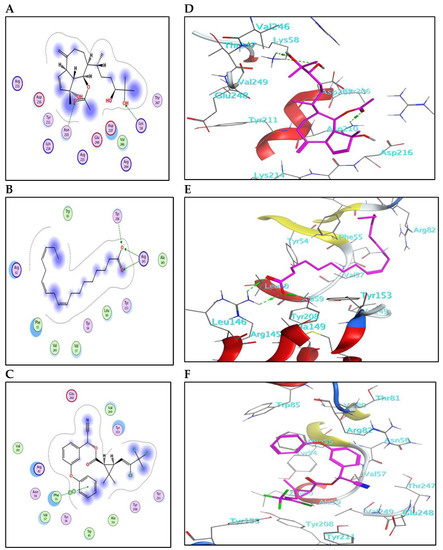
Figure 6.
(A–C) The 2D-binding interaction profile for compounds 10, 12, and λ-cyhalothrin, respectively, with the pocket of GST protein. (D–F) In-depth 3D ligand-GST interaction mode for compounds 10, 12, and λ-cyhalothrin, respectively.
The 2D-binding interaction profile for compounds 1, 2, 4, 7, 8, and 11 with the active pocket of GST enzyme are represented in (Supplementary Figures S7 and S8).
3. Materials and Methods
3.1. Plant Material
The three algae samples, Cystoseira myrica, C. trinodis, and C. tamariscifolia, were collected in October 2020 from the Gulf of Suez shores, Ras Sedr city, Egypt 29°50′19.7″ N and 32°37′36.8″ E. A voucher sample was identified according to Ibraheem et al. 2014 [63] and was deposited at the Pharmacognosy Department, Faculty of Pharmacy, Badr University in Cairo under codes BUC-PHG-CM-3, BUC-PHG-CT-4 and BUC-PHG-CT-5, respectively. The collected algal samples were brought to the laboratory in sterilized plastic bags containing seawater to prevent evaporation. They were also cleaned of epiphytes and rock and sand debris and then given a quick freshwater rinse to remove any adhered surface salts or residues. Photos of the three samples are included in (Supplementary Figure S9).
3.2. Preparation of the Plant Extract
The grounded air-dried powder of each sample (100 g), Cystoseira myrica, C. trinodis, and C. tamariscifolia were extracted with pure methanol by maceration method three times at room temperature (3 × 500 mL) followed by concentration by evaporation in vacuo at low temperature (45 °C) to yield a dark brown residue 18.2, 26, and 30 g, respectively [27].
3.3. UPLC-ESI-MS Analysis
UPLC/MS analysis was performed at the Centre of Drug Discovery Research and Development, Department of Pharmacognosy, Faculty of Pharmacy, Ain Shams University, Egypt, using Waters®TQD UPLC-MS with an ESI source using waters® acquity UPLC RP-C18 column, (100 × 2 mm, ID), and particle size of 1.7 µm, with an integrated pre-column. A gradient of water and acetonitrile (with 0.1% formic acid) was applied from 2% to 100% acetonitrile. The flow rate was 1 or 0.5 mL/min, and one run took 35 min. The MS was operated at −10 V for ESI-, 240 °C source temperature, and high purity nitrogen as a sheath and auxiliary gas at flow rates of 80 and 40 (arbitrary units), respectively. The injection volume was 5 μL. The spray voltage was 4.48 kV, the tube lens voltage was 10.00 V, and the capillary voltage was 39.6 V. A full scan mode was adjusted in the mass range of 100–2000 m/z [64].
Tentative metabolite assignments were done based on the MS data (in the negative ionization mode) by comparison with the previously reported compounds from the genus and the family [41,42,43,44,50,65,66] alongside online public databases. Data acquisitions and analyses were executed by XcaliburTM 2.0.7 software (Thermo Scientific, Karlsruhe, Germany).
3.4. Insect Culture
Egg rafts of Cx. pipiens were obtained from colonies maintained at the Research and Training Center of Vector Disease, Ain Shams University, Cairo. The eggs were transferred to white metal enamel plates containing dechlorinated tap water and reared in the insectary for 3rd instar larvae collection for further bioassay experiments. Mosquitoes were maintained at 25 ± 2 °C and 75% RH under a 12:12 L/D photoperiod. Newly-hatched larvae were fed a diet of tetramine. Pupae were transferred to cups containing dechlorinated tap water and placed in rearing cages (40 × 30 × 25 cm), where adults emerged. Cotton pieces soaked in a 10% sucrose solution were used to feed adults. Females were provided with a pigeon placed on the mesh of the cage for blood feeding (Supplementary Figure S10). This research paper was approved by the research ethics committee from the Faculty of Science, Ain Shams University (ASU-SCI/ENTO/2023/1/2).
3.5. Larvicidal Bioassay
An amount of 10 g of each crude extract of Cystoseira myrica, C. trinodis, and C. tamariscifolia was dissolved in 100 mL of ethanol as a 10 percent stock solution. Distilled water was used to prepare five different concentrations of each extract (50, 100, 150, 200, and 250 ppm). Twenty-five larvae of the third instar were placed in plastic cups containing five different concentrations of each extract, while in the control, ethanol was used as calculated from the highest concentration preparation [10,67]. The experiment was replicated three times. Mortality data were recorded after 24, 48, and 72 post-treatment. The mortality percentages were corrected using Abbott’s formula [68].
3.6. Biochemical Assay
Insects were smashed in a chilled glass Teflon tissue homogenizer. Homogenized samples were centrifuged at 8000 rpm for 15 min at 5 °C in a refrigerated centrifuge. Supernatants were kept in a deep freezer at −20 °C until use in biochemical assays.
3.6.1. Estimation of Total Carbohydrates
By using the phenol sulfuric acid method, total carbohydrates were calculated, as stated in [69]. A 100 µL sample was mixed with 200 µL of sulfuric acid and 100 µL of phenol (5 g/100 mL). After 30 min of incubation, the resultant absorbance at 490 nm was measured.
3.6.2. Estimation of Total Lipids
Total lipids were estimated by the method of Knight et al. (1972) using the phospho-vanillin reagent (20%) [70]. A 250 μL sample was mixed with 5 mL of sulfuric acid in a test tube and heated for 10 min in a boiling water bath. After cooling to room temperature, the sample was added to the phospho-vanillin reagent (6 mL). After 45 min, the absorbance of the obtained color was measured at 525 nm [71].
3.6.3. Estimation of Total Proteins
Total protein was determined according to Bradford (1976) using a solution of Coomassie Brilliant Blue dissolved in 95% ethanol. 100 mL of the sample was mixed with 5 mL of Bradford reagent. The absorbance was measured at 595 nm [72].
3.7. Enzyme Activities
3.7.1. Glutathione S-transferase (GST)
The activity of glutathione S-transferase (GST) was evaluated depending on Kao et al. (2016) method [73] CDNB (1- chloro-2,4-dinitrobenzene) was used as a substrate.
3.7.2. Acetylcholine Esterase (AChE)
The activity of acetylcholinesterase (AChE) was measured in untreated and treated larval samples previously treated with LC50 of tested compounds using the substrate acetylcholine bromide (AChBr) [74].
3.8. Statistical Analysis
The results obtained were statistically calculated by using probit analysis [75] with the statistics program (LDP-line). The biochemical results were analyzed by one-way analysis of variance (One-way ANOVA). The means were compared by Tukey’s multiple comparisons tests. The significance of the differences between the tested and control groups was determined for every tested extract. A p-value of ≤ 0.05 was considered statistically significant.
3.9. In Silico Studies
The 3D-crystal structures for the macromolecules AChE (PDB ID: 6XYS) and GST (PDB ID: 1M0U) from Drosophila melanogaster [76,77] were retrieved from the protein data bank website (www.rcsb.org accessed on 1 December 2022). The major compounds identified in C. myrica, C. trinodis, and C. tamariscifolia methanol fraction and λ-cyhalothrin (reference insecticide) were docked separately into the active pocket of the receptors, AChE, and GST. All docking calculations and results visualizations were performed using a molecular operating environment (MOE) 2015.10 software package [78].
The preparation module in the MOE’s receptors was used to fix the protein structure for any missing atoms or residues and make any necessary adjustments. This process comprised applying partial charges through the MMFF94 (modified) forcefield, 3D-protonation, and minimizing the protein structures to a chosen gradient using default parameters. The binding pockets of AChE and GST were defined using the dummy atoms that were generated at the binding location using MOE’s site finder option. The docking algorithm was configured to use the triangle matcher placement approach with rigid receptor refinement. The triangular match algorithm was programmed to create 50 poses, but the induced fit refinement approach produced five poses. Furthermore, the default GBVI/WSA dG technique was used as a docking function in MOE [79]. Docking conformations with the lowest binding energies were chosen. Validation of the docking protocol was done by redocking ligands into the active pocket of AChE and GST proteins. Only those docking poses were considered successful whose root-mean-square deviation (RMSD) values were less than 2.0 Å [80].
4. Conclusions
The present study showed the UPLC/ESI/MS analysis of the methanol fraction of three algae samples, Cystoseira myrica, C. trinodis, and C. tamariscifolia, and revealed the presence of flavonoids and terpenoids as main compounds. Also, they showed promising larvicidal activity against third-instar larvae of Cx. pipiens. Moreover, the three fractions showed a reduction in acetylcholinesterase and an elevation in glutathione-S-transferase enzymes that enhance the detoxification process of Cx. pipiens larvae. The promising larvicidal activity of the tested samples was confirmed by in silico studies of the identified compounds towards two tested enzymes. Compounds, (2E, l0E) 1-hydroxy-6,13-diketo-7-methylene-3,11,15-trimethyl-hexadeca-2,l0,l4-triene, bifuhalol hexacetate, oxocrinol, cystoseirol monoacetate, and α-linolenic acid showed good binding affinities to the AChE enzyme. Besides, bifuhalol hexacetate and cystoseirol monoacetate showed good binding affinities to the GST enzyme. Further investigations are required to investigate the chemistry of the methanol extract of three species in-depth. To sum up, the findings of this study can be used to introduce a biodegradable and novel organic larvicide to control mosquitoes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md21020117/s1, Figure S1: UPLC-ESI-MS base peak chromatogram of Cystoseira myrica total methanol extract in the negative ion mode; Figure S2: UPLC-ESI-MS base peak chromatogram of Cystoseira trinodis total methanol extract in the negative ion mode; Figure S3: UPLC-ESI-MS base peak chromatogram of Cystoseira tamariscifolia total methanol extract in the negative ion mode; Figure S4: The three tested extracts induced abnormalities in the treated larvae and also in the pupae that resulted from treated larvae; Figure S5: (A–C) 2D-binding interaction profile for compounds 1 and 2, respectively, with the active pocket of the AChE enzyme. (D–F) In-depth 3D ligand-AChE interaction mode for compounds 1 and 2, respectively; Figure S6: (A–D) 2D-binding interaction profile for compounds 4, 7, 8, and 11, respectively, with the active pocket of the AChE enzyme. (E–H) In-depth 3D ligand-AChE interaction mode for compounds 4, 7, 8, and 11, respectively; Figure S7: (A–C) 2D-binding interaction profile for compounds 1, 2, and 4, respectively, with the active pocket of GST enzyme. (D–F) In-depth 3D ligand-GST interaction mode for compounds 1, 2, and 4, respectively; Figure S8: (A–C) 2D-binding interaction profile for compounds 7, 8, and 11, respectively, with the active pocket of GST enzyme. (D–F) In-depth 3D ligand- GST interaction mode for compounds 7, 8, and 11, respectively; Figure S9: High-resolution images of the three samples of the work; A. Cystoseira myrica, B. C. trinodis, C. C. tamariscifolia; Figure S10: Laboratory Maintenance of Cx. Pipiens.
Author Contributions
Conceptualization, S.H.A., A.M.E., S.M.F., O.H.Z. and A.N.B.S.; formal analysis, S.H.A., A.M.E., S.M.F., H.I.M. and O.H.Z.; methodology, S.H.A., A.M.E., S.M.F. and H.I.M.; validation, S.H.A., D.S., N.A.A. and A.N.B.S.; software, H.I.M., D.S. and N.A.A.; visualization, S.H.A. and A.N.B.S.; Writing—original draft, S.H.A., S.M.F., H.I.M. and O.H.Z.; Writing—review and editing, S.H.A., A.M.E., S.M.F., O.H.Z. and A.N.B.S.; funding acquisition, N.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This research paper was approved by the research ethics committee from the Faculty of Science, Ain Shams University (ASU-SCI/ENTO/2023/1/2).
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kurane, I. The Effect of Global Warming on Infectious Diseases. Osong Public Health Res. Perspect. 2010, 1, 4–9. [Google Scholar] [CrossRef]
- Tibbetts, J. Driven to Extremes Health Effects of Climate Change. Environ. Health Perspect. 2007, 115, A196–A203. [Google Scholar] [CrossRef]
- Martinet, J.-P.; Ferté, H.; Failloux, A.-B.; Schaffner, F.; Depaquit, J. Mosquitoes of North-Western Europe as Potential Vectors of Arboviruses: A Review. Viruses 2019, 11, 1059. [Google Scholar] [CrossRef] [PubMed]
- Harbach, R.E. Culex pipiens: Species versus Species Complex Taxonomic History and Perspective. J. Am. Mosq. Control Assoc. 2012, 28, 10–23. [Google Scholar] [CrossRef]
- Pitasawat, B.; Champakaew, D.; Choochote, W.; Jitpakdi, A.; Chaithong, U.; Kanjanapothi, D.; Rattanachanpichai, E.; Tippawangkosol, P.; Riyong, D.; Tuetun, B.; et al. Aromatic Plant-Derived Essential Oil: An Alternative Larvicide for Mosquito Control. Fitoterapia 2007, 78, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, S.; Inbaneson, S.J.; Suganthi, P.; Gokulakrishnan, R.; Venkatesan, M. In Vitro Antiplasmodial Activity of Ethanolic Extracts of Seaweed Macroalgae against Plasmodium falciparum. Parasitol. Res. 2011, 108, 1411–1416. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Maggi, F.; Petrelli, R.; Nicoletti, M. Commentary: Making Green Pesticides Greener? The Potential of Plant Products for Nanosynthesis and Pest Control. J. Clust. Sci. 2017, 28, 3–10. [Google Scholar] [CrossRef]
- Paeporn, P.; Komalamisra, N.; Deesin, V.; Rongsriyam, Y.; Eshita, Y.; Thongrungkiat, S. Temephos Resistance in Two Forms of Aedes aegypti and Its Significance for the Resistance Mechanism. Southeast Asian J. Trop. Med. Public Health 2003, 34, 786–792. [Google Scholar]
- Abdel Haleem, D.R.; El Tablawy, N.H.; Ahmed Alkeridis, L.; Sayed, S.; Saad, A.M.; El-Saadony, M.T.; Farag, S.M. Screening and Evaluation of Different Algal Extracts and Prospects for Controlling the Disease Vector Mosquito Culex pipiens L. Saudi J. Biol. Sci. 2022, 29, 933–940. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Allam, A.E.; Farag, S.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. New Quinolizidine Alkaloid and Insecticidal Activity of Sophora secundiflora and Sophora tomentosa against Culex pipiens (Diptera: Culicidae). Nat. Prod. Res. 2021, 36, 2722–2734. [Google Scholar] [CrossRef]
- Tantawy, A.H.; Farag, S.M.; Abdel-Haleem, D.R.; Mohamed, H.I. Facile Synthesis, Larvicidal Activity, Biological Effects, and Molecular Docking of Sulfonamide-Incorporating Quaternary Ammonium Iodides as Acetylcholinesterase Inhibitors against Culex pipiens L. Bioorg. Chem. 2022, 128, 106098. [Google Scholar] [CrossRef]
- Singab, A.N.B.; Mostafa, N.M.; Elkhawas, Y.A.; Al-Sayed, E.; Bishr, M.M.; Elissawy, A.M.; Elnaggar, M.S.; Fawzy, I.M.; Salama, O.M.; Tsai, Y.H.; et al. Cyclodepsipeptides: Isolation from Endophytic Fungi of Sarcophyton ehrenbergi and Verification of Their Larvicidal Activity via In-Vitro and In-Silico Studies. Mar. Drugs 2022, 20, 331. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K. Biologically Active Compounds in Seaweed Extracts—The Prospects for the Application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef]
- Dias, C.N.; Moraes, D.F.C. Essential Oils and Their Compounds as Aedes aegypti L. (Diptera: Culicidae) Larvicides: Review. Parasitol. Res. 2014, 113, 565–592. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, K.A.; Dunbar, D.C.; Perry, T.L.; Wilkins, S.P.; Hamann, M.T.; Greenplate, J.T.; Wideman, M.A. Marine Natural Products as Prototype Insecticidal Agents. J. Agric. Food Chem. 1997, 45, 2735–2739. [Google Scholar] [CrossRef]
- Elbrense, H.; Gheda, S. Evaluation of the Insecticidal and Antifeedant Activities of Some Seaweed Extracts against the Egyptian Cotton Leaf Worm, Spodoptera littoralis, and the Lesser Grain Borer Rhyzopertha dominica. Egypt. J. Exp. Biol. 2021, 17, 1. [Google Scholar] [CrossRef]
- Fatima Manzelat, S.; Mohammed Mufarrah, A.; Ahmed Hasan, B.; Ali Hussain, N.; Shuqaiq, A.; Huraidha, A.; Qahma, A.; Birk, A. Macro Algae of the Red Sea from Jizan, Saudi Arabia. Phykos 2018, 48, 88–108. [Google Scholar] [CrossRef]
- Pati, M.P.; Sharma, S.D.; Nayak, L.; Panda, C.R. Uses of Seaweed and Its Application to Human Welfare: A Review. Int. J. Pharm. Pharm. Sci. 2016, 8, 12–20. [Google Scholar] [CrossRef]
- Stranska-Zachariasova, M.; Kurniatanty, I.; Gbelcova, H.; Jiru, M.; Rubert, J.; Nindhia, T.G.T.; D’Acunto, C.W.; Sumarsono, S.H.; Tan, M.I.; Hajslova, J.; et al. Bioprospecting of Turbinaria Macroalgae as a Potential Source of Health Protective Compounds. Chem. Biodivers. 2017, 14, e1600192. [Google Scholar] [CrossRef]
- Argandoña, V.; Del Pozo, T.; San-Martín, A.; Rovirosa, J. Insecticidal Activity of Plocamium cartilagineum Monoterpene. Boletín la Soc. Chil. Química 2000, 45, 371–376. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Mary Jeeva, Y. Eficacia Ninfcida y Ovicida de Una Alga Marina Sargassum tenerrimum (J. Agardh), Contra Dysdercus cingulatus (Fab.) (Pyrrhocoridae). Chil. J. Agric. Res. 2012, 72, 152–156. [Google Scholar] [CrossRef]
- Salvador-Neto, O.; Gomes, S.A.; Soares, A.R.; Da Silva Machado, F.L.; Samuels, R.I.; Fonseca, R.N.D.; Souza-Menezes, J.; Da Cunha Moraes, J.L.; Campos, E.; Mury, F.B.; et al. Larvicidal Potential of the Halogenated Sesquiterpene (+)-Obtusol, Isolated from the Alga Laurencia dendroidea J. Agardh (Ceramiales: Rhodomelaceae), against the Dengue Vector Mosquito Aedes aegypti (Linnaeus) (Diptera: Culicidae). Mar. Drugs 2016, 14, 20. [Google Scholar] [CrossRef]
- Yu, K.-X.; Jantan, I.; Ahmad, R.; Wong, C.-L. The Major Bioactive Components of Seaweeds and Their Mosquitocidal Potential. Parasitol. Res. 2014, 113, 3121–3141. [Google Scholar] [CrossRef] [PubMed]
- Abbassy, M. Insecticidal and Fungicidal Activity of Ulva lactuca Linnaeus (Chlorophyta) Extracts and Their Fractions. Annu. Res. Rev. Biol. 2014, 4, 2252–2262. [Google Scholar] [CrossRef]
- Manilal, A.; Selvin, J.; Thajuddin, N.; Sujith, S.; Panikkar, M.V.N.; Idhayadhulla, A.; Kumar, R.S. Biopotentials of Marine Alga, Lobophora variegate Collected from the South Indian Littoral. Thalassas 2012, 28, 47–54. [Google Scholar]
- Ishii, T.; Nagamine, T.; Nguyen, B.C.Q.; Tawata, S. Insecticidal and Repellent Activities of Laurinterol from the Okinawan Red Alga Laurencia nidifica. Rec. Nat. Prod. 2017, 11, 63–68. [Google Scholar]
- González-Castro, A.L.; Muñoz-Ochoa, M.; Hernández-Carmona, G.; López-Vivas, J.M. Evaluation of Seaweed Extracts for the Control of the Asian Citrus Psyllid diaphorina citri. J. Appl. Phycol. 2019, 31, 3815–3821. [Google Scholar] [CrossRef]
- Elbanna, S.; Hegazi, M. Screening of Some Seaweeds Species from South Sinai, Red Sea as Potential Bioinsecticides against Mosquito Larvae, Culex pipiens. Egypt. Acad. J. Biol. Sci. A Entomol. 2011, 4, 21–30. [Google Scholar] [CrossRef]
- Saber, A.A.; Hamed, S.M.; Abdel-Rahim, E.F.M.; Cantonati, M. Insecticidal Prospects of Algal and Cyanobacterial Extracts against the Cotton Leafworm Spodoptera littoralis. Vie Milieu 2018, 68, 199–212. [Google Scholar]
- Banaigs, B.; Francisco, C.; Gonzalez, E.; Fenical, W. Diterpenoid Metabolites from the Marine Alga Cystoseira elegans. Tetrahedron 1983, 39, 629–638. [Google Scholar] [CrossRef]
- Apostolova, E.; Lukova, P.; Baldzhieva, A.; Delattre, C.; Molinié, R.; Petit, E.; Elboutachfaiti, R.; Nikolova, M.; Iliev, I.; Murdjeva, M.; et al. Structural Characterization and In Vivo Anti-Inflammatory Activity of Fucoidan from Cystoseira crinita (Desf.) Borry. Mar. Drugs 2022, 20, 714. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, G.; Fontana, M.; Asnaghi, V.; Chiantore, M.; Mirata, S.; Salis, A.; Damonte, G.; Scarfì, S. The Remarkable Antioxidant and Anti-Inflammatory Potential of the Extracts of the Brown Alga Cystoseira amentacea Var. Stricta. Mar. Drugs 2021, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Zbakh, H.; Zubía, E.; de los Reyes, C.; Calderón-Montaño, J.M.; López-Lázaro, M.; Motilva, V. Meroterpenoids from the Brown Alga Cystoseira usneoides as Potential Anti-Inflammatory and Lung Anticancer Agents. Mar. Drugs 2020, 18, 207. [Google Scholar] [CrossRef]
- Čagalj, M.; Skroza, D.; Razola-Díaz, M.D.C.; Verardo, V.; Bassi, D.; Frleta, R.; Mekinić, I.G.; Tabanelli, G.; Šimat, V. Variations in the Composition, Antioxidant and Antimicrobial Activities of Cystoseira compressa during Seasonal Growth. Mar. Drugs 2022, 20, 64. [Google Scholar] [CrossRef] [PubMed]
- Saber, F.R.; Aly, S.H.; Khallaf, M.A.; El-Nashar, H.A.S.; Fahmy, N.M.; El-Shazly, M.; Radha, R.; Prakash, S.; Kumar, M.; Taha, D.; et al. Hyphaene thebaica (Areceaeae) as a Promising Functional Food: Extraction, Analytical Techniques, Bioactivity, Food, and Industrial Applications. Food Anal. Methods 2022. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Eldehna, W.M.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Aly, S.H. GC/MS Analysis of Essential Oil and Enzyme Inhibitory Activities of Syzygium cumini (Pamposia) Grown in Docking Studies. Molecules 2021, 26, 6984. [Google Scholar] [CrossRef]
- El-Nashar, H.A.S.; Aly, S.H.; Ahmadi, A.; El-Shazly, M. The Impact of Polyphenolics in the Management of Breast Cancer: Mechanistic Aspects and Recent Patents. Recent Pat. Anticancer Drug Discov. 2021, 17, 358–379. [Google Scholar] [CrossRef]
- Aly, S.H.; Eldahshan, O.A.; Al-rashood, S.T.; Binjubair, F.A.; El Hassab, M.A.; Eldehna, W.M.; Acqua, S.D.; Zengin, G. Chemical Constituents, Antioxidant, and Enzyme Inhibitory Activities Supported by In-Silico Study of n-Hexane Extract and Essential Oil of Guava Leaves. Molecules 2022, 27, 8979. [Google Scholar] [CrossRef]
- Ads, E.N.; Hassan, S.I.; Rajendrasozhan, S.; Hetta, M.H.; Aly, S.H.; Ali, M.A. Isolation, Structure Elucidation and Antimicrobial Evaluation of Natural Pentacyclic Triterpenoids and Phytochemical Investigation of Different Fractions of Ziziphus spina-christi (L.) Stem Bark Using LCHRMS Analysis. Molecules 2022, 27, 1805. [Google Scholar] [CrossRef]
- Aly, S.H.; El-hassab, M.A.; Elhady, S.S.; Gad, H.A. Comparative Metabolic Study of Tamarindus Indica L.’s Various Organs Based on GC / MS Analysis, In Silico and In Vitro Anti-Inflammatory and Wound Healing Activities. Plants 2022, 12, 87. [Google Scholar] [CrossRef]
- Mokrini, R.; Ben Mesaoud, M.; Daoudi, M.; Hellio, C.; Maréchal, J.P.; El Hattab, M.; Ortalo-Magné, A.; Piovetti, L.; Culioli, G. Meroditerpenoids and Derivatives from the Brown Alga Cystoseira baccata and Their Antifouling Properties. J. Nat. Prod. 2008, 71, 1806–1811. [Google Scholar] [CrossRef]
- Amico, V.; Oriente, G.; Piattelli, M.; Ruberto, G.; Tringali, C. Novel Acyclic Diterpenes from the Brown Alga Cystoseira crinita. Phytochemistry 1981, 20, 1085–1088. [Google Scholar] [CrossRef]
- Fattorusso, E.; Magno, S.; Mayol, L.; Santacroce, C.; Sica, D.; Amico, V.; Oriente, G.; Piattelli, M.; Tringali, C. Oxocrinol and Crinitol, Novel Linear Terpenoids from the Brown Alga Cystoseira crinita. Tetrahedron Lett. 1976, 17, 937–940. [Google Scholar] [CrossRef]
- Ayyad, S.E.N.; Abdel-Halim, O.B.; Shier, W.T.; Hoye, T.R. Cytotoxic Hydroazulene Diterpenes from the Brown Alga Cystoseira myrica. Zeitschrift Fur Naturforsch.—Sect. C J. Biosci. 2003, 58, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Oliyaei, N.; Moosavi-Nasab, M. Ultrasound-Assisted Extraction of Fucoxanthin from Sargassum angustifolium and Cystoseira indica Brown Algae. J. Food Process. Preserv. 2021, 45, e15929. [Google Scholar] [CrossRef]
- Bharadwaj, S.; Sheeja, L.; Lakshmi, D.; Parveen, S.K. 1 H NMR Analysis and Bioautography Screening of Methanol Extract of Sargassum wightii by Chromatographic Separation. Res. J. Pharm. Technol. 2017, 10, 473. [Google Scholar] [CrossRef]
- El-Khateeb, A.; Hamed, E.; Ibrahim, F.; Hamed, S. Eco-Friendly Synthesis of Selenium and Zinc Nanoparticles with Biocompatible Sargassum latifolium Algae Extract in Preservation of Edible Oils. J. Food Dairy Sci. 2019, 10, 141–146. [Google Scholar] [CrossRef]
- Sun, J.; Liang, F.; Bin, Y.; Li, P.; Duan, C. Screening Non-Colored Phenolics in Red Wines Using Liquid Chromatography/Ultraviolet and Mass Spectrometry/Mass Spectrometry Libraries. Molecules 2007, 12, 679–693. [Google Scholar] [CrossRef]
- Savarese, M.; De Marco, E.; Sacchi, R. Food Chemistry Characterization of Phenolic Extracts from Olives (Olea europaea Cv. Pisciottana) by Electrospray Ionization Mass Spectrometry. Food Chem. 2007, 105, 761–770. [Google Scholar] [CrossRef]
- Glombitza, K.W.; Rosener, H.U.; Müller, D. Bifuhalol und Diphlorethol aus Cystoseira tamariscifolia. Phytochemistry 1975, 14, 1115–1116. [Google Scholar] [CrossRef]
- Faheem, S.A.; Saeed, N.M.; El-Naga, R.N.; Ayoub, I.M.; Azab, S.S. Hepatoprotective Effect of Cranberry nutraceutical Extract in Non-Alcoholic Fatty Liver Model in Rats: Impact on Insulin Resistance and Nrf-2 Expression. Front. Pharmacol. 2020, 11, 218. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; El-Nashar, H.A.S.; Al-Mohammadi, A.G.A.; Eldahshan, O.A. Orange Fruit (Citrus sinensis) Peel Extract Attenuates Chemotherapy-Induced Toxicity in Male Rats. Food Funct. 2021, 12, 9443–9455. [Google Scholar] [CrossRef]
- Koul, S.K.; Dhar, K.L.; Thakur, R.S. A New Coumarin Glucoside from Prangos pabularia. Phytochemistry 1979, 18, 1762–1763. [Google Scholar] [CrossRef]
- Emam, M.; Abdel-Haleem, D.R.; Salem, M.M.; Abdel-Hafez, L.J.M.; Latif, R.R.A.; Farag, S.M.; Sobeh, M.; Raey, M.A. El Phytochemical Profiling of Lavandula coronopifolia poir. Aerial Parts Extract and Its Larvicidal, Antibacterial, and Antibiofilm Activity against Pseudomonas aeruginosa. Molecules 2021, 26, 1710. [Google Scholar] [CrossRef] [PubMed]
- Segarra, G.; Jáuregui, O.; Casanova, E.; Trillas, I. Simultaneous Quantitative LC-ESI-MS/MS Analyses of Salicylic Acid and Jasmonic Acid in Crude Extracts of Cucumis sativus under Biotic Stress. Phytochemistry 2006, 67, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Minicante, S.A.; Carlin, S.; Stocco, M.; Sfriso, A.; Capelli, G.; Montarsi, F. Preliminary Results on the Efficacy of Macroalgal Extracts Against Larvae of Aedes albopictus. J. Am. Mosq. Control Assoc. 2017, 33, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Eid, A.M.; Abdel-Rahman, M.A.; EL-Belely, E.F.; Awad, M.A.; Hassan, S.E.D.; AL-Faifi, Z.E.; Hamza, M.F. Enhanced Antimicrobial, Cytotoxicity, Larvicidal, and Repellence Activities of Brown Algae, Cystoseira crinita-Mediated Green Synthesis of Magnesium Oxide Nanoparticles. Front. Bioeng. Biotechnol. 2022, 10, 849921. [Google Scholar] [CrossRef]
- Waliwitiya, R.; Nicholson, R.A.; Kennedy, C.J.; Lowenberger, C.A. The Synergistic Effects of Insecticidal Essential Oils and Piperonyl Butoxide on Biotransformational Enzyme Activities in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2012, 49, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Boily, M.; Sarrasin, B.; Deblois, C.; Aras, P.; Chagnon, M. Acetylcholinesterase in Honey Bees (Apis mellifera) Exposed to Neonicotinoids, Atrazine and Glyphosate: Laboratory and Field Experiments. Environ. Sci. Pollut. Res. Int. 2013, 20, 5603–5614. [Google Scholar] [CrossRef]
- Rajashekar, Y.; Raghavendra, A.; Bakthavatsalam, N. Acetylcholinesterase Inhibition by Biofumigant (Coumaran) from Leaves of Lantana camara in Stored Grain and Household Insect Pests. Biomed Res. Int. 2014, 2014, 187019. [Google Scholar] [CrossRef]
- Huang, C.S.; Lii, C.K.; Lin, A.H.; Yeh, Y.W.; Yao, H.T.; Li, C.C.; Wang, T.S.; Chen, H.W. Protection by Chrysin, Apigenin, and Luteolin against Oxidative Stress Is Mediated by the Nrf2-Dependent up-Regulation of Heme Oxygenase 1 and Glutamate Cysteine Ligase in Rat Primary Hepatocytes. Arch. Toxicol. 2013, 87, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Farag, S.M.; Hussein, M.A.; Hafez, S.E.; Khaled, A.S.; Kamel, O.M.; Zyaan, O.H. Larvicidal, Biological, and Histopathological Alterations Induced by Pomegranate Peel Extract, Punica granatum against Culex pipiens L. (Diptera: Culicidae). Egypt. J. Aquat. Biol. Fish. 2021, 25, 139–161. [Google Scholar] [CrossRef]
- Ibraheem, I.B.M.; Alharbi, R.M.; Abdel-Raouf, N.; Al-Enazi, N.M. Contributions to the Study of the Marine Algae Inhabiting Umluj Seashore, Red Sea. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 278–285. [Google Scholar] [CrossRef]
- Aly, S.H.; Elissawy, A.M.; Fayez, A.M.; Eldahshan, O.A.; Elshanawany, M.A.; Singab, A.N.B. Neuroprotective Effects of Sophora secundiflora, Sophora tomentosa Leaves and Formononetin on Scopolamine-Induced Dementia. Nat. Prod. Res. 2020, 35, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, H.; Li, P.; Zhu, J.; Xiong, M. Identification and Quantification of Atractylenolide I and Atractylenolide III in Rhizoma Atractylodes Macrocephala by Liquid Chromatography-Ion Trap Mass Spectrometry. Biomed. Chromatogr. 2013, 27, 699–707. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Huang, Y.; Wen, X.; Wu, Y.; Zhao, Y.; Ni, Y. Extraction, Purification, and Hydrolysis Behavior of Apigenin-7-O-Glucoside from Chrysanthemum morifolium Tea. Molecules 2018, 23, 2933. [Google Scholar] [CrossRef]
- Elhawary, E.A.; Mostafa, N.M.; Shehata, A.Z.I.; Labib, R.M.; Singab, A.N.B. Comparative Study of Selected Rosa Varieties’ Metabolites through UPLC-ESI-MS/MS, Chemometrics and Investigation of Their Insecticidal Activity against Culex pipiens L. Jordan J. Pharm. Sci. 2021, 14, 417–433. [Google Scholar]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. 1925. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar]
- Saad, A.M.; El-Saadony, M.T.; Mohamed, A.S.; Ahmed, A.I.; Sitohy, M.Z. Impact of Cucumber Pomace Fortification on the Nutritional, Sensorial and Technological Quality of Soft Wheat Flour-Based Noodles. Int. J. Food Sci. Technol. 2021, 56, 3255–3268. [Google Scholar] [CrossRef]
- Knight, J.A.; Anderson, S.; Rawle, J.M. Chemical Basis of the Sulfo-Phospho-Vanillin Reaction for Estimating Total Serum Lipids. Clin. Chem. 1972, 18, 199–202. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Stepnowski, P.; Gołębiowski, M. Identification and Quantitative Analysis of Lipids and Other Organic Compounds Contained in Eggs of Colorado Potato Beetle (Leptinotarsa decemlineata). J. Plant Dis. Prot. 2019, 126, 379–384. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kao, C.-W.; Bakshi, M.; Sherameti, I.; Dong, S.; Reichelt, M.; Oelmüller, R.; Yeh, K.-W. A Chinese Cabbage (Brassica campetris subsp. Chinensis) τ-Type Glutathione-S-Transferase Stimulates Arabidopsis Development and Primes against Abiotic and Biotic Stress. Plant Mol. Biol. 2016, 92, 643–659. [Google Scholar] [CrossRef]
- Simpson, D.R.; Bull, D.L.; Lindquist, D.A. A Semimicrotechnique for the Estimation of Cholinesterase Activity in Boll Weevils1. Ann. Entomol. Soc. Am. 1964, 57, 367–371. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1971. [Google Scholar]
- Nachon, F.; Rosenberry, T.L.; Silman, I.; Sussman, J.L. A Second Look at the Crystal Structures of Drosophila melanogaster Acetylcholinesterase in Complex with Tacrine Derivatives Provides Insights Concerning Catalytic Intermediates and the Design of Specific Insecticides. Molecules 2020, 25, 1198. [Google Scholar] [CrossRef]
- Agianian, B.; Tucker, P.A.; Schouten, A.; Leonard, K.; Bullard, B.; Gros, P. Structure of a Drosophila Sigma Class Glutathione S-Transferase Reveals a Novel Active Site Topography Suited for Lipid Peroxidation Products. J. Mol. Biol. 2003, 326, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Kidachi, Y.; Kamiie, K.; Noshita, T.; Umetsu, H. Structural Insight into the Ligand-Receptor Interaction between Glycyrrhetinic Acid (GA) and the High-Mobility Group Protein B1 (HMGB1)-DNA Complex. Bioinformation 2012, 8, 1147–1153. [Google Scholar] [CrossRef]
- Aziz, M.; Ejaz, S.A.; Tamam, N.; Siddique, F.; Riaz, N.; Qais, F.A.; Chtita, S.; Iqbal, J. Identification of Potent Inhibitors of NEK7 Protein Using a Comprehensive Computational Approach. Sci. Rep. 2022, 12, 6404. [Google Scholar] [CrossRef]
- Morris, G.M.; Lim-Wilby, M. Molecular Docking. In Methods in Molecular Biology; Kukol, A., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 443, pp. 365–382. ISBN 9781588298645. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).