Red Marine Algae Lithothamnion calcareum Supports Dental Enamel Mineralization
Abstract
1. Introduction
2. Results
2.1. Experiment 1—In Vitro Study
2.2. Experiment 2—In Situ/Ex Vivo Co-Twin Control Study
3. Discussion
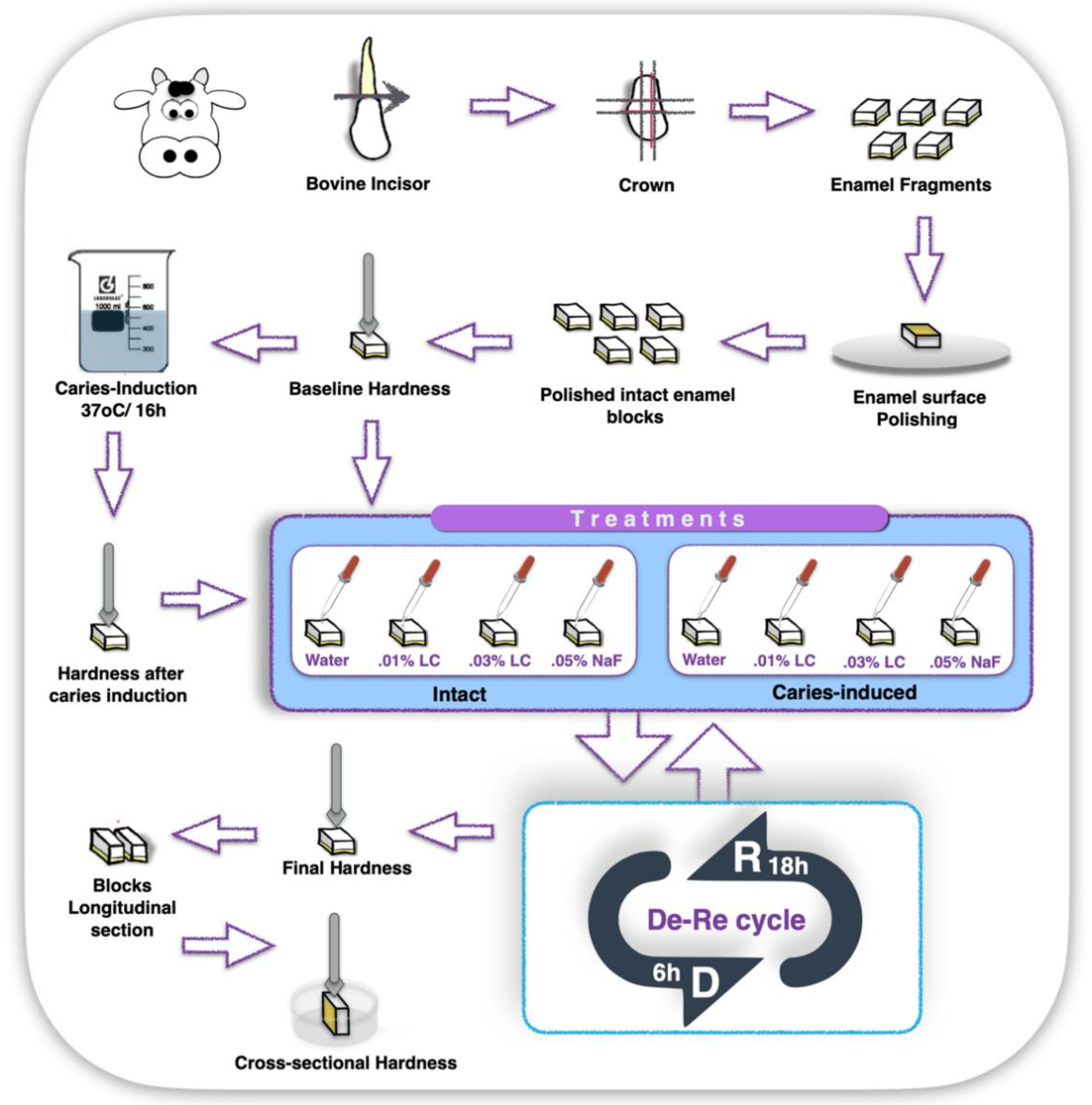
4. Materials and Methods
4.1. LC Solubilization and Ion Content Characterization
4.2. Experiment 1—In Vitro Study
4.2.1. Enamel Blocks Preparation and Baseline Measurements
4.2.2. Enamel Blocks Chemical Demineralizing-Challenge Protocol
4.2.3. Treatment
4.2.4. Post-Treatment Microhardness and Gravimetrical Measurements
4.2.5. Statistical Analysis
4.3. Experiment 2—In Situ/Ex Vivo, Co-Twin Control Study
4.3.1. Experimental Design and Ethical Approval
4.3.2. Demographics and Caries Risk Assessment of the Study Population
4.3.3. Intraoral Appliances Preparation and Assembling
4.3.4. In Situ Phase
4.3.5. Enamel Final Hardness and QLF measurements
4.3.6. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Oral Disorders Collaborators. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Schlueter, N.; Amaechi, B.T.; Bartlett, D.; Buzalaf, M.A.R.; Carvalho, T.S.; Ganss, C.; Hara, A.T.; Huysmans, M.D.N.J.M.; Lussi, A. Terminology of erosive tooth wear: Consensus report of a workshop organized by the ORCA and the cariology research group of the IADR. Caries Res. 2020, 54, 2–6. [Google Scholar] [CrossRef]
- Baig, A.A.; Fox, J.L.; Young, R.A.; Wang, Z.; Hsu, J.; Higuchi, W.I.; Chhettry, A.; Zhuang, H.; Otsuka, M. Relationships among carbonated apatite solubility, crystallite size, and microstrain parameters. Calcif. Tissue Int. 1999, 64, 37–49. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. Engl. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Hara, A.T.; Ando, M.; Gonzalez-Cabezas, C.; Cury, J.A.; Serra, M.C.; Zero, D.T. Protective effect of the dental pellicle against erosive challenges in situ. J. Dent. Res. 2006, 85, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Buchalla, W.; Attin, T.; Schulte-Monting, J.; Hellwig, E. Fluoride uptake, retention, and remineralization efficacy of a highly concentrated fluoride solution on enamel lesions in situ. J. Dent. Res. 2002, 81, 329–333. [Google Scholar] [CrossRef]
- Wiegand, A.; Krieger, C.; Attin, R.; Hellwig, E.; Attin, T. Fluoride uptake and resistance to further demineralisation of demineralised enamel after application of differently concentrated acidulated sodium fluoride gels. Clin. Oral. Investig. 2005, 9, 52–57. [Google Scholar] [CrossRef]
- Huysmans, M.C.; Young, A.; Ganss, C. The role of fluoride in erosion therapy. Monogr. Oral. Sci. 2014, 25, 197–205. [Google Scholar] [CrossRef]
- Lussi, A.; Carvalho, T.S. The future of fluorides and other protective agents in Erosion Prevention. Caries Res. 2015, 1, 18–29. [Google Scholar] [CrossRef]
- Carey, C.M. Focus on fluorides: Update on the use of fluoride for the prevention of dental caries. J. Evid Based Dent. Pract 2014, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Slade, G.D.; Grider, W.Z.; Maas, W.R.; Sanders, A.E. Water Fluoridation and Dental Caries in U.S. Children and Adolescents. J. Dent. Res. 2018, 97, 1122–1128. [Google Scholar] [CrossRef]
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride Revolution and Dental Caries: Evolution of Policies for Global Use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef]
- James, P.; Harding, M.; Beecher, T.; Browne, D.; Cronin, M.; Guiney, H.; O’Mullane, D.; Whelton, H. Impact of Reducing Water Fluoride on Dental Caries and Fluorosis. J. Dent. Res. 2021, 100, 507–514. [Google Scholar] [CrossRef]
- Epple, M.; Enax, J.; Meyer, F. Prevention of Caries and Dental Erosion by Fluorides-A Critical Discussion Based on Physico-Chemical Data and Principles. Dent. J. 2022, 10, 6. [Google Scholar] [CrossRef]
- Iheozor-Ejiofor, Z.; Worthington, H.V.; Walsh, T.; O’Malley, L.; Clarkson, J.E.; Macey, R.; Alam, R.; Tugwell, P.; Welch, V.; Glenny, A.M. Water fluoridation for the prevention of dental caries. Cochrane Database Syst. Rev. 2015, 2015, CD010856. [Google Scholar] [CrossRef]
- CADTH Rapid Response Reports. Community Water Fluoridation Exposure: A Review of Neurological and Cognitive Effects—A 2020 Update. [Internet]; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2020. [Google Scholar]
- Aoun, A.; Darwiche, F.; Al Hayek, S.; Doumit, J. The Fluoride Debate: The Pros and Cons of Fluoridation. Prev. Nutr. Food Sci. 2018, 23, 171–180. [Google Scholar] [CrossRef]
- Lee, N.; Kang, S.; Lee, W.; Hwang, S.S. The Association between Community Water Fluoridation and Bone Diseases: A Natural Experiment in Cheongju, Korea. Int. J. Environ. Res. Public Health 2020, 17, 9170. [Google Scholar] [CrossRef]
- Fluoridation Facts. Practical Series Guide; American Dental Association: Chicago, IL, USA, 2018. [Google Scholar]
- British Fluoridation Society. One in a Million: The Facts about Water Fluoridation. Archives. Available online: https://bfsweb.org/one-in-a-million (accessed on 10 October 2022).
- Unde, M.P.; Patil, R.U.; Dastoor, P.P. The Untold Story of Fluoridation: Revisiting the Changing Perspectives. Indian J. Occup Environ. Med. 2018, 22, 121–127. [Google Scholar] [CrossRef]
- Adey, W.H.; McKibbin, D.L. Studies on the Maerl Species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnium corallioides Crouan in the Ria de Vigo. Bot Marina (1970): In Gargominy O (2022). Phymatolithon calcareum (Pallas) TAXREF. Version 4.8. UMS PatriNat (OFB-CNRS-MNHN), Paris. Checklist Dataset. Available online: https://doi.org/10.15468/vqueam (accessed on 10 October 2022).
- Aslam, M.N.; Kreider, J.M.; Paruchuri, T.; Bhagavathula, N.; DaSilva, M.; Zernicke, R.F.; Goldstein, S.A.; Varani, J. A mineral-rich extract from the red marine algae Lithothamnion calcareum preserves bone structure and function in female mice on a Western-style diet. Calcif. Tissue Int. 2010, 86, 313–324. [Google Scholar] [CrossRef]
- Aslam, M.N.; Bergin, I.; Jepsen, K.; Kreider, J.M.; Graf, K.H.; Naik, M.; Goldstein, S.A.; Varani, J. Preservation of bone structure and function by Lithothamnion sp. derived minerals. Biol. Trace Elem. Res. 2013, 156, 210–220. [Google Scholar] [CrossRef]
- Aslam, M.N.; Jepsen, K.J.; Khoury, B.; Graf, K.H.; Varani, J. Bone structure and function in male C57BL/6 mice. Effects of a high-fat Western-style diet with or without trace minerals. Bone Rep. 2016, 5, 141–149. [Google Scholar] [CrossRef]
- O’Gorman, D.M.; Tierney, C.M.; Brennan, O.; O’Brien, F.J. The marine-derived, multi-mineral formula, Aquamin, enhances mineralisation of osteoblast cells in vitro. Phytother. Res. 2012, 26, 375–380. [Google Scholar] [CrossRef]
- Brennan, O.; Sweeney, J.; O’Meara, B.; Widaa, A.; Bonnier, F.; Byrne, H.J.; O’Gorman, D.M.; O’Brien, F.J. A Natural, Calcium-Rich Marine Multi-mineral. Complex Preserves Bone Structure, Composition and Strength in an Ovariectomised Rat Model of Osteoporosis. Calcif. Tissue Int. 2017, 101, 445–455. [Google Scholar] [CrossRef]
- Zenk, J.L.; Frestedt, J.L.; Kuskowski, M.A. Effect of Calcium Derived from Lithothamnion sp. on Markers of Calcium Metabolism in Premenopausal Women. J. Med. Food 2018, 21, 154–158. [Google Scholar] [CrossRef]
- Ehrlich, H.; Koutsoukos, P.G.; Demadis, K.D.; Pokrovsky, O.S. Principles of demineralization: Modern strategies for the isolation of organic frameworks. Part I. Common definitions and history. Micron 2008, 39, 1062–1091. [Google Scholar] [CrossRef]
- Ehrlich, H.; Koutsoukos, P.G.; Demadis, K.D.; Pokrovsky, O.S. Principles of demineralization: Modern strategies for the isolation of organic frameworks. Part II. Decalcification. Micron 2009, 40, 169–193. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomedicine 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Magalhães, A.C.; Comar, L.P.; Rios, D.; Delbem, A.C.; Buzalaf, M.A. Effect of a 4% titanium tetrafluoride (TiF4) varnish on demineralisation and remineralisation of bovine enamel in vitro. J. Dent. 2008, 36, 158–162. [Google Scholar] [CrossRef]
- Queiroz, C.S.; Hara, A.T.; Paes Leme, A.F.; Cury, J.A. pH-cycling models to evaluate the effect of low fluoride dentifrice on enamel de- and remineralization. Braz Dent. J. 2008, 19, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rantonen, P.J.; Meurman, J.H. Correlations between total protein, lysozyme, immunoglobulins, amylase, and albumin in stimulated whole saliva during daytime. Acta Odontol Scand. 2000, 58, 160–165. [Google Scholar] [CrossRef]
- Guerrieri, A.; Gaucher, C.; Bonte, E.; Lasfargues, J.J. Minimal intervention dentistry: Part 4. Detection and diagnosis of initial caries lesions. Br. Dent. J. 2012, 213, 551–557. [Google Scholar] [CrossRef]
- Pretty, I.A.; Ellwood, R.P. The caries continuum: Opportunities to detect, treat and monitor the re-mineralization of early caries lesions. J. Dent. 2013, 41, S12–S21. [Google Scholar] [CrossRef]
- Ko, H.Y.; Kang, S.M.; Kim, H.E.; Kwon, H.K.; Kim, B.I. Validation of quantitative light-induced fluorescence-digital (QLF-D) for the detection of approximal caries in vitro. J. Dent. 2015, 43, 568–575. [Google Scholar] [CrossRef]
- Gomez, J.; Pretty, I.A.; Santarpia, R.P., 3rd; Cantore, B.; Rege, A.; Petrou, I.; Ellwood, R.P. Quantitative light-induced fluorescence to measure enamel remineralization in vitro. Caries Res. 2014, 48, 223–227. [Google Scholar] [CrossRef]
- Jallad, M.; Zero, D.; Eckert, G.; Ferreira Zandona, A. In vitro Detection of Occlusal Caries on Permanent Teeth by a Visual, Light-Induced Fluorescence and Photothermal Radiometry and Modulated Luminescence Methods. Caries Res. 2015, 49, 523–530. [Google Scholar] [CrossRef]
- Lenzi, T.L.; Piovesan, C.; Mendes, F.M.; Braga, M.M.; Raggio, D.P. In vitro performance of QLF system and conventional methods for detection of occlusal caries around tooth-colored restorations in primary molars. Int. J. Paediatr. Dent. 2016, 26, 26–34. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Reynolds, E.C. Calcium Phosphopeptides—Mechanisms of action and evidence for clinical efficacy. Adv. Dent. Res. 2012, 24, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Fischer, M. Co-twin Control Methods. In Encyclopedia of Statistics in Behavioral Science, 1st ed.; Everitt, B.S., Howell, D.C., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2005; Volume 1, pp. 415–418. [Google Scholar] [CrossRef]
- Spencer, P.; Barnes, C.; Martini, J.; Garcia, R.; Elliott, C.; Doremus, R. Incorporation of magnesium into rat dental enamel and its influence on crystallization. Arch. Oral Biol. 1989, 34, 767–771. [Google Scholar] [CrossRef]
- Aoba, T.; Shimoda, S.; Moreno, E.C. Labile or surface pools of magnesium, sodium, and potassium in developing porcine enamel mineral. J. Dent. Res. 1992, 71, 1826–1831. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, J.H.; Kim, Y.T.; Riu, D.H.; Jung, S.J.; Lee, Y.J.; Chung, S.C.; Kim, Y.H. Synthesis of Si, Mg substituted hydroxyapatites and their sintering behaviors. Biomaterials 2003, 24, 1389–1398. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; TenHuisen, K.S. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical-hydrothermal method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef]
- Ren, F.; Leng, Y.; Xin, R.; Ge, X. Synthesis, characterization and ab initio simulation of magnesium-substituted hydroxyapatite. Acta Biomater. 2010, 6, 2787–2796. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.N.; Eimar, H.; Bassett, D.C.; Schnabel, M.; Ciobanu, O.; Nelea, V.; McKee, M.D.; Cerruti, M.; Tamimi, F. Diagenesis-inspired reaction of magnesium ions with surface enamel mineral modifies properties of human teeth. Acta Biomater. 2016, 37, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Crowley, E.K.; Long-Smith, C.M.; Murphy, A.; Patterson, E.; Murphy, K.; O’Gorman, D.M.; Stanton, C.; Nolan, Y.M. Dietary Supplementation with a Magnesium-Rich Marine Mineral Blend Enhances the Diversity of Gastrointestinal Microbiota. Mar. Drugs 2018, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Swaminath, S.; Um, C.Y.; Prizment, A.E.; Lazovich, D.; Bostick, R.M. Combined Mineral Intakes and Risk of Colorectal Cancer in Postmenopausal Women. Cancer Epidemiol. Biomark Prev. 2019, 28, 392–399. [Google Scholar] [CrossRef]
- Featherstone, J.D.B. Remineralization, the Natural Caries Repair Process—The Need for New Approaches. Adv. Dent. Res. 2009, 21, 4–7. [Google Scholar] [CrossRef]
- World Health Organization. Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 18 November 2022).
- Saravanan, C.; Easwaramoorthi, S.; Wang, L. Colorimetric detection of fluoride ion by 5-arylidenebarbituric acids: Dual interaction mode for fluoride ion with single receptor. Dalton Trans. 2014, 43, 5151–5157. [Google Scholar] [CrossRef]
- Sium, M.; Kareru, P.; Keriko, J.; Girmay, B.; Medhanie, G.; Debretsion, S. Profile of Trace Elements in Selected Medicinal Plants Used for the Treatment of Diabetes in Eritrea. Sci. World J. 2016, 2016, 2752836. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
R. Carrilho, M.; Bretz, W. Red Marine Algae Lithothamnion calcareum Supports Dental Enamel Mineralization. Mar. Drugs 2023, 21, 109. https://doi.org/10.3390/md21020109
R. Carrilho M, Bretz W. Red Marine Algae Lithothamnion calcareum Supports Dental Enamel Mineralization. Marine Drugs. 2023; 21(2):109. https://doi.org/10.3390/md21020109
Chicago/Turabian StyleR. Carrilho, Marcela, and Walter Bretz. 2023. "Red Marine Algae Lithothamnion calcareum Supports Dental Enamel Mineralization" Marine Drugs 21, no. 2: 109. https://doi.org/10.3390/md21020109
APA StyleR. Carrilho, M., & Bretz, W. (2023). Red Marine Algae Lithothamnion calcareum Supports Dental Enamel Mineralization. Marine Drugs, 21(2), 109. https://doi.org/10.3390/md21020109





