Production of Bioactive Peptides from Hake By-Catches: Optimization and Scale-Up of Enzymatic Hydrolysis Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Initial Screening: Enzyme Selection
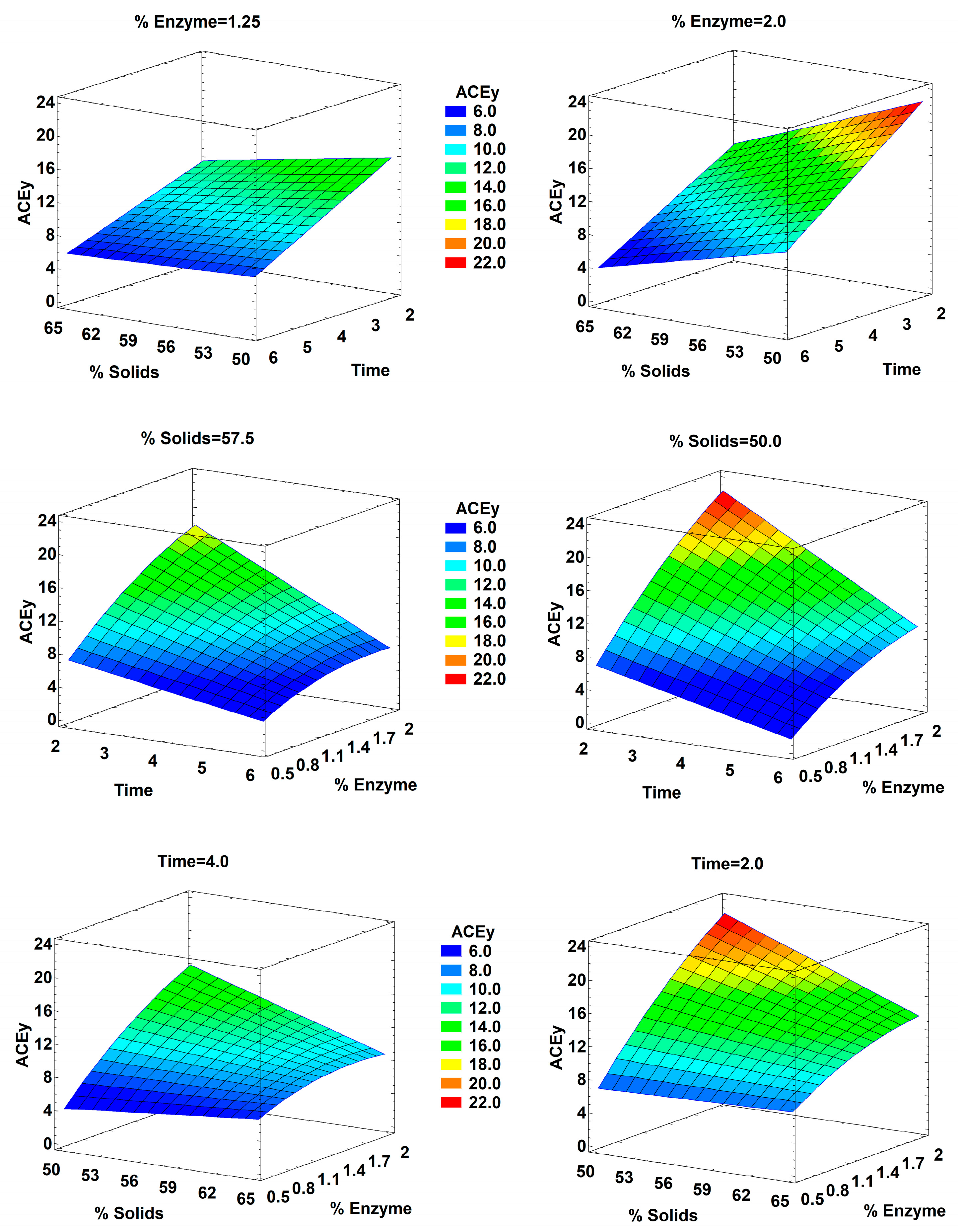
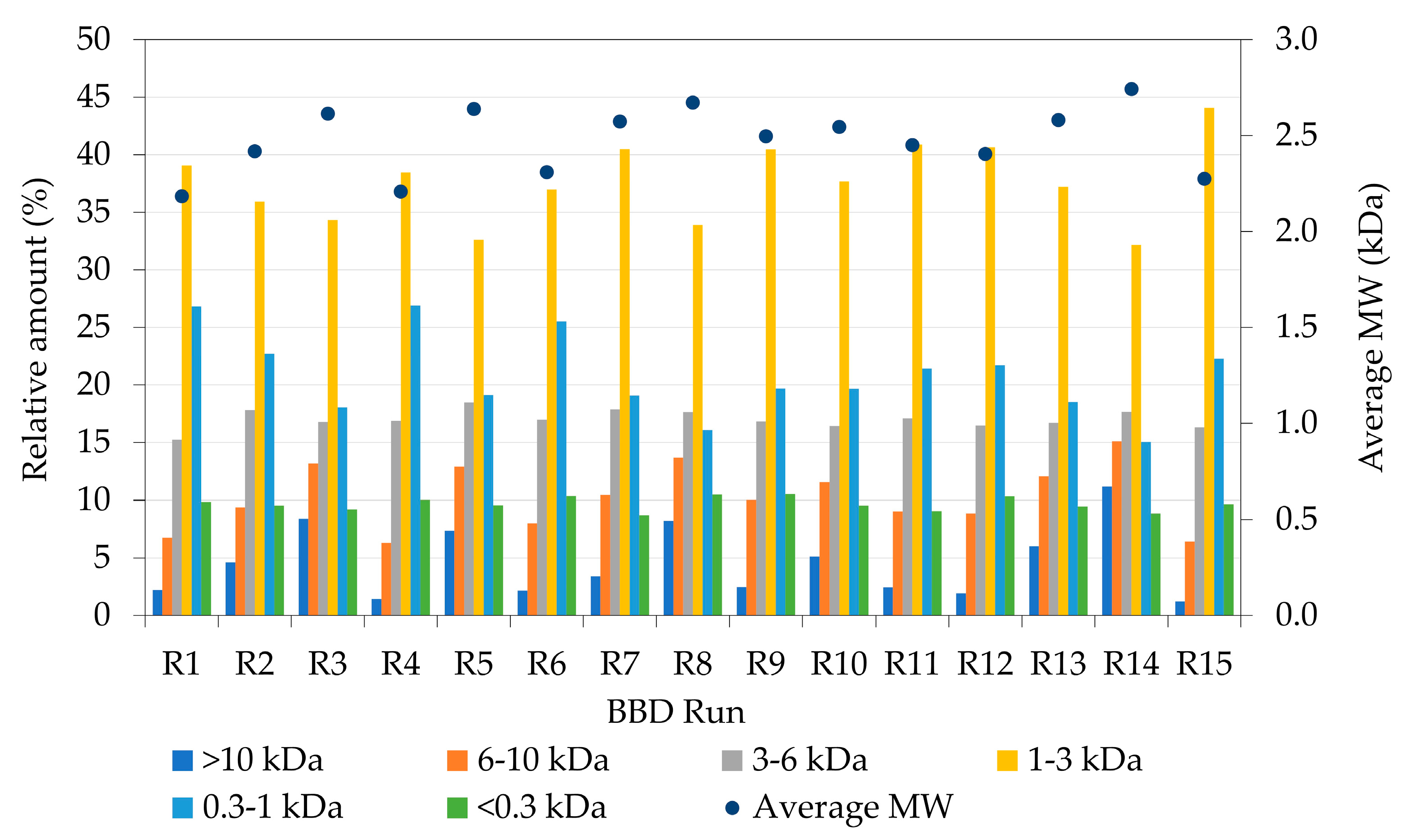
2.2. Hydrolysis Optimization
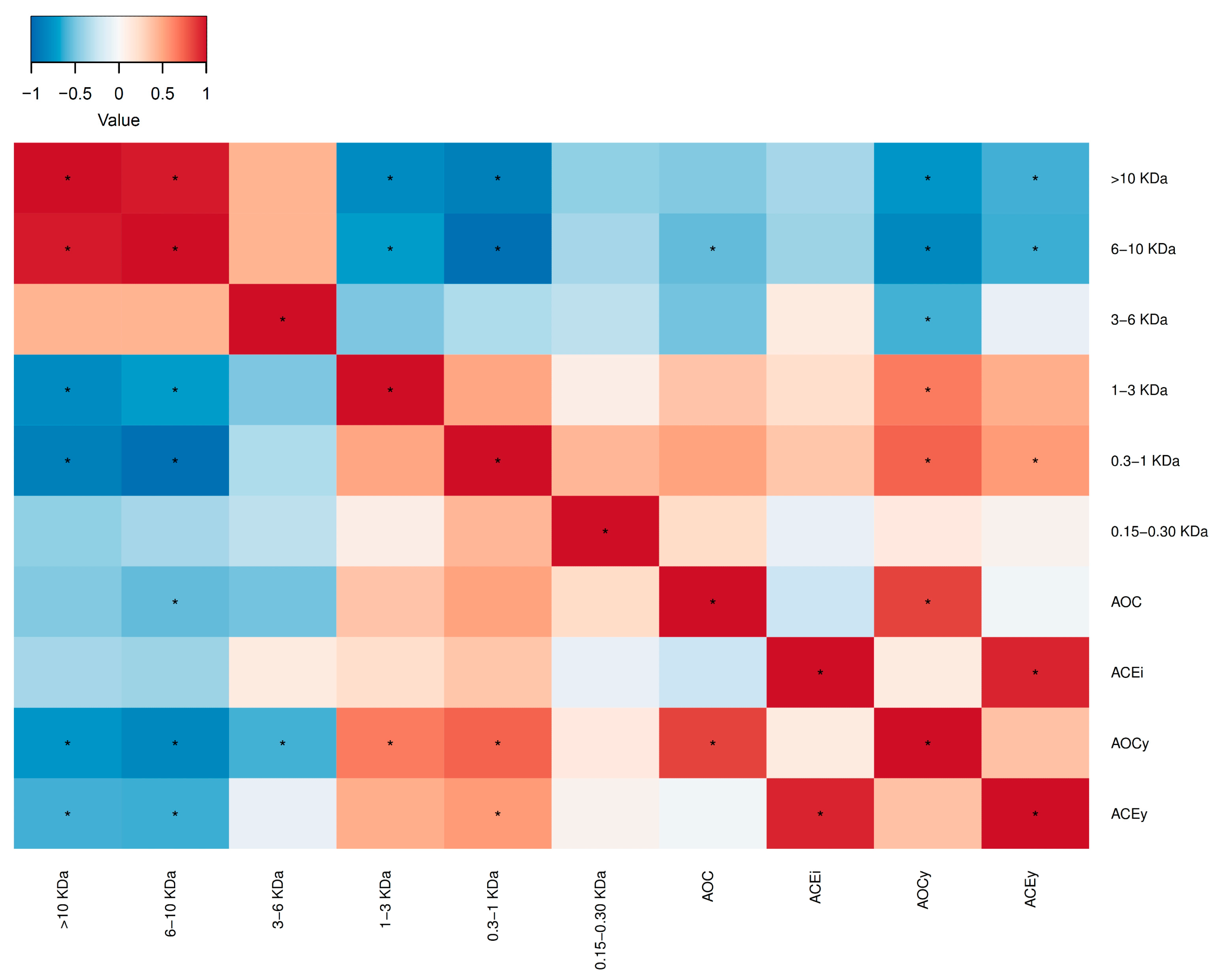
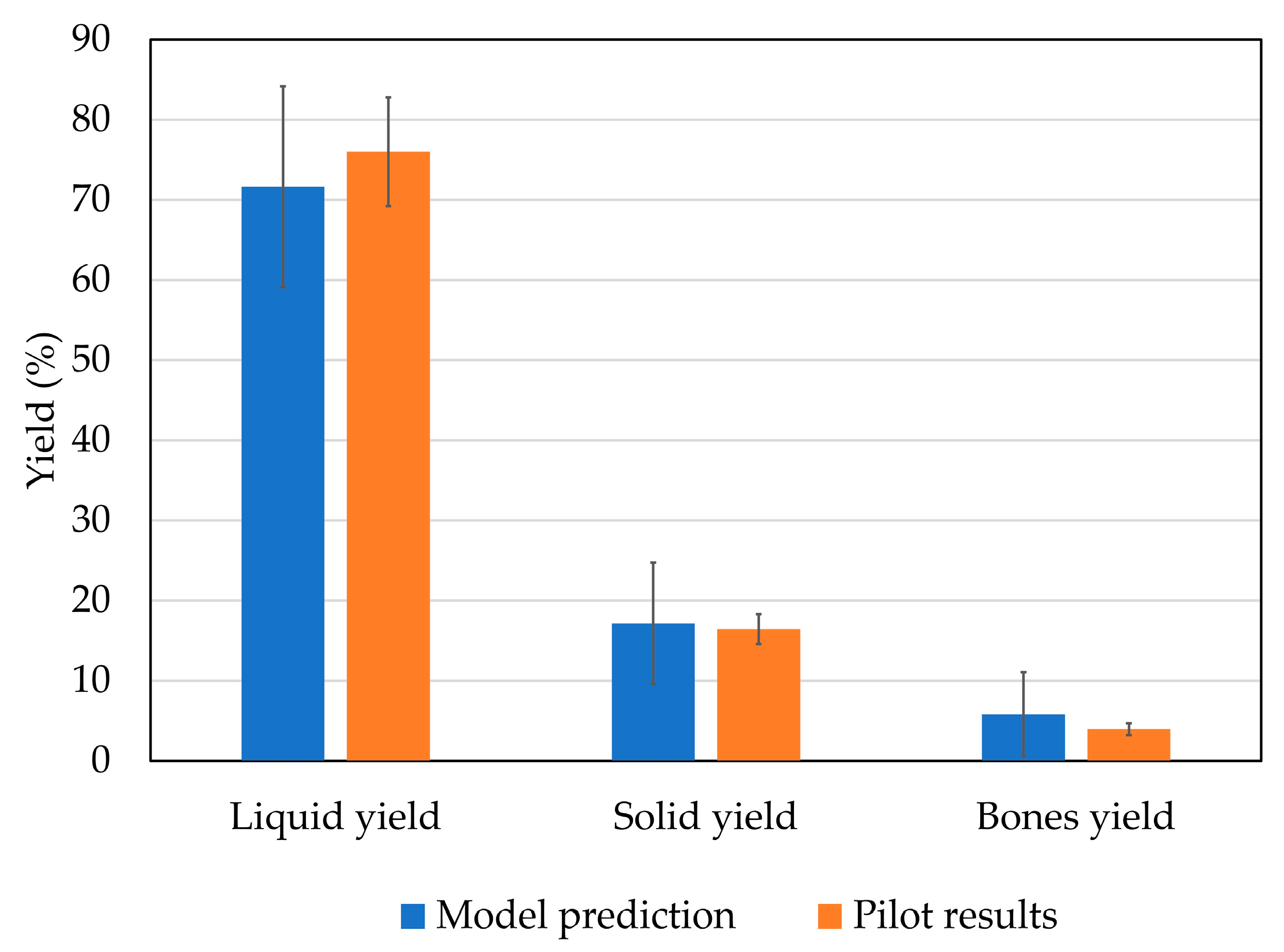
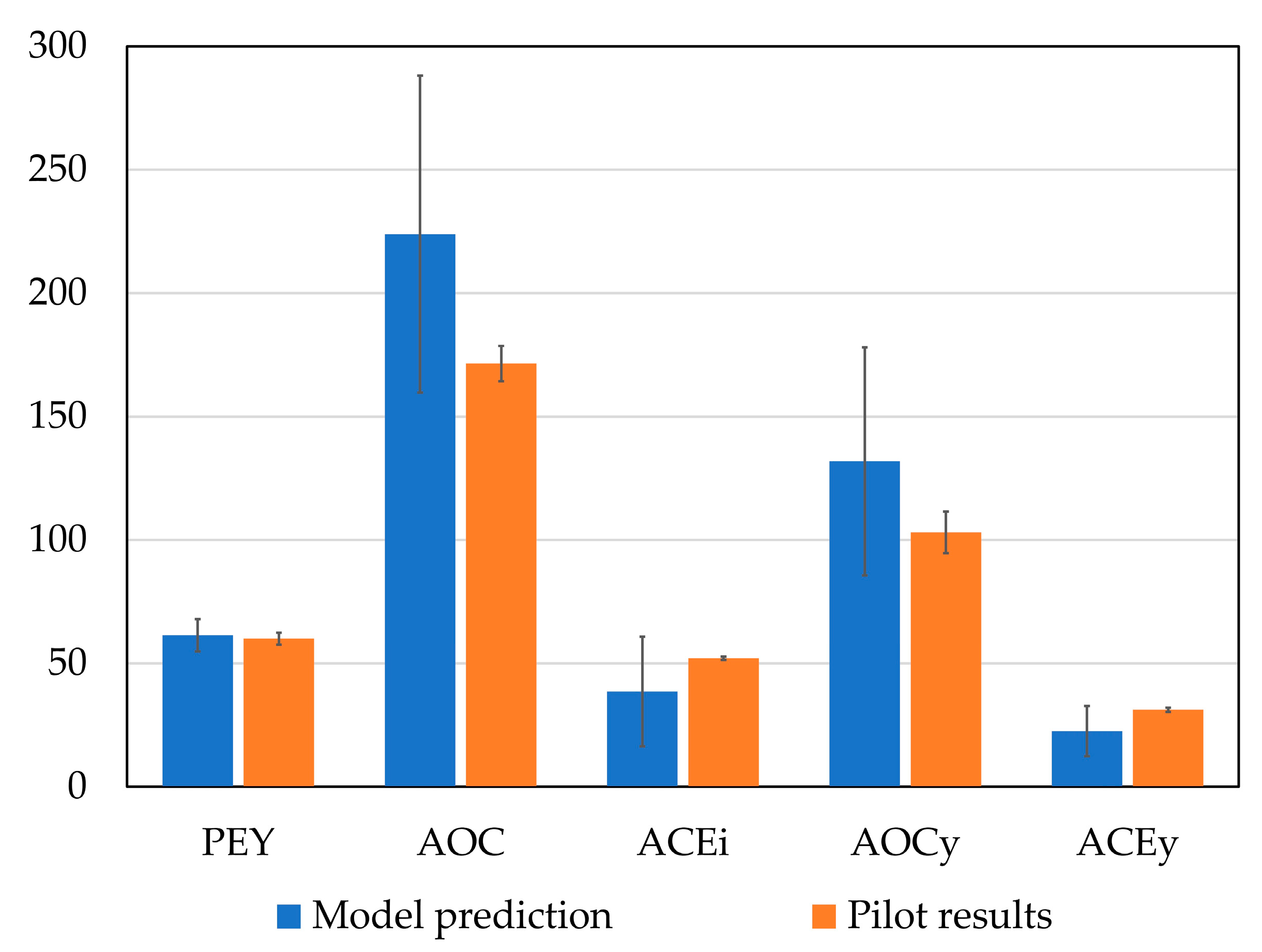
2.3. Hydrolysis Scaling-Up
3. Materials and Methods
3.1. Raw Material
3.2. Enzymatic Hydrolysis at Laboratory Scale
3.2.1. Initial Screening
3.2.2. Optimization via Box–Behnken Design (BBD)
3.3. Process Scale-Up
3.4. Analytical Determination
3.4.1. Chemical Analysis
3.4.2. Degree of Hydrolysis
3.4.3. Molecular Weight Distribution
3.4.4. Antioxidant Capacity Test
3.4.5. Antihypertensive Activity Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- WaSeaBi: Optimal Utilization of Seafood Side-Streams through the Design of New Holistic Process Lines Project. Available online: https://www.waseabi.eu/ (accessed on 8 May 2023).
- Iñarra, B.; Bald, C.; Cebrián, M.; Peral, I.; Llorente, R.; Zufía, J. Evaluation of unavoidable unwanted catches valorisation options: The Bay of Biscay case study. Mar. Policy 2020, 116, 103680. [Google Scholar] [CrossRef]
- Serrano, E.; Simpfendorfer, R.; Paillaman, J.; Sánchez, J.C. Comparative studies on nutritional composition of captive and wild southern hake Merluccius australis broodstocks. Rev. De Biol. Mar. Y Oceanogr. 2021, 55, 177–182. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of Valuable Compounds and Bioactive Metabolites from By-Products of Fish Discards Using Chemical Processing, Enzymatic Hydrolysis, and Bacterial Fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Noriega, D.; Ramos, P.; Valcarcel, J.; Novoa-Carballal, R.; Pastrana, L.; Reis, R.L.; Pérez-Martín, R.I. Optimization of high purity chitin and chitosan production from Illex argentinus pens by a combination of enzymatic and chemical processes. Carbohydr. Polym. 2017, 174, 262–272. [Google Scholar] [CrossRef]
- Sousa, S.C.; Vázquez, J.A.; Pérez-Martín, R.I.; Carvalho, A.P.; Gomes, A.M. Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins. Molecules 2017, 22, 1545. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef]
- Idowu, A.T.; Benjakul, S. Bitterness of fish protein hydrolysate and its debittering prospects. J. Food Biochem. 2019, 43, e12978. [Google Scholar] [CrossRef]
- Se-Kwon, K.; You-Jin, J.; Byun, H.-G.; Park, P.-J.; Gyu-Hyung, K.; Yong-Ri, C.; Yeon-Sook, L. Calcium Absorption Acceleration Effect on Phosphorylated and Non-phosphorylated Peptides from Hoki (Johnius Belengeri) Frame. Korean J. Fish. Aquat. Sci. 1999, 32, 713–717. [Google Scholar]
- Idowu, A.T.; Igiehon, O.O.; Idowu, S.; Olatunde, O.O.; Benjakul, S. Bioactivity Potentials and General Applications of Fish Protein Hydrolysates. Int. J. Pept. Res. Ther. 2021, 27, 109–118. [Google Scholar] [CrossRef]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported Foodborne Outbreaks Due to Fresh Produce in the United States and European Union: Trends and Causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Jackson, C.-J.C.; Connelly, P.W.; Parker, T.; Faulkner, D.; Vidgen, E.; Cunnane, S.C.; Leiter, L.A.; Josse, R.G. Effects of high- and low-isoflavone soyfoods on blood lipids, oxidized LDL, homocysteine, and blood pressure in hyperlipidemic men and women. Am. J. Clin. Nutr. 2002, 76, 365–372. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.A.; Emire, S.A.; Barea, P.; Illera, A.E.; Melgosa, R.; Beltrán, S.; Sanz, M.T. Valorisation of low-valued ray-finned fish (Labeobarbus nedgia) by enzymatic hydrolysis to obtain fish-discarded protein hydrolysates as functional foods. Food Bioprod. Process. 2023, 141, 167–184. [Google Scholar] [CrossRef]
- Alahmad, K.; Noman, A.; Xia, W.; Jiang, Q.; Xu, Y. Influence of the Enzymatic Hydrolysis Using Flavourzyme Enzyme on Functional, Secondary Structure, and Antioxidant Characteristics of Protein Hydrolysates Produced from Bighead Carp (Hypophthalmichthys nobilis). Molecules 2023, 28, 519. [Google Scholar] [CrossRef]
- Podpora, B.; Świderski, F.; Sadowska, A.; Rakowska, R.; Wasiak-Zys, G. Spent brewer’s yeast extracts as a new component of functional food. Czech J. Food Sci. 2016, 34, 554–563. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Machado, M.T.d.C.; da Silva Zandonadi, F.; Barros, H.D.d.F.Q.; Maróstica Júnior, M.R.; Sussulini, A.; Hubinger, M.D. Proteolytic enzymes positively modulated the physicochemical and antioxidant properties of spent yeast protein hydrolysates. Process Biochem. 2020, 91, 34–45. [Google Scholar] [CrossRef]
- Phanturat, P.; Benjakul, S.; Visessanguan, W.; Roytrakul, S. Use of pyloric caeca extract from bigeye snapper (Priacanthus macracanthus) for the production of gelatin hydrolysate with antioxidative activity. LWT-Food Sci. Technol. 2010, 43, 86–97. [Google Scholar] [CrossRef]
- Raghavan, S.; Kristinsson, H.G.; Leeuwenburgh, C. Radical Scavenging and Reducing Ability of Tilapia (Oreochromis niloticus) Protein Hydrolysates. J. Agric. Food Chem. 2008, 56, 10359–10367. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G. Effect of pretreatments on chemical compositions of mince from Nile tilapia (Oreochromis niloticus) and fishy odor development in protein hydrolysate. Int. Aquat. Res. 2012, 4, 7. [Google Scholar] [CrossRef][Green Version]
- Morales-Medina, R.; Pérez-Gálvez, R.; Guadix, A.; Guadix, E.M. Multiobjective optimization of the antioxidant activities of horse mackerel hydrolysates produced with protease mixtures. Process Biochem. 2017, 52, 149–158. [Google Scholar] [CrossRef]
- Chi, C.-F.; Cao, Z.-H.; Wang, B.; Hu, F.-Y.; Li, Z.-R.; Zhang, B. Antioxidant and Functional Properties of Collagen Hydrolysates from Spanish Mackerel Skin as Influenced by Average Molecular Weight. Molecules 2014, 19, 11211–11230. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Kishimura, H.; Shahidi, F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011, 124, 1354–1362. [Google Scholar] [CrossRef]
- Taheri, A.; Sabeena Farvin, K.H.; Jacobsen, C.; Baron, C.P. Antioxidant activities and functional properties of protein and peptide fractions isolated from salted herring brine. Food Chem. 2014, 142, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Blanco, M.; Massa, A.E.; Amado, I.R.; Pérez-Martín, R.I. Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology. Mar. Drugs 2017, 15, 306. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel, J. Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules 2020, 10, 310. [Google Scholar] [CrossRef]
- Hayes, M.; Mora, L.; Hussey, K.; Aluko, R.E. Boarfish protein recovery using the pH-shift process and generation of protein hydrolysates with ACE-I and antihypertensive bioactivities in spontaneously hypertensive rats. Innov. Food Sci. Emerg. Technol. 2016, 37, 253–260. [Google Scholar] [CrossRef]
- Geirsdottir, M.; Sigurgisladottir, S.; Hamaguchi, P.Y.; Thorkelsson, G.; Johannsson, R.; Kristinsson, H.G.; Kristjansson, M.M. Enzymatic Hydrolysis of Blue Whiting (Micromesistius poutassou); Functional and Bioactive Properties. J. Food Sci. 2011, 76, C14–C20. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, N.; Abidi, F.; Hardouin, J.; Abdelkafi, Z.; Marrakchi, N.; Jouenne, T.; Marzouki, M.N. Two novel peptides with angiotensin I converting enzyme inhibitory and antioxidative activities from Scorpaena notata muscle protein hydrolysate. Biotechnol. Appl. Biochem. 2017, 64, 201–210. [Google Scholar] [CrossRef]
- Bougatef, H.; de la Vega-Fernández, C.; Sila, A.; Bougatef, A.; Martínez-Alvarez, O. Identification of ACE I-Inhibitory Peptides Released by the Hydrolysis of Tub Gurnard (Chelidonichthys lucerna) Skin Proteins and the Impact of Their In Silico Gastrointestinal Digestion. Mar. Drugs 2023, 21, 131. [Google Scholar] [CrossRef]
- Korkmaz, K.; Tokur, B. Optimization of hydrolysis conditions for the production of protein hydrolysates from fish wastes using response surface methodology. Food Biosci. 2022, 45, 101312. [Google Scholar] [CrossRef]
- Ramakrishnan, V.V.; Ghaly, A.E.; Brooks, M.S.-L.; Budge, S.M. Extraction of Proteins from Mackerel Fish Processing Waste Using Alcalase Enzyme. J. Bioprocess. Biotech. 2013, 3, 1–9. [Google Scholar] [CrossRef]
- Rodrigues, D.P.; Calado, R.; Ameixa, O.M.C.C.; Valcarcel, J.; Vázquez, J.A. Valorisation of Atlantic codfish (Gadus morhua) frames from the cure-salting industry as fish protein hydrolysates with in vitro bioactive properties. LWT 2021, 149, 111840. [Google Scholar] [CrossRef]
- Zamorano-Apodaca, J.C.; Garcia-Sifuentes, C.O.; Carvajal-Millan, E.; Vallejo-Galland, B.; Scheuren-Acevedo, S.M.; Lugo-Sanchez, M.E. Biological and functional properties of peptide fractions obtained from collagen hydrolysate derived from mixed by-products of different fish species. Food Chem. 2020, 331, 127350. [Google Scholar] [CrossRef] [PubMed]
- Himaya, S.W.A.; Ngo, D.-H.; Ryu, B.; Kim, S.-K. An active peptide purified from gastrointestinal enzyme hydrolysate of Pacific cod skin gelatin attenuates angiotensin-1 converting enzyme (ACE) activity and cellular oxidative stress. Food Chem. 2012, 132, 1872–1882. [Google Scholar] [CrossRef]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Qian, Z.-J.; Ryu, B.; Park, J.W.; Kim, S.-K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Yathisha, U.G.; Ishani, B.; Iddya, K.; Mamatha, B.S. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar] [CrossRef]
- García-Moreno, P.J.; Espejo-Carpio, F.J.; Guadix, A.; Guadix, E.M. Production and identification of angiotensin I-converting enzyme (ACE) inhibitory peptides from Mediterranean fish discards. J. Funct. Foods 2015, 18, 95–105. [Google Scholar] [CrossRef]
- Ahmad, A.; Rehman, M.U.; Wali, A.F.; El-Serehy, H.A.; Al-Misned, F.A.; Maodaa, S.N.; Aljawdah, H.M.; Mir, T.M.; Ahmad, P. Box–Behnken Response Surface Design of Polysaccharide Extraction from Rhododendron arboreum and the Evaluation of Its Antioxidant Potential. Molecules 2020, 25, 3835. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Association of Official Analytical Chemists. Official Methods of Analysis; AOAC International: Rockville, MD, USA, 1996. [Google Scholar]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Tejano, L.A.; Peralta, J.P.; Yap, E.E.S.; Chang, Y.-W. Bioactivities of enzymatic protein hydrolysates derived from Chlorella sorokiniana. Food Sci. Nutr. 2019, 7, 2381–2390. [Google Scholar] [CrossRef]
- Sillero Ortigosa, L.; Prado, R.; Labidi, J. Optimization of Different Extraction Methods to Obtaining Bioactive Compounds from Larix Decidua Bark. Chem. Eng. Trans. 2018, 70, 1369–1374. [Google Scholar] [CrossRef]
- Sentandreu, M.A.; Toldrá, F. A fluorescence-based protocol for quantifying angiotensin-converting enzyme activity. Nat. Protoc. 2006, 1, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Coscueta, E.R.; Campos, D.A.; Osório, H.; Nerli, B.B.; Pintado, M. Enzymatic soy protein hydrolysis: A tool for biofunctional food ingredient production. Food Chem. X 2019, 1, 100006. [Google Scholar] [CrossRef]
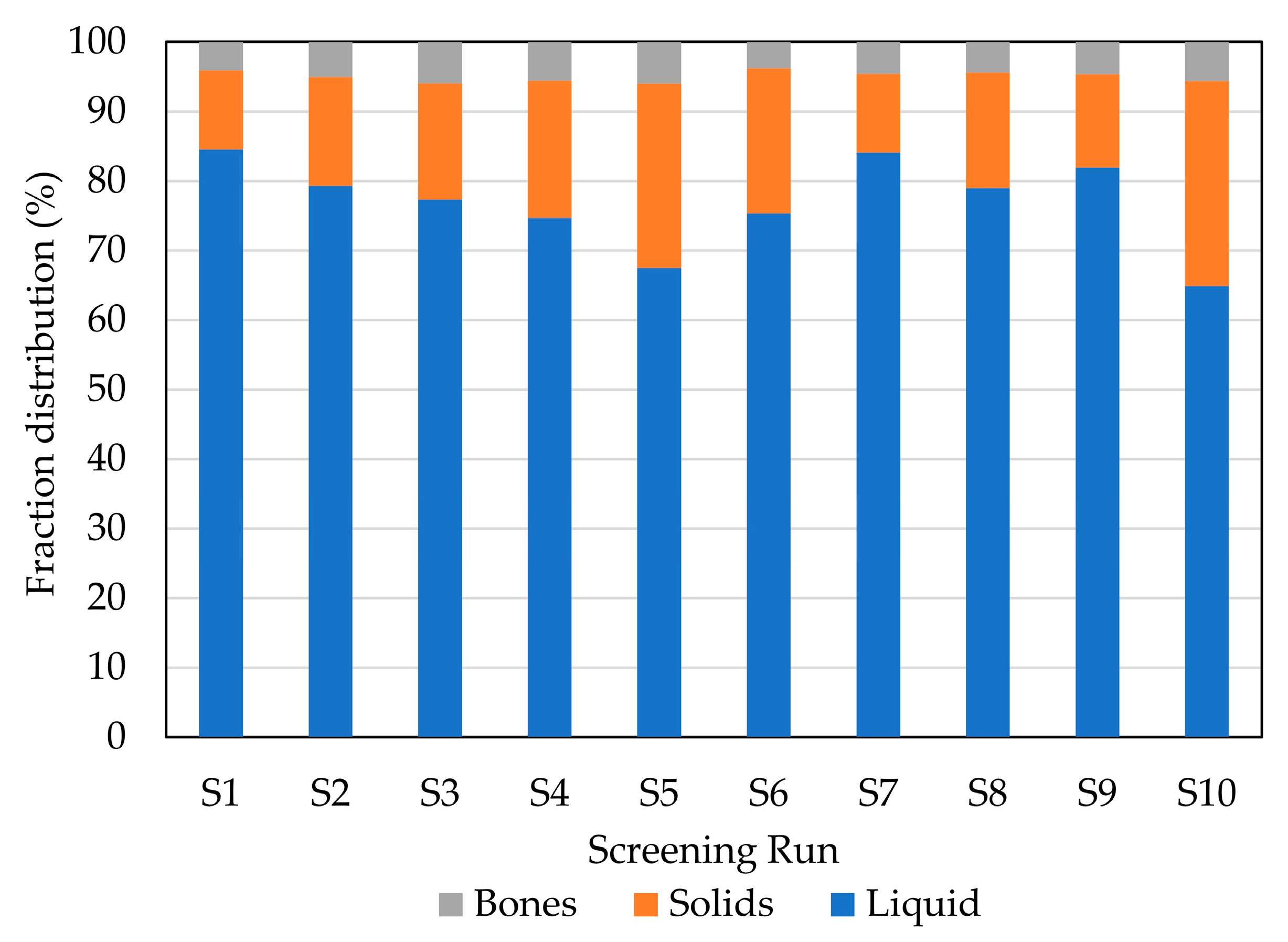
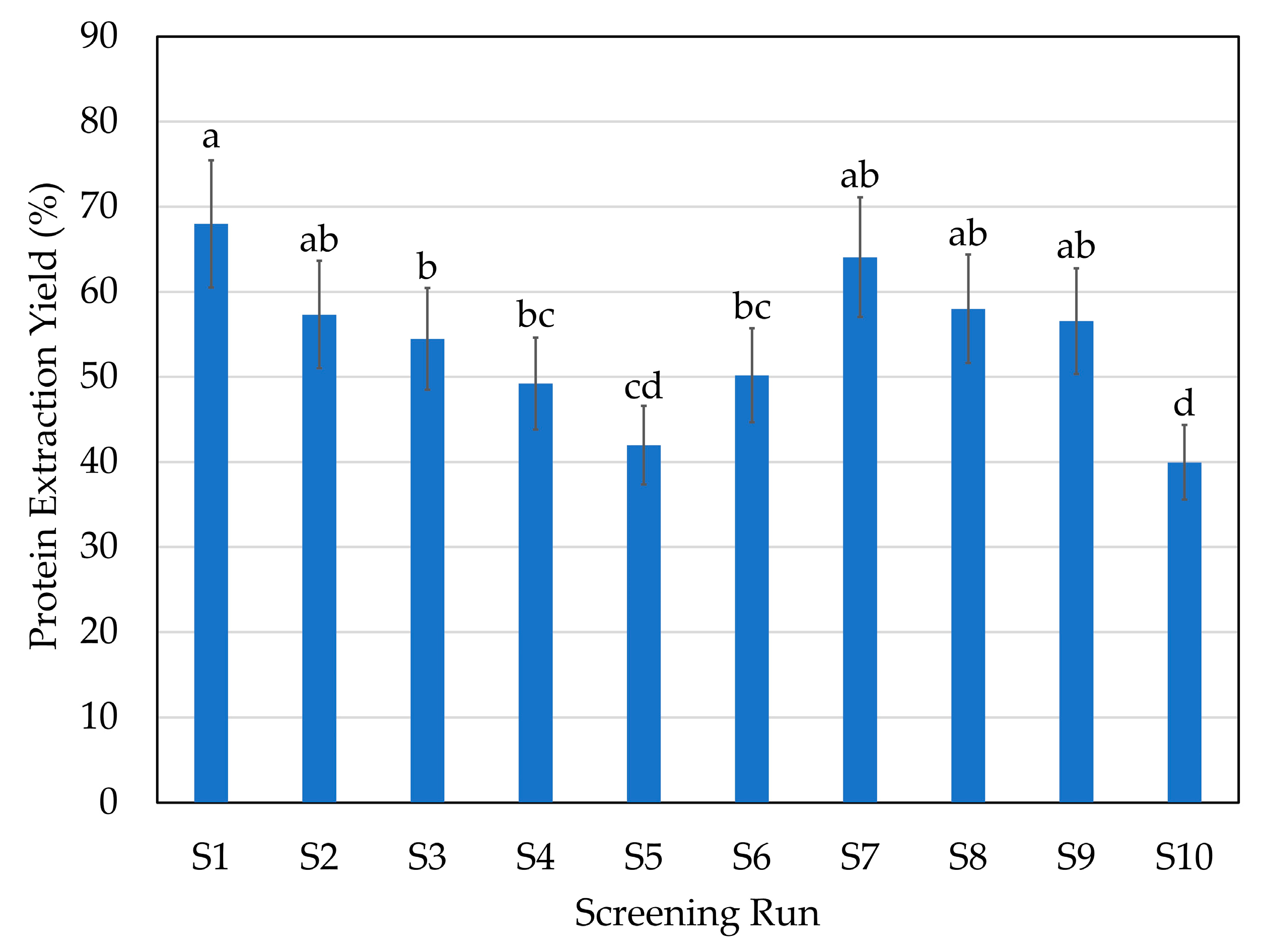
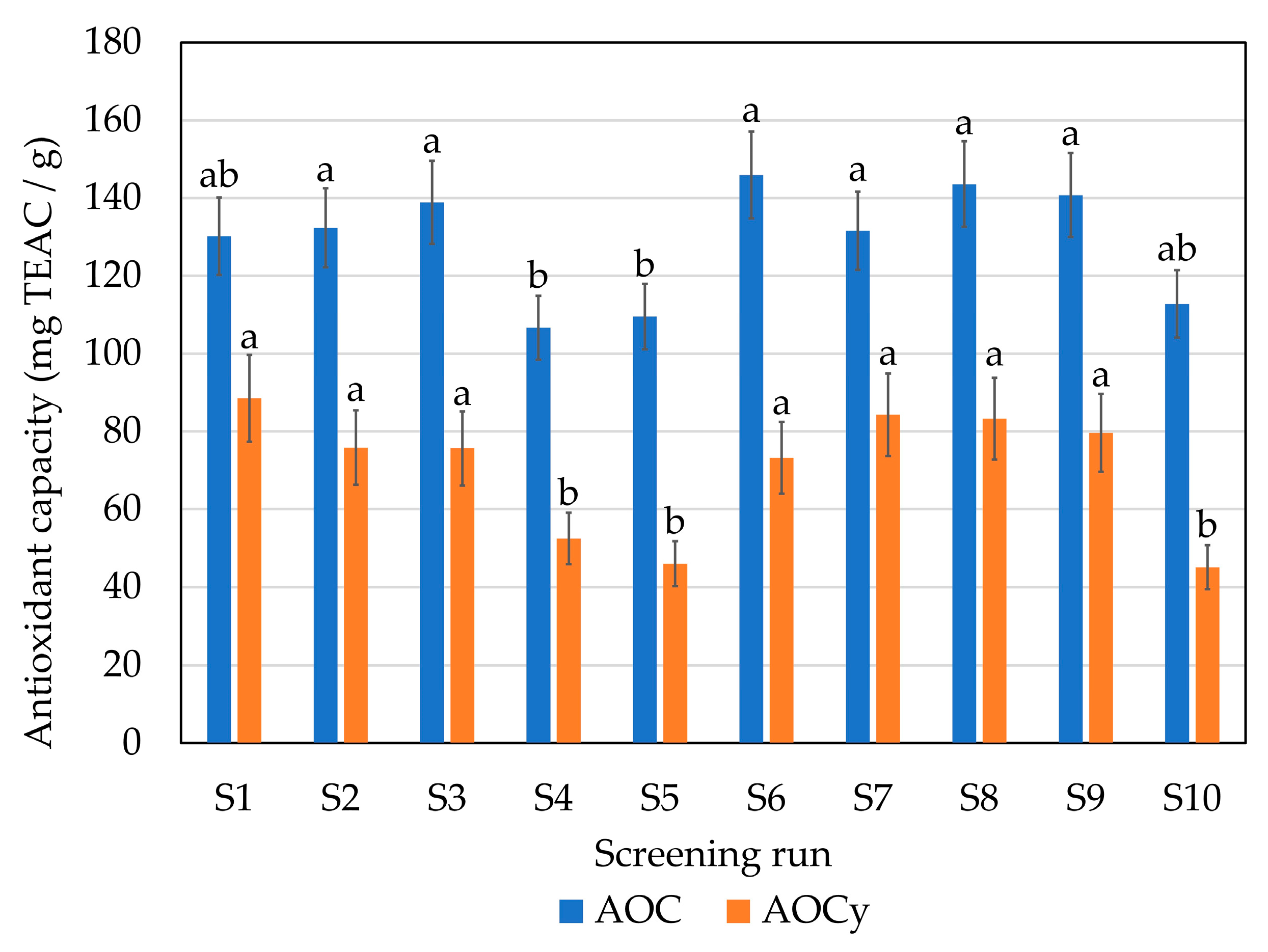
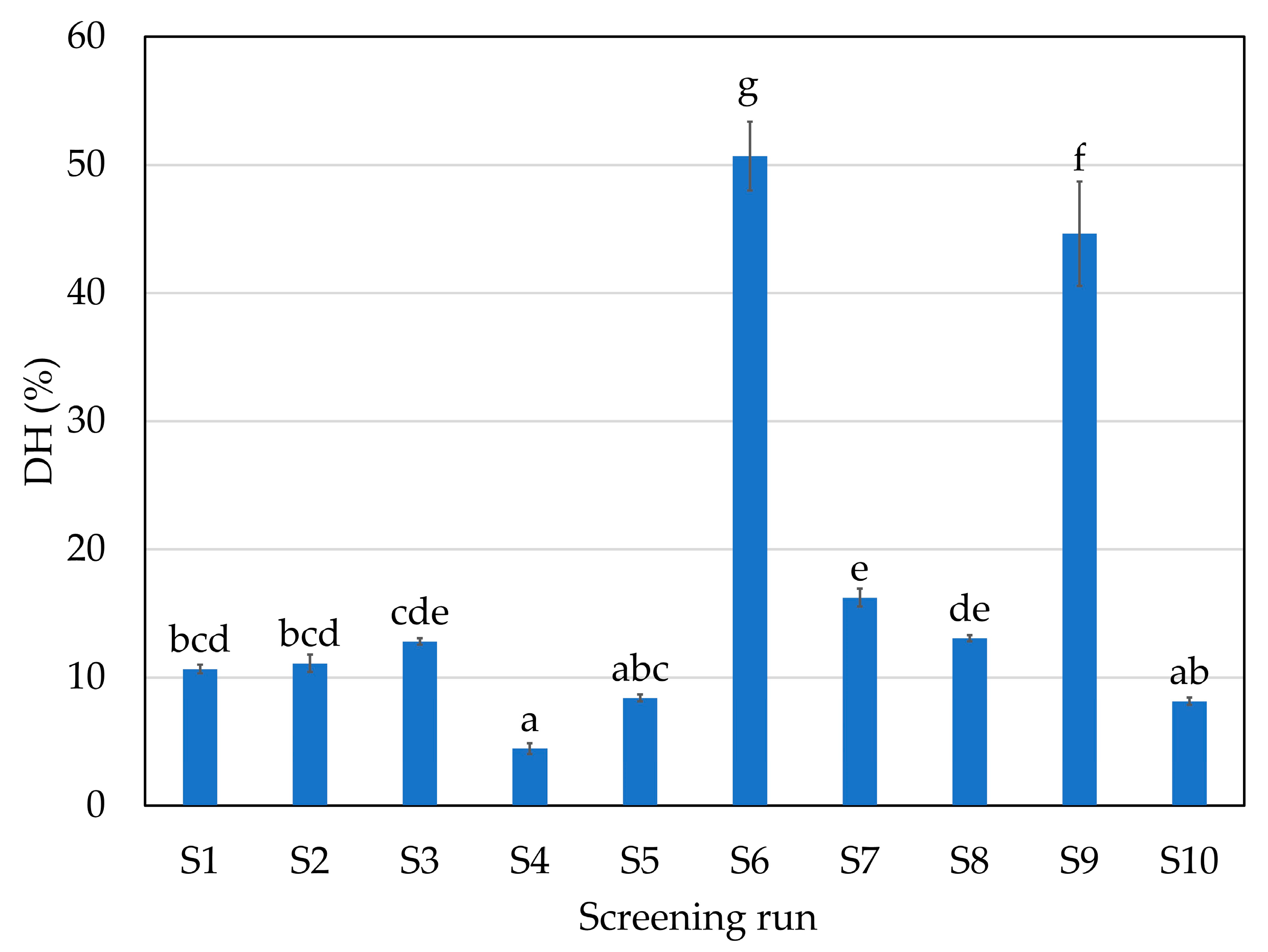

| Screening Run | Enzyme | Hydrolysis Conditions |
|---|---|---|
| S1 | A: endoprotease of the serine type | 1% 70 °C pH 9 |
| S2 | A: endoprotease of the serine type | 1% 50 °C pH 6 |
| S3 | P: broad-spectrum endoprotease | 1% 50 °C pH 6 |
| S4 | T: trypsin-specific protease | 1% 45 °C pH 6 |
| S5 | C: chymotrypsin-like protease | 1% 70 °C pH 6 |
| S6 | G: glutamic acid-specific protease | 1% 50 °C pH 6 |
| S7 | A + F: endoprotease of the serine type + blend of endo- and exopeptidases | 1% of each enzyme 50 °C pH 6 |
| S8 | P + F: broad-spectrum endoprotease + blend of endo- and exopeptidases | 1% of each enzyme 50 °C pH 6 |
| S9 | P + G: broad-spectrum endoprotease + glutamic acid-specific protease | 1% of each enzyme 50 °C pH 6 |
| S10 | CTR: without enzyme | 50 °C pH 6 |
| BBD Run | Enzyme–Substrate Ratio (%) | Time (h) | Solid (%) | PEY (%) | DH (%) | AOC (mg TEAC/g) | AOCy (mg TEAC/g) | ACEi (% at 1 mg/mL) | ACEy (% at 1 mg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| R1 | 2.00 | 4 | 50.0 | 61.64 | 7.94 | 160.79 | 99.11 | 21.50 | 13.25 |
| R2 | 1.25 | 6 | 65.0 | 43.80 | 11.33 | 169.69 | 74.32 | 7.44 | 3.54 |
| R3 | 0.50 | 4 | 50.0 | 43.30 | 8.11 | 174.96 | 75.75 | 6.74 | 2.92 |
| R4 | 2.00 | 2 | 57.5 | 53.56 | 5.22 | 255.00 | 136.59 | 34.73 | 18.6 |
| R5 | 0.50 | 4 | 65.0 | 35.89 | 9.90 | 125.02 | 44.87 | 24.50 | 8.79 |
| R6 | 1.25 | 4 | 57.5 | 50.72 | 9.56 | 130.09 | 65.97 | 32.73 | 16.6 |
| R7 | 1.25 | 2 | 65.0 | 44.97 | 4.32 | 125.97 | 56.66 | 23.62 | 10.62 |
| R8 | 0.50 | 6 | 57.5 | 29.36 | 11.31 | 150.39 | 44.16 | 11.32 | 3.32 |
| R9 | 1.25 | 6 | 50.0 | 49.09 | 12.64 | 150.15 | 73.71 | 16.53 | 8.12 |
| R10 | 2.00 | 6 | 57.5 | 46.36 | 8.84 | 145.65 | 67.52 | 17.98 | 8.34 |
| R11 | 1.25 | 2 | 50.0 | 54.71 | 4.57 | 150.88 | 82.54 | 33.59 | 18.38 |
| R12 | 1.25 | 4 | 57.5 | 51.50 | 9.00 | 158.41 | 81.58 | 10.89 | 5.61 |
| R13 | 1.25 | 4 | 57.5 | 42.16 | 6.74 | 142.71 | 60.16 | 19.01 | 7.59 |
| R14 | 0.50 | 2 | 57.5 | 35.78 | 4.86 | 126.21 | 45.16 | 17.02 | 6.09 |
| R15 | 2.00 | 4 | 65.0 | 54.27 | 11.50 | 191.14 | 103.72 | 17.76 | 9.64 |
| Coefficients | p-Value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEY | DH | AOC | ACEi | AOCy | ACEy | PEY | DH | AOC | ACEi | AOCy | ACEy | |
| Intercept | 281.496 | 40.849 | 535.329 | −121.479 | 555.637 | −6.323 | ||||||
| A: % Solids | −8.487 | −1.586 | −7.840 | 4.0604 | −14.896 | 0.147 | 0.0088 | 0.4759 | 0.7126 | 0.8408 | 0.2842 | 0.3840 |
| B: Time | 1.954 | 5.201 | −29.255 | −3.568 | −10.098 | −3.385 | 0.0434 | 0.0037 | 0.5381 | 0.0596 | 0.2138 | 0.0266 |
| C: % Enzyme | 25.759 | −3.354 | −174.499 | 74.267 | −41.763 | 39.325 | 0.0001 | 0.8955 | 0.0345 | 0.2263 | 0.0059 | 0.0332 |
| AA | 0.067 | 0.014 | −0.026 | 0.090 | 0.0431 | 0.4210 | 0.7552 | 0.5522 | ||||
| AB | 0.074 | −0.018 | 0.741 | 0.442 | 0.053 | 0.4727 | 0.7729 | 0.3682 | 0.4228 | 0.6911 | ||
| AC | 0.079 | 3.568 | −0.956 | 1.577 | −0.421 | 0.6326 | 0.1294 | 0.2527 | 0.2964 | 0.2567 | ||
| BB | −0.937 | −0.253 | 1.480 | −0.617 | 0.0437 | 0.3146 | 0.6353 | 0.7961 | 0.7683 | |||
| BC | −0.472 | −22.255 | −1.842 | −11.345 | −1.248 | 0.4530 | 0.0265 | 0.5396 | 0.0753 | 0.3617 | ||
| CC | −5.537 | 0.241 | 35.067 | −2.623 | 11.712 | −2.128 | 0.0806 | 0.8866 | 0.1472 | 0.7500 | 0.4432 | 0.5658 |
| R2 | 94.10 | 85.70 | 79.65 | 61.44 | 86.76 | 72.76 | ||||||
| Adj R2 | 88.16 | 59.96 | 52.52 | 10.02 | 62.93 | 45.51 | ||||||
| Dependent Variables | Solids (%) | Time (h) | Enzyme–Substrate Ratio (%) |
|---|---|---|---|
| PEY | 50 | 3.07 | 2.0 |
| DH | 65.0 | 6.0 | 0.5 |
| AOC | 65.0 | 2.0 | 2.0 |
| ACEi | 50.0 | 2.0 | 2.0 |
| AOCy | 50.0 | 2.0 | 2.0 |
| ACEy | 50.0 | 2.0 | 2.0 |
| AOCy + ACEy | 50.0 | 2.0 | 2.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iñarra, B.; Bald, C.; Gutierrez, M.; San Martin, D.; Zufía, J.; Ibarruri, J. Production of Bioactive Peptides from Hake By-Catches: Optimization and Scale-Up of Enzymatic Hydrolysis Process. Mar. Drugs 2023, 21, 552. https://doi.org/10.3390/md21110552
Iñarra B, Bald C, Gutierrez M, San Martin D, Zufía J, Ibarruri J. Production of Bioactive Peptides from Hake By-Catches: Optimization and Scale-Up of Enzymatic Hydrolysis Process. Marine Drugs. 2023; 21(11):552. https://doi.org/10.3390/md21110552
Chicago/Turabian StyleIñarra, Bruno, Carlos Bald, Monica Gutierrez, David San Martin, Jaime Zufía, and Jone Ibarruri. 2023. "Production of Bioactive Peptides from Hake By-Catches: Optimization and Scale-Up of Enzymatic Hydrolysis Process" Marine Drugs 21, no. 11: 552. https://doi.org/10.3390/md21110552
APA StyleIñarra, B., Bald, C., Gutierrez, M., San Martin, D., Zufía, J., & Ibarruri, J. (2023). Production of Bioactive Peptides from Hake By-Catches: Optimization and Scale-Up of Enzymatic Hydrolysis Process. Marine Drugs, 21(11), 552. https://doi.org/10.3390/md21110552









