Soy Protein Isolate–Chitosan Nanoparticle-Stabilized Pickering Emulsions: Stability and In Vitro Digestion for DHA
Abstract
:1. Introduction
2. Results and Discussion
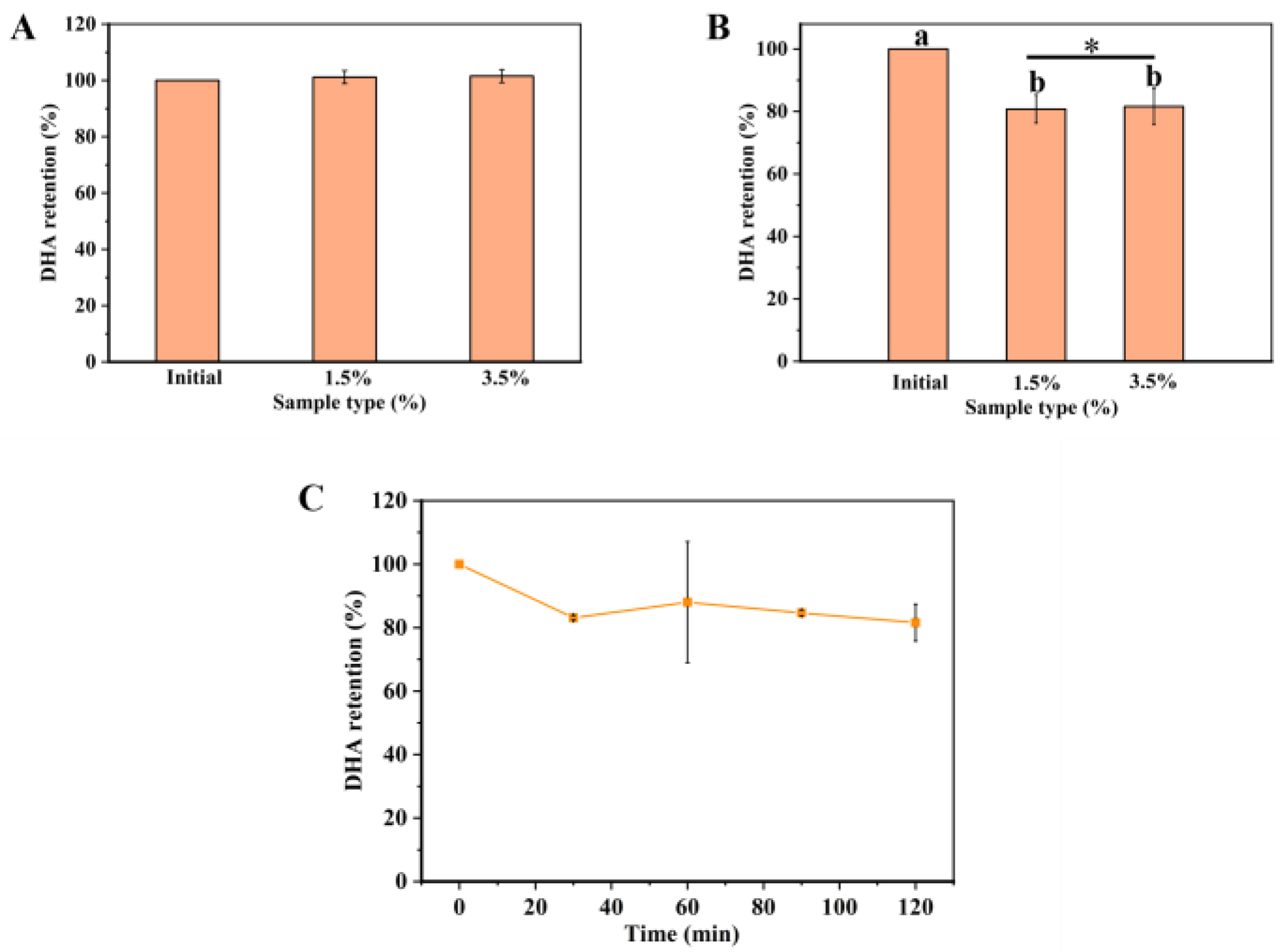
2.1. Stability of DHA-Encapsulated Pickering Emulsions
2.2. In Vitro Oral Digestion of DHA-Encapsulated Pickering Emulsions
2.3. In Vitro Gastric Digestion of DHA-Encapsulated Pickering Emulsions
2.4. In Vitro Small Intestine Digestion of DHA-Encapsulated Pickering Emulsions
2.5. Cell Viability of SPI-CS Pickering Emulsions
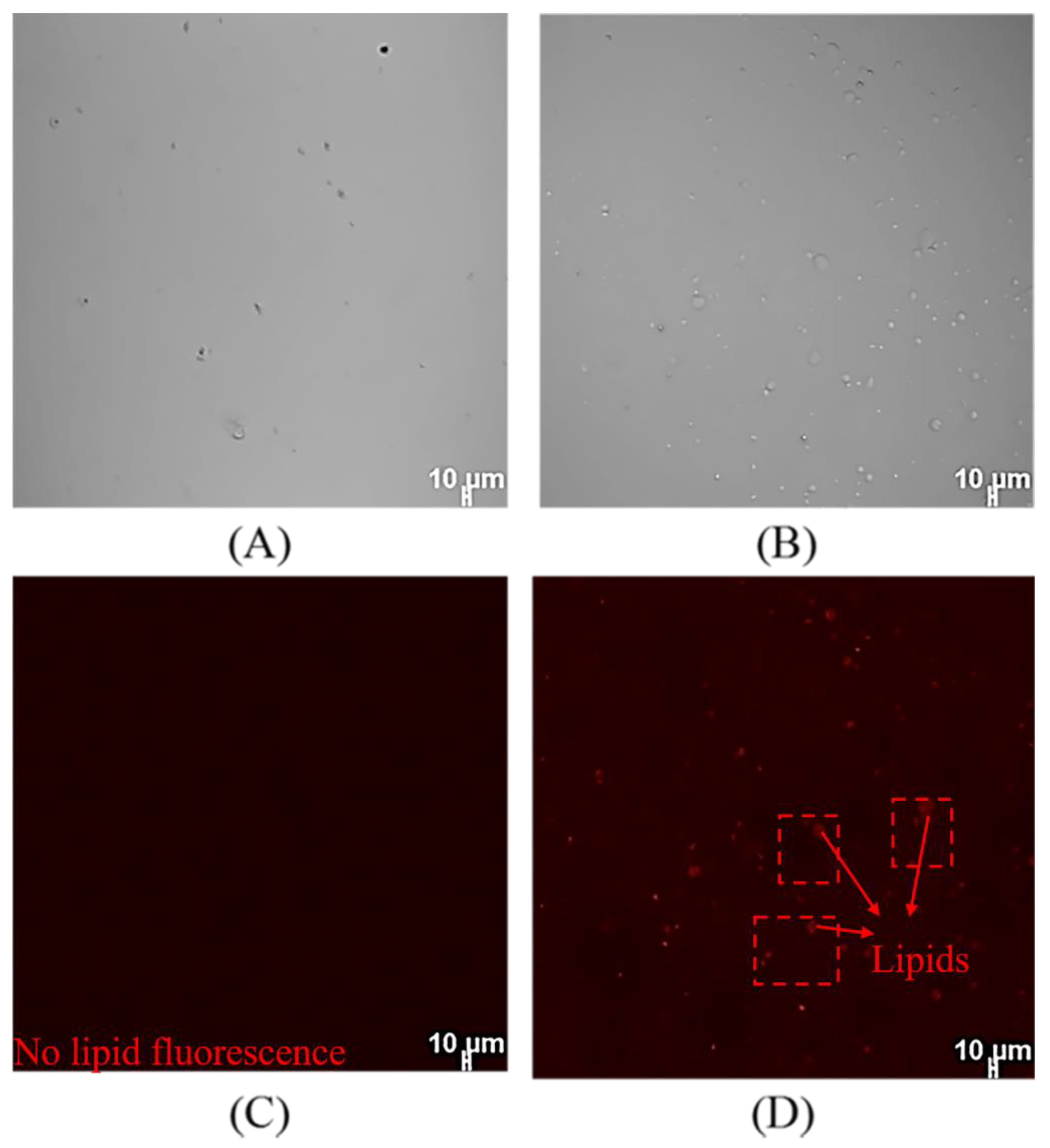
2.6. Cellular Uptake of SPI-CS Pickering Emulsions
3. Materials and Methods
3.1. Materials
3.2. Preparation of DHA-Encapsulated Pickering Emulsions
3.3. Cell Culture
3.4. Microscopy of DHA-Encapsulated Pickering Emulsions
3.5. Determination of DHA Retention Rate of Pickering Emulsions
3.5.1. Gas Chromatography (GC) Quantification of DHA
3.5.2. Extraction and Determination of DHA in Pickering Emulsions
3.6. Stability of DHA-Encapsulated SPI-CS Pickering Emulsions
3.6.1. Storage Stability of DHA-Encapsulated Pickering Emulsions
3.6.2. Thermal Stability of DHA-Encapsulated SPI-CS Pickering Emulsions
3.6.3. Ionic Strength Stability of DHA-Encapsulated SPI-CS Pickering Emulsions
3.7. In Vitro Digestion Model
3.8. Cytotoxicity of Pickering Emulsions
3.9. Cell uptake Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Narayan, B.; Miyashita, K.; Hosakawa, M. Physiological effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)—A review. Food Rev. Int. 2006, 22, 291–307. [Google Scholar] [CrossRef]
- Chang, Y.; Mcclements, D.J. Influence of emulsifier type on the in vitro digestion of fish oil-in-water emulsions in the presence of an anionic marine polysaccharide (fucoidan): Caseinate, whey protein, lecithin, or Tween 80. Food Hydrocoll. 2016, 61, 92–101. [Google Scholar] [CrossRef]
- Lee, M.C.; Tan, C.; Abbaspourrad, A. Combination of internal structuring and external coating in an oleogel-based delivery system for fish oil stabilization. Food Chem. 2019, 277, 213–221. [Google Scholar] [CrossRef]
- Hosseini, E.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A.; Jahanbin, K. Preparation of Pickering flaxseed oil-in-water emulsion stabilized by chitosan-myristic acid nanogels and investigation of its oxidative stability in presence of clove essential oil as antioxidant. Food Biophys. 2020, 15, 216–228. [Google Scholar] [CrossRef]
- Ziani, K.; Fang, Y.; Mcclements, D.J. Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: Vitamin E, vitamin D, and lemon oil. Food Chem. 2012, 134, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J. Enhancing nutraceutical bioavailability through food matrix design. Curr. Opin. Food Sci. 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Winuprasith, T.; Khomein, P.; Mitbumrung, W.; Suphantharika, M.; Nitithamyong, A.; Mcclements, D.J. Encapsulation of vitamin D3 in Pickering emulsions stabilized by nanofibrillated mangosteen cellulose: Impact on in vitro digestion and bioaccessibility. Food Hydrocoll. 2018, 83, 153–164. [Google Scholar] [CrossRef]
- Pal, A.; Brasseur, J.G.; Abrahamsson, B. A stomach road or “magenstrasse” for gastric emptying. J. Biomech. 2007, 40, 1202–1210. [Google Scholar] [CrossRef]
- Shah, B.R.; Zhang, C.; Li, Y.; Li, B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016, 89, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, D.; Liu, F.; Gao, Y. Emulsion design for the delivery of beta-carotene in complex food systems. Crit. Rev. Food Sci. Nutr. 2018, 58, 770–784. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–192. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.; Chevalier, Y. Effects of solid particle content on properties of o/w Pickering emulsions. J. Colloid Interface Sci. 2010, 351, 348–356. [Google Scholar] [CrossRef]
- Wijaya, W.; Van der Meeren, P.; Wijaya, C.H.; Patel, A.R. High internal phase emulsions stabilized solely by whey protein isolate-low methoxyl pectin complexes: Effect of ph and polymer concentration. Food Funct. 2017, 8, 584–594. [Google Scholar] [CrossRef]
- Liu, W.; Gao, H.; Mcclements, D.J.; Zhou, L.; Wu, J.; Zou, L. Stability, rheology, and β-carotene bioaccessibility of high internal phase emulsion gels. Food Hydrocoll. 2019, 88, 210–217. [Google Scholar] [CrossRef]
- Yi, J.; Huang, H.; Liu, Y.; Lu, Y.; Fan, Y.; Zhang, Y. Fabrication of curcumin-loaded pea protein-pectin ternary complex for the stabilization and delivery of beta-carotene emulsions. Food Chem. 2020, 313, 126118. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Wang, D.; Chen, Y.; Zhu, S.; Zhang, J.; Mao, L.; Gao, Y.; Yuan, F. Pickering emulsion gels stabilized by novel complex particles of high-pressure-induced wpi gel and chitosan: Fabrication, characterization and encapsulation. Food Hydrocoll. 2020, 108, 105992. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, D.; Li, F.; Li, D.; Huang, Q. Cinnamon essential oil Pickering emulsion stabilized by zein-pectin composite nanoparticles: Characterization, antimicrobial effect and advantages in storage application. Int. J. Biol. Macromol. 2020, 148, 1280–1289. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Y.; Zhu, J.; Wang, T.; Wang, D.; Xu, Y. Zein/soluble soybean polysaccharide composite nanoparticles for encapsulation and oral delivery of lutein. Food Hydrocoll. 2020, 103, 105715. [Google Scholar] [CrossRef]
- Yang, H.; Su, Z.; Meng, X.; Zhang, X.; Kennedy, J.F.; Liu, B. Fabrication and characterization of Pickering emulsion stabilized by soy protein isolate-chitosan nanoparticles. Carbohydr. Polym. 2020, 247, 116712. [Google Scholar] [CrossRef] [PubMed]
- Gayoso, L.; Ansorena, D.; Astiasaran, I. DHA rich algae oil delivered by O/W or gelled emulsions: Strategies to increase its bioaccessibility. J. Sci. Food Agric. 2019, 99, 2251–2258. [Google Scholar] [CrossRef] [PubMed]
- Sjoo, M.; Emek, S.C.; Hall, T.; Rayner, M.; Wahlgren, M. Barrier properties of heat treated starch Pickering emulsions. J. Colloid Interface Sci. 2015, 450, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xue, S.J.; Wang, B.; Wang, W.; Ye, X.; Quek, S.Y. Optimization of formulation and influence of environmental stresses on stability of lycopene-microemulsion. LWT-Food Sci. Technol. 2015, 60, 999–1008. [Google Scholar] [CrossRef]
- Hosseini, R.S.; Rajaei, A. Potential Pickering emulsion stabilized with chitosan-stearic acid nanogels incorporating clove essential oil to produce fish-oil-enriched mayonnaise. Carbohydr. Polym. 2020, 241, 116340. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.T.; Singh, R.P.; Singh, H. Behavior of an oil-in-water emulsion stabilized by β-lactoglobulin in an in vitro gastric model. Food Hydrocoll. 2009, 23, 1563–1569. [Google Scholar] [CrossRef]
- Vingerhoeds, M.H.; Blijdenstein, T.; Zoet, F.D.; van Aken, G.A. Emulsion flocculation induced by saliva and mucin. Food Hydrocoll. 2005, 19, 915–922. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Preparation of chitosan/gum arabic nanoparticles and their use as novel stabilizers in oil/water Pickering emulsions. Carbohydr. Polym. 2019, 224, 115190. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Tang, C. Gel-like pea protein Pickering emulsions at ph3.0 as a potential intestine-targeted and sustained-release delivery system for β-carotene. Food Res. Int. 2016, 79, 64–72. [Google Scholar] [CrossRef]
- Leal-Calderon, F.; Cansell, M. The design of emulsions and their fate in the body following enteral and parenteral routes. Soft Matter 2012, 8, 10213–10225. [Google Scholar] [CrossRef]
- Qiu, C.; Zhao, M.; Decker, E.A.; Mcclements, D.J. Influence of anionic dietary fibers (xanthan gum and pectin) on oxidative stability and lipid digestibility of wheat protein-stabilized fish oil-in-water emulsion. Food Res. Int. 2015, 74, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Verkempinck, S.H.E.; Sun, L.; Van Loey, A.M.; Grauwet, T.; Hendrickx, M.E. Lipid digestion, micelle formation and carotenoid bioaccessibility kinetics: Influence of emulsion droplet size. Food Chem. 2017, 229, 653–662. [Google Scholar] [CrossRef]
- Wang, J.; Ossemond, J.; Jardin, J.; Briard-Bion, V.; Henry, G.; Le Gouar, Y.; Menard, O.; Le, S.; Madadlou, A.; Dupont, D.; et al. Encapsulation of dha oil with heat-denatured whey protein in Pickering emulsion improves its bioaccessibility. Food Res. Int. 2022, 162, 112112. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, D.; Mackie, A.; Yang, S.; Dai, L.; Zhang, L.; Mao, L.; Gao, Y. Stability, interfacial structure, and gastrointestinal digestion of beta-carotene-loaded Pickering emulsions co-stabilized by particles, a biopolymer, and a surfactant. J. Agric. Food Chem. 2021, 69, 1619–1636. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Wu, Z.; Wang, Y.; Cheng, J.; Wang, T.; Zhang, B. Chitosan hydrochloride/carboxymethyl starch complex nanogels stabilized Pickering emulsions for oral delivery of beta-carotene: Protection effect and in vitro digestion study. Food Chem. 2020, 315, 126288. [Google Scholar] [CrossRef] [PubMed]
- Mcclements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef]
- Lu, X.; Zhu, J.; Pan, Y.; Huang, Q. Assessment of dynamic bioaccessibility of curcumin encapsulated in milled starch particle stabilized Pickering emulsions using TNO’s gastrointestinal model. Food Funct. 2019, 10, 2583–2594. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Xie, X.; Li, C.; Zhen, B.; Cai, X.; Zhang, G.; Zhou, C.; Wang, L. Medium-chain triglyceride/water Pickering emulsion stabilized by phosphatidylcholine-kaolinite for encapsulation and controlled release of curcumin. Colloid Surf. B-Biointerfaces 2019, 183, 110414. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Grieger, J.A.; Etherton, T.D. Dietary reference intakes for dha and epa. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Glahn, R.P.; Liu, R.H. Assessment of carotenoid bioavailability of whole foods using a caco-2 cell culture model coupled with an in vitro digestion. J. Agric. Food Chem. 2004, 52, 4330–4337. [Google Scholar] [CrossRef]
- Puyol, P.; Perez, M.D.; Sanchez, L.; Ena, J.M.; Calvo, M. Uptake and passage of beta-lactoglobulin, palmitic acid and retinol across the caco-2 monolayer. Biochim. Biophys. Acta 1995, 1236, 149–154. [Google Scholar] [CrossRef]
- Low, L.E.; Tan, L.T.; Goh, B.; Tey, B.T.; Ong, B.H.; Tang, S.Y. Magnetic cellulose nanocrystal stabilized Pickering emulsions for enhanced bioactive release and human colon cancer therapy. Int. J. Biol. Macromol. 2019, 127, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, C.; Huang, Q. Combining in vitro digestion model with cell culture model: Assessment of encapsulation and delivery of curcumin in milled starch particle stabilized Pickering emulsions. Int. J. Biol. Macromol. 2019, 139, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Yang, X.; Gao, S.; Yao, J.; Mcclements, D.J. Influence of dietary fibers on lipid digestion: Comparison of single-stage and multiple-stage gastrointestinal models. Food Hydrocoll. 2017, 69, 382–392. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Ji, Y.; Yang, H.; Meng, X.; Liu, B. Soy Protein Isolate–Chitosan Nanoparticle-Stabilized Pickering Emulsions: Stability and In Vitro Digestion for DHA. Mar. Drugs 2023, 21, 546. https://doi.org/10.3390/md21100546
Zhao P, Ji Y, Yang H, Meng X, Liu B. Soy Protein Isolate–Chitosan Nanoparticle-Stabilized Pickering Emulsions: Stability and In Vitro Digestion for DHA. Marine Drugs. 2023; 21(10):546. https://doi.org/10.3390/md21100546
Chicago/Turabian StyleZhao, Pengcheng, Yuan Ji, Han Yang, Xianghong Meng, and Bingjie Liu. 2023. "Soy Protein Isolate–Chitosan Nanoparticle-Stabilized Pickering Emulsions: Stability and In Vitro Digestion for DHA" Marine Drugs 21, no. 10: 546. https://doi.org/10.3390/md21100546
APA StyleZhao, P., Ji, Y., Yang, H., Meng, X., & Liu, B. (2023). Soy Protein Isolate–Chitosan Nanoparticle-Stabilized Pickering Emulsions: Stability and In Vitro Digestion for DHA. Marine Drugs, 21(10), 546. https://doi.org/10.3390/md21100546









