Abstract
The silver-cheeked toadfish (Lagocephalus sceleratus), an invasive alien pufferfish species that has rapidly settled throughout the Mediterranean region, poses significant threats not only to native marine species and fisheries but also to public health due to the tetrodotoxin (TTX) they harbor. In this study, TTX concentrations in L. sceleratus from Antalya Bay in the Northeastern Mediterranean Sea were investigated using Q-TOF-LC-MS on a monthly basis over a one-year period. Pufferfish were caught by angling from May 2018 to April 2019. The TTX levels in three different tissues (gonads, liver, and muscle) of 110 pufferfish in total were determined in both male and female individuals caught for 11 months. The highest TTX mean levels generally occurred in the gonads and the lowest in the muscle samples. As regards the maximum TTX contents, the highest concentrations determined were 68.2, 34.2, and 7.8 µg/g in the gonad, liver, and muscle tissues, respectively. The highest levels were generally observed in late autumn to winter (especially in November and December) in all tissues from both genders. Female individuals were generally found to be more toxic than male individuals. The TTX levels found confirm that the consumption of L. sceleratus from Antalya Bay remains dangerous throughout the year, and thus L. sceleratus constantly constitutes an important risk source for public health.
1. Introduction
Lessepsian species migrating from the Red Sea to the Mediterranean via the Suez Canal are increasing. While some of these alien species are of economic value, species such as pufferfish are considered invasive and poisonous. There are currently eleven established pufferfish species in the Mediterranean [1], among which Lagocephalus sceleratus is considered to be the most dangerous and invasive one due to its negative effects on autochthonous species, fishing activities and public health [2].
L. sceleratus was first reported in 2005 off the coast of Türkiye [3] and then spread rapidly. In a short time, relevant occurrence reports followed from Greece, Croatia, Spain, Libya, Tunisia, Egypt, and Italy [4,5,6,7,8,9,10]. The rapid spread of L. sceleratus has negatively affected native species in the Mediterranean ecosystem [11]. A significant part of the ecological success of L. sceleratus is due to the fact that it has one of the most advanced types of teeth in the animal kingdom. The ‘first generation teeth’ are covered with recurrent bands of teeth that are continuously renewed by stem cells [12]. These tooth bands fuse to form the upper and lower plates, which together form a beak. This strong beak-like structure allows them to crush and slice even very tough prey organisms such as decapods and bivalves [1,13]. Owing to this unique tooth structure, it can also damage the fishing gear of commercial and recreational fisheries, resulting in significant socio-economic effects on fishermen in affected countries, such as Türkiye [14].
In the wider Mediterranean area, pufferfish species have attracted remarkable attention in the last few decades due to their invasion potential as well as the economic damage and poisoning they can cause. For example, the impact of pufferfish on small-scale fisheries in Türkiye is estimated to lead to an annual loss of EUR 2 million (loss of fishing gear and labor) [15]. Similarly, in Cyprus, pufferfish expansion is estimated to generate an annual economic loss of EUR 4173.53 per small-scale fisher. It is also noteworthy that many small-scale commercial fishers in Cyprus have changed their fishing strategies (e.g., using larger nets, fishing for shorter periods of time, and relocating) due to the damage caused by pufferfish to fishing gear [16].
In addition to the ecological and economic impacts of pufferfish, the risks posed by L. sceleratus to public health are even more worrisome, as this species has also been implicated in poisonings in the Mediterranean, some of which have resulted in patient deaths [17,18,19,20]. This is attributed to tetrodotoxin (TTX), one of the most potent toxins known in the world, which is commonly present in many pufferfish. As such, in the context of public health protection, the EU and many other Mediterranean countries have introduced legislation to ban the placing on the market of fishery products derived from poisonous fish belonging to the families Tetraodontidae, Molidae, Diodontidae, and Canthigasteridae [2,21,22,23].
TTX is a compound with a low molecular weight (319.27) and non-protein structure. It is a water-soluble, colorless, odorless heat-stable toxin. In case of poisoning, symptoms begin to be observed within 2–4 h, and poisoning can result in death because there is no known antidote for this toxin [17]. The origin of TTX is still debated. It is thought to have its origins in bacteria belonging to the Proteobacteria phylum, which includes Pseudomonas, Pseudoalteromonas, and Vibrio. However, there are occasional reports of several other bacterial phyla (Actinobacteria, Bacterioides, Firmicutes, and Proteobacteria) being considered as potential sources of TTX [24,25]. These TTX-producing bacteria, such as Vibrio, Pseudomonas, Aeromonas, Alteromonas, Nocardiopsis, Bacillus, Shewanella, and Roseobacter, have been discovered in various locations within several aquatic species, including subcutaneous mucus, ovaries, and the gastrointestinal tract [26,27,28,29]. Additionally, some evidence in the literature suggests their association with specific dinoflagellate blooms, such as Alexandrium tamarense or Prorocentrum cordatum [30,31,32].
TTX and its analogues (TTXs) are found in a taxonomically diverse group of animals, living in both terrestrial and aquatic (marine, freshwater, and brackish) environments; however, pufferfish constitute the most prominent vectors of this toxin [17,33,34]. At the worldwide level, pufferfish are represented by 29 different genera and about 200 different species, and most of these species contain TTX [35]. Some of these pufferfish species that inhabit the Mediterranean also possess TTX at levels that are toxic and potentially deadly [17,36,37,38,39]. To date, most studies on pufferfish toxicity in the Mediterranean have focused particularly on L. sceleratus [2,8,33,36,38,39,40,41,42,43,44]. However, there are also scientific studies on the TTX levels of T. flavimaculosus, L. guentheri, L. suezensis, and Sphoeroides pachygaster [2,8,37,45], whereas poisoning cases caused by the consumption of pufferfish have also been reported in different Mediterranean countries [17,46,47]. These poisoning cases mainly stemmed from misidentification and a lack of knowledge on pufferfish. People living in the Mediterranean coastal regions often confuse the pufferfish species encountered in this area with the Japanese pufferfish, which constitute a delicacy (fugu). Nevertheless, it is worth emphasizing that the pufferfish species prevalent in the Mediterranean region do not comply with the classification endorsed by the Japanese Ministry of Health, Labor, and Welfare, designating the pufferfish that are safe for human consumption [48].
The studies conducted so far on the toxin content of pufferfish in the Mediterranean region have generally focused on investigating single-sampling or seasonal TTX contents [2,8,33,36,38,39,40,41,42,43,44]. Considering, however, the extremely harmful effects that pufferfish may have on public health, it would also be beneficial to determine the monthly changes in TTX levels of these organisms. Poisoning cases in many countries appear on an occasional basis, indicating the importance of monitoring the variation of TTX levels in pufferfish over time. On the other hand, clinical trials of anti-cancer and anti-pain drugs containing TTX are ongoing [42]. As such, exploitation of L. sceleratus for TTX purification could be of help to stabilize their population and reduce their impact on the environment. It would therefore be useful to investigate the variation in TTX levels in pufferfish throughout the year in order to target fishing efforts towards the months when TTX levels are highest. To address these considerations, the present study investigated the monthly and sex-related changes in TTX levels of the population of L. sceleratus in Antalya Bay.
2. Results and Discussion
Only sexually mature pufferfish were included in this study. The maximum mean weights of the female and male samples were 2062.2 g and 1665.4 g, respectively, while the maximum mean lengths of the females and males were 54.5 cm and 52.5 cm, respectively (Table 1).

Table 1.
Monthly length and weight of pufferfish samples (n = 5, mean ± SD).
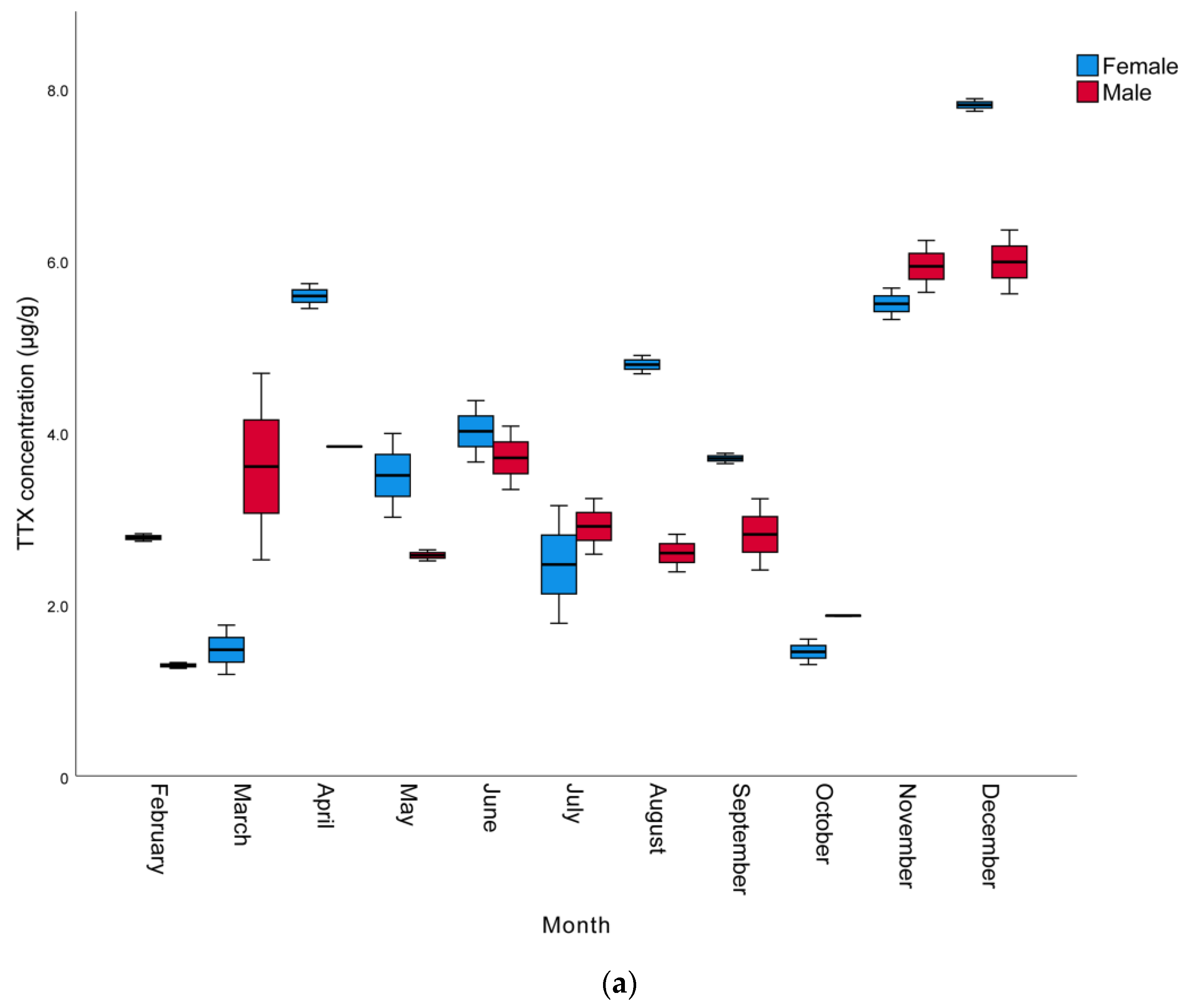
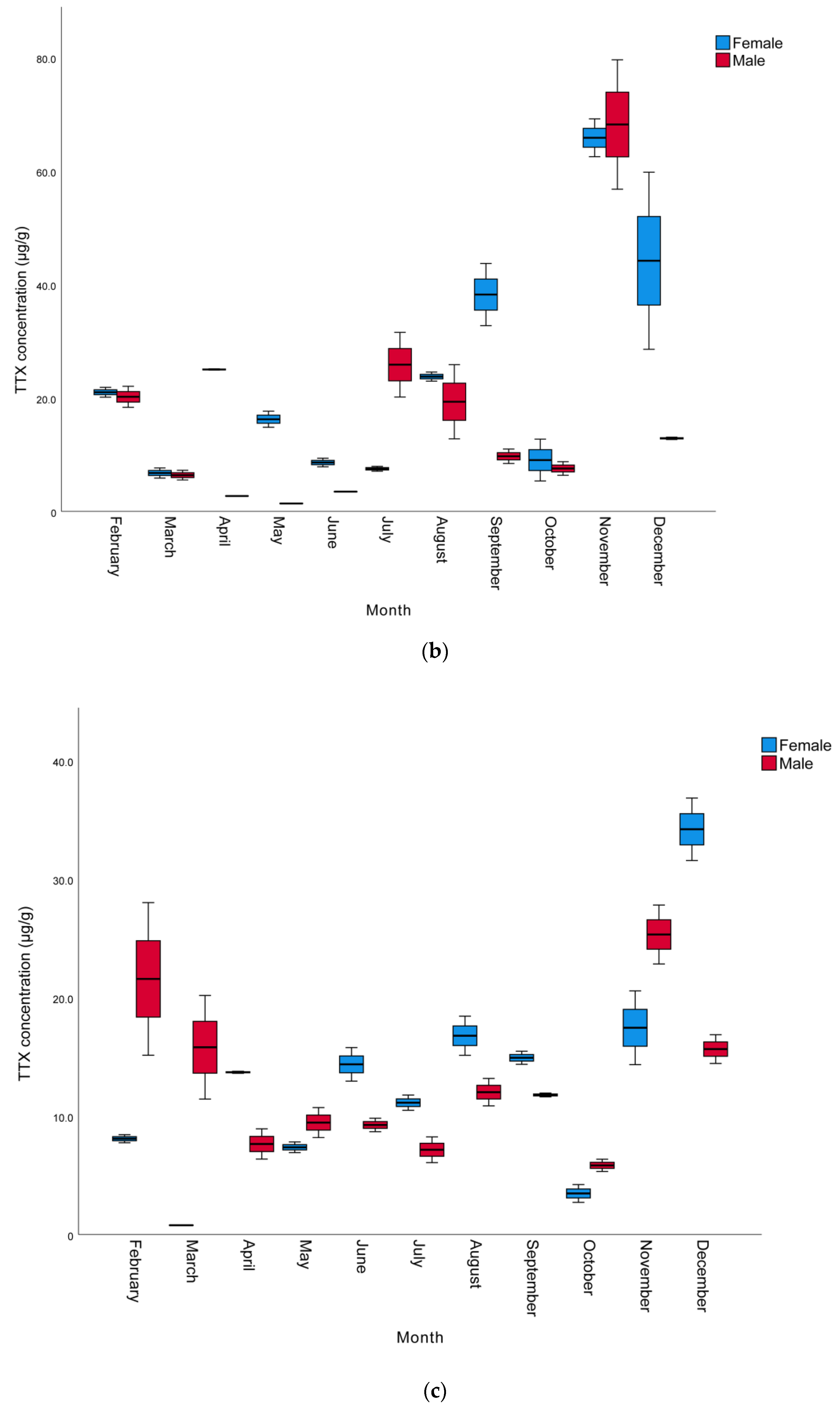
The monthly and sexual variation of TTX levels in the muscle, liver, and gonads of L. sceleratus caught in Antalya Bay are presented in Table 2, whereas a graphical comparison of TTX contents in different tissues of male and female individuals is shown in Figure 1. The TTX levels measured were in the range of 0.8 to 68.2 µg/g. The highest TTX mean levels were present in the gonads and the lowest in the muscle samples. The maximum toxin concentrations determined per tissue were 68.2, 34.2, and 7.8 µg TTX/g in the gonad, liver, and muscle, respectively. The highest TTX concentrations for all tissues and both sexes were observed in November, with female individuals generally harboring more toxins than male ones. However, in some months, especially between October and March, liver TTX contents were higher in males than females. In terms of monthly toxin levels, the TTX levels of most female individuals were found to be higher than those of males.

Table 2.
Monthly TTX levels (µg/g) in different tissues of L. sceleratus (mean ± SD).
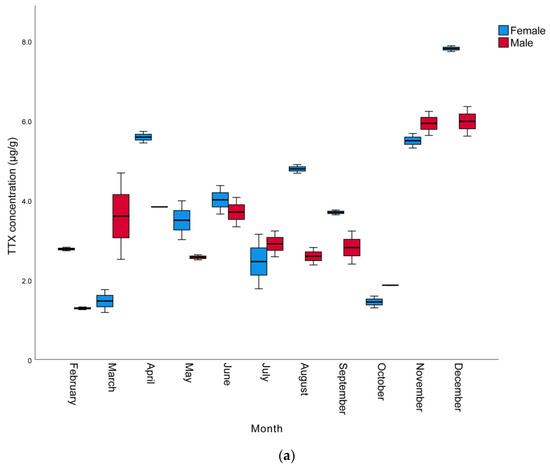
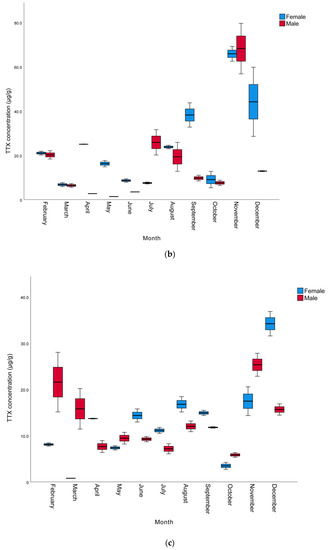
Figure 1.
Monthly variation of TTX levels (µg/g) of male and female L. sceleratus individuals in (a) muscle, (b) liver, and (c) gonads (n = 5, error bars represent SD).
In terms of the TTX levels measured in the tissues throughout the year, the order was found to be gonad > liver > muscle. This is in agreement with other studies conducted by Christidis et al. [39], Rambla-Alegre et al. [8], Alkassar et al. [49] and Kosker et al. [2,42], where the highest toxin concentrations were also observed in the gonads and the lowest toxin levels were detected in the muscle tissue. The TTX levels in the gonads were within the range of 1.4–68.2 µg/g, similarly to the ranges reported for L. sceleratus individuals from the Aegean Sea [33,36,41,50], the Adriatic Sea [51], the Eastern Mediterranean [42,43], the Western Mediterranean [8] and the Southern Mediterranean [39]. The TTX levels in the liver were in the range of 0.78–34.19 µg/g, also in accordance with the levels detected in L. sceleratus from the Aegean Sea [36,41], the Eastern Mediterranean [40,42] and the Southern Mediterranean [39]. However, as can be seen in Table 3, the TTX levels in the livers of pufferfish caught in Antalya Bay were higher than the values reported by Reverté et al. from the Aegean Sea [33], Kosker et al. from the North East Mediterranean [2], Rambla-Alegre et al. from the West Mediterranean [8] and Acar et al. from the East Mediterranean [43], while they were lower than those reported in the livers of L. sceleratus from the southern Aegean by Anastasiou et al. [50]. Finally, the muscle tissue TTX concentrations (1.3–7.80 µg/g) were equivalent to those reported by Katikou et al. from the Aegean Sea [41], Kosker et al. from the North East Mediterranean [2] and Christidis et al. from the South Mediterranean [39]. On the other hand, the TTX levels determined in the present study in the gonads were lower compared to some other studies [40,43,49,50] conducted nearby. This could be attributed to differences in the sampling period, which in the other studies was only seasonal and probably involved one single sample per season, in contrast to the monthly sampling of the present study. This hypothesis is further supported by the notable monthly fluctuations in TTX concentrations, indicating that the sampling intervals’ periodicity may hold a significant role. However, differences in environmental conditions in the respective sampling periods/years, such as temperature, availability of nutrients, etc., as well as specimens’ sizes and ages, may well account for the variation of toxin levels observed between individual studies.

Table 3.
TTX levels in different tissues of L. sceleratus from the Mediterranean (μg/g), reported using different liquid chromatography–mass spectrometry (LC-MS) methods.
Considering monthly changes, a statistically significant increase was observed in the TTX levels in all tissues, especially towards the late autumn to early winter months (p < 0.05). The mean TTX levels in the muscle, liver, and gonads of male pufferfish in November were 5.9, 25.3 and 68.2 µg/g, respectively. In females, the mean TTX levels in the gonads was 65.9 µg/g in November, while the highest TTX levels in the muscle and liver tissues (7.8 and 34.2 µg/g), respectively, were observed in December. The spawning season of L. sceleratus in the Mediterranean includes a very wide period and can last from April to September [1,44,52]. It has been reported that the TTX molecule is a reproductive adaptation for pufferfish and that they show maximum toxicity in the spring and summer seasons, which are the breeding seasons of pufferfish [53,54]. However, Yu and Yu [55] reported that Takifugu niphobles and Takifugu alboplumbeus pufferfish are not toxic during their spawning period. Similarly, according to the findings of the studies conducted by Katikou et al. [41], Kosker et al. [2,42] and Acar et al. [43], L. sceleratus living in the Mediterranean seem to contain higher TTX in the late autumn months after the spawning period. Interestingly, in these cases, pufferfish show higher toxicity outside of their breeding season, a phenomenon that may be specific to the Mediterranean ecosystem.
Overall, the TTX levels of L. sceleratus caught in Antalya Bay are generally in agreement with those reported from different parts of the Mediterranean by means of analytical methods (Table 3). On the other hand, specifically where potential poisoning caused by muscle tissue consumption in humans and wild animals or pets is concerned, almost all of the pufferfish examined in this study were toxic, that is, exceeding the sole existing safety threshold of 10 mouse units (MU) TTX eq/g (equivalent to 2.2 µg TTX eq/g) established by the Japanese authorities [56]. Similarly, high concentrations of TTX have been reported in other Mediterranean pufferfish species, L. suezensis and T. flavimaculosus [2,37]. However, the main responsible species for poisoning cases in the Mediterranean is L. sceleratus [17]. At earlier times, these latter pufferfish were sold at fish stalls, probably due to their larger individual size and their widespread presence in almost all Mediterranean countries [57]. Currently, the trade of pufferfish is restricted by legal regulations in Türkiye, the EU, and other Mediterranean countries [22,23], but still, several poisoning cases resulting from the consumption of L. sceleratus have occurred in some Mediterranean countries [17,19,46]. These cases are commonly the result of accidental consumption of pufferfish caught during amateur fishing by people living in coastal areas. Nevertheless, some of the poisoning incidents have been caused by pufferfish caught professionally by fishing lines, especially in the Antalya Bay [17].
As aforementioned, L. sceleratus is not listed as an edible pufferfish species in Japan [28], mainly due to the fact that its muscle commonly contains TTX at levels surpassing the safety threshold of 2.2 μg TTX eq/g. This limit was established to ensure the safe consumption of pufferfish meat in Japan but has also been employed as a toxicity assessment criterion for L. sceleratus samples from the Mediterranean Sea [17,20]. Taking into account this threshold value, the TTX levels found in the present study generally indicate a substantial risk of foodborne poisoning associated with the potential ingestion of L. sceleratus edible tissues (muscle, liver, and gonads) caught by angling in Antalya Bay, regardless of the time period of sampling. As such, L. sceleratus should continue to be considered as an important source of risk in terms of public health in addition to its ecological and economic consequences.
It is noteworthy to mention that currently, TTX levels in seafood are a subject of debate, especially in the European Union. The European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM) has concluded that a concentration of 44 µg TTX/kg of shellfish meat, as regards marine bivalves and gastropods, is not expected to lead to adverse effects in humans [29]. This threshold value is notably more conservative compared to the relevant Japanese safety limit for the assessment of edible pufferfish. Subsequently, newer toxicological data obtained by administering TTX in mice through feeding, instead of gavage, but following the same calculation logic as that of the EFSA Panel, indicated that a higher concentration of 560 µg TTX/kg of shellfish meat would be expected not to lead to adverse effects in humans [58]. In addition, the EFSA opinion has also suggested exploring whether paralytic shellfish poisoning toxins (saxitoxins, STXs) and TTXs should be combined into one health-based guidance value due to their similarities as regards toxic effects and mode of action [29]. In this context, a recent study has concluded that the current regulatory threshold of 800 μg STX eq./kg [22], which is much higher than the EFSA proposed limit for TTXs alone, would be fit for purpose for the combined presence of STXs and TTXs [59]. Nevertheless, regardless of which of the above threshold values is used, almost all the pufferfish tested in our study would have been deemed unsuitable for human consumption.
3. Materials and Methods
3.1. Tetrodotoxin Standard
The TTX standard (1 mg powder, purity 99%) used for the instrumental toxin analysis was purchased from Abcam Biochemicals (Cambridge, UK). The TTX standard was diluted with methanol (Merck, Darmstadt, Germany) containing 0.01M acetic acid to obtain the stock TTX standard. Working standard solutions at concentrations of 0.05, 0.1, 0.5, and 2 μg/mL were prepared from the stock solution to obtain a calibration curve (R2 = 0.9994). The standards were kept at −20 °C until used.
3.2. Fish Collection, Measurements, and Identification
The pufferfish were caught by angling at monthly intervals between May 2018 and April 2019, except for January 2019, where sampling was not possible due to weather conditions and technical difficulties. The fish were transported to the laboratory on ice. Length–weight measurements of the pufferfish caught in all months were obtained (Table 1), and sex determinations were made by examining the gonads of fish with the help of a microscope. A total of 110 pufferfish, 5 male and 5 female, were examined for each month. Only individuals that had reached sexual maturity and sizes likely to be caught by amateur fishermen were used.
3.3. Preparation of Samples and Toxin Extraction
Fish samples were dissected to obtain their dorsal muscle (carefully avoiding the gastrointestinal tract), gonads, and liver. ΤΤΧ extractions in the liver, gonads, and muscle tissues were performed according to the method of Silva et al. [34]. Briefly, 1 g of each tissue was extracted by adding 3 mL of methanol containing 1% acetic acid, and the mixture was homogenized with an Ultra Turrax (IKA T25 Digital Ultra Turrax, Staufen, Germany) at 7200 rpm for 10 min. Then, the homogenized extracts were placed in an ultrasonic bath (Bandelin Sonorex RK 100, Berlin, Germany) at 100 Hz for 10 min. The extracts were kept at room temperature for 15 min and were then centrifuged at 4500 rpm and 4 °C, for 20 min (Hettich Zentrifugen, Universal 32R, Tuttlingen, Germany). The supernatants were collected, and the same procedure was repeated for the pellet residue. The supernatants from both extractions were combined, and the extracts were made up to 7 mL. The extracts were vortex-mixed before proceeding to solid–phase extraction (SPE). After that, 1 mL of the extract was cleaned by running it through a 500 mg/3 mL C18 solid-phase extraction (SPE) cartridge (Supelco, Bellefonte, PA, USA). The sample was eluted with 10 mL of 100% methanol and diluted with the same solvent to a final volume of 12 mL. It was then vortex-mixed and evaporated to dryness using a rotary evaporator. The dry residue was reconstituted with 1 mL of methanol, filtered through 0.45 µm membrane filters, and transferred to vials for analysis.
3.4. Tetrodotoxin Analysis
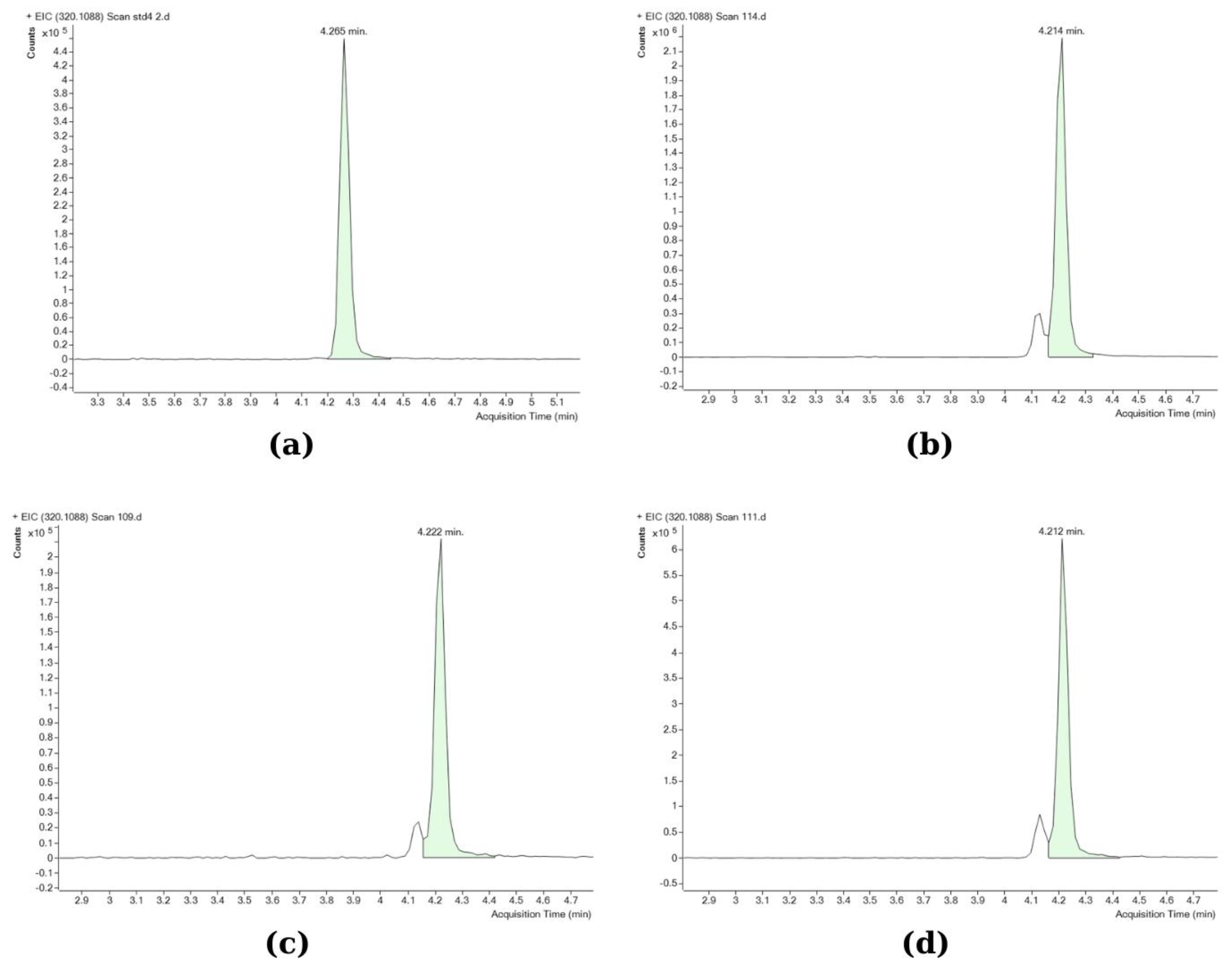
TTX analyses were performed using an Agilent 6545 Accurate-Mass Q-TOF mass spectrometer coupled to an Agilent 1260 HPLC system (Agilent Technologies, Inc., Santa Clara, CA, USA). The Q-TOF LC/MS analysis was performed according to Kosker et al. [37]. A Poroshell 120 HILIC (3.0 × 50 mm; 2.7 µm) column (Agilent Technologies, Inc., Santa Clara, CA, USA) was used for the analyses. The toxin was separated in the column using two different mobile phases. Mobile phase A was 20 mM ammonium acetate in distilled water (Sigma-Aldrich), and mobile phase B was 20 mM ammonium acetate in acetonitrile (Sigma-Aldrich). The analysis was completed in 8 min, with the retention time of TTX being 4.2 min (Figure 2). A gradient program was used as follows: the first 2.5 min were set as 3% mobile phase A, 97% mobile phase B, then for 2 min 30% mobile phase A, 70% mobile phase B, and again between 4.5 and 8 min 3% mobile phase A, 97% mobile phase B. The column temperature was 20 °C, and the injection volume was 10 µL. The LC system was operated in the positive ion mode with the ESI (electrospray ionization) interface using the following parameters: drying gas flow, 10.0 L/min; nebulizer pressure, 40 psi; gas drying temperature, 325 °C; sheath gas temperature, 400 °C; sheath gas flow, and nitrogen at 12 L/min. The scanning was performed in the range of m/z 50–500. Only the parent TTX compound was determined, as other previous studies by Christidis et al. [39] and Bane et al. [60] have confirmed that it is the most abundant among all TTX analogues in L. sceleratus pufferfish, whereas the limited information on the toxic potential of individual TTX analogues, although probably present in the samples, would not allow for a solid evaluation of their contribution in overall toxicity. The Q-TOF LC/MS operating conditions were first optimized using the TTX standard. The method’s limit of detection (LOD; S/N > 3) was calculated at 0.008 μg/g and the limit of quantification (LOQ; S/N > 10) at 0.026 μg/g. The working mass range was from m/z 50 to 500 in full-scan acquisition mode. Quantitation was performed by using the MS mode; the accurate measured mass of the detected ion was [M + H]+, m/z 320.1088. The peaks were identified by retention time (4200–4270 min) and exact mass (mass window ±5 ppm, mass accuracy 0.93 ppm, monoisotopic pattern of the signals). Data processing was carried out using the Mass Hunter software. All Q-TOF LC/MS analyses were performed in triplicate.
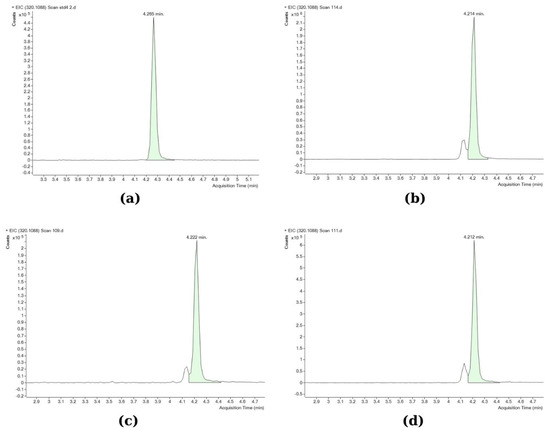
Figure 2.
Q-TOF chromatograms of standard and different samples: (a) TTX standard (2 μg/mL); (b) gonad; (c) muscle; (d) liver (the smaller peak left to TTX in the tissue samples corresponds to 4-epiTTX, which was not included in the analysis). Accurate mass m/z: 320.1088; retention time window: 4200–4270 min; mass accuracy: 0.93 ppm; mass window: ± 5ppm.
3.5. Statistical Analysis
Results are reported as the mean and standard deviation of the measurements. SPSS version 17.0 (SPSS Inc., Chicago, IL USA) was used for the statistical evaluations of the monthly changes of TTX values among tissues and between males and females captured in the same month (Figure S1). To identify significant differences between monthly values of TTX levels in tissues, a one-way analysis of variance (ANOVA) combined with Duncan’s multiple range test comparisons at p < 0.05 were performed. The t-test was applied between different genders belonging to the same month.
4. Conclusions
The present study extends our knowledge on the TTX contents of L. sceleratus at monthly intervals and provides further data on toxin levels required for the establishment of more efficient risk management practices for both public health and the fishing industry around Mediterranean countries. Our results demonstrate that the Mediterranean L. sceleratus remains poisonous and unsafe for human consumption throughout the year. As such, authorities should carefully consider the risks posed by pufferfish, especially of this species, and carry out awareness activities to prevent their consumption under any circumstances. In addition, considering that pufferfish are the most important source of purified TTX to be used as a drug [61], it is possible that Antalya Bay could constitute an appropriate potential production site for TTX with the purpose of alleviating the detrimental effects of pufferfish expansion through overfishing. In this context, this study provides further insights into the months during which TTX levels are elevated in L. sceleratus. To summarize, the findings highlight the toxin levels of L. sceleratus in Antalya Bay by offering valuable information regarding the temporal distribution of TTX in this ecosystem. Further research on the monthly variation of TTX and its analogues could also be necessary to obtain more solid information on their individual contribution to the overall toxic burden of L. sceleratus and their occurrence patterns, as well as on TTX metabolism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md21100527/s1, Figure S1: TTX spectra of different pufferfish samples.
Author Contributions
Conceptualization, A.R.K., P.K., M.K. and F.Ö.; methodology, A.R.K., M.K., İ.D., D.A. and Y.U.; writing—original draft preparation, A.R.K., P.K. and M.D.; writing—review and editing, A.R.K., P.K., M.D. and F.Ö.; supervision, P.K. and A.R.K.; funding acquisition, P.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Republic of Türkiye Ministry of Agriculture and Forestry, General Directorate of Agricultural Research and Policies (Project No. TAGEM/HAYSÜD/B/17/A6/P1/507).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Acknowledgments
We would like to thank Sedat Gündoğdu for his contribution of preparing the figures and Bahar Meryemoğlu for her contribution of the Q-TOF-LC-MS analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ulman, A.; Yildiz, T.; Demirel, N.; Canak, O.; Yemişken, E.; Pauly, D. The biology and ecology of the invasive silver-cheeked toadfish (Lagocephalus sceleratus), with emphasis on the Eastern Mediterranean. NeoBiota 2021, 68, 145–175. [Google Scholar] [CrossRef]
- Kosker, A.R.; Özogul, F.; Ayas, D.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels of three pufferfish species (Lagocephalus sp.) caught in the North-Eastern Mediterranean sea. Chemosphere 2019, 219, 95–99. [Google Scholar] [CrossRef]
- Akyol, O.; Ünal, V.; Ceyhan, T.; Bilecenoglu, M. First confirmed record of Lagocephalus sceleratus(Gmelin, 1789) in the Mediterranean Sea. J. Fish Biol. 2005, 66, 1183–1186. [Google Scholar] [CrossRef]
- Kasapidis, P.; Peristeraki, P.; Tserpes, G.; Magoulas, A. First record of the Lessepsian migrant Lagocephalus sceleratus (Gmelin 1789) (Osteichthyes: Tetraodontidae) in the Cretan Sea (Aegean, Greece). Aquat. Invasions 2007, 2, 71–73. [Google Scholar] [CrossRef]
- Jribi, I.; Bradai, M.N. First record of the lessepsian migrant species Lagocephalus sceleratus (Gmelin, 1789) (actinopterygii: Tetraodontidae) in the central mediterranean. BioInvasions Rec. 2012, 1, 49–52. [Google Scholar] [CrossRef]
- Milazzo, M.; Azzurro, E.; Badalamenti, F. On the occurrence of the silverstripe blaasop Lagocephalus sceleratus (Gmelin, 1789) along the Libyan coast. BioInvasions Rec. 2012, 1, 125–127. [Google Scholar] [CrossRef]
- Sulić Šprem, J.; Dobroslavić, T.; Kožul, V.; Kuzman, A.; Dulčić, J. First record of Lagocephalus sceleratus in the Adriatic Sea (Croatian coast), a Lessepsian migrant. Cybium 2014, 38, 147–148. [Google Scholar]
- Rambla-Alegre, M.; Reverté, L.; del Río, V.; de la Iglesia, P.; Palacios, O.; Flores, C.; Caixach, J.; Campbell, K.; Elliott, C.T.; Izquierdo-Muñoz, A.; et al. Evaluation of tetrodotoxins in puffer fish caught along the Mediterranean coast of Spain. Toxin profile of Lagocephalus sceleratus. Environ. Res. 2017, 158, 1–6. [Google Scholar] [CrossRef]
- Azzurro, E.; Castriota, L.; Falautano, M.; Giardina, F.; Andaloro, F. The silver-cheeked toadfish Lagocephalus sceleratus (Gmelin, 1789) reaches Italian waters. J. Appl. Ichthyol. 2014, 30, 1050–1052. [Google Scholar] [CrossRef]
- Guardone, L.; Gasperetti, L.; Maneschi, A.; Ricci, E.; Susini, F.; Guidi, A.; Armani, A. Toxic invasive pufferfish (Tetraodontidae family) along Italian coasts: Assessment of an emerging public health risk. Food Control 2018, 91, 330–338. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Thiery, A.P.; Shono, T.; Kurokawa, D.; Britz, R.; Johanson, Z.; Fraser, G.J. Spatially restricted dental regeneration drives pufferfish beak development. Proc. Natl. Acad. Sci. USA. 2017, 114, E4425–E4434. [Google Scholar] [CrossRef]
- Turingan, R.G. Ecomorphological relationships among Caribbean tetraodontiform fishes. J. Zool. 1994, 233, 493–521. [Google Scholar] [CrossRef]
- Ünal, V.; Göncüoğlu Bodur, H. The socio-economic impacts of the silver-cheeked toadfish on small-scale fishers: A comparative study from the Turkish coast. Ege J. Fish. Aquat. Sci. 2017, 34, 119–127. [Google Scholar] [CrossRef]
- Ünal, V.; Göncüoğlu, H.; Durgun, D.; Tosunoğlu, Z.; Deval, M.C.; Turan, C. Silver-cheeked toadfish, Lagocephalus sceleratus (Actinopterygii: Tetraodontiformes: Tetraodontidae), causes a substantial economic losses in the Turkish Mediterranean coast: A call for decision makers. Acta Ichthyol. Piscat. 2015, 45, 231–237. [Google Scholar] [CrossRef]
- Kleitou, P.; Moutopoulos, D.K.; Giovos, I.; Kletou, D.; Savva, I.; Cai, L.L.; Hall-Spencer, J.M.; Charitou, A.; Elia, M.; Katselis, G.; et al. Conflicting interests and growing importance of non-indigenous species in commercial and recreational fisheries of the Mediterranean Sea. Fish. Manag. Ecol. 2022, 29, 169–182. [Google Scholar] [CrossRef]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campàs, M.; Ozogul, F. An Updated Review of Tetrodotoxin and Its Peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Bentur, Y.; Spanier, E. Ciguatoxin-like substances in edible fish on the eastern Mediterranean. Clin. Toxicol. 2007, 45, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Kheifets, J.; Rozhavsky, B.; Girsh Solomonovich, Z.; Marianna, R.; Soroksky, A. Severe Tetrodotoxin Poisoning after Consumption of Lagocephalus sceleratus (Pufferfish, Fugu) Fished in Mediterranean Sea, Treated with Cholinesterase Inhibitor. Case Rep. Crit. Care 2012, 2012, 1–3. [Google Scholar] [CrossRef]
- Abd Rabou, A.F.N. On the occurrence and health risks of the silver-cheeked Toadfish (Lagocephalus sceleratus Gmelin, 1789) in the marine ecosystem of the Gaza Strip, Palestine. Biodiversitas 2019, 20, 2620–2627. [Google Scholar] [CrossRef]
- Nader, M.; Indary, S.; Boustany, L. The Puffer Fish Lagocephalus Sceleratus (Gmelin, 1789) in the Eastern Mediterranean. Available online: http://www.fao.org/3/ap967e/ap967e.pdf (accessed on 11 August 2023).
- European Commission Regulation (EC) No 853/2004 of the European Parlamient and of the Council. Off. J. Eur. Union 2004, L 139, 55.
- Republic of Türkiye Ministry of Agriculture and Forestry. Hayvansal Gidalar için Özel Hijyen Kurallari Yönetmeliği, Sayı: 28155, 27 Aralık 2011; Canlı çift kabuklu yumuşakçalar için sağlık standartları: Madde 49. Available online: https://www.resmigazete.gov.tr/eskiler/2011/12/20111227-10.htm (accessed on 11 August 2023).
- Jal, S.; Khora, S.S. An overview on the origin and production of tetrodotoxin, a potent neurotoxin. J. Appl. Microbiol. 2015, 119, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Magarlamov, T.Y.; Melnikova, D.I.; Chernyshev, A.V. Tetrodotoxin-producing bacteria: Detection, distribution and migration of the toxin in aquatic systems. Toxins 2017, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, Y.; Xie, L.; Xia, G.; Hu, J.; Wang, S.; Zhang, R. Toxicity and distribution of tetrodotoxin-producing bacteria in puffer fish Fugu rubripes collected from the Bohai Sea of China. Toxicon 2005, 46, 471–476. [Google Scholar] [CrossRef]
- Noguchi, T.; Arakawa, O.; Takatani, T. TTX accumulation in pufferfish. Comp. Biochem. Physiol.—Part D Genom. Proteom. 2006, 1, 145–152. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for public health related to the presence of tetrodotoxin (TTX) and TTX analogues in marine bivalves and gastropods. EFSA J. 2017, 15, e04752. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Sato, S.; Sakamoto, S.; Ogata, T. Occurrence of tetrodotoxin in Alexandrium tamarense, a causative dinoflagellate of paralytic shellfish poisoning. Toxicon 1996, 34, 1101–1105. [Google Scholar] [CrossRef]
- Vlamis, A.; Katikou, P.; Rodriguez, I.; Rey, V.; Alfonso, A.; Papazachariou, A.; Zacharaki, T.; Botana, A.M.; Botana, L.M. First detection of tetrodotoxin in greek shellfish by UPLC-MS/MS potentially linked to the presence of the dinoflagellate prorocentrum minimum. Toxins 2015, 7, 1779–1807. [Google Scholar] [CrossRef]
- Rodríguez, I.; Alfonso, A.; Alonso, E.; Rubiolo, J.A.; Roel, M.; Vlamis, A.; Katikou, P.; Jackson, S.A.; Menon, M.L.; Dobson, A.; et al. The association of bacterial C 9 -based TTX-like compounds with Prorocentrum minimum opens new uncertainties about shellfish seafood safety. Sci. Rep. 2017, 7, 40880. [Google Scholar] [CrossRef]
- Reverté, L.; De La Iglesia, P.; Del Río, V.; Campbell, K.; Elliott, C.T.; Kawatsu, K.; Katikou, P.; Diogène, J.; Campàs, M. Detection of Tetrodotoxins in Puffer Fish by a Self-Assembled Monolayer-Based Immunoassay and Comparison with Surface Plasmon Resonance, LC-MS/MS, and Mouse Bioassay. Anal. Chem. 2015, 87, 10839–10847. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Azevedo, J.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Vasconcelos, V. New gastropod vectors and tetrodotoxin potential expansion in temperate waters of the Atlantic Ocean. Mar. Drugs 2012, 10, 712–726. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.S. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 978-0-471-25031-9. [Google Scholar]
- Rodríguez, P.; Alfonso, A.; Otero, P.; Katikou, P.; Georgantelis, D.; Botana, L.M. Liquid chromatography–mass spectrometry method to detect Tetrodotoxin and Its analogues in the puffer fish Lagocephalus sceleratus (Gmelin, 1789) from European waters. Food Chem. 2012, 132, 1103–1111. [Google Scholar] [CrossRef]
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Šimat, V.; Özogul, Y. First report on TTX levels of the yellow spotted pufferfish (Torquigener flavimaculosus) in the Mediterranean Sea. Toxicon 2018, 148, 101–106. [Google Scholar] [CrossRef]
- Leonardo, S.; Kiparissis, S.; Rambla-Alegre, M.; Almarza, S.; Roque, A.; Andree, K.B.; Christidis, A.; Flores, C.; Caixach, J.; Campbell, K.; et al. Detection of tetrodotoxins in juvenile pufferfish Lagocephalus sceleratus (Gmelin, 1789) from the North Aegean Sea (Greece) by an electrochemical magnetic bead-based immunosensing tool. Food Chem. 2019, 290, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Christidis, G.; Mandalakis, M.; Anastasiou, T.I.; Tserpes, G.; Peristeraki, P.; Somarakis, S. Keeping lagocephalus sceleratus off the table: Sources of variation in the quantity of ttx, ttx analogues, and risk of tetrodotoxication. Toxins 2021, 13, 896. [Google Scholar] [CrossRef]
- Hassoun, A.E.R.; Ujević, I.; Jemaa, S.; Roje-Busatto, R.; Mahfouz, C.; Fakhri, M.; Nazlić, N. Concentrations of Tetrodotoxin (TTX) and Its Analogue 4,9-Anhydro TTX in Different Tissues of the Silver-Cheeked Pufferfish (Lagocephalus sceleratus, Gmelin, 1789) Caught in the South-Eastern Mediterranean Sea, Lebanon. Toxins 2022, 14, 123. [Google Scholar] [CrossRef]
- Katikou, P.; Georgantelis, D.; Sinouris, N.; Petsi, A.; Fotaras, T. First report on toxicity assessment of the Lessepsian migrant pufferfish Lagocephalus sceleratus (Gmelin, 1789) from European waters (Aegean Sea, Greece). Toxicon 2009, 54, 50–55. [Google Scholar] [CrossRef]
- Kosker, A.R.; Özogul, F.; Durmus, M.; Ucar, Y.; Ayas, D.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin levels in pufferfish (Lagocephalus sceleratus) caught in the Northeastern Mediterranean Sea. Food Chem. 2016, 210, 332–337. [Google Scholar] [CrossRef]
- Acar, C.; Ishizaki, S.; Nagashima, Y. Toxicity of the Lessepsian pufferfish Lagocephalus sceleratus from eastern Mediterranean coasts of Türkiye and species identification by rapid PCR amplification. Eur Food Res Technol 2017, 243, 49–57. [Google Scholar] [CrossRef]
- Akbora, H.D.; Kunter, İ.; Erçeti N, T.; Elagöz, A.M.; Çiçek, B.A. Determination of tetrodotoxin (TTX) levels in various tissues of the silver cheeked puffer fish (Lagocephalus sceleratus (Gmelin, 1789)) in Northern Cyprus Sea (Eastern Mediterranean). Toxicon 2020, 175, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Malloggi, C.; Rizzo, B.; Giusti, A.; Guardone, L.; Gasperetti, L.; Dall’Ara, S.; Armani, A. First Toxicological Analysis of the Pufferfish Sphoeroides pachygaster Collected in Italian Waters (Strait of Sicily): Role of Citizens Science in Monitoring Toxic Marine Species. Animals 2023, 13, 1873. [Google Scholar] [CrossRef]
- Bentur, Y.; Ashkar, J.; Lurie, Y.; Levy, Y.; Azzam, Z.S.; Litmanovich, M.; Golik, M.; Gurevych, B.; Golani, D.; Eisenman, A. Lessepsian migration and tetrodotoxin poisoning due to Lagocephalus sceleratus in the eastern Mediterranean. Toxicon 2008, 52, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Chamandi, S.C.; Kallab, K.; Mattar, H.; Nader, E. Human Poisoning after ingestion of puffer fish caught from Mediterranean sea. Middle East J. Anesthesiol. 2009, 20, 285–288. [Google Scholar] [PubMed]
- Ministry of Health, Labour and Welfare of Japan. Natural Toxin Risk Profile: Fish: Pufferfish Toxin. Available online: https://www.mhlw.go.jp/topics/syokuchu/poison/animal_det_01.html (accessed on 11 August 2023).
- Alkassar, M.; Sanchez-Henao, A.; Reverté, J.; Barreiro, L.; Rambla-Alegre, M.; Leonardo, S.; Mandalakis, M.; Peristeraki, P.; Diogène, J.; Campàs, M. Evaluation of Toxicity Equivalency Factors of Tetrodotoxin Analogues with a Neuro-2a Cell-Based Assay and Application to Puffer Fish from Greece. Mar. Drugs 2023, 21, 432. [Google Scholar] [CrossRef]
- Anastasiou, T.I.; Kagiampaki, E.; Kondylatos, G.; Tselepides, A.; Peristeraki, P.; Mandalakis, M. Assessing the Toxicity of Lagocephalus sceleratus Pufferfish from the Southeastern Aegean Sea and the Relationship of Tetrodotoxin with Gonadal Hormones. Mar. Drugs 2023, 21, 520. [Google Scholar] [CrossRef]
- Ujević, I.; Roje-Busatto, R.; Dragičević, B.; Dulčić, J. Tetrodotoxin in Invasive Silver-cheeked Toadfish Lagocephalus sceleratus (Gmelin, 1789) in the Adriatic Sea. Handb. Environ. Chem. 2021, 110, 141–149. [Google Scholar] [CrossRef]
- Farrag, M.M.S.; El-Haweet, A.E.A.K.; Osman, A.G.M.; Akel, E.S.K.A.; Moustafa, M.A. Reproductive behaviour of the silver-stripe blaasop; lagocephalus sceleratus (Gmelin, 1789) from the mediterranean coast, egypt. Egypt. J. Aquat. Biol. Fish. 2019, 23, 441–454. [Google Scholar] [CrossRef][Green Version]
- El-Sayed, M.; Yacout, G.A.; El-Samra, M.; Ali, A.; Kotb, S.M. Toxicity of the Red Sea pufferfish Pleuranacanthus sceleratus “El-Karad. ” Ecotoxicol. Environ. Saf. 2003, 56, 367–372. [Google Scholar] [CrossRef]
- Hwang, D.F.; Noguchi, T. Tetrodotoxin Poisoning. Adv. Food Nutr. Res. 2007, 52, 141–236. [Google Scholar] [CrossRef]
- Yu, C.F.; Yu, P.H.F. The annual toxicological profiles of two common puffer fish, Takifugu niphobles (Jordan and Snyder) and Takifugu alboplumbeus (Richardson), collected along Hong Kong coastal waters. Toxicon 2002, 40, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, T. The Manual for the Methods of Food Sanitation Tests; Japan Food Hygiene Association, Environmental Health Bureau: Tokyo, Japan, 1978. [Google Scholar]
- Köşker, A.R.; Özoğul, F.; Ayas, D.; Durmuş, M.; Uçar, Y. The new toxin of Mediterranean:Tetrodotoxin. Ege J. Fish. Aquat. Sci. 2015, 32, 15–24. [Google Scholar] [CrossRef]
- Finch, S.C.; Boundy, M.J.; Harwood, D.T. The acute toxicity of tetrodotoxin and tetrodotoxin–saxitoxin mixtures to mice by various routes of administration. Toxins 2018, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.C.; Webb, N.G.; Boundy, M.J.; Harwood, D.T.; Munday, J.S.; Sprosen, J.M.; Somchit, C.; Broadhurst, R.B. A Sub-Acute Dosing Study of Saxitoxin and Tetrodotoxin Mixtures in Mice Suggests That the Current Paralytic Shellfish Toxin Regulatory Limit Is Fit for Purpose. Toxins 2023, 15, 437. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Hutchinson, S.; Sheehan, A.; Brosnan, B.; Barnes, P.; Lehane, M.; Furey, A. LC-MS/MS method for the determination of tetrodotoxin (TTX) on a triple quadruple mass spectrometer. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1728–1740. [Google Scholar] [CrossRef]
- Bucciarelli, G.M.; Lechner, M.; Fontes, A.; Kats, L.B.; Eisthen, H.L.; Shaffer, H.B. From poison to promise: The evolution of tetrodotoxin and its potential as a therapeutic. Toxins 2021, 13, 517. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).