Optimization of Heterotrophic Culture Conditions for the Microalgae Euglena gracilis to Produce Proteins
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effects of Nitrogen Sources on the Protein Content and Protein Yield
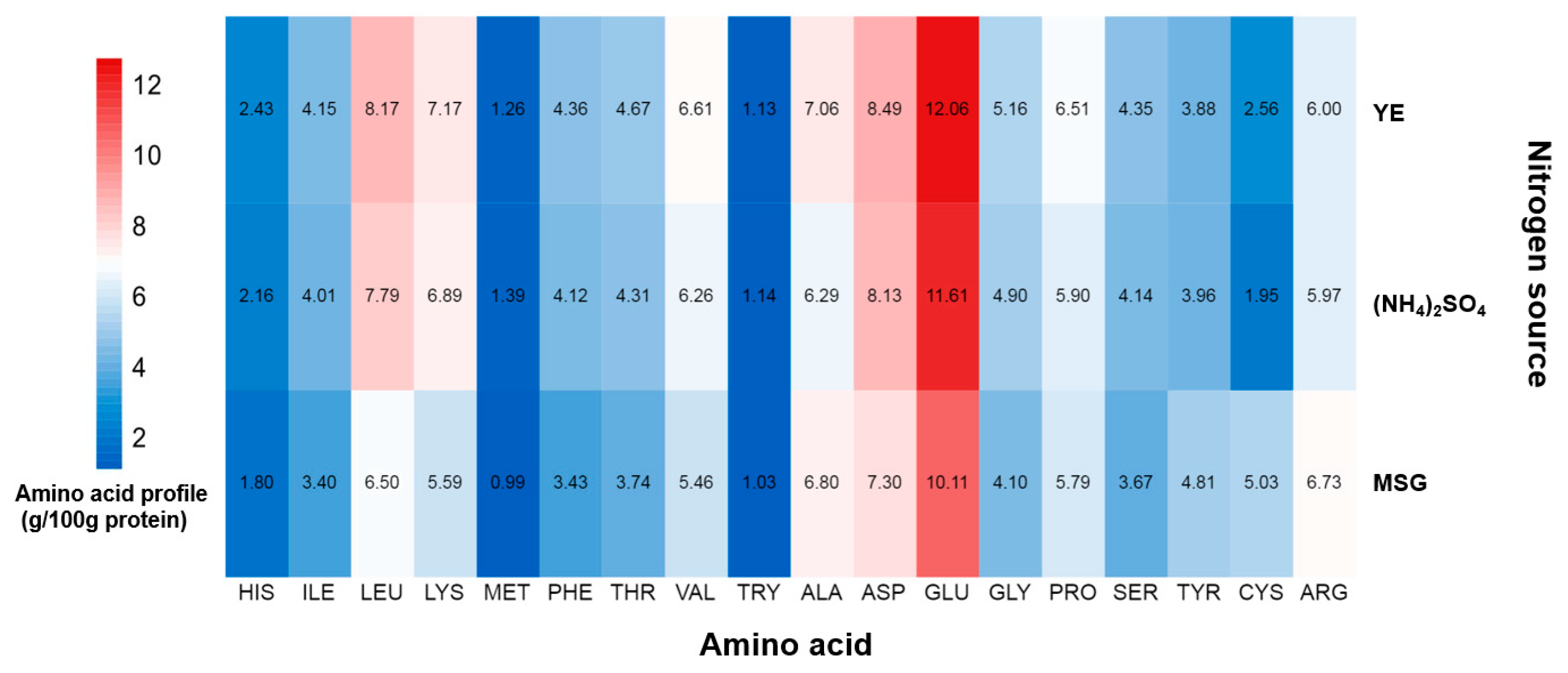
2.2. The Effects of Nitrogen Sources on the Amino Acid Profile
2.3. The Effect of Initial pH on Protein Content and Protein Yield
2.4. The Effect of Temperature on Protein Content and Protein Yield
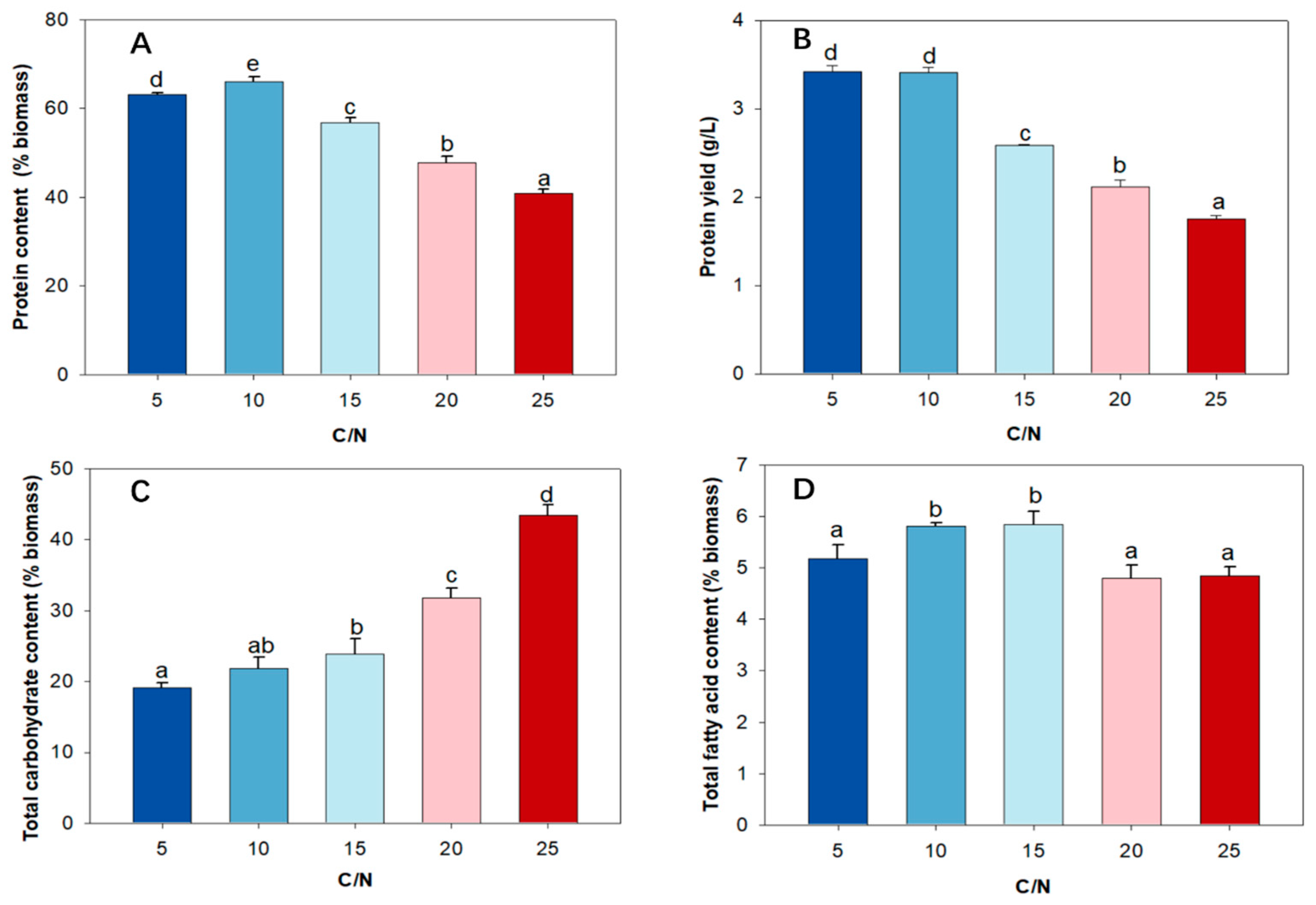
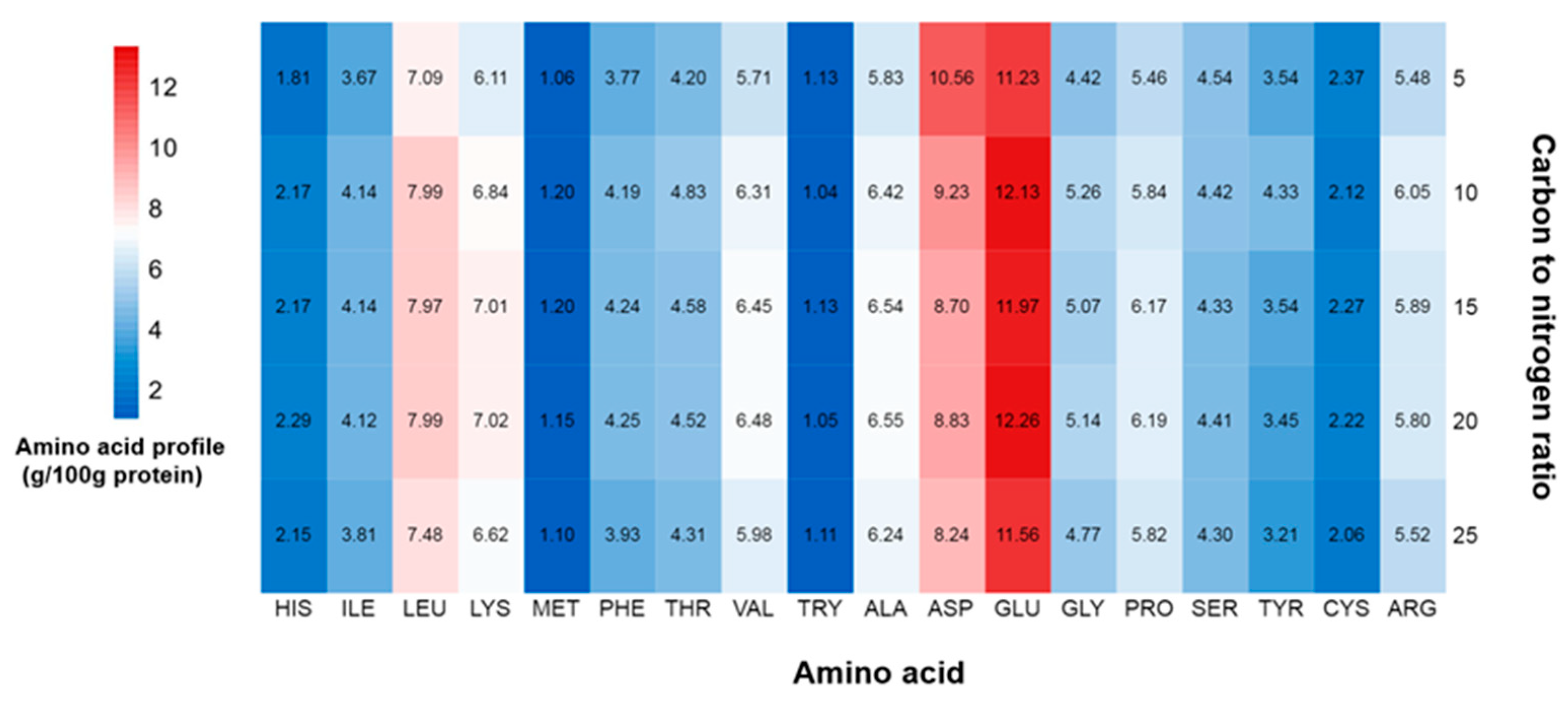
2.5. Effect of C/N Ratio on Protein Content, Protein Yield, and Amino Acid Profile
3. Materials and Methods
3.1. Microalgae Strain and Culture Conditions
3.2. Optimization of Culture Conditions
3.3. Determination of Biomass Concentration
3.4. Protein Content Analysis
3.5. Photosynthetic Pigments Analysis
3.6. Amino Acid Composition Analysis
3.7. Total Fatty Acid Content Analysis
3.8. Total Carbohydrate Content Analysis
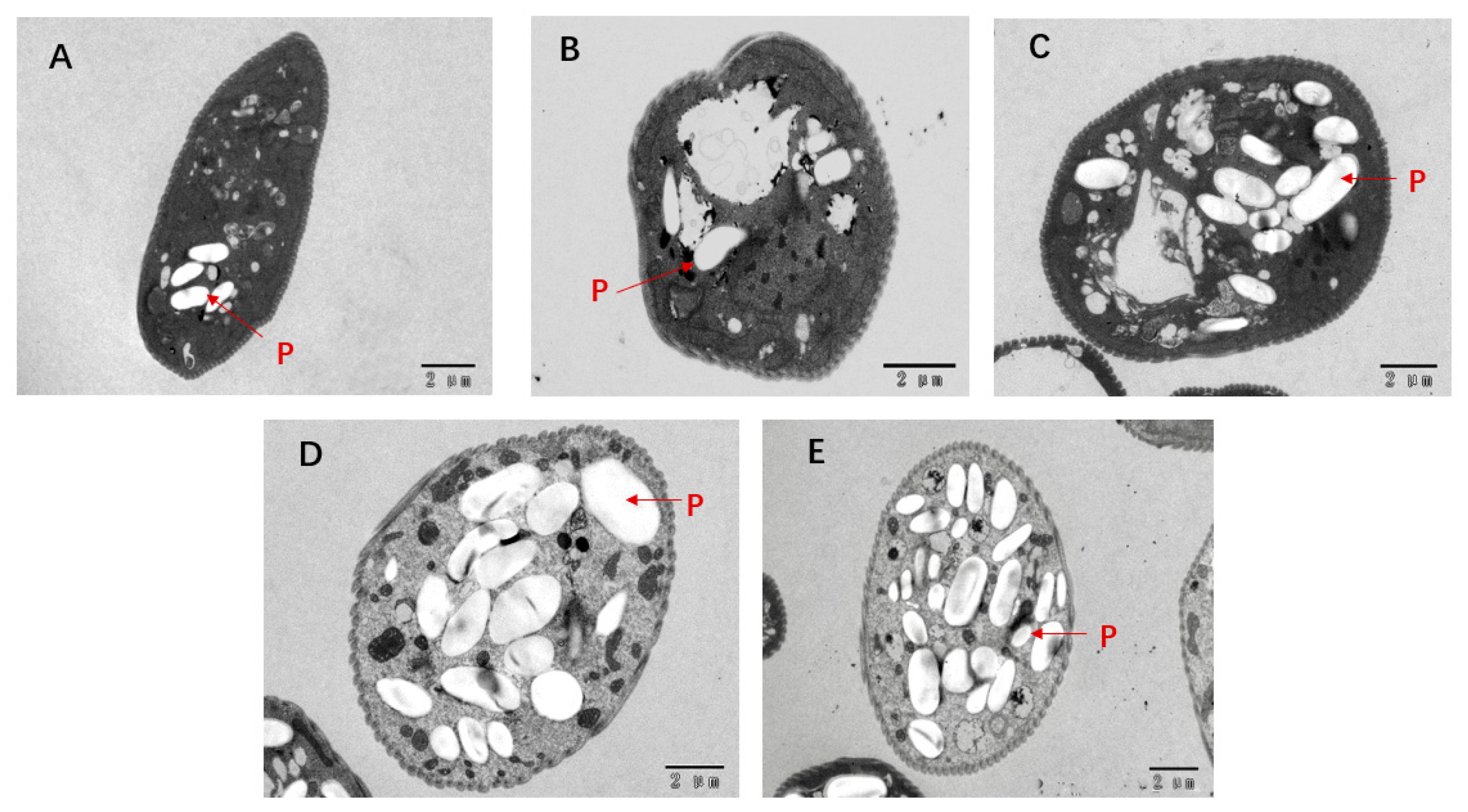
3.9. Transmission Electron Microscopy (TEM)
3.10. Statistics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single cell protein—State-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Rubio, N.R.; Xiang, N.; Kaplan, D.L. Plant-based and cell-based approaches to meat production. Nat. Commun. 2020, 11, 6276. [Google Scholar] [CrossRef] [PubMed]
- Boukid, F. Plant-based meat analogues: From niche to mainstream. Eur. Food Res. Technol. 2021, 247, 297–308. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.d.R.; Leite, M.d.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The potentials and challenges of using microalgae as an ingredient to produce meat analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Gissibl, A.; Sun, A.; Care, A.; Nevalainen, H.; Sunna, A. Bioproducts From Euglena gracilis: Synthesis and Applications. Front. Bioeng. Biotechnol. 2019, 7, 108. [Google Scholar] [CrossRef]
- Kitaoka, S. Determination of the Nutritive Value of Euglena gracilis Protein by in vitro Digestion Experiments and Rat Feeding Tests. Nougei Kagaku Kaishi 1977, 51, 483–488. [Google Scholar]
- Ebenezer, T.E.; Zoltner, M.; Burrell, A.; Nenarokova, A.; Novák Vanclová, A.M.; Prasad, B.; Soukal, P.; Santana-Molina, C.; O’Neill, E.; Nankissoor, N.N. Transcriptome, proteome and draft genome of Euglena gracilis. BMC Biol. 2019, 17, 11. [Google Scholar] [CrossRef]
- Chae, S.; Hwang, E.; Shin, H.-S. Single cell protein production of Euglena gracilis and carbon dioxide fixation in an innovative photo-bioreactor. Bioresour. Technol. 2006, 97, 322–329. [Google Scholar] [CrossRef]
- Kitaoka, S.; Hosotani, K. Studies on culture conditions for the determination of the nutritive value of Euglena gracilis protein and the general and amino acid compositions of the cells. J. Agric. Chem. Soc. Jpn. 1977, 51, 477–482. [Google Scholar]
- Wu, M.; Qin, H.; Deng, J.; Liu, Y.; Lei, A.; Zhu, H.; Hu, Z.; Wang, J. A new pilot-scale fermentation mode enhances Euglena gracilis biomass and paramylon (β-1,3-glucan) production. J. Clean. Prod. 2021, 321, 128996. [Google Scholar] [CrossRef]
- Reihani, S.F.S.; Khosravi-Darani, K. Influencing factors on single-cell protein production by submerged fermentation: A review. Electron. J. Biotechnol. 2019, 37, 34–40. [Google Scholar] [CrossRef]
- Xu, Q.; Hou, G.; Chen, J.; Wang, H.; Yuan, L.; Han, D.; Hu, Q.; Jin, H. Heterotrophically ultrahigh-cell-density cultivation of a high protein-yielding unicellular alga Chlorella with a novel nitrogen-supply strategy. Front. Bioeng. Biotechnol. 2021, 9, 774854. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Eladel, H.; Battah, M.; Abd-Elal, S. Effect of different nitrogen sources on growth and biochemical composition of the green microalgae Scenedesmus obliquus and Chlorella kessleri. Int. Confer. Biol. Sci. 2004, 3, 419–432. [Google Scholar]
- Ogbonna, J.C.; Tomiyamal, S.; Tanaka, H. Heterotrophic cultivation of Euglena gracilis Z for efficient production of α-tocopherol. J. Appl. Phycol. 1998, 10, 67–74. [Google Scholar] [CrossRef]
- Oda, Y.; Miyatake, K.; Kitaoka, S. Inability of Euglena gracilis Z to utilize nitrate, nitrite and urea as the nitrogen sources. Bull. Univ. Osaka Prefect. Ser. B Agric. Biol. 1979, 31, 43–48. [Google Scholar]
- Sui, Y.; Vlaeminck, S.E. Dunaliella microalgae for nutritional protein: An undervalued asset. Trends Biotechnol. 2020, 38, 10–12. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Chen, C.; Zhao, S.; Sun, M.; Gao, Z.; Wu, H.; Fan, J. Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour. Technol. 2020, 309, 123362. [Google Scholar] [CrossRef]
- Regnault, A.; Piton, F.; Calvayrac, R. Growth, proteins and chlorophyll in Euglena adapted to various C/N balances. Phytochemistry 1990, 29, 3711–3715. [Google Scholar] [CrossRef]
- Richter, P.; Liu, Y.; An, Y.; Li, X.; Nasir, A.; Strauch, S.; Becker, I.; Krüger, J.; Schuster, M.; Ntefidou, M. Amino acids as possible alternative nitrogen source for growth of Euglena gracilis Z in life support systems. Life Sci. Space Res. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Gao, B.; Wang, F.; Huang, L.; Liu, H.; Zhong, Y.; Zhang, C. Biomass, lipid accumulation kinetics, and the transcriptome of heterotrophic oleaginous microalga Tetradesmus bernardii under different carbon and nitrogen sources. Biotechnol. Biofuels. 2021, 14, 4. [Google Scholar] [CrossRef]
- Bernard, S.M.; Habash, D.Z. The importance of cytosolic glutamine synthetase in nitrogen assimilation and recycling. New Phytol. 2009, 182, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Otero, D.M.; Mendes, G.d.R.L.; da Silva Lucas, A.J.; Christ-Ribeiro, A.; Ribeiro, C.D.F. Exploring alternative protein sources: Evidence from patents and articles focusing on food markets. Food Chem. 2022, 394, 133486. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhai, L.; Fang, X.; Wu, K.; Liu, Y.; Cui, X.; Wang, Y.; Yu, Z.; Ruan, R.; Liu, T. Effects of C/N ratio on the growth and protein accumulation of heterotrophic Chlorella in broken rice hydrolysate. Biofuels Bioprod. 2022, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, O.; Escalante, F.M.; De-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Marquez, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Basis for the calculation of specific nitrogen-to-protein conversion factors. J. Phycol. 1998, 34, 798–811. [Google Scholar] [CrossRef]
- Templeton, D.W.; Laurens, L.M. Nitrogen-to-protein conversion factors revisited for applications of microalgal biomass conversion to food, feed and fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef]
- Tabuchi, T.; Ueoka, Y.; Nakano, Y.; Kitaoka, S.; Shigeoka, S.; Ohnishi, T.; Murakami, T.; Iizuka, Y. Utilization of molasses on production of Euglena protein. J. Jpn. Soc. Nutr. Food Sci. 1984, 37, 525–534. [Google Scholar] [CrossRef]
- Ras, M.; Steyer, J.-P.; Bernard, O. Temperature effect on microalgae: A crucial factor for outdoor production. Rev. Environ. Sci. Biotechnol. 2013, 12, 153–164. [Google Scholar] [CrossRef]
- Kitaya, Y.; Azuma, H.; Kiyota, M. Effects of temperature, CO2/O2 concentrations and light intensity on cellular multiplication of microalgae, Euglena gracilis. Adv. Space Res. 2005, 35, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Villareal, J.; Liu, K.; Ling, M. Investigating the effects of temperature on Euglena gracilis growth rate. Exped. 2021, 12. [Google Scholar]
- Salbitani, G.; Carfagna, S. Ammonium utilization in microalgae: A sustainable method for wastewater treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Wang, Y.; Seppänen-Laakso, T.; Rischer, H.; Wiebe, M.G. Euglena gracilis growth and cell composition under different temperature, light and trophic conditions. PLoS ONE 2018, 13, e0195329. [Google Scholar] [CrossRef] [PubMed]
- Thanapornpoonpong, S.-N.; Vearasilp, S.; Pawelzik, E.; Gorinstein, S. Influence of various nitrogen applications on protein and amino acid profiles of amaranth and quinoa. J. Agric. Food Chem. 2008, 56, 11464–11470. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lu, J.-C.; Chang, Y.-H.; Chen, J.-H.; Nagarajan, D.; Lee, D.-J.; Chang, J.-S. Optimizing heterotrophic production of Chlorella sorokiniana SU-9 proteins potentially used as a sustainable protein substitute in aquafeed. Bioresour. Technol. 2023, 370, 128538. [Google Scholar] [CrossRef]
- Bombo, G.; Cristofoli, N.L.; Santos, T.F.; Schüler, L.; Maia, I.B.; Pereira, H.; Barreira, L.; Varela, J. Dunaliella viridis TAV01: A Halotolerant, Protein-Rich Microalga from the Algarve Coast. Appl. Sci. 2023, 13, 2146. [Google Scholar] [CrossRef]
- Takeyama, H.; Kanamaru, A.; Yoshino, Y.; Kakuta, H.; Kawamura, Y.; Matsunaga, T. Production of antioxidant vitamins, β-carotene, vitamin C, and vitamin E, by two-step culture of Euglena gracilis Z. Biotechnol. Bioeng. 1997, 53, 185–190. [Google Scholar] [CrossRef]
- Grossmann, L.; Ebert, S.; Hinrichs, J.; Weiss, J. Effect of precipitation, lyophilization, and organic solvent extraction on preparation of protein-rich powders from the microalgae Chlorella protothecoides. Algal Res. 2018, 29, 266–276. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Chang, C.; Chen, J.; Cao, F.; Zhao, J.; Zheng, Y.; Zhu, J. Physicochemical and functional properties of proteins extracted from three microalgal species. Food Hydrocoll. 2019, 96, 510–517. [Google Scholar] [CrossRef]
- Beneragama, C.; Goto, K. Chlorophyll a: B ratio increases under low-light in ‘shade-tolerant’Euglena gracilis. Trop. Agric. Res. 2010, 22, 12–25. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Xu, M.; Ohm, J.-B.; Rao, J.; Chen, B. The impact of hempseed dehulling on chemical composition, structure properties and aromatic profile of hemp protein isolate. Food Hydrocoll. 2020, 106, 105889. [Google Scholar] [CrossRef]
- Landry, J.; Delhaye, S.; Jones, D. Determination of tryptophan in feedstuffs: Comparison of two methods of hydrolysis prior to HPLC analysis. J. Sci. Food Agric. 1992, 58, 439–441. [Google Scholar] [CrossRef]
- Mossé, J.; Huet, J.; Baudet, J. The amino acid composition of wheat grain as a function of nitrogen content. J. Cereal Sci. 1985, 3, 115–130. [Google Scholar] [CrossRef]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition. World Health Organ. Tech. Rep. Ser. 2007, (935), 1–265. Available online: https://pubmed.ncbi.nlm.nih.gov/18330140/ (accessed on 20 September 2023).
- Mao, X.; Wang, X.; Ge, M.; Chen, F.; Liu, B. New Insights into Xanthophylls and Lipidomic Profile Changes Induced by Glucose Supplementation in the Marine Diatom Nitzschia laevis. Mar. Drugs 2022, 20, 456. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]


| Yeast Extract | Ammonium Sulfate | MSG | |
|---|---|---|---|
| Sum of EAA (g/100 g sample) | 9.79 ± 0.50 a | 19.90 ± 0.80 c | 11.54 ± 0.55 b |
| Sum of NEAA (g/100 g sample) | 13.74 ± 0.74 a | 27.64 ± 1.28 c | 19.63± 0.89 b |
| Sum AA (g/100 g sample) | 23.53 ± 1.24 a | 47.53 ± 2.08 c | 31.16± 1.38 b |
| E/T (%) | 41.61 ± 0.13 a | 41.87 ± 0.21 a | 37.02 ± 0.59 b |
| kA | 6.16 ± 0.01 b | 6.17 ± 0.00 b | 6.11 ± 0.01 a |
| kP | 5.14 ± 0.34 a | 4.87 ± 0.13 a | 4.62 ± 0.13 a |
| NTP | 5.65 ± 0.17 b | 5.52 ± 0.06 ab | 5.37 ± 0.06 a |
| C/N = 5 | C/N = 10 | C/N = 15 | C/N = 20 | C/N = 25 | |
|---|---|---|---|---|---|
| kP | 4.71 ± 0.31a | 5.07 ± 0.08 a | 5.00 ± 0.10 a | 5.02 ± 0.53 a | 4.72 ± 0.20 a |
| kA | 6.26 ± 0.07 b | 6.20 ± 0.05 ab | 6.18 ± 0.01 ab | 6.18 ± 0.01 ab | 6.17 ± 0.01 a |
| NTP | 5.49 ± 0.13 a | 5.63 ± 0.02 a | 5.59 ± 0.04 a | 5.60 ± 0.26 a | 5.45 ± 0.10 a |
| EAAI | 1.49 ± 0.14 ab | 1.62 ± 0.05 ab | 1.63 ± 0.03 b | 1.62 ± 0.17 ab | 1.39 ± 0.05 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Li, X.; Xu, H.; Chen, F.; Cheng, K.-W.; Liu, H.; Liu, B. Optimization of Heterotrophic Culture Conditions for the Microalgae Euglena gracilis to Produce Proteins. Mar. Drugs 2023, 21, 519. https://doi.org/10.3390/md21100519
Xie W, Li X, Xu H, Chen F, Cheng K-W, Liu H, Liu B. Optimization of Heterotrophic Culture Conditions for the Microalgae Euglena gracilis to Produce Proteins. Marine Drugs. 2023; 21(10):519. https://doi.org/10.3390/md21100519
Chicago/Turabian StyleXie, Weiying, Xiaojie Li, Huo Xu, Feng Chen, Ka-Wing Cheng, Hongbin Liu, and Bin Liu. 2023. "Optimization of Heterotrophic Culture Conditions for the Microalgae Euglena gracilis to Produce Proteins" Marine Drugs 21, no. 10: 519. https://doi.org/10.3390/md21100519
APA StyleXie, W., Li, X., Xu, H., Chen, F., Cheng, K.-W., Liu, H., & Liu, B. (2023). Optimization of Heterotrophic Culture Conditions for the Microalgae Euglena gracilis to Produce Proteins. Marine Drugs, 21(10), 519. https://doi.org/10.3390/md21100519









