Fused Tricyclic Guanidine Alkaloids: Insights into Their Structure, Synthesis and Bioactivity
Abstract
1. Introduction
2. Methodology
3. Synthesis
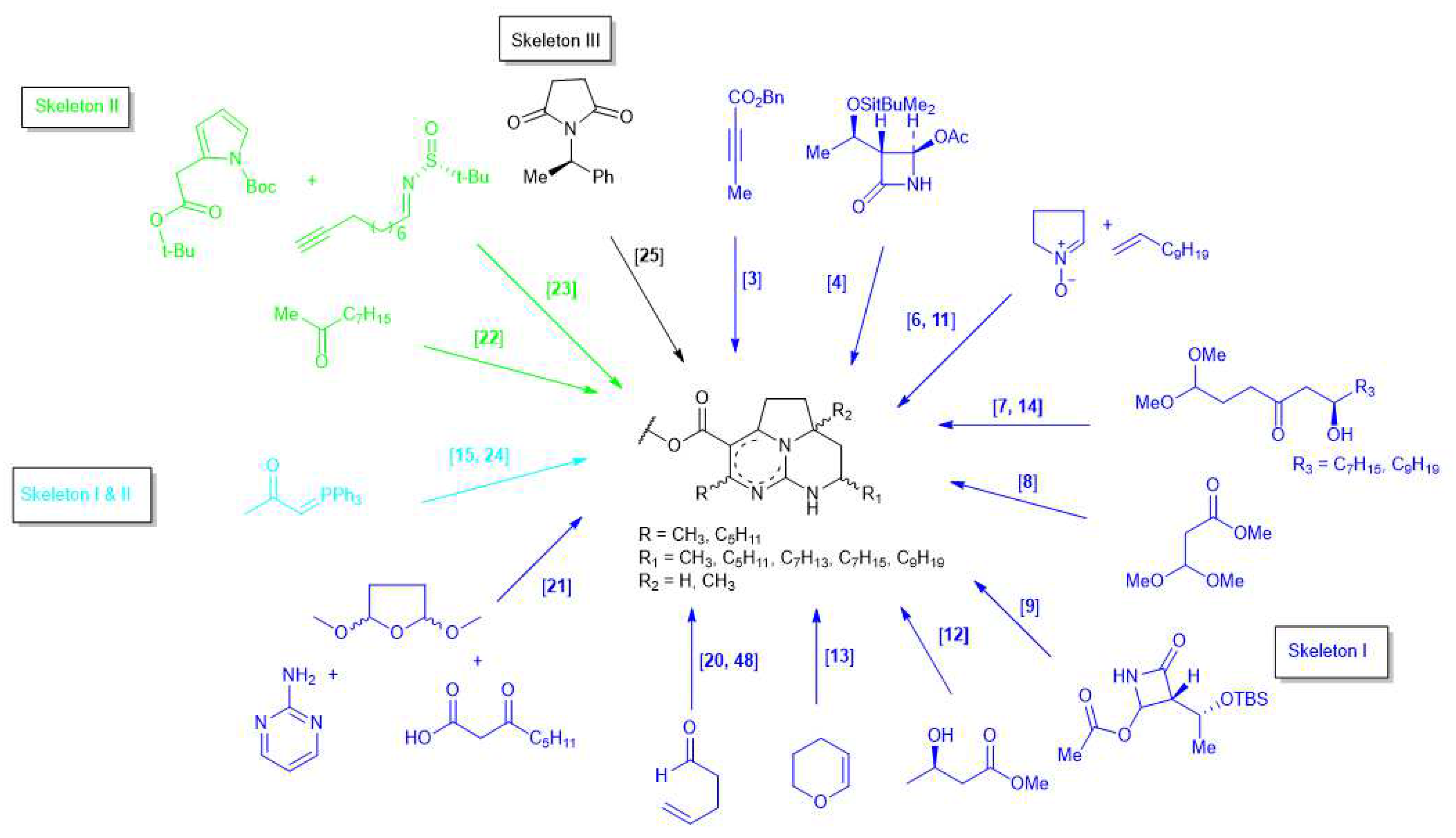
3.1. Batzelladine Skeleton
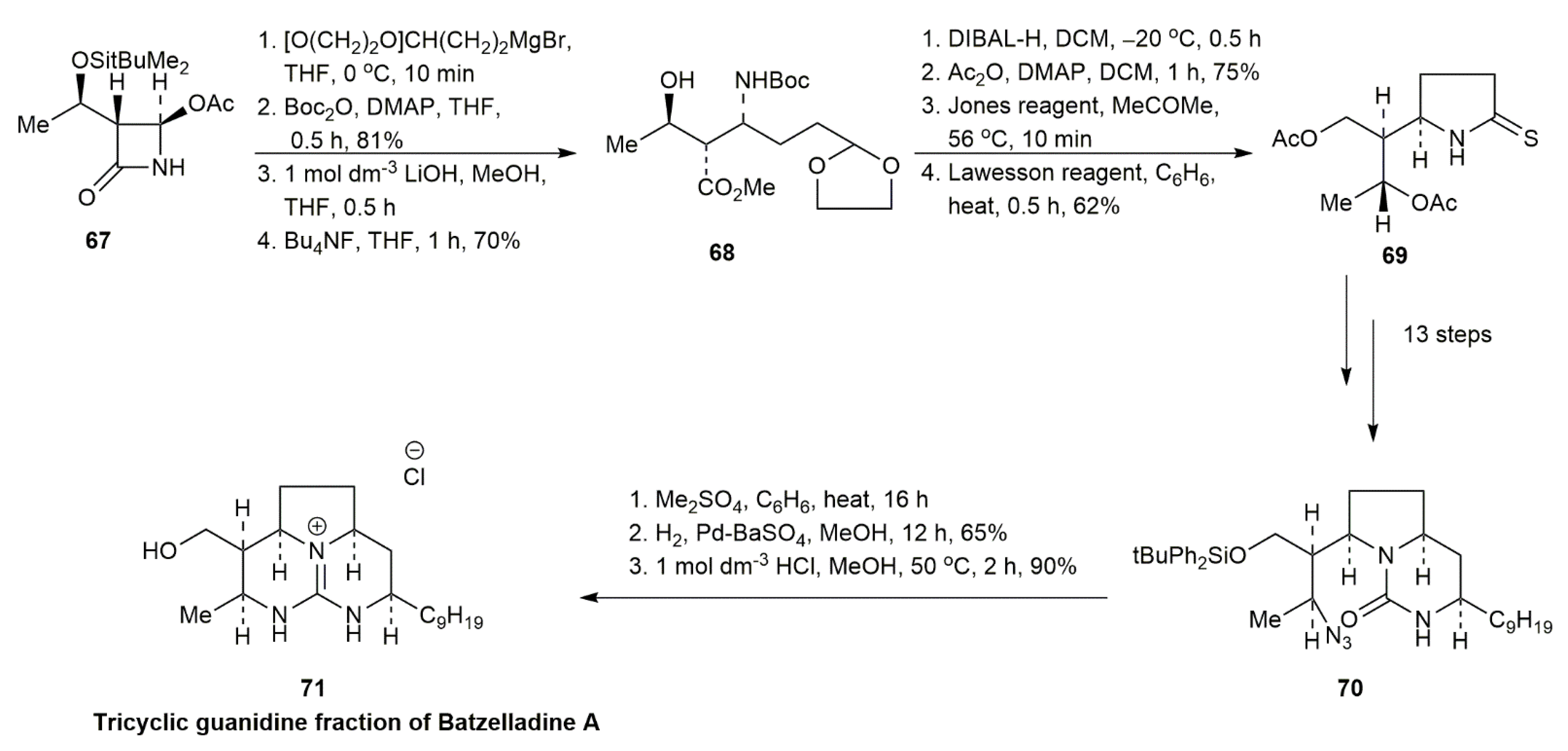
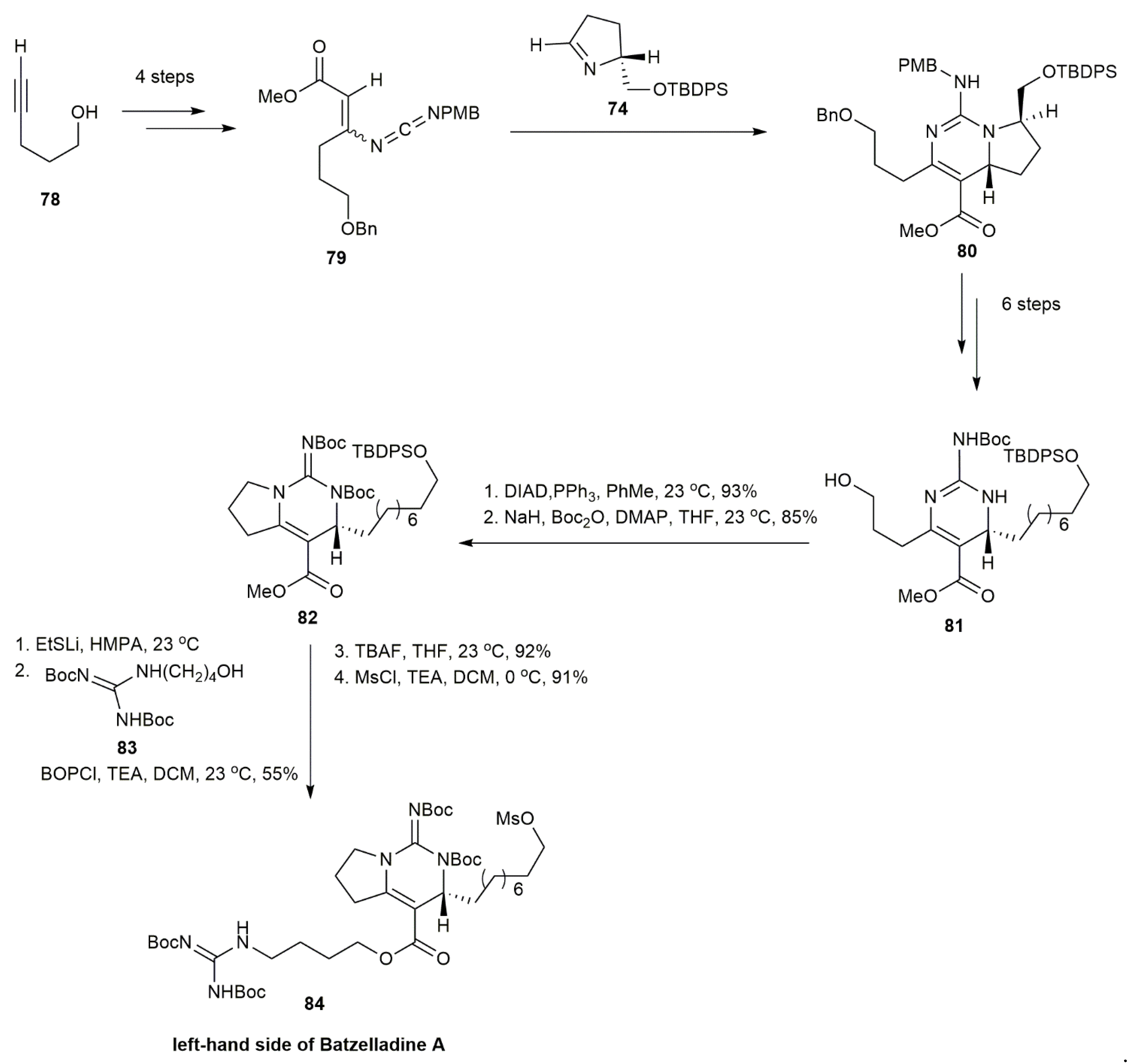
3.1.1. Batzelladine A
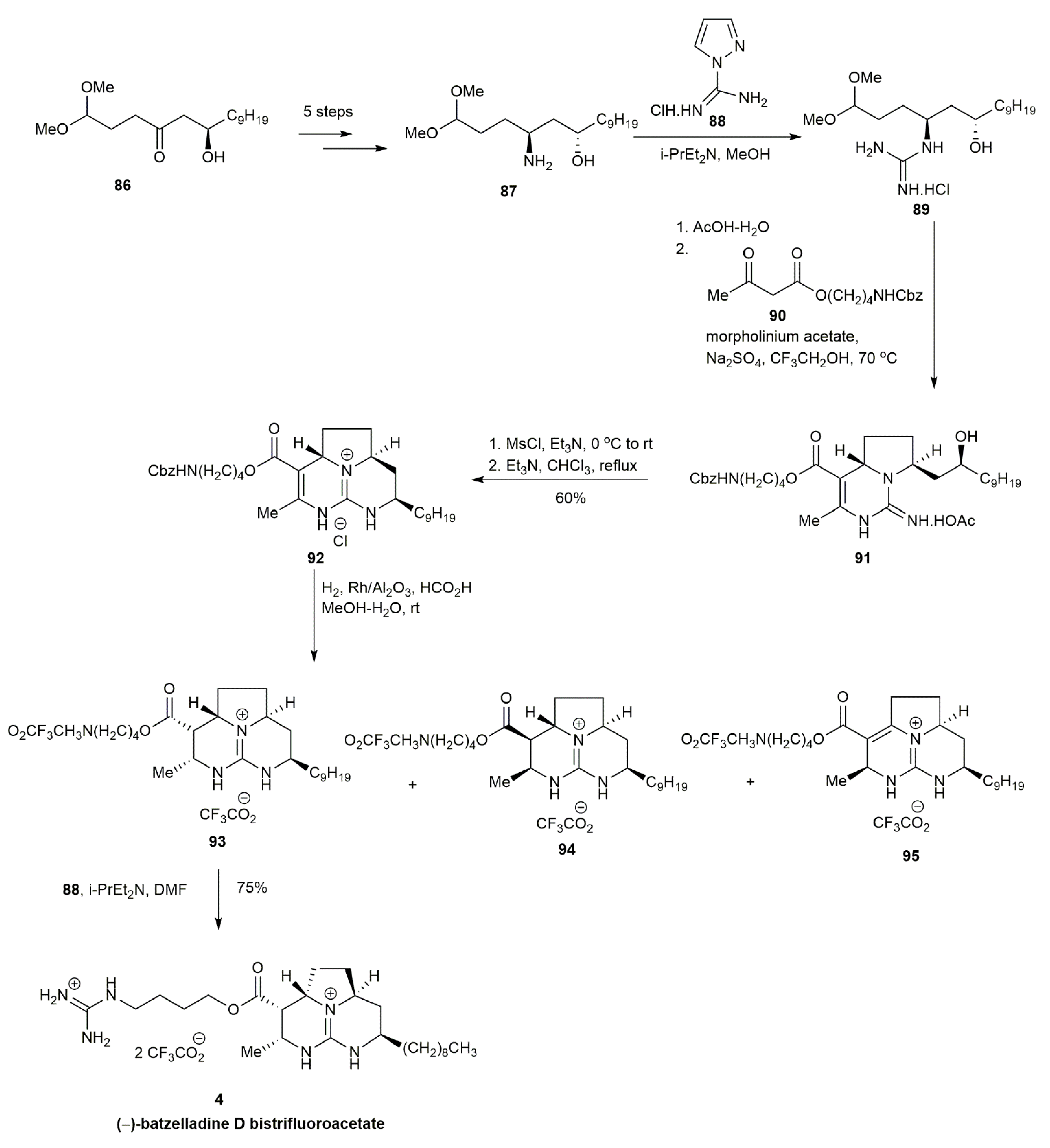
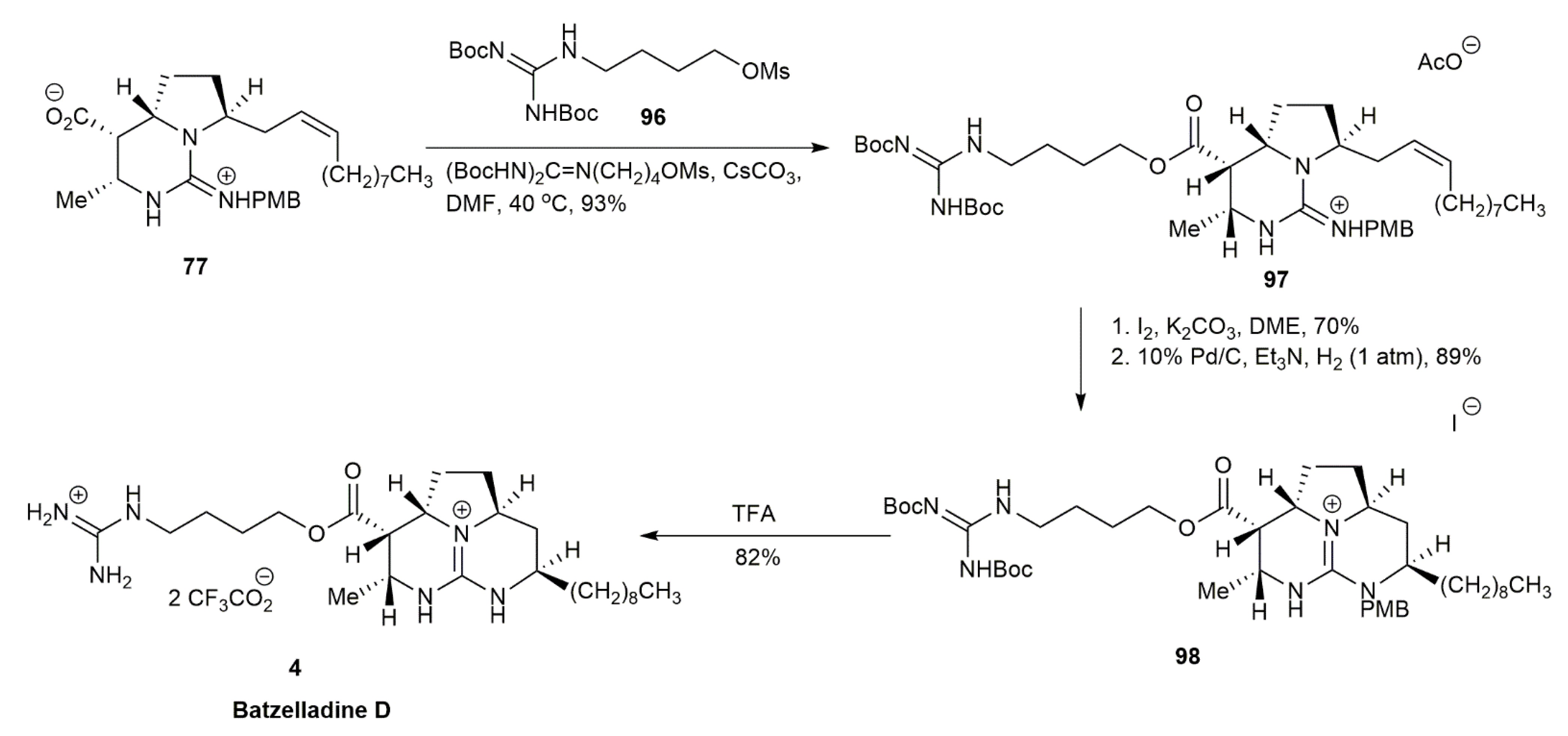
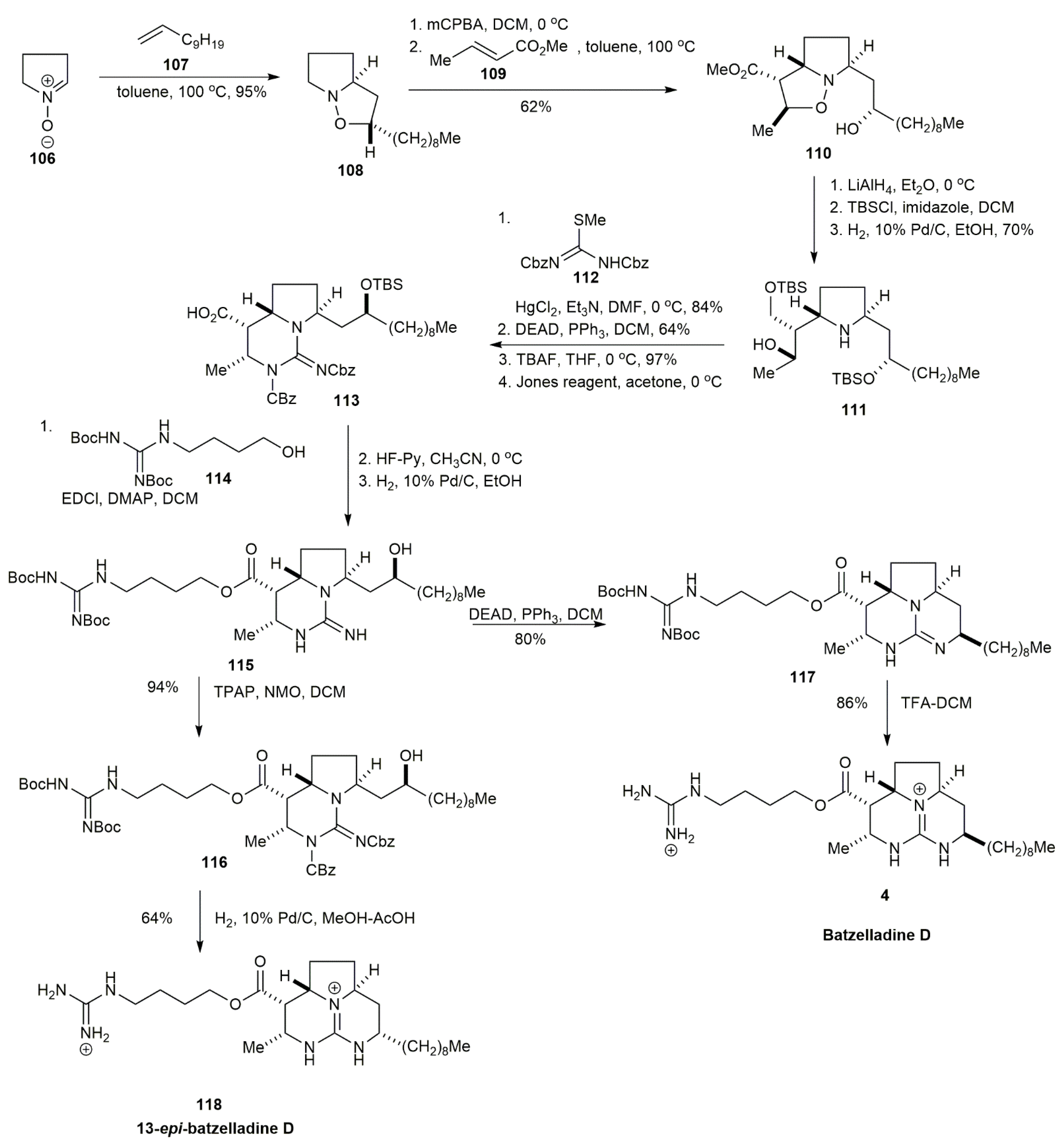
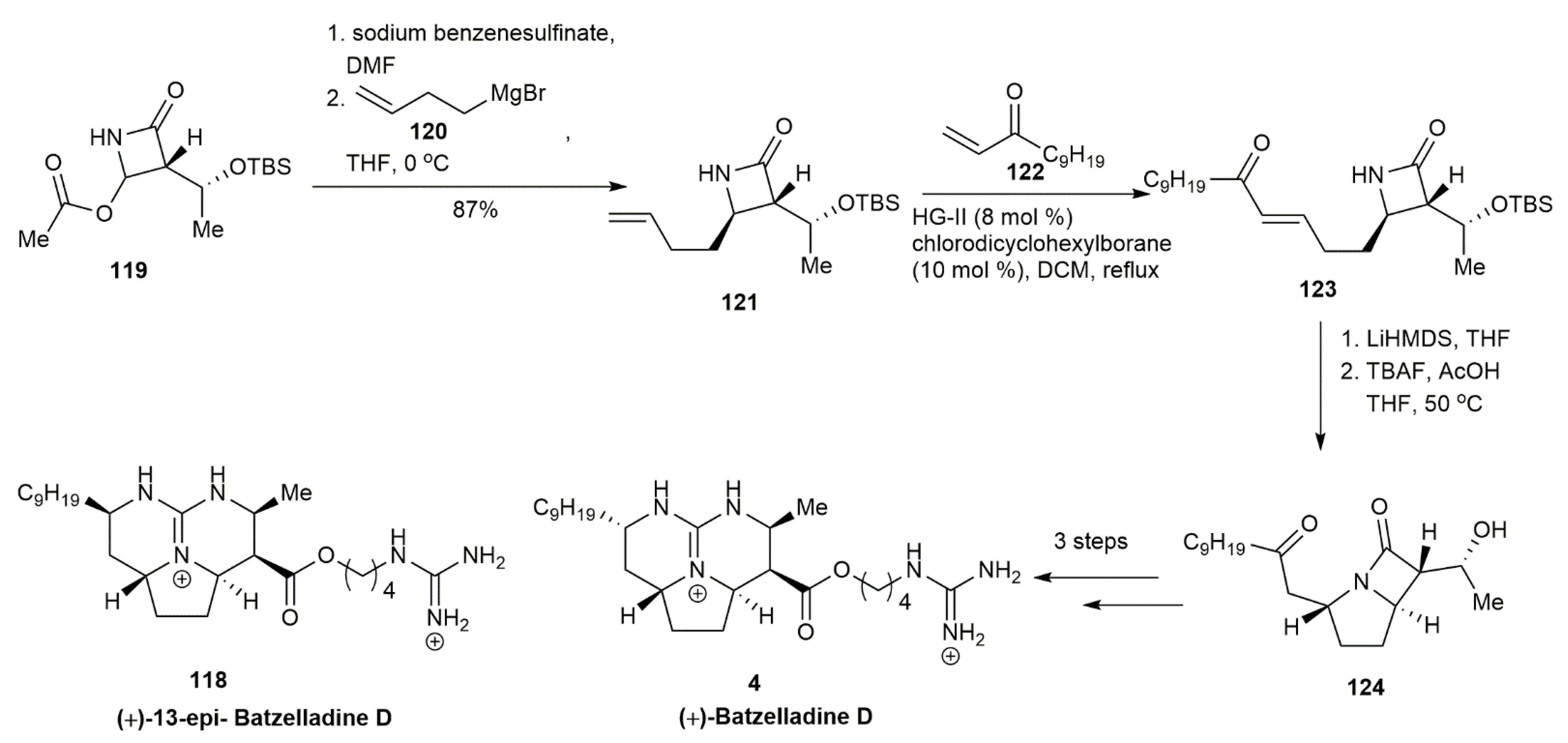
3.1.2. Batzelladine D
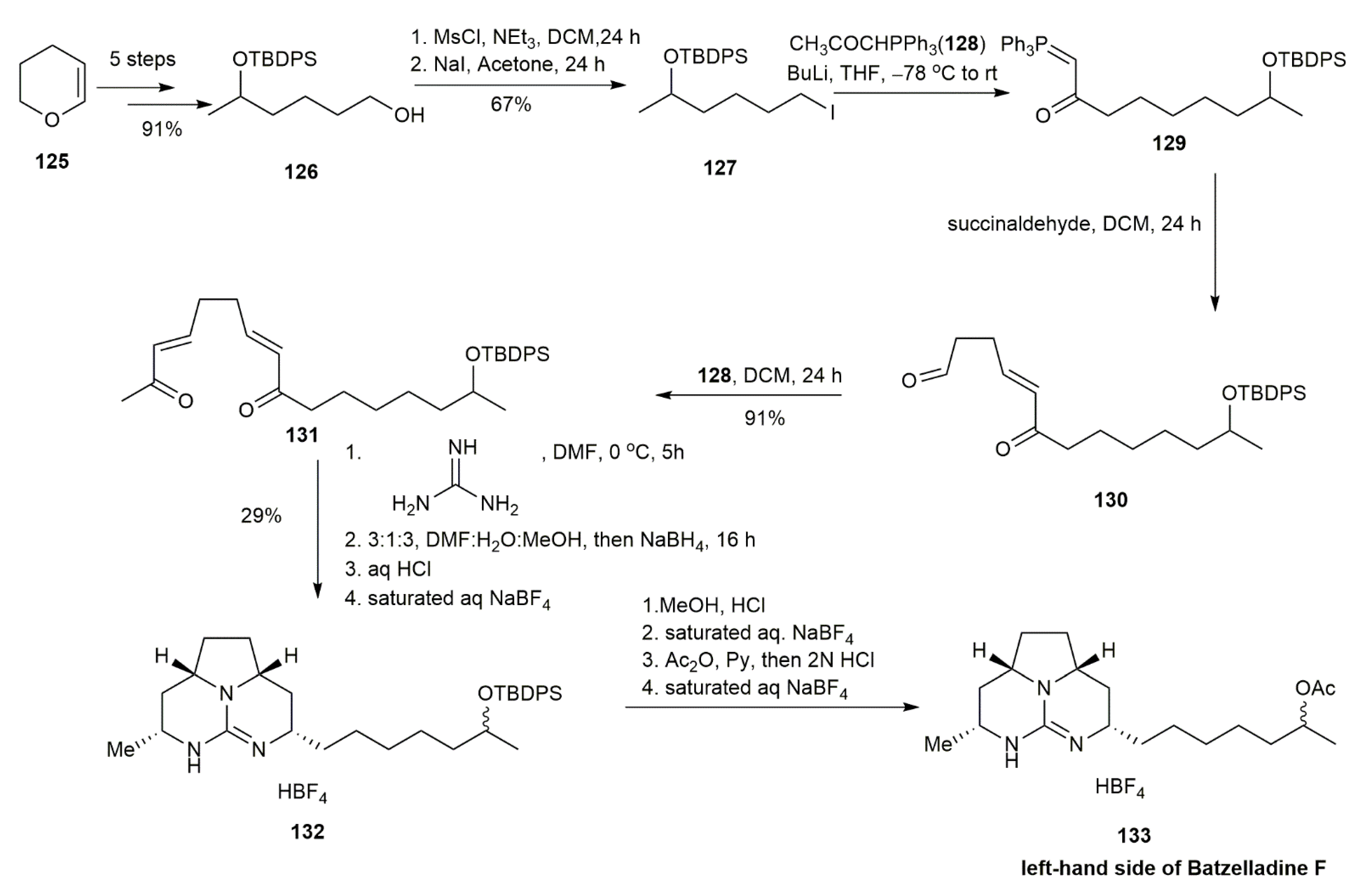
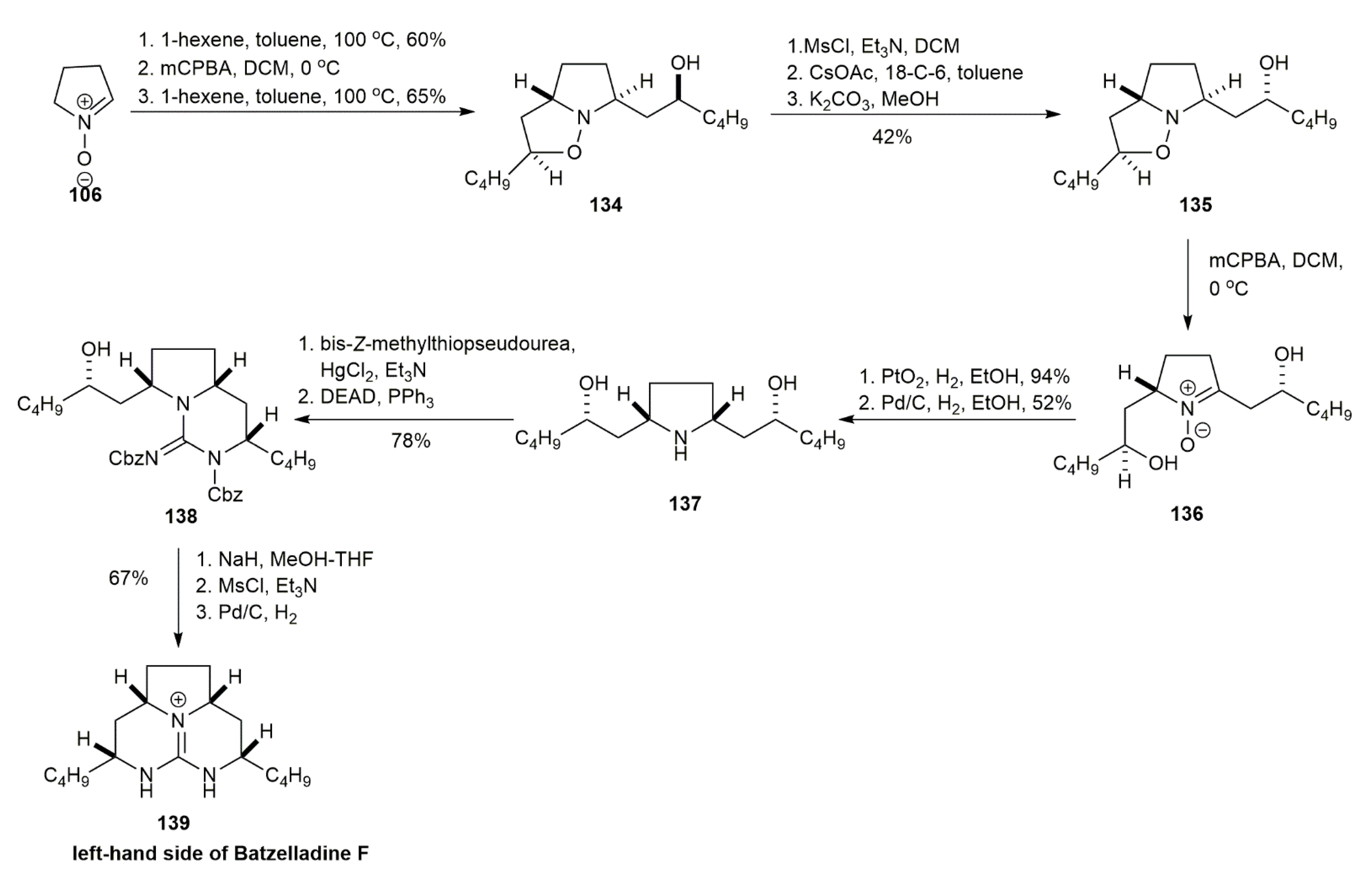
3.1.3. Batzelladine F
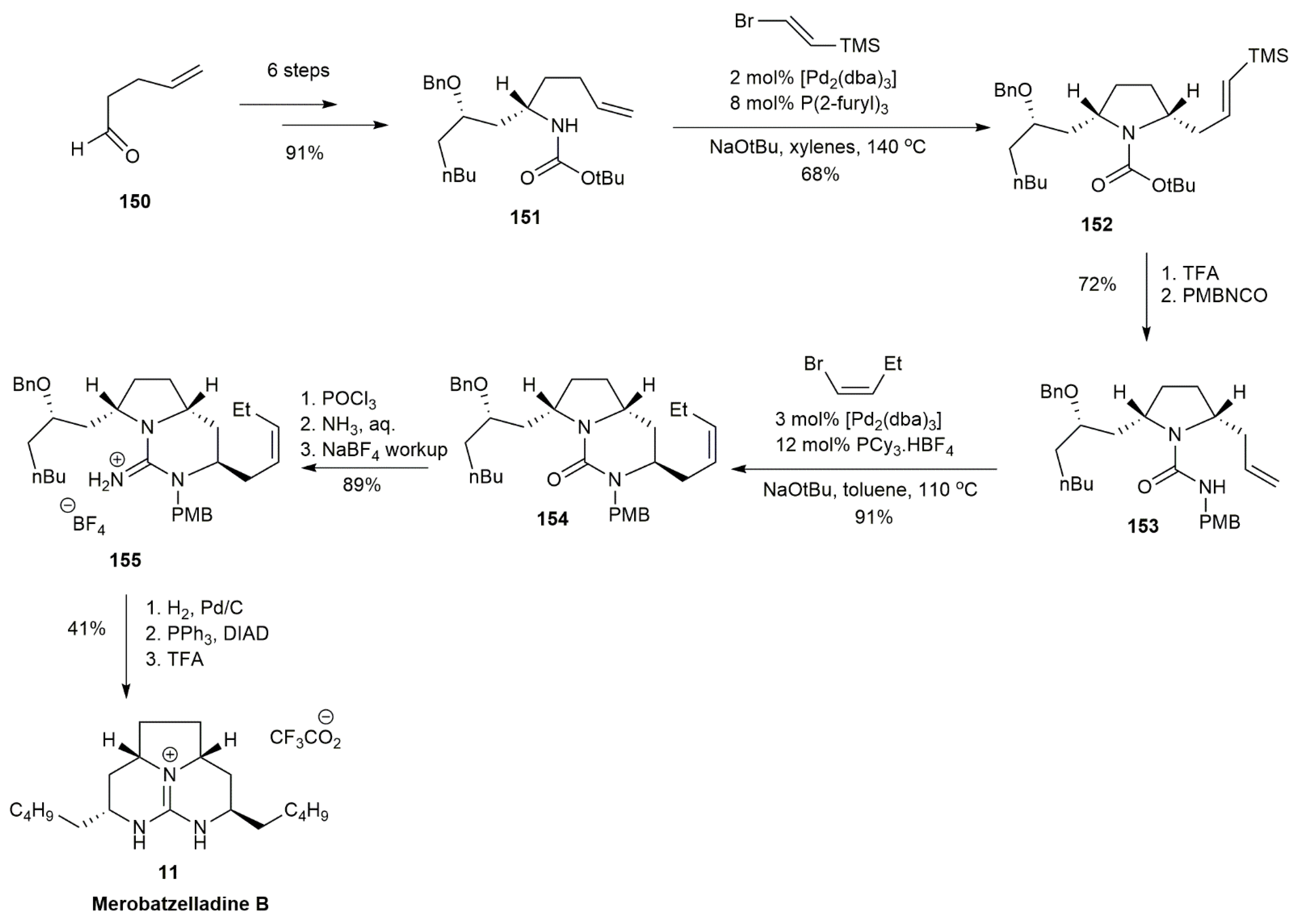
3.1.4. Merobatzelladine B
3.1.5. 9-Epi-batzelladine K
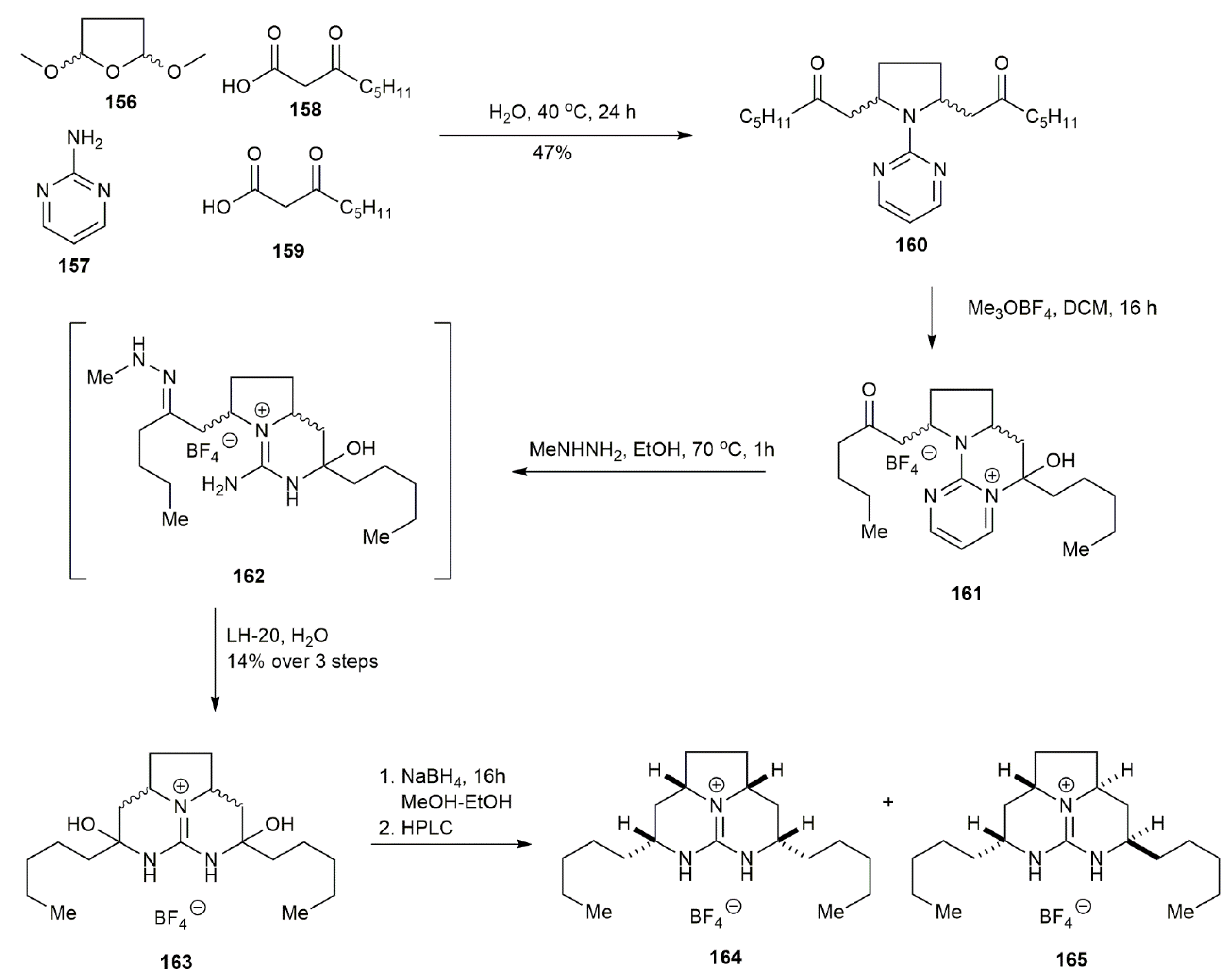
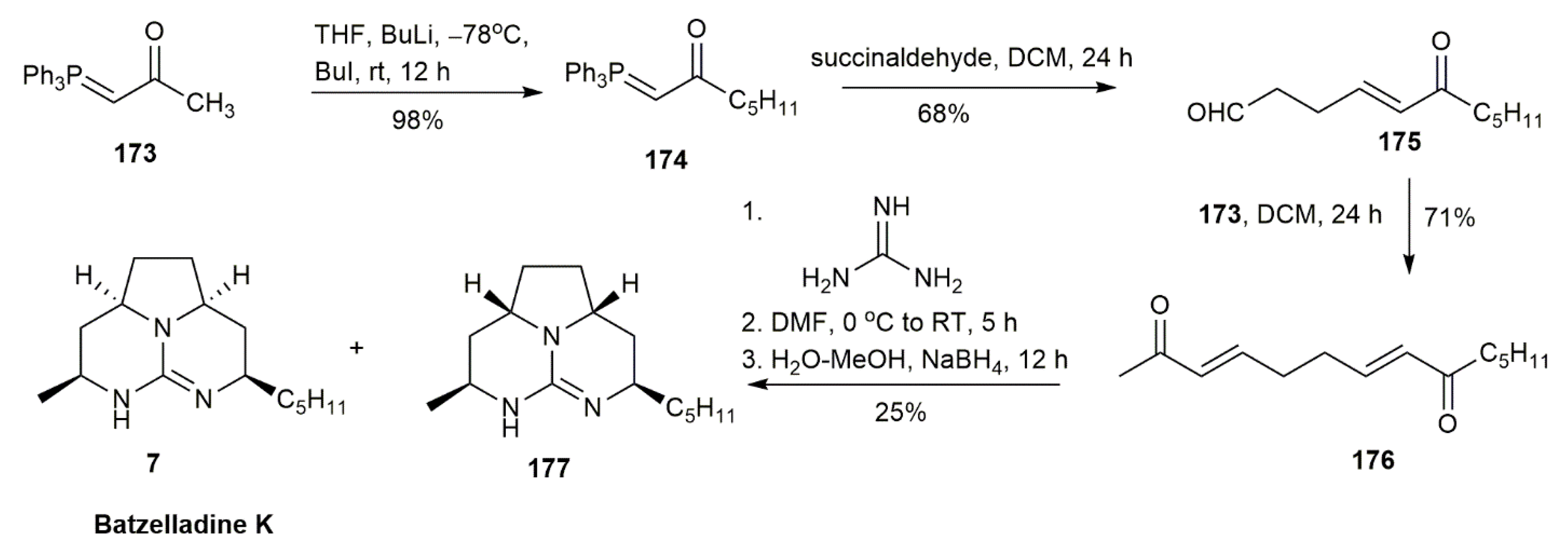
3.1.6. Batzelladine K
3.1.7. Batzelladine E
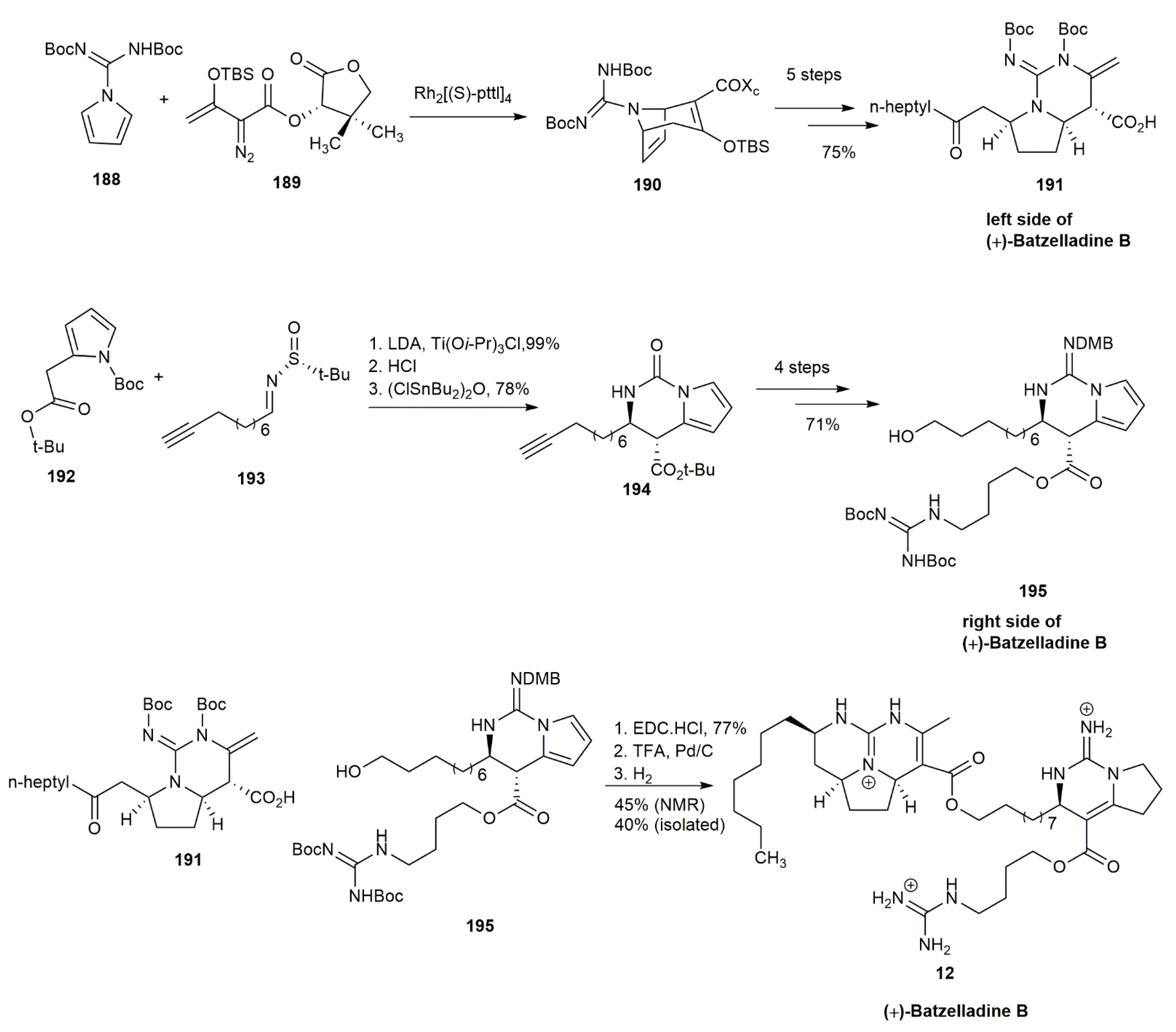
3.1.8. Batzelladine B
3.1.9. Batzelladine C Methyl Ester
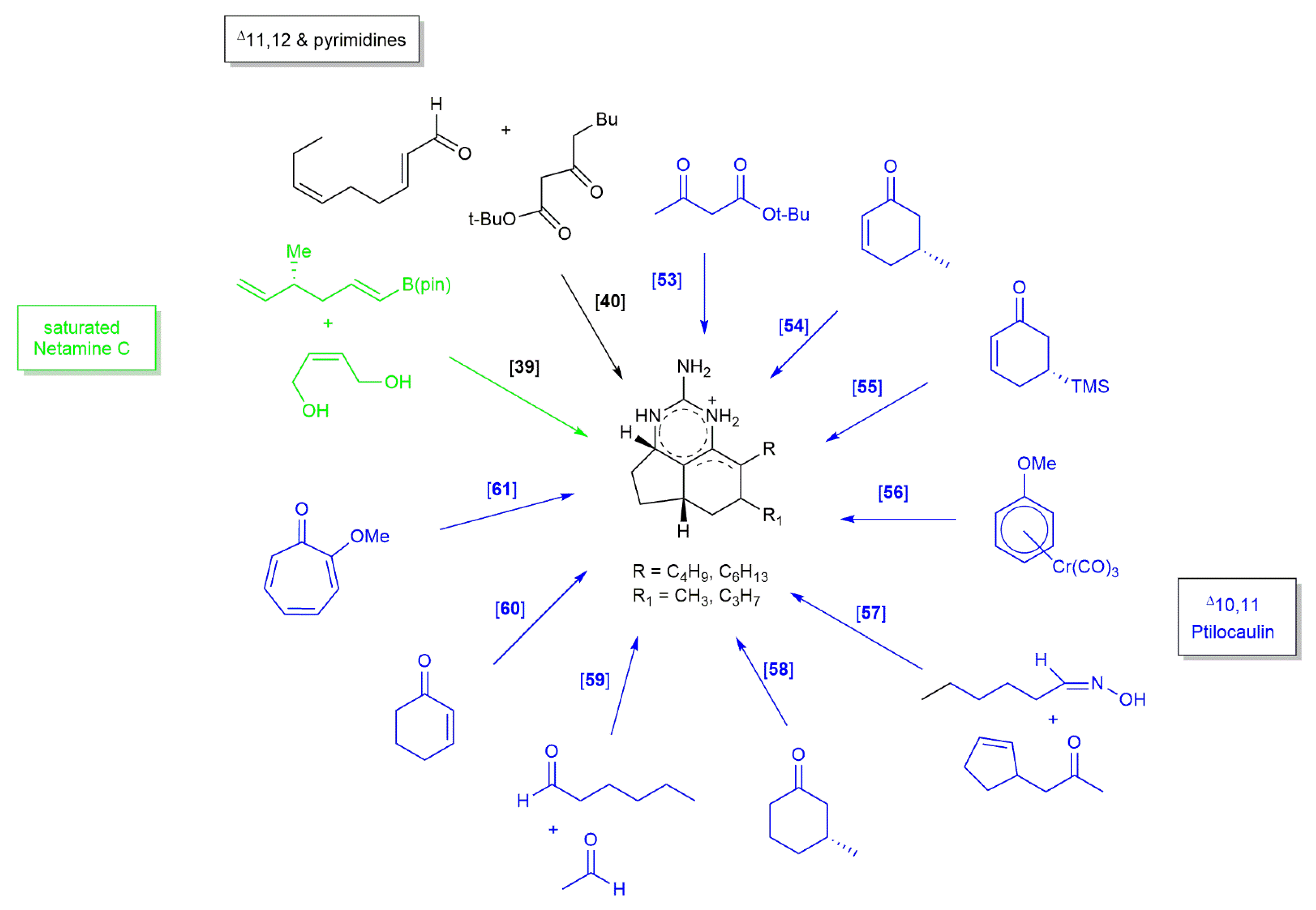
3.2. Ptilocaulin and Its Derivatives Skeleton
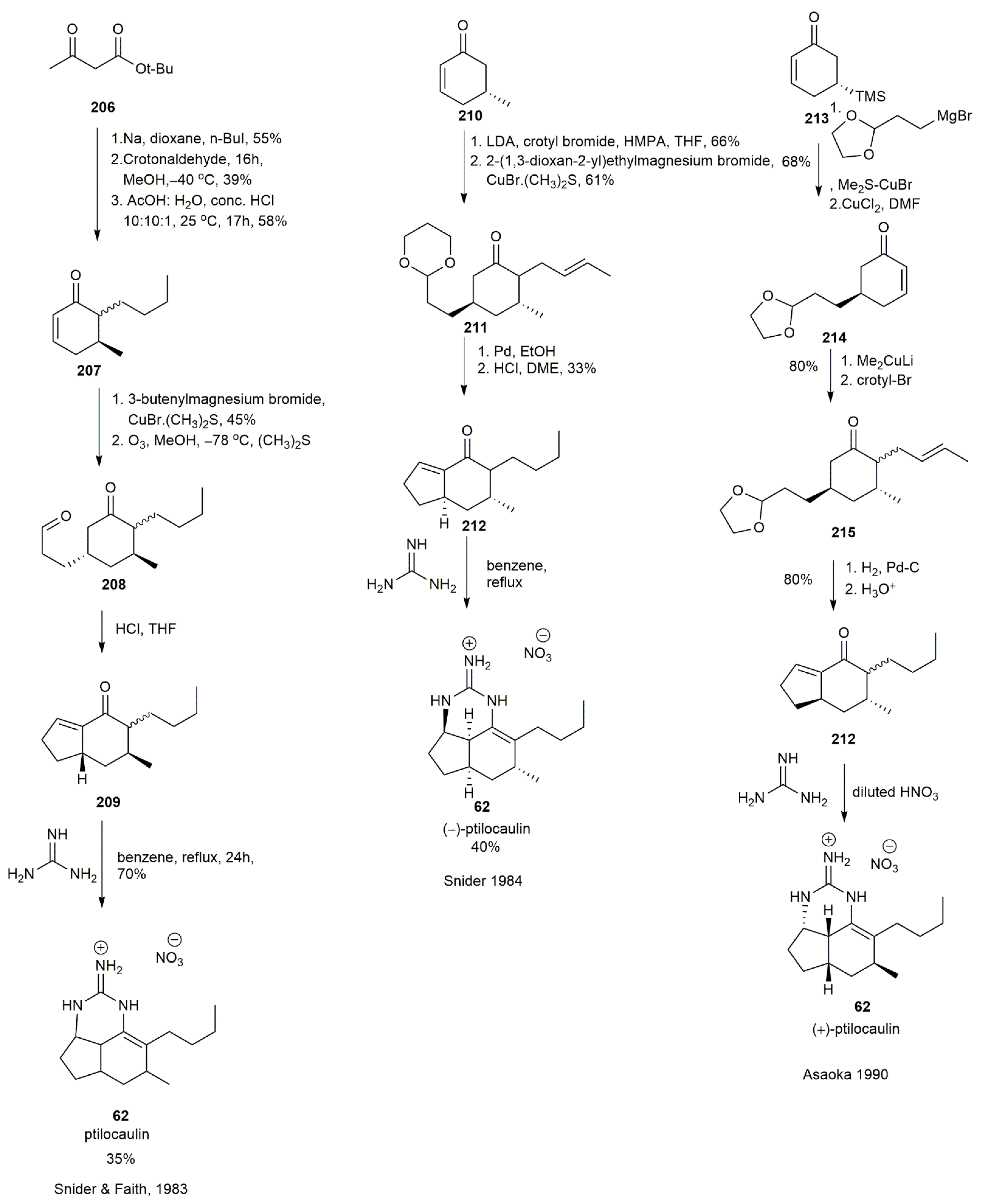
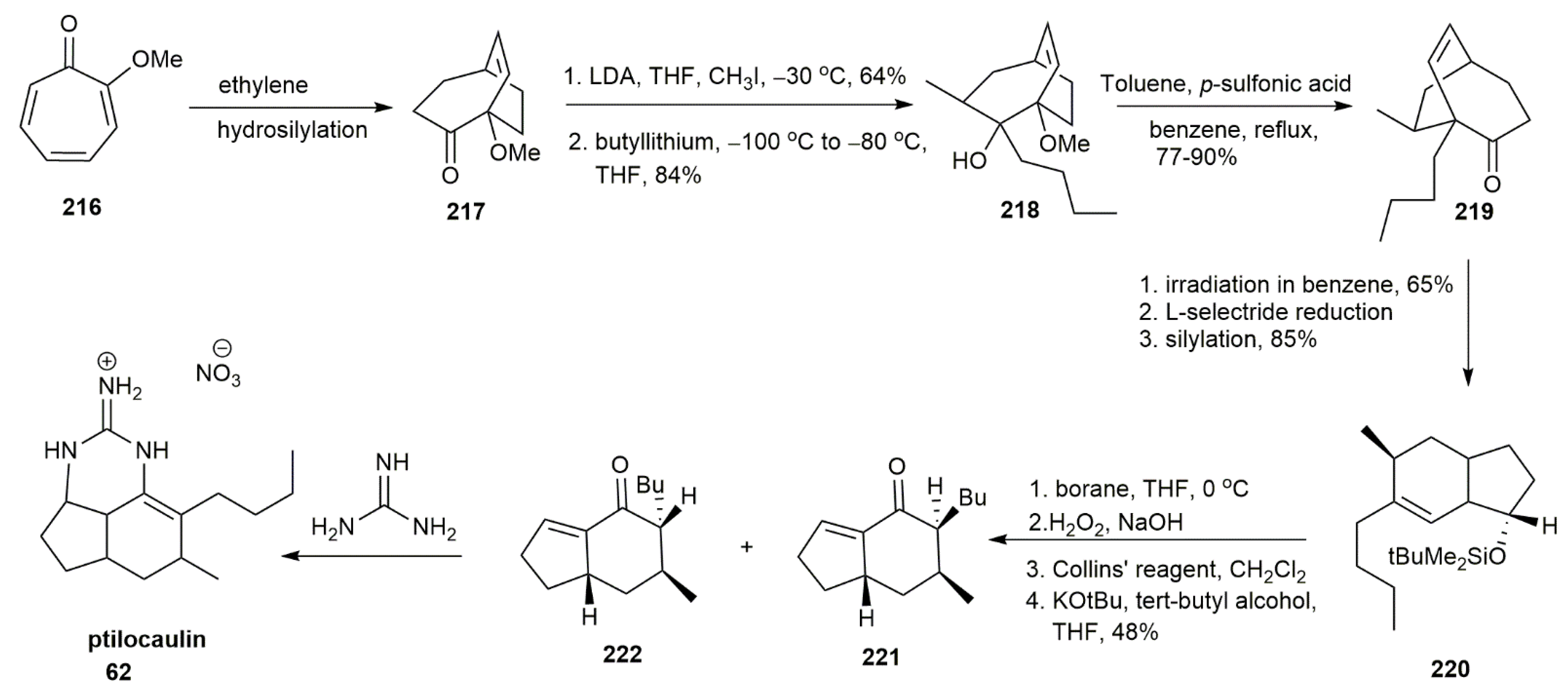
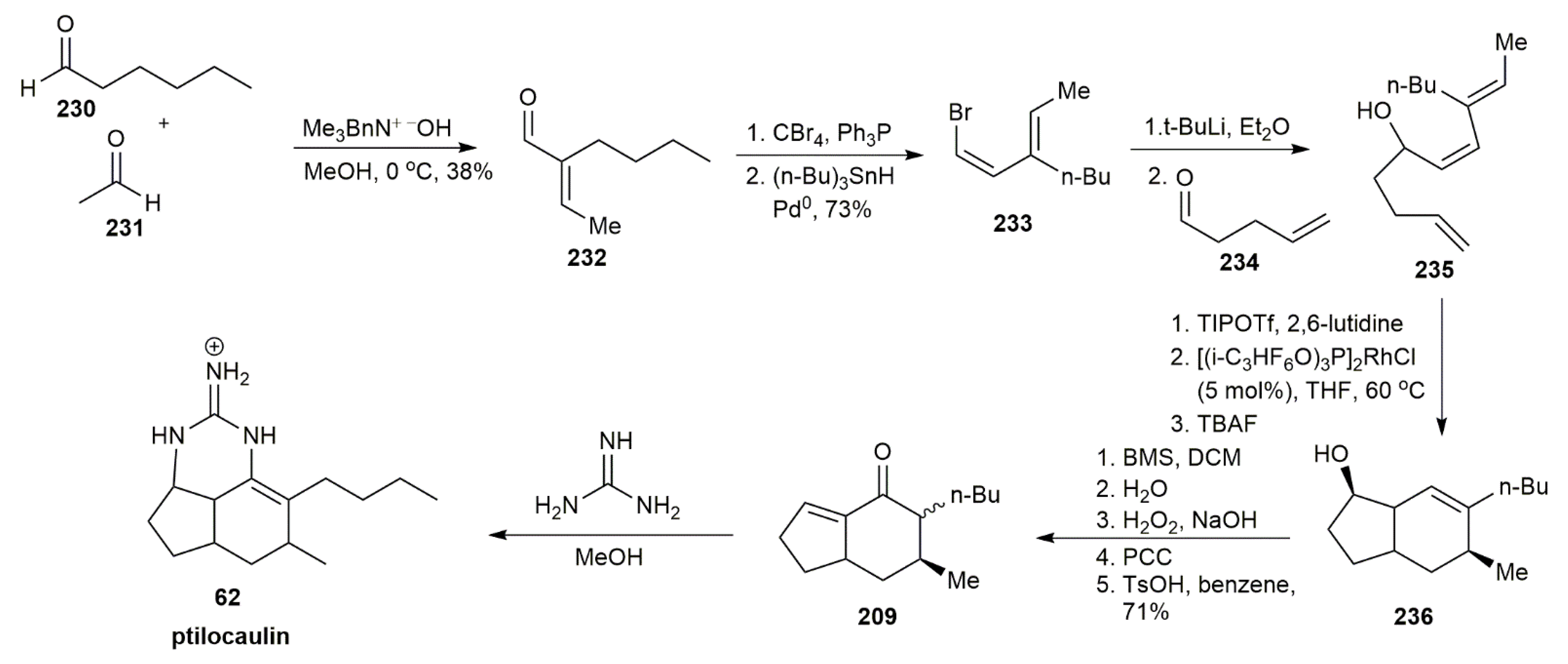
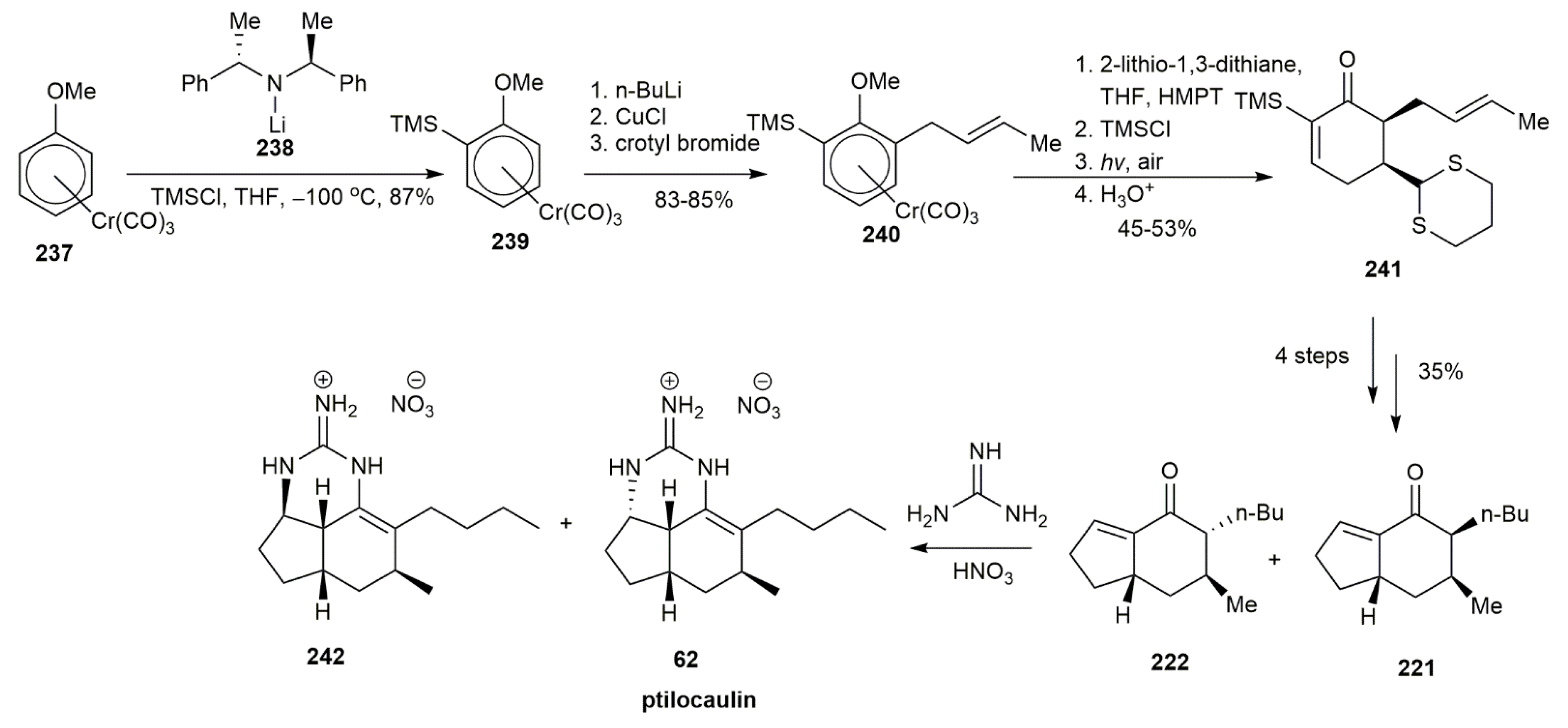
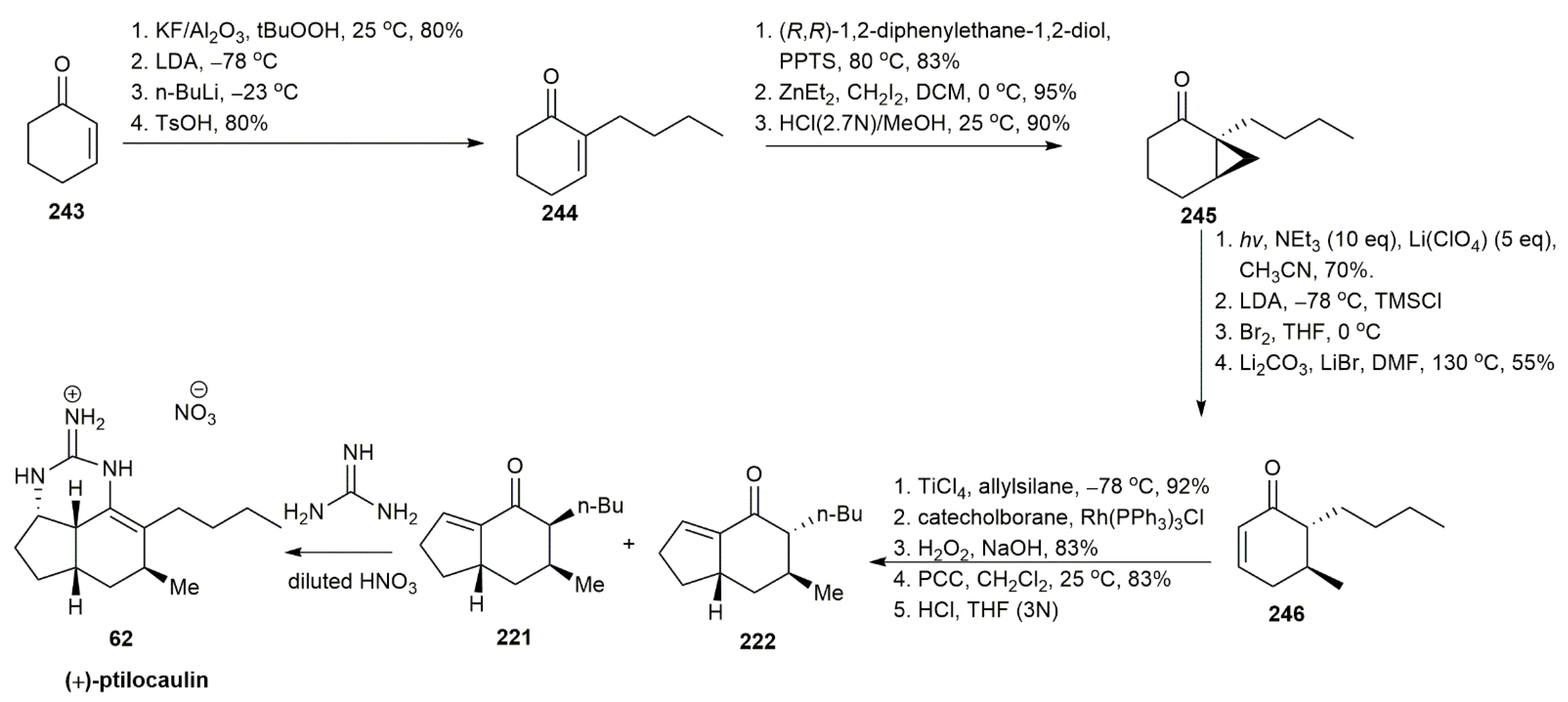
3.2.1. Ptilocaulin
3.2.2. 7-Epineoptilocaulin and Mirabilin B
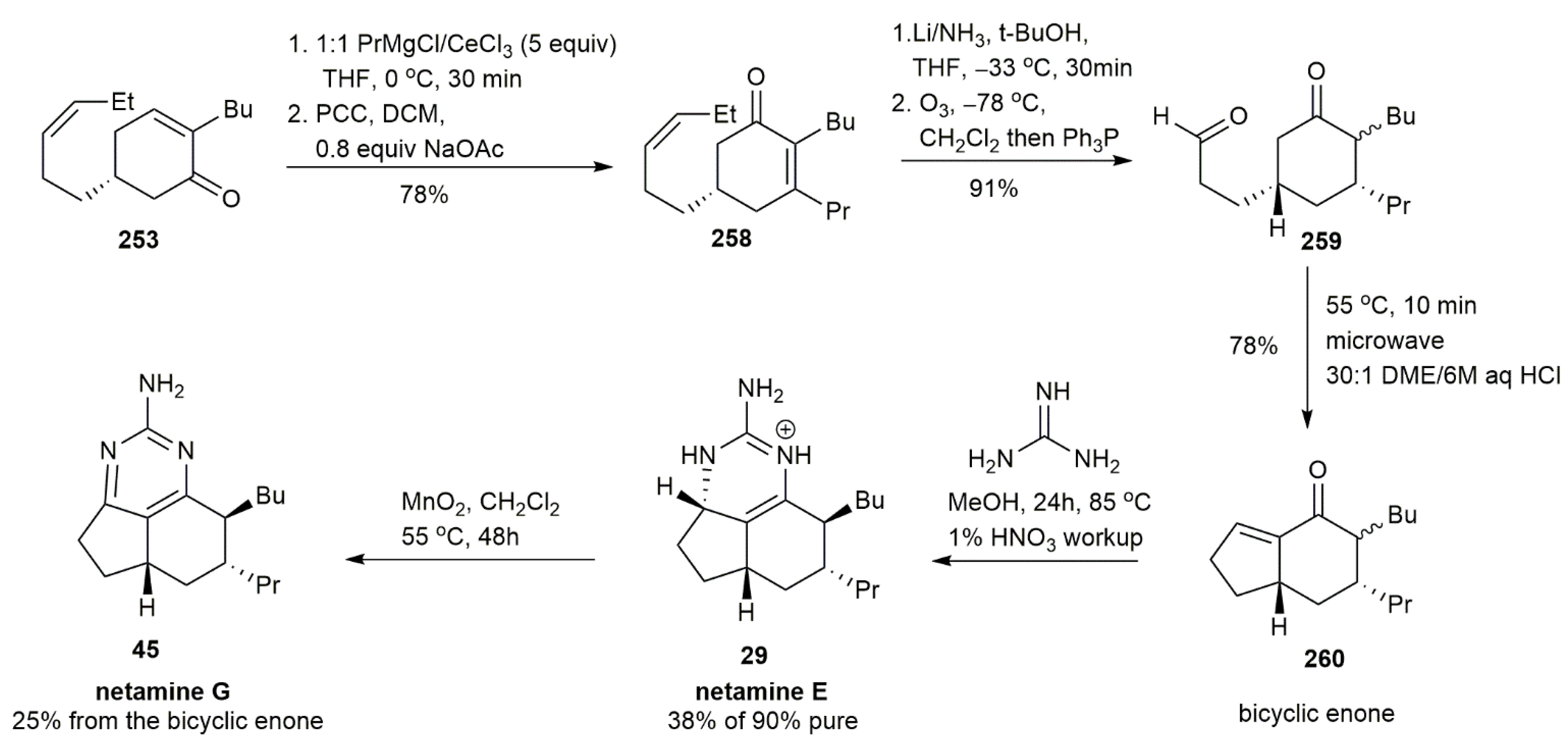
3.2.3. Netamine E and Netamine G
3.2.4. Netamine C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Patil, A.D.; Kumar, N.V.; Kokke, W.C.; Bean, M.F.; Freyer, A.J.; Brosse, C.D.; Mai, S.; Truneh, A.; Faulkner, D.J.; Carte, B.; et al. Novel Alkaloids from the Sponge Batzella sp. Inhibitors of HIV gp120-Human CD4 Binding. J. Org. Chem. 1995, 60, 1182–1188. [Google Scholar] [CrossRef]
- Laville, R.; Thomas, O.P.; Berrue, F.; Marquez, D.; Vacelet, J.; Amade, P. Bioactive Guanidine Alkaloids from Two Caribbean Marine Sponges. J. Nat. Prod. 2009, 72, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Day, K.A.; Duron, S.G.; Gin, D.Y. Total Synthesis of (+)-Batzelladine A and (−)-Batzelladine D via [4+2]-Annulation of Vinyl Carbodiimides with N-Alkyl Imines. J. Am. Chem. Soc. 2006, 128, 13255–13260. [Google Scholar] [CrossRef]
- Rama Rao, A.V.; Gurjar, M.K.; Vasudevan, J. An enantiospecific synthesis of the tricyclic guanidine segment of the anti-HIV marine alkaloid batzelladine A. J. Chem. Soc. Chem. Commun. 1995, 1369–1370. [Google Scholar] [CrossRef]
- Santos, M.F.C.; Harper, P.M.; Williams, D.E.; Mesquita, J.T.; Pinto, E.G.; da Costa-Silva, T.A.; Hajdu, E.; Ferreira, A.G.; Santos, R.A.; Murphy, P.J.; et al. Anti-parasitic Guanidine and Pyrimidine Alkaloids from the Marine Sponge Monanchora arbuscula. J. Nat. Prod. 2015, 78, 1101–1112. [Google Scholar] [CrossRef]
- Ishiwata, T.; Hino, T.; Koshino, H.; Hashimoto, Y.; Nakata, T.; Nagasawa, K. Total Synthesis of Batzelladine D. Org. Lett. 2002, 4, 2921–2924. [Google Scholar] [CrossRef]
- Cohen, F.; Overman, L.E.; Ly Sakata, S.K. Asymmetric Total Synthesis of Batzelladine D. Org. Lett. 1999, 1, 2169–2172. [Google Scholar] [CrossRef]
- Evans, P.A.; Qin, J.; Robinson, J.E.; Bazin, B. Enantioselective Total Synthesis of the Polycyclic Guanidine Containing Marine Alkaloid (-)-Batzelladine D. Angew. Chem. 2007, 46, 7417–7419. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Ribaucourt, A.; Moazami, Y.; Pierce, J.G. Concise Synthesis and Antimicrobial Evaluation of the Guanidinium Alkaloid Batzelladine D: Development of a Stereodivergent Strategy. J. Am. Chem. Soc. 2020, 142, 9850–9857. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Taylor, P.B.; Carte, B.; Zuber, G.; Johnson, R.K.; Faulkner, D.J. Batzelladines F-I, Novel Alkaloids from the Sponge Batzella sp.: Inducers of p56lck-CD4 Dissociation. J. Org. Chem. 1997, 62, 1814–1819. [Google Scholar] [CrossRef]
- Nagasawa, K.; Koshino, H.; Nakata, T. Stereoselective synthesis of tricyclic guanidine systems: Confirmation of the stereochemistry of batzelladine left-hand tricyclic guanidine portion. Tetrahedron Lett. 2001, 42, 4155–4158. [Google Scholar] [CrossRef]
- Cohen, F.; Overman, L.E. Evolution of a Strategy for the Synthesis of Structurally Complex Batzelladine Alkaloids. Enantioselective Total Synthesis of the Proposed Structure of Batzelladine F and Structural Revision. J. Am. Chem. Soc. 2006, 128, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Black, G.P.; Murphy, P.J.; Thornhill, A.J.; Walshe, N.D.A.; Zanetti, C. Synthesis of the left hand unit of batzelladine F; Revision of the reported relative stereochemistry. Tetrahedron 1999, 55, 6547–6554. [Google Scholar] [CrossRef]
- Cohen, F.; Overman, L.E. Enantioselective Total Synthesis of Batzelladine F and Definition of Its Structure. J. Am. Chem. Soc. 2006, 128, 2604–2608. [Google Scholar] [CrossRef]
- Ahmed, N.; Brahmbhatt, K.G.; Singh, I.P.; Bhutani, K.K. Total Synthesis of (±)-Batzelladine K: A Biomimetic Approach. Synthesis 2010, 2010, 2567–2570. [Google Scholar]
- Hua, H.M.; Peng, J.; Dunbar, D.C.; Schinazi, R.F.; Andrews, A.G.D.C.; Cuevas, C.; Garcia-Fernandez, L.F.; Kelly, M.; Hamann, M.T. Batzelladine alkaloids from the caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron 2007, 63, 11179–11188. [Google Scholar] [CrossRef]
- Takishima, S.; Ishiyama, A.; Iwatsuki, M.; Otoguro, K.; Yamada, H.; Omura, S.; Kobayashi, H.; van Soest, R.W.M.; Matsunaga, S. Merobatzelladines A and B, Anti-Infective Tricyclic Guanidines from a Marine Sponge Monanchora sp. Org. Lett. 2009, 11, 2655–2658. [Google Scholar] [CrossRef]
- Domingos, L.T.S.; Santos, M.F.C.; de Moraes, D.C.; de Sa, L.F.R.; da Silva, V.A.D.; Meuren, L.M.; Berlinck, R.G.S.; Ferreira-Pereira, A. Batzelladine D and Norbatzelladine L purified from marine sponge Monanchora arbuscula induce the reversal of fluconazole. Bioorganic Chem. 2020, 105, 104402. [Google Scholar] [CrossRef]
- Elgohary, A.M.; Elfiky, A.A.; Pereira, F.; Abd El-Aziz, T.M.; Sobeh, M.; Arafa, R.K.; El-Demerdash, A. Investigating the structure-activity relationship of marine polycyclic batzelladine alkaloids as promising inhibitors for SARS-CoV-2 main protease (Mpro). Comput. Biol. Med. 2022, 147, 105738. [Google Scholar] [CrossRef]
- Babij, N.R.; Wolfe, J.P. Asymmetric Total Synthesis of (+)-Merobatzelladine B. Angew. Chem. Int. Ed. 2012, 51, 4128–4130. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Ermolenko, L.; Gros, E.; Retailleau, P.; Thanh, B.N.; Gauvin-Bialecki, A.; Al-Mourabit, A. Short-Cut Bio-Inspired Synthesis of Tricyclic Guanidinic Motifs of Crambescidins and Batzelladines Marine Alkaloids. Eur. J. Org. Chem. 2020, 2020, 5677–5684. [Google Scholar] [CrossRef]
- Franklin, A.S.; Ly, S.K.; Mackin, G.H.; Overman, L.E.; Shaka, A.J. Application of the Tethered Biginelli Reaction for Enantioselective Synthesis of Batzelladine Alkaloids. Absolute Configuration of the Tricyclic Guanidine Portion of Batzelladine B. J. Org. Chem. 1999, 64, 1512–1519. [Google Scholar] [CrossRef]
- Parr, B.T.; Economou, C.; Herzon, S.B. A concise synthesis of (+)-batzelladine B from simple pyrrole-based starting materials. Nature 2015, 525, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Snider, B.B.; Chen, J. Synthesis of Batzelladine E and its E Isomer. Tetrahedron Lett. 1998, 39, 5697–5700. [Google Scholar] [CrossRef]
- Butters, M.; Davies, C.D.; Elliott, M.C.; Hill-Cousins, J.; Kariuki, B.M.; Ooi, L.; Wood, J.L.; Wordingham, S.V. Synthesis and stereochemical determination of batzelladine C methyl ester. Org. Biomol. Chem. 2009, 7, 5001–5009. [Google Scholar] [CrossRef]
- Gallimore, W.A.; Kelly, M.; Scheuer, P.J. Alkaloids from the Sponge Monanchora unguifera. J. Nat. Prod. 2005, 68, 1420–1423. [Google Scholar] [CrossRef]
- Harbour, G.C.; Tymiak, A.A.; Rinehart, K.L.; Shaw, P.D.; Hughes, R.G.; Mizsak, S.A.; Coats, J.H.; Zurenko, G.E.; Li, L.H.; Kuentzel, S.L. Ptilocaulin and isoptilocaulin, antimicrobial and cytotoxic cyclic guanidines from the Caribbean sponge Ptilocaulis aff. P. spiculifer (Lamarck, 1814). J. Am. Chem. Soc. 1981, 103, 5604–5606. [Google Scholar] [CrossRef]
- Tavares, R.; Daloze, D.; Braekman, J.C. 8b-hydroxyptilocaulin, A New Guanidine Alkaloid from the Sponge Monanchora Arbuscula. J. Nat. Prod. 1995, 58, 1139–1142. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Offen, P.; Bean, M.F.; Johnson, R.K. Three New Tricyclic Guanidine Alkaloids from the Sponge Batzella sp. J. Nat. Prod. 1997, 60, 704–707. [Google Scholar] [CrossRef]
- Hua, H.M.; Peng, J.; Fronczek, F.R.; Kelly, M.; Hamann, M.T. Crystallographic and NMR studies of antiinfective tricyclic guanidine alkaloids from the sponge Monanchora unguifera. Bioorganic Med. Chem. 2004, 12, 6461–6464. [Google Scholar] [CrossRef]
- Barrow, R.A.; Murray, L.M.; Lim, T.K.; Capon, R.J. Mirabilins (A-F): New Alkaloids from a Southern Australian Marine Sponge, Arenochalina mirabilis. Aust. J. Chem. 1996, 49, 767–773. [Google Scholar] [CrossRef]
- Capon, R.J.; Miller, M.; Rooney, F. Mirabilin G: A New Alkaloid from a Southern Australian Marine Sponge, Clathria species. J. Nat. Prod. 2001, 64, 643–644. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.; Conte, M.; Capon, R.J. Mirabilins revisited: Polyketide alkaloids from a southern Australian marine sponge, Clathria sp. Org. Biomol. Chem. 2010, 8, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Sorek, H.; Rudi, A.; Gueta, S.; Reyes, F.; Martin, M.J.; Aknin, M.; Gaydou, E.; Vacelet, J.; Kashman, Y. Netamines A-G: Seven new tricyclic guanidine alkaloids from the marine sponge Biemna laboutei. Tetrahedron 2006, 62, 8838–8843. [Google Scholar] [CrossRef]
- Gros, E.; Al-Mourabit, A.; Martin, M.T.; Corres, J.; Vacelet, J.; Frederich, M.; Aknin, M.; Kashman, Y.; Gauvin-Bialecki, A. Netamines H-N, Tricyclic Alkaloids from the Marine Sponge Biemna laboutei and Their Antimalarial Activity. J. Nat. Prod. 2014, 77, 818–823. [Google Scholar] [CrossRef]
- Gros, E.; Martin, M.T.; Sorres, J.; Moriou, C.; Vacelet, J.; Frederich, M.; Aknin, M.; Kashman, Y.; Gauvin-Bialecki, A.; Al-Mourabit, A. Netamines O-S, Five New Tricyclic Guanidine Alkaloids from the Madagascar Sponge Biemna laboutei, and Their Antimalarial Activities. Chem. Biodivers. 2015, 12, 1725–1733. [Google Scholar] [CrossRef]
- Grkovic, T.; Blees, J.S.; Bayer, M.M.; Colburn, N.H.; Thomas, C.L.; Henrich, C.J.; Peach, M.L.; McMahon, J.B.; Schmid, T.; Gustafson, K.R. Tricyclic Guanidine Alkaloids from the Marine Sponge Acanthella cavernosa that Stabilize the Tumor Suppressor PDCD4. Mar. Drugs 2014, 12, 4593–4601. [Google Scholar] [CrossRef]
- Ramadhan, D.S.F.; Siharis, F.; Abdurrahman, S.; Isrul, M.; Fakih, T.M. In silico analysis of marine natural product from sponge (Clathria Sp.) for their activity as inhibitor of SARS-CoV-2 Main Protease. J. Biomol. Struct. Dyn. 2021. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Y.; Shi, Y.; del Pozo, J.; Torker, S.; Hoveyda, A.H. Copper-Hydride-Catalyzed Enantioselective Processes with Allenyl Boronates. Mechanistic Nuances, Scope, and Utility in Target-Oriented Synthesis. J. Am. Chem. Soc. 2019, 141, 12087–12099. [Google Scholar] [CrossRef]
- Yu, M.; Pochapsky, S.S.; Snider, B.B. Synthesis of 7-Epineoptilocaulin, Mirabilin B. and Isoptilocaulin. A Unified Biosynthetic Proposal for the Ptilocaulin and Batzelladine Alkaloids. Synthesis and Structure Revision of Netamines E and G. J. Org. Chem. 2008, 73, 9065–9074. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.G.; Wilke, D.V.; Jimenez, P.C.; Oliveira, J.R.D.; Pessoa, O.D.L.; Silveira, E.R.; Viana, F.A.; Pessoa, C.; Moraes, M.O.D.; Hajdu, E.; et al. Guanidine Alkaloids from Monanchora arbuscula: Chemistry and Antitumor Potential. Chem. Biodivers. 2011, 8, 1433–1445. [Google Scholar] [CrossRef]
- Ruben, R.L.; Snider, B.B.; Hobbs, F.W., Jr.; Confalone, P.N.; Dusak, B.A. Cytotoxicity of synthetic racemic ptilocaulin: A novel cyclic guanidine. Investig. New Drugs 1989, 7, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Snider, B.B.; Shi, Z. Biomimetic synthesis of the bicyclic guanidine moieties of crambines A and B. J. Org. Chem. 1992, 57, 2526–2528. [Google Scholar] [CrossRef]
- Saito, T.; Ohkubo, T.; Kuboki, H.; Maeda, M.; Tsuda, K.; Karakasa, T.; Satsumabayashi, S. Thermal or Lewis acid-promoted electrocyclisation and hetero Diels–Alder cycloaddition of α,β-unsaturated(conjugated) carbodiimides: A facile synthesis of nitrogen-containing heterocycles. J. Chem. Soc. Perkin Trans. 1 1998, 18, 3065–3080. [Google Scholar] [CrossRef]
- Murphy, P.J.; Williams, H.L.; Hibbs, D.E.; Hursthouse, M.B.; Abdul Malik, K.M. Biomimetic Model Studies towards Ptilomycalin A. Tetrahedron 1996, 52, 8315–8332. [Google Scholar] [CrossRef]
- Black, G.P.; Murphy, P.J.; Walshe, N.D. A short synthetic route to the tricyclic guanidium core of the batzelladine alkaloids. Tetrahedron 1998, 54, 9481–9488. [Google Scholar] [CrossRef]
- Robinson, R. LXIII.—A synthesis of tropinone. J. Chem. Soc. Trans. 1917, 111, 762–768. [Google Scholar] [CrossRef]
- Babij, N.R.; Wolfe, J.P. Desymmetrization of meso-2,5-Diallylpyrrolidinyl Ureas through Asymmetric Palladium-Catalyzed Carboamination: Stereocontrolled Synthesis of Bicyclic Ureas. Angew. Chem. 2013, 125, 9417–9420. [Google Scholar] [CrossRef][Green Version]
- Snider, B.B.; Shi, Z. Biomimetic synthesis of the pentacyclic nucleus of ptilomycalin A. J. Am. Chem. Soc. 1994, 116, 549–557. [Google Scholar] [CrossRef]
- Hong, C.Y.; Kishi, Y. Enantioselective Total Synthesis of (−)-Decarbamoylsaxitoxin. J. Am. Chem. Soc. 1992, 114, 7001–7006. [Google Scholar] [CrossRef]
- Arnold, M.A.; Duron, S.G.; Gin, D.Y. Diastereoselective [4+2] Annulation of Vinyl Carbodiimides with N-Alkyl Imines. Asymmetric Synthetic Access to the Batzelladine Alkaloids. J. Am. Chem. Soc. 2005, 127, 6924–6925. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.C.; Long, M.S. Studies towards the total synthesis of Batzelladine A: Synthesis of a model pyrrolo[1,2-c]pyrimidine. Tetrahedron Lett. 2002, 43, 9191–9194. [Google Scholar] [CrossRef]
- Snider, B.B.; Faith, W.C. The total synthesis of (±)-ptilocaulin. Tetrahedron Lett. 1983, 24, 861–864. [Google Scholar] [CrossRef]
- Snider, B.B.; Faith, W.C. Total Synthesis of (±)- and (−)-Ptilocaulin. J. Am. Chem. Soc. 1984, 106, 1443–1445. [Google Scholar] [CrossRef]
- Asaoka, M.; Sakurai, M.; Takei, H. Total Synthesis of (+)-Ptilocaulin. Tetrahedron Lett. 1990, 31, 4759–4760. [Google Scholar] [CrossRef]
- Schellhaas, K.; Schmalz, H.-G.; Bats, J.W. Chiral [η6-Arene–Cr(CO)3] Complexes as Synthetic Building Blocks: A Short Enantioselective Total Synthesis of (+)-Ptilocaulin. Chem. Eur. J. 1998, 4, 57–66. [Google Scholar] [CrossRef]
- Murthy, K.S.K.; Hassner, A. Stereoselective Total Synthesis of (±)-Ptilocaulin and its 7-Epimer. A Strategy Based On the Use of an Intramolecular Nitrile Oxide Olefin Cycloaddition (INOC) Reaction. Isr. J. Chem. 1991, 31, 239–246. [Google Scholar] [CrossRef]
- Roush, W.R.; Walts, A.E. Total Synthesis of (−)-Ptilocaulin. J. Am. Chem. Soc. 1984, 106, 721–723. [Google Scholar] [CrossRef]
- Shen, K.; Livinghouse, T. A Stereocontrolled Synthesis of (±)-Ptilocaulin via a Rh(I)-Catalyzed Intramolecular [4+2] Cycloaddition. Synlett 2010, 2010, 247–249. [Google Scholar]
- Cossy, J.; BouzBouz, S. A Short Access to (+)-Ptilocaulin. Tetrahedron Lett. 1996, 37, 5091–5094. [Google Scholar] [CrossRef]
- Uyehara, T.; Furuta, T.; Kabasawa, Y.; Yamada, J.; Kato, T. Total synthesis of (±)-ptilocaulin starting from tropolone. J. Chem. Soc. Chem. Commun. 1986, 539–540. [Google Scholar] [CrossRef]
- Cossy, J.; Furet, N. Photochemical ring opening of cyclopropyl ketones induced by electron transfer. Tetrahedron Lett. 1993, 34, 8107–8110. [Google Scholar] [CrossRef]
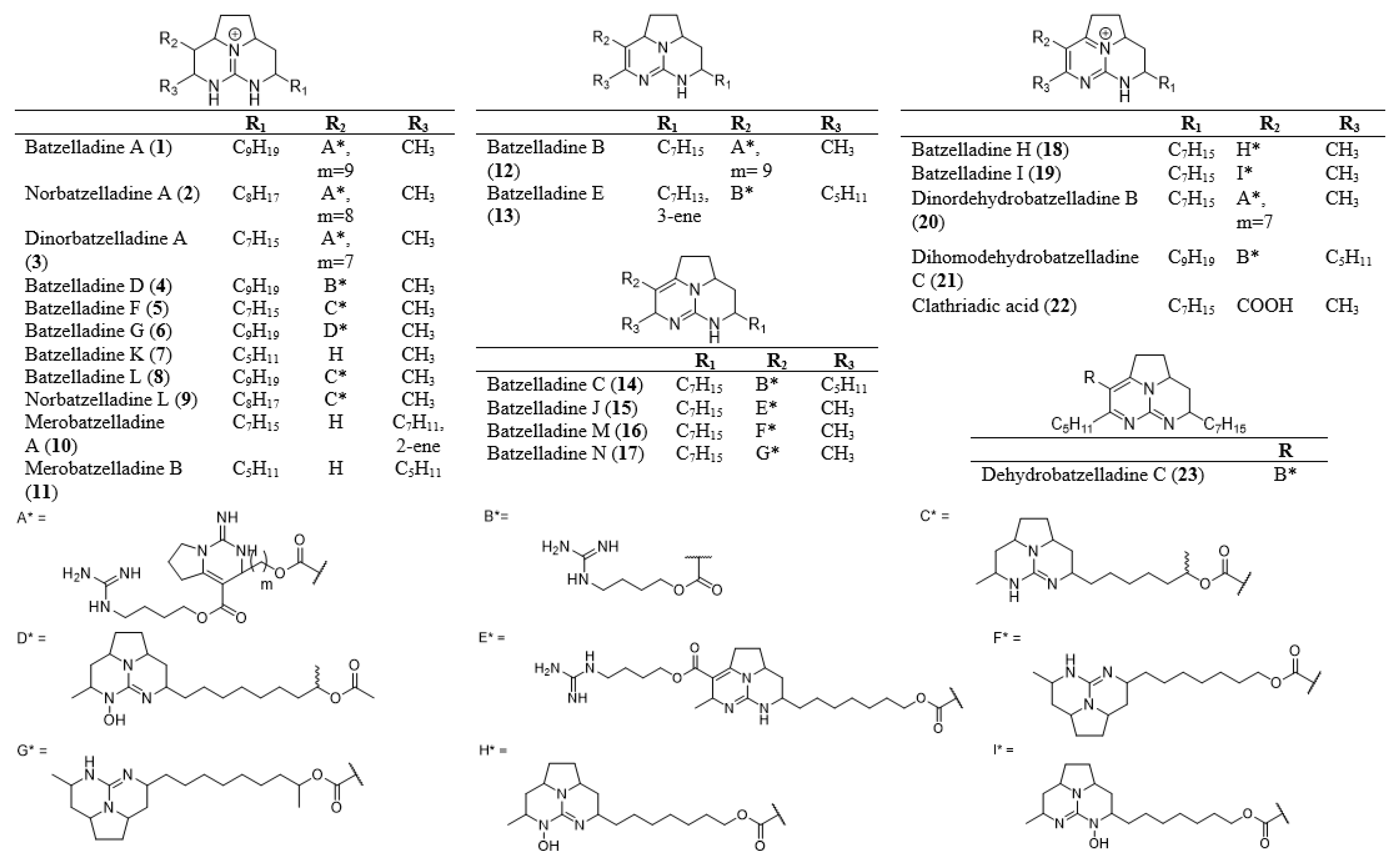













| Sponge Source | Synthesized | Anti-Cancer | Anti-Malarial | Anti- Microbial | HIV Inhibitor | Reference | |
|---|---|---|---|---|---|---|---|
| Skeleton 1 | |||||||
| Batzelladine A (1) | Batzella sp., Monanchora arbusculla, Clathria calla | / | / | / | [1,2,3,4] | ||
| Norbatzelladine A (2) | M. arbuscula, C. calla | / | / | [2] | |||
| Dinorbatzelladine A (3) | M. arbuscula, C. calla | / | / | [2] | |||
| Batzelladine D (4) | Batzella sp., M. arbuscula | / | / | / | [1,3,5,6,7,8,9] | ||
| Batzelladine F (5) | Batzella sp., M. arbuscula | / | / | / | [5,10,11,12,13,14] | ||
| Batzelladine G (6) | Batzella sp. | / | [10] | ||||
| Batzelladine K (7) | M. unguifera | / | [15,16] | ||||
| Batzelladine L (8) | M. unguifera, M. arbuscula, C. calla | / | / | / | / | [2,5,16] | |
| Norbatzelladine L (9) | M. arbuscula, C. calla | / | / | / | [2,5] | ||
| Merobatzelladine A (10) | Monanchora sp. | / | / | [17] | |||
| Merobatzelladine B (11) | Monanchora sp. | / | / | / | [17,20,21] | ||
| Skeleton 2 | |||||||
| Batzelladine B (12) | Batzella sp. | / | / | [1,22,23] | |||
| Batzelladine E (13) | Batzella sp. | / | [1,24] | ||||
| Skeleton 3 | |||||||
| Batzelladine C (14) | Batzella sp., M. unguifera | / | / | / | / | / | [1,16,25] |
| Batzelladine J (15) | M. unguifera | / | [26] | ||||
| Batzelladine M (16) | M. unguifera | / | / | / | / | [16] | |
| Batzelladine N (17) | M. unguifera | / | / | / | [16] | ||
| Skeleton 4 | |||||||
| Batzelladine H (18) | Batzella sp. | / | [10] | ||||
| Batzelladine I (19) | Batzella sp. | / | [10] | ||||
| Dinordehydrobatzelladine B (20) | M. arbuscula, C. calla | / | / | [2] | |||
| Dihomodehydrobatzelladine C (21) | M. arbuscula, C. calla | / | / | [2] | |||
| Clathriadic acid (22) | M. arbuscula, C. calla | / | / | [2] | |||
| Skeleton 5 | |||||||
| Dehydrobatzelladine C (23) | M. unguifera | / | / | / | / | [16] | |
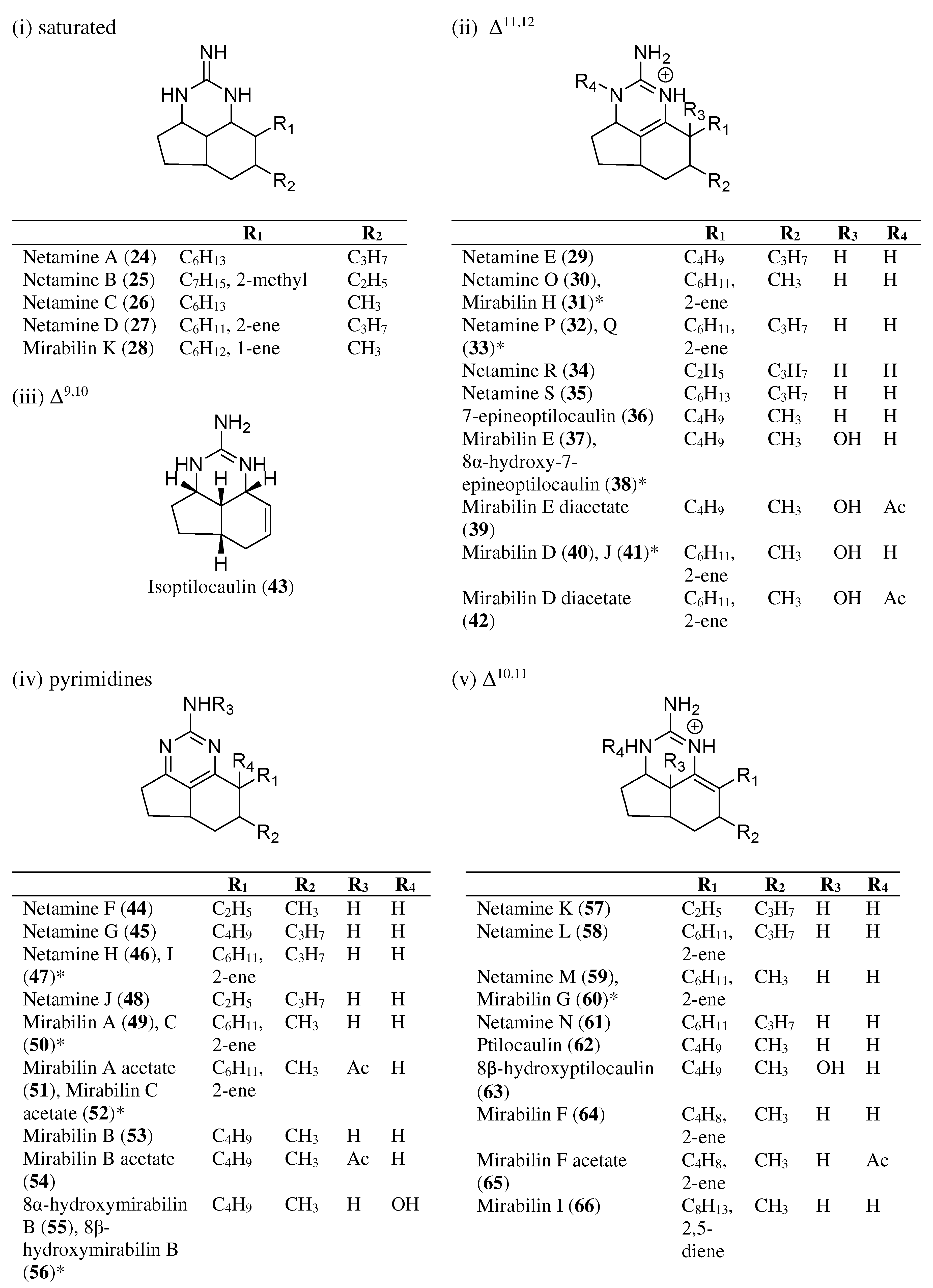
| Sponge Source | Synthesized | Anti-Cancer | Anti-Malarial | Antimicrobial | Hemolytic Activities | Reference | |
|---|---|---|---|---|---|---|---|
| Saturated | |||||||
| Netamine A (24) | Biemna laboutei | [34] | |||||
| Netamine B (25) | Biemna laboutei | [34] | |||||
| Netamine C (26) | Biemna laboutei | / | / | [34,39] | |||
| Netamine D (27) | Biemna laboutei | / | [34] | ||||
| Mirabilin K (28) | Acanthella cavernosa | [37] | |||||
| Δ 11,12 | |||||||
| Netamine E (29) | Biemna laboutei | / | [34,40] | ||||
| Netamine O (30) | Biemna laboutei | / | / | [36] | |||
| Mirabilin H (31) | Clathria sp. | / | [33] | ||||
| Netamine P (32) | Biemna laboutei | / | [36] | ||||
| Netamine Q (33) | Biemna laboutei | / | / | [36] | |||
| Netamine R (34) | Biemna laboutei | [36] | |||||
| Netamine S (35) | Biemna laboutei | [36] | |||||
| 7-Epineoptilocaulin (36) | Batzella sp. | / | [29,40] | ||||
| Mirabilin E (37) | Arenochalina mirabilis | [31] | |||||
| 8α-Hydroxy-7-epineoptilocaulin (38) | Batzella sp. | [29] | |||||
| Mirabilin E diacetate (39) | Arenochalina mirabilis | [31] | |||||
| Mirabilin D (40) | Arenochalina mirabilis | [31] | |||||
| Mirabilin J (41) | Clathria sp. | / | [33] | ||||
| Mirabilin D diacetate (42) | Arenochalina mirabilis | [31] | |||||
| Δ 9,10 | |||||||
| Isoptilocaulin (43) | Ptilocaulis aff. Ptilocaulis spiculifer | / | / | [27] | |||
| Pyrimidines | |||||||
| Netamine F (44) | Biemna laboutei | [34] | |||||
| Netamine G (45) | Biemna laboutei | / | [34,40] | ||||
| Netamine H (46) | Biemna laboutei | [35] | |||||
| Netamine I (47) | Biemna laboutei | [35] | |||||
| Netamine J (48) | Biemna laboutei | [35] | |||||
| Mirabilin A (49) | Arenochalina mirabilis, Biemna laboutei | / | [31,35] | ||||
| Mirabilin C (50) | Arenochalina mirabilis, Biemna laboutei, Clathria sp. | / | [31,33,35] | ||||
| Mirabilin A acetate (51) | Arenochalina mirabilis | [31] | |||||
| Mirabilin C acetate (52) | Arenochalina mirabilis | [31] | |||||
| Mirabilin B (53) | Arenochalina mirabilis, Monanchora unguifera | / | X | / | [30,31,41] | ||
| Mirabilin B acetate (54) | Arenochalina mirabilis | [31] | |||||
| 8α-Hydroxymirabilin (55) | Batzella sp., Monanchora unguifera | [29,30] | |||||
| 8β-Hydroxymirabilin (56) | Monanchora unguifera | [30] | |||||
| Δ 10,11 | |||||||
| Netamine K (57) | Biemna laboutei | / | [35] | ||||
| Netamine L (58) | Biemna laboutei | [35] | |||||
| Netamine M (59) | Biemna laboutei | / | [35,37] | ||||
| Mirabilin G (60) | Clathria sp. | / | / | [32,33,37] | |||
| Netamine N (61) | Biemna laboutei | [35] | |||||
| Ptilocaulin (62) | Ptilocaulis aff., Ptilocaulis spiculifer | / | / | / | / | [27,41,42] | |
| 8β-Hydroxyptilocaulin (63) | Monanchora arbuscula | / | / | [28,41] | |||
| Mirabilin F (64) | Arenochalina mirabilis | / | [31,33] | ||||
| Mirabilin F acetate (65) | Arenochalina mirabilis | [31] | |||||
| Mirabilin I (66) | Clathria sp. | / | [33] | ||||
| Common Names | Mode of Action/Cells Inhibited | References |
|---|---|---|
| HIV inhibitor | ||
| Batzelladine A (1) | Inhibits gp120 binding to CD4, protein kinase C activity, binding of interleukin-8 (IL8) and calcitonin gene-related peptide (CGRP) to their receptors, inhibits Vero cells | [1] |
| Batzelladine D (4) | Vero cells | [1] |
| Batzelladine F (5) | Induces p56lck-CD4 dissociation | [10] |
| Batzelladine G (6) | ||
| Batzelladine L (8) | Shows inhibitory activity against human HIV-1 virus | [16] |
| Batzelladine B (12) | Inhibits gp120 binding to CD4, protein kinase C activity, binding of interleukin-8 (IL8) and calcitonin gene-related peptide (CGRP) to their receptors, inhibits Vero cells | [1] |
| Batzelladine C (14) | Vero cells, shows inhibitory activity against the human HIV-1 virus | [1,16] |
| Batzelladine M (16) | Shows inhibitory activity against human HIV-1 virus | [16] |
| Batzelladine N (17) | ||
| Batzelladine H (18) | Induces p56lck-CD4 dissociation when combined with batzelladine I | [10] |
| Batzelladine I (19) | Induces p56lck-CD4 dissociation when combined with batzelladine H | |
| Dehydrobatzelladine C (23) | Shows inhibitory activity against human HIV-1 virus | [16] |
| SARS-CoV-2 inhibitor | ||
| Batzelladine H (18) | Inhibits SARS-CoV-2 main protease (Mpro) | |
| Batzelladine I (19) | ||
| Mirabilin G (60) | Inhibits SARS-CoV-2 main protease (Mpro) | |
| Anti-cancer | ||
| Norbatzelladine A (2) | MDA-MB-231, A549, HT29 | [2] |
| Dinorbatzelladine A (3) | ||
| Batzelladine L (8) | DU-145, IGROV, SK-BR3, leukemia L-562, PANCL, HeLa, SK-MEL-28, A549, HT-29, LOVO, and LOVO-DOX | [16] |
| Norbatzelladine L (9) | MDA-MB-231, A549, HT29 | [2] |
| Batzelladine C (14) | DU-145, IGROV, SK-BR3, leukemia L-562, PANCL, HeLa, SK-MEL-28, A549, HT-29, LOVO, and LOVO-DOX | [16] |
| Batzelladine J (15) | P-388, A-549, HT-29, MEL-28, DU-145 | [26] |
| Batzelladine M (16) | DU-145, IGROV, SK-BR3, leukemia L-562, PANCL, HeLa, SK-MEL-28, A549, HT-29, LOVO, and LOVO-DOX | [16] |
| Batzelladine N (17) | ||
| Dinordehydrobatzelladine B (20) | A549, HT29 | [2] |
| Dihomodehydrobatzelladine C (21) | MDA-MB-231, A549, HT29 | |
| Clathriadic acid (22) | ||
| Dehydrobatzelladine C (23) | DU-145, IGROV, SK-BR3, leukemia L-562, PANCL, HeLa, SK-MEL-28, A549, HT-29, LOVO, and LOVO-DOX | [16] |
| Netamine C (26) | A549, HT29, MDA-MB-231 | [34] |
| Netamine D (27) | ||
| Netamine O (30) | KB tumor cell | [36] |
| Mirabilin H (31) | SH-SY5Y, AGS, HT29, Intestine-407 | [33] |
| Netamine Q (33) | KB tumor cells | [36] |
| Mirabilin J (41) | SH-SY5Y, AGS, HT29, Intestine-407 | [33] |
| Isoptilocaulin (43) | L1210 leukemia cells | [27] |
| Mirabilin C (50) | SH-SY5Y, AGS, HT29, Intestine-407 | [33] |
| Netamine M (59) | KB cell, HEK 293 cells. Inhibits TPA-induced degradation of PDCD4 | [35,37] |
| Mirabilin G (60) | HEK 293, SH-SY5Y, AGS, HT29, Intestine-407, stabilizes TPA-induced degradation of PDCD4 | [33,37] |
| Ptilocaulin (62) | L1210, MCF-7, B16F10, HL-60, and MDA-MB-435 | [27,41,42] |
| 8b-Hydroxyptilocaulin (63) | HL-60 and MDA-MB-435 | [41] |
| Mirabilin F (64) | SH-SY5Y, AGS, HT29, Intestine-407 | [33] |
| Mirabilin I (66) | ||
| Anti-malarial | ||
| Batzelladine A (1) | P. falciparum (FcB1) | [2] |
| Norbatzelladine A (2) | ||
| Dinorbatzelladine A (3) | ||
| Batzelladine L (8) | P. falciparum (FcB1) and its D6 clone and W2 clone | [2,16] |
| Norbatzelladine L (9) | P. falciparum (FcB1) | [2] |
| Merobatzelladine A (10) | P. falciparum | [17] |
| Merobatzelladine B (11) | ||
| Batzelladine C (14) | Against P. falciparum D6 clone and W2 clone | [16] |
| Batzelladine M (16) | ||
| Dinordehydrobatzelladine B (20) | P. falciparum (FcB1) | [2] |
| Dihomodehydrobatzelladine C (21) | ||
| Clathriadic acid (22) | ||
| Dehydrobatzelladine C (23) | Against P. falciparum D6 clone and W2 clone | [16] |
| Netamine O (30) | P. falciparum | [36] |
| Netamine P (32) | ||
| Netamine Q (33) | ||
| Mirabilin A (49) | [35] | |
| Netamine K (57) | ||
| Antimicrobial | ||
| Batzelladine D (4) | Trypanosoma cruzi trypomastigotes, Leishmania infantum promastigotes, Saccharomyces cerevisiae | [5,18] |
| Batzelladine F (5) | Trypanosoma cruzi trypomastigotes, Leishmania infantum promastigotes | [5] |
| Batzelladine L (8) | T. cruzi trypomastigotes, L. infantum promastigotes Strong activities against AIDS-OIs Candida albicans, Cryptococcus neoformans, S. aureus, methicillin-resistant S. aureus (MRS), Pseudomonas aeruginosa, and M. intracellulare, as well as Aspergillus fumigatus, M. tuberculosis, and Leishmania donovani | [5,16] |
| Norbatzelladine L (9) | T. cruzi trypomastigotes, L. infantum promastigotes, S. cerevisiae | [5] |
| Merobatzelladine A (10) | Vibrio anguillarum, Trypanosoma brucei brucei | [17] |
| Merobatzelladine B (11) | ||
| Batzelladine C (14) | Strong activities against AIDS-OIs C. albicans, C. neoformans, S. aureus, methicillin-resistant S. aureus (MRS), P. aeruginosa, M. intracellulare, and A. fumigatus | [16] |
| Batzelladine M (16) | ||
| Batzelladine N (17) | M. tuberculosis | [16] |
| Dehydrobatzelladine C (23) | Strong activities against AIDS-OIs C. albicans, C. neoformans, S. aureus, methicillin-resistant S. aureus (MRS), P. aeruginosa, M. intracellulare, and A. fumigatus | [16] |
| Isoptilocaulin (43) | S. pyogenes, S. pneumoniae, E. faecalis, S. aureus, E. coli | [27] |
| Mirabilin B (53) | C. neoformans, L. donovani | [30] |
| Mirabilin G (60) | E. coli, Serratia marcescens, and S. cerevisiae | [32] |
| Ptilocaulin (62) | Streptococcus pyogenes, Streptococcus pneumoniae, E. faecalis, S. aureus, E. coli | [27] |
| Hemolytic activities | ||
| Ptilocaulin (62) | The plasma membrane of mouse erythrocytes | [41] |
| 8β-Hydroxyptilocaulin (63) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Rani, N.Z.; Lee, Y.K.; Ahmad, S.; Meesala, R.; Abdullah, I. Fused Tricyclic Guanidine Alkaloids: Insights into Their Structure, Synthesis and Bioactivity. Mar. Drugs 2022, 20, 579. https://doi.org/10.3390/md20090579
Abd Rani NZ, Lee YK, Ahmad S, Meesala R, Abdullah I. Fused Tricyclic Guanidine Alkaloids: Insights into Their Structure, Synthesis and Bioactivity. Marine Drugs. 2022; 20(9):579. https://doi.org/10.3390/md20090579
Chicago/Turabian StyleAbd Rani, Nur Zahirah, Yean Kee Lee, Sarfraz Ahmad, Ramu Meesala, and Iskandar Abdullah. 2022. "Fused Tricyclic Guanidine Alkaloids: Insights into Their Structure, Synthesis and Bioactivity" Marine Drugs 20, no. 9: 579. https://doi.org/10.3390/md20090579
APA StyleAbd Rani, N. Z., Lee, Y. K., Ahmad, S., Meesala, R., & Abdullah, I. (2022). Fused Tricyclic Guanidine Alkaloids: Insights into Their Structure, Synthesis and Bioactivity. Marine Drugs, 20(9), 579. https://doi.org/10.3390/md20090579





