Abstract
The marine is a highly complex ecosystem including various microorganisms. Bacillus species is a predominant microbialflora widely distributed in marine ecosystems. This review aims to provide a systematic summary of the newly reported metabolites produced by marine-derived Bacillus species over recent years covering the literature from 2014 to 2021. It describes the structural diversity and biological activities of the reported compounds. Herein, a total of 87 newly reported metabolites are included in this article, among which 49 compounds originated from marine sediments, indicating that marine sediments are majority sources of productive strains of Bacillus species Therefore, marine-derived Bacillus species are a potentially promising source for the discovery of new metabolites.
1. Introduction
The ocean is a highly complex ecosystem, a rich and underdeveloped treasure house containing a wide variety of biological resources including aquatic species and various microorganisms [1,2,3]. Natural products, especially small molecules isolated from biological sources, have long been regarded for their huge potential in human medicine and are still gaining traction [4]. Marine microorganisms produce many undiscovered molecules with unprecedented structures and pharmacological activities in an extreme living environment [2]. Therefore, it is commonly recognized that marine microbes constitute a promising source of novel metabolites with considerable therapeutically potential for new drug screening and development [3,5,6,7,8].
Bacillus species is a predominant microbialflora widely distributed in marine ecosystems [9,10]. Bacillus species can grow rapidly and tolerate extremely adverse environmental conditions such as extreme ambient temperature, salinity and pH, high pressure and nutrient deficiency [11]. B. subtilis can adopt several responses when faced with the depletion of essential nutrients, including motility, secretion of extracellular enzymes, genetic transformation, antibiotic production, and finally sporulation [12]. The genus Bacillus is a prolific producer of bioactive metabolites, including more than 350 kinds of rod-shaped and Gram-positive bacteria [13]. Thereinto, B. subtilis, B. licheniformis and B. amyloliquefaciens possess potential value as therapeutic agent candidates on account of their ability to produce bioactive secondary metabolites [14,15,16,17].
In recent years, a variety of secondary metabolites of marine Bacillus species have been studied, including lipopeptides [18], polyketides [19], non-ribosomal peptides [20], macrolides [16,21], and glycopeptides [9]. Several natural products were isolated from the marine organisms for drug development by traditional means of bioactivity-guided methods and chemical structure elucidation. More precise analytical methods, such as LC-MS and NMR spectroscopy guided metabolic profiling and dereplication of a crude extract, also promoted the emergence of new secondary metabolites [22,23,24]. Some novel technologies, such as improved genome mining methods, have propelled natural products in the field of drug discovery [25]. The marine hosts a huge variety of organisms adapted to the specific environment, which should result in the production of a wide range of unique biomolecules [7]. The production of secondary metabolites is normally associated with the bacterium’s response to a growth-limiting environment; hence, exploring the high diversity of marine environments may uncover multiple compounds with unique structures and biological activities [26]. For example, most of this industrial Bacillus pumilus group (Bp group) have been isolated from terrestrial ecosystems at present. By contrast, members of the Bp group are ubiquitous and diverse in marine environments, but less explored [27].
According to studies, these compounds have a wide range of biological activities, viz., antimicrobial [28,29], anticancer [30,31,32], antivirus [33], antifungal, promotion of plant growth [34], immunosuppressive, antituberculosis, antimycoplasmic and exceptional surfactant [35], indicating their promising medicinal, agricultural and industrial potential. Over the past decades, Firmicutes phylum were found to be the marine-derived bacteria producers of the most antimicrobial activity, Bacillus strains specifically [26]. Sequel of genomic analyses demonstrated the prospect of marine Bacillus species producing wide-ranging polyketide classes of antibiotic agents, such as macrocyclic lactones, bacillaene, macrolactins, and difficidins [36,37]. Polyketide, as conspicuous bactericidal agents in the human health area, was reported to produce by multifunctional microbial polyketide synthase (pks) complex [38]. Difficidins are a polyketide class of polyenes, which were reported to biosynthesize by type I pks encrypted in the dif operon and were recognized to hinder bacterial pathogens [35]. Moreover, polyketides are the most structurally diverse and pharmacologically relevant natural products with low toxicity and high efficacy, many of which exhibit cytotoxic effects on cancer cells.
While numerous articles address Bacillus species and their secondary metabolites in general or the renewal biological functionalities, an overview of recently discovered compounds produced by marine Bacillus is missing. Only one review of Bacillus species was reported, by Mondol MA et al. in 2013 [39]. Therefore, we delved into the literature from the beginning of 2014 to the end of 2021. To the best of our knowledge, a summary of such literature is provided along with the novel chemical structures, diverse producing strains, environmental sources, pharmacological activities, employed experimental and putative biosynthetic pathways as well.
The literature retrieval was performed using a previously reported method [40,41]. All original articles were collected in the present review by searching various databases, including PubMed, Web of Science, China National Knowledge Infrastructure (CNKI) between 2014 and 2021. The search strategy was as follows: “Title: (Bacillus); Refined by: Topic (marine) and Document types (article); Timespan: 2014–2021”. A total of 127 hits were made. Only papers with reports about isolation of secondary metabolites are considered in this review. Other reports, including genome sequencing, industry, and ecological environmental studies, are touched upon but are not described comprehensively. It should be noted that this review was initially planned for the end of 2021. The studies published or being submitted in the current year might not be indexed in the PubMed in a timely manner; thus, the time span of literature search is from 2014 till 2021. During this period, a total of 87 secondary metabolites were isolated and characterized from marine-derived Bacillus species, which were reported in 33 original articles and account for 25.98% of these reported publications. The numbers of articles, strains and compounds were collected and collated according to the year they were published. Results indicated that the numbers of strains and compounds reported to be produced by marine-derived Bacillus species have been fluctuating over the past eight years (between 2014 and 2021). In particular, data showed that there were no articles reporting compounds isolated from marine-derived Bacillus species in 2019 (Figure 1).
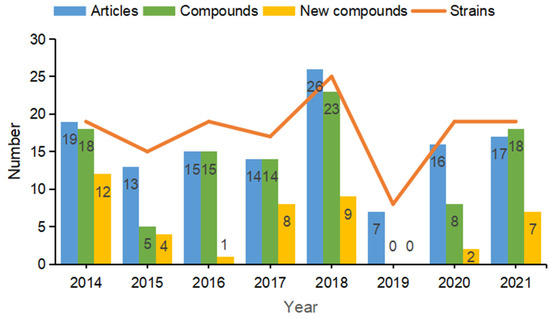
Figure 1.
The numbers of articles, compounds, and strains reported by marine-derived Bacillus species from 2014–2021.
2. Structural Diversity
This review summarizes the newly reported secondary metabolites (1–87) produced by marine-derived Bacillus species from 2014 till 2021. These secondary metabolites could be classified into six major categories: cyclic lipopeptides (1–19); diketopiperazines (20–27); linear lipopeptides (28–38); polyketides (39–44); nonribosomal peptides (45–57); macrolactins (58–72); and other compounds (73–87) based on their structural patterns.
2.1. Cyclic Lipopeptides
Cyclic lipopeptides (CLPs) are common secondary metabolites isolated from marine-derived Bacillus. The CLPs are a class of metabolites with structural diversity produced by multifarious bacterial genera [42]. There are three families of CLPs being of particular importance, namely surfactins, iturins and plipastatins, all consisting of a short cyclic oligopeptide linked to the tail of a fatty acid [14]. Surfactin sequences comprise of seven amino acids and a β-hydroxy fatty acid chain containing 12–16 carbons [43]. The iturin family sequences are composed of heptapeptides and a β-amino fatty acid chain of 14–17 carbon atoms, which consists of bacillomycin D, F, L, Lc, iturin A, AL, C and mycosubtilin (Figure 2) [44]. The plipastatin family comprise of ten amino acids and a β-hydroxy fatty acid containing 14–18 carbon atoms [45]. Figure 3 lists the structures of cyclic lipopeptides produced by marine-derived Bacillus species.
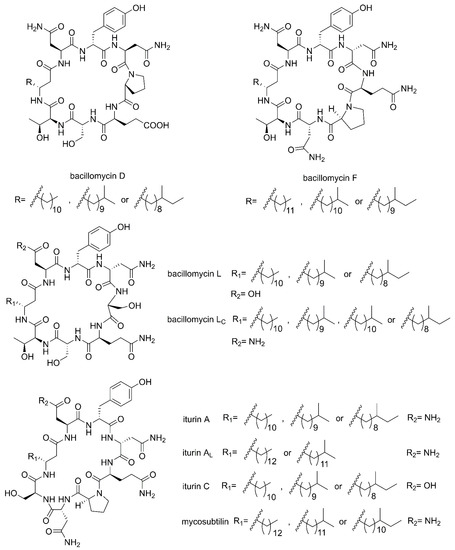
Figure 2.
The structures of iturin family members (bacillomycin D, F, L, Lc, iturin A, AL, C and mycosubtilin).
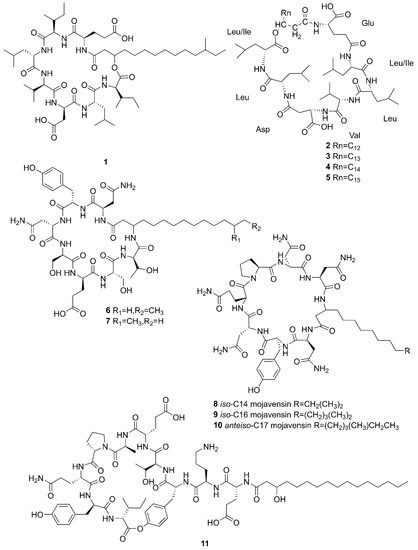
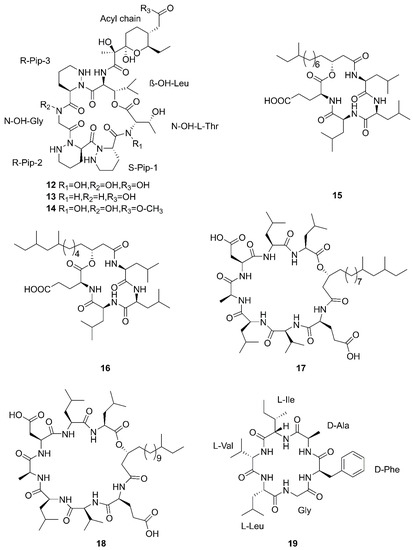
Figure 3.
Cyclic lipopeptides produced by marine-derived Bacillus species.
Compounds 1–5 belong to surfactin family. A new CLP surfactin named anteiso-C15 Ile2,7 surfactin (1) was isolated from B. velezensis SH-B74 in the China Center for Type Culture Collection (CCTCC), which collected from the marine sediments, comprising of an anteiso-C15 type saturated fatty acid chain, and a peptidic backbone of L-Glu1, L-Ile2, D-Leu3, L-Val4, L-Asp5, D-Leu6, L-Ile7 [46]. Rn-Glu1-Leu/Ile2-Leu3-Val4-Asp5-Leu6-Leu/Ile7 (2–5) belonging to surfactin homolog were isolated from B. licheniformis MB01 collected from sediments in the Bohai Sea, China [47]. Compounds 6–10 belong to the iturin family. A novel lipopeptide antibiotic bacillopeptin named bacillopeptin B1 (6) and a known compound, bacillopeptin B (7) were detected in the fermentation broth of a marine sediment-derived B. amyloliquefaciens SH-B74 collected from sediments in the South China Sea. More precisely, compound 6 as a member of bacillopeptin family has the same amino-acid sequence and the same molecular weight as compound 7, but has a different fatty-acid residue [22]. Compounds 8–10 were characterized as cyclic lipopeptides with saturated β-amino fatty acid chain residues, iso-C14 mojavensin, iso-C16 mojavensin, and anteiso-C17 mojavensin, all of which were produced by a marine-derived B. mojavensis B0621A obtained from the mantle of a pearl oyster Pinctada martensii in the South China Sea [48]. In addition, plipastatin A1 (11), belonging to the plipastatin family, was obtained by solidphase extraction and reversed-phase high-performance liquid chromatograph (RP-HPLC) from the fermentation broth of a marine sediment-derived B. amyloliquefaciens SH-B74 in the CCTCC [49]. A new cyclic hexapeptide with three piperazic acids (N-OH-Thr, N-OH-Gly, β-OH-Leu) named dentigerumycin E (12) and two reported derivatives, 2-N, 16-N-deoxydenteigerumycin E (13) and dentigerumycin E methyl ester (14), were isolated from coculture of marine Streptomyces and Bacillus strains collected together from the intertidal mudflat in Wando, Republic of Korea. It is worth mentioning that only compound 12 showed antiproliferative and antimetastatic activities against human cancer cells, suggesting that 2-N-OH, 16-N-OH, and 37-OH (carboxylic acid) are essential for the activities [25]. Two novel cyclic lipopeptides, bacilotetrin A (15) and bacilotetrin B (16), possessing three leucines and one glutamic residue cyclized with a lipophilic 3-hydroxyl fatty acid, were isolated from B. subtilis 109GGC020 in the sediments from the Gageocho of southern reef, Republic of Korea [12]. Additionally, gageopeptins A (17) and B (18), two novel cyclic lipopeptides, were isolated from the same strain in the same sediments as above [50]. A new cyclic hexapeptide named bacicyclin (19) was purified from Bacillus sp. BC028 associated with the blue mussel Mytilus edulis collected from the western shore of the Baltic Sea in Germany [51].
2.2. Diketopiperazines
Cyclicpeptide diketopiperazines consist of residues of two amino acids and mevalonic acid [52]. Figure 4 lists the structures of diketopiperazines that were produced by marine-derived Bacillus species.
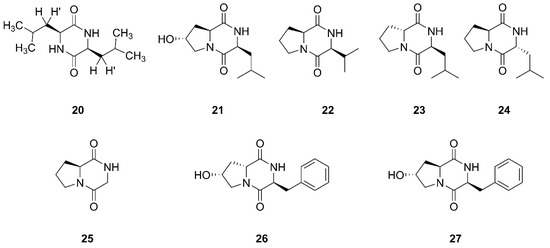
Figure 4.
Diketopiperazines produced by marine-derived Bacillus species.
Compound 20 was established as a diketopiperazine (3S, 6S)-3,6-diisobutylpiperazine-2,5-dione, which was isolated from the ethyl acetate extract of the culture broth of Bacillus sp. SPB7. This strain SPB7 was obtained from marine sponge Spongia officinalis collected from the Palk Bay of Bengal, India. In particular, this is the first time that compound 20 had been isolated from a sponge-associated microbe [53]. Finally, seven cyclic dipeptides compounds 21–27 were identified and characterized as cyclo (L-leu-trans-8-hydroxy-L-pro), cyclo (L-val-L-pro), cyclo (D-pro-L-leu), cyclo (L-pro-D-leu), cyclo (gly-L-pro), cyclo (L-phe-cis-8-hydroxy-D-pro), and cyclo (L-phe-trans-8-hydroxy-L-pro), respectively, which were isolated from Bacillus sp. UST050418-715 collected from sponge in the sea near St. Juan Island, Washington, USA [54]. Compound 25 was also found from sponge-endosymbiotic Bacillus species collected from Agatti island located in the Arabian Sea, in the Laksha Archipelago of India [55].
2.3. Linear Lipopeptides
Linear lipopeptide is a kind of lipopeptide, in which amino acids are connected in turn into linear, unconnected head and tail and no cyclic structure. Fatty acids are connected to α-amino groups or other hydroxyl groups at the N-terminal of the peptide chain [56]. Figure 5 lists the structures of linear lipopeptides that were produced by marine-derived Bacillus species.
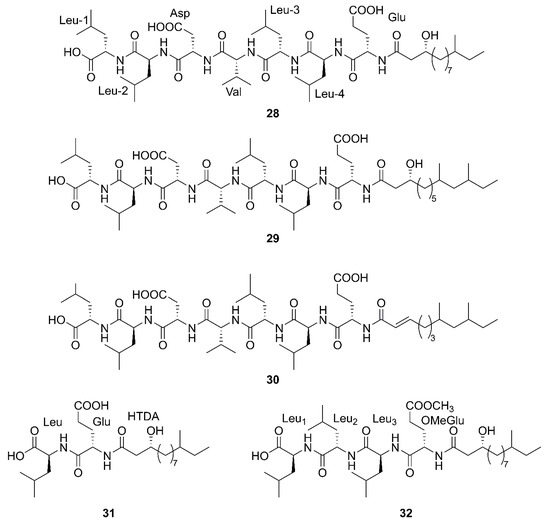
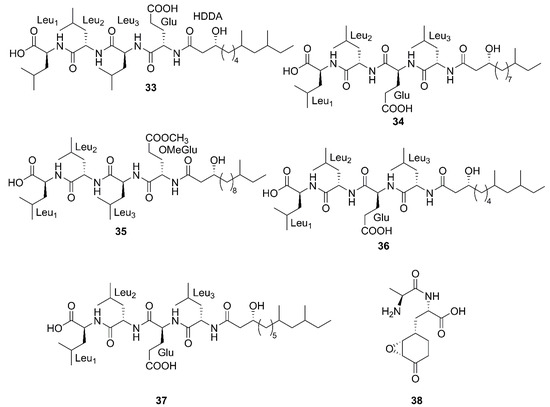
Figure 5.
Linear lipopeptides produced by marine-derived Bacillus species.
Three newfound linear lipopeptides named gageostatins A (28), B (29) and C (30), comprising of hepta-peptides and new 3-β-hydroxy fatty acids yielded by a marine-derived bacterium B. subtilis from the culture broth [35]. Furthermore, three novel linear lipopeptides possessing di- and tetrapeptides and a new fatty acid, gageotetrins A (31), B (32) and C (33), were isolated from a marine B. subtilis [57]. Four unreported lipopeptides, gageopeptides A (34), B (35), C (36), and D (37) were isolated and identified from a marine-derived bacterium B. subtilis, which consisted of tetrapeptides and 3-β-hydroxy fatty acids [58]. The fatty acid of 28, 31, 32 and 34 was identical and determined as a 3-β-hydroxy-11-methyltridecanoic acid. Likewise, compounds 29 and 37 both possessed the same fatty acid, 3-β-hydroxy-9,11-dimethyltridecanoic acid. Moreover, the fatty acid unit of 33 and 36 was 3-β-hydroxy-8,10-dimethyldodecanoic acid. In particular, the absolute stereochemistry at C-3 of the fatty acids of linear lipopeptides 28–37 is R configuration except 30. Additionally, the configuration of the amino acid residues in 28–37 was found to be L-form, while Val in 28–30 was D-form. Besides, bacilysin (38), another identified dipeptide, was isolated from seaweed-associated B. amyloliquefaciens MTCC 10456 in Microbial Type Culture Collection and Gene Bank (MTCC) of Chandigarh in India. Notably, this is the first report on the co-production and isolation of anti- Malassezia spp. chemicals from marine Bacillus species [44]. In conclusion, all linear Lipopeptide mentioned above were obtained from the Gageocho in the southern reef (Republic of Korea) except 38.
2.4. Nonribosomal Peptides
Nonribosomal peptides (NRPs) are large enzyme complexes with a modular structure responsible for binding a particular amino acid. NRPSs of Bacillus are synthesized by large multimodular nonribosomal peptide-synthetase (NRPS) through prolonging the active monomers of amino acid building blocks [59]. Figure 6 lists the structures of nonribosomal peptides produced by marine-derived Bacillus species.
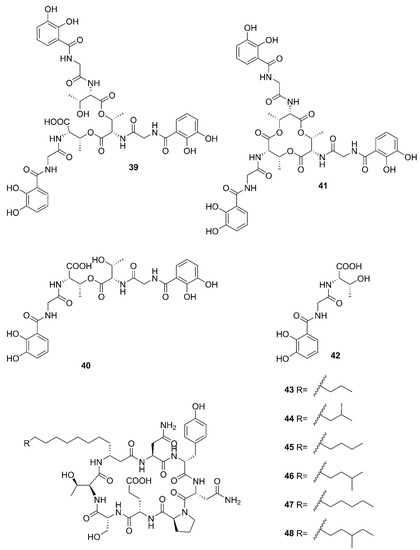
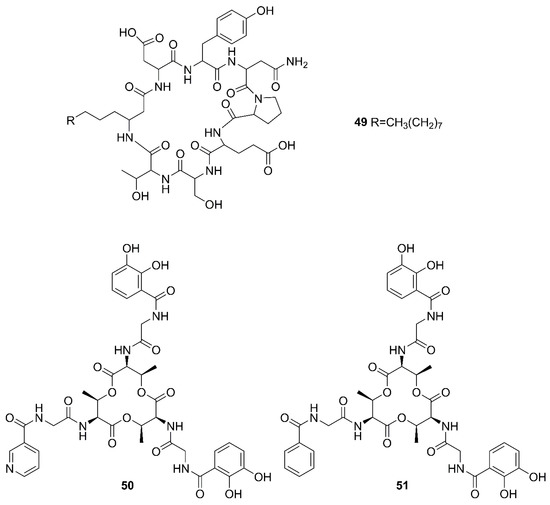
Figure 6.
Nonribosomal peptides produced by marine-derived Bacillus species.
Two unreported compounds, bacillibactin B (39) and bacillibactin C (40), along with the known compounds Bacillibactin (41) and SVK21 (42), were discovered from Bacillus sp. named PKU-MA00093 from sponges, corals and sediments in the South China Sea. Additionally, compounds 43–48 were characterized as bacillomycin D, iso-C15 bacillomycin D, C15 bacillomycin D, iso-C16 bacillomycin D, C16 bacillomycin D, and anteiso-C17 bacillomycin D, respectively. They were isolated from Bacillus sp. PKU-MA00092 collected from sponges, corals and sediments in the South China Sea. Notably, this was the first time to report the structures of 45 and 47 with fully specified 1H NMR and 13C NMR data; their structures are highly similar except for the fatty acid moieties [60]. Compounds 45 and 47 in company with C14 bacillomycin D (49), were obtained from seaweed-associated B. amyloliquefaciens MTCC 10456 collected from seaweed in the MTCC, of Chandigarh, India [44]. Moreover, compounds 45 and 49 were also isolated from the methanol extract harvested from marine-derived B. megaterium CGMCC7086 obtained from the intestines of marine fish in the Yellow Sea of East China by two-step ultrafiltration and liquid chromatography-electronic spray ionization-tandem mass spectrometry (LC-ESI-MS/MS). Using a highly-efficient separation technique and identification method, more than 40 lipopeptides variants were identified from a Bacillus strain [23]. Besides, compound 47 was isolated from B. subtilis B38 strain [61]. Two unique bacillibactins, bacillibactins E (50) and F (51) were the first bacterial siderophores containing nicotinic and benzoic acid moieties isolated from a marine sponge Cinachyrella apion associated Bacillus sp. WMMC1349 collected from the west shore of Ramrod Key in Florida [24].
2.5. Polyketides
Polyketides are a class of extremely large secondary metabolites assembled from simple acyl-coA compounds [62]. Bacillus species of marine origin was a potential source of bioactive compounds of polyketides and bacteriocins with significant antimicrobial activity against human pathogens [63]. Figure 7 lists the structures of polyketides that were produced by marine-derived Bacillus species.
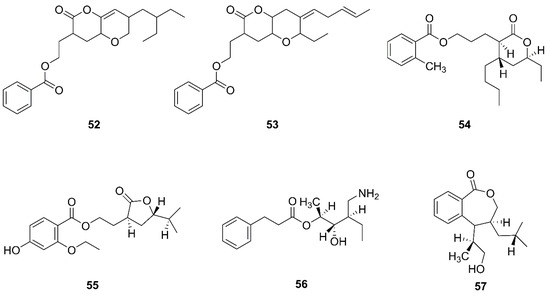
Figure 7.
Polyketides produced by marine-derived Bacillus species.
Two novel compounds, O-heterocycle pyrans, 2-(7-(2-Ethylbutyl)-2,3,4,4a,6,7-hexahydro-2-oxopyrano-[3,2b]-pyran-3-yl)-ethyl benzoate (52) and 2-((4Z)-2-ethyl-octahydro-6-oxo-3-((E)-pent-3-enylidene)-pyrano-[3,2b]-pyran-7-yl)-ethyl benzoate (53) were obtained by repeated chromatography from the heterotrophic bacterium B. subtilis MTCC 10407 associated with brown seaweed Sargassum myriocystum on the southeast coast of India [64]. Additionally, 11-(15-butyl-13-ethyl-tetrahydro-12-oxo-2H-pyran-13-yl) propyl-2-methylbenzoate (54), 9-(tetrahydro-2-isopropyl-11-oxofuran-10-yl)-ethyl-4-ethoxy-2-hydroxybenzoate (55), 12-(aminomethyl)-11-hydroxyhexanyl-10-phenylpropanoate (56), and 7-(14-hydroxypropan-13-yl)-8-isobutyl-7, and 8 dihydrobenzo[c]oxepin-1(3H)-one (57) were isolated from a heterotrophic marine bacterium B. amyloliquefaciens. This strain was isolated from the brown seaweed Padina gymnospora collected from the intertidal zone of the Mannar Gulf in Peninsular India [63].
2.6. Macrolactins
Marine Bacillus species produce abundant polyketide classes of antibiotic agents, such as macrolactins, difficidins, and bacillaenes [13,36,65]. Diffcidin is a highly unsaturated macrocyclic polyene with a 22-membered carbon skeleton and a phosphate moiety, which is rarely found in secondary metabolites of Bacillus species. Bacillaene is a linear structure consisting of a conjugated hexaene. Carbon skeleton of most macrolactins contains three diene groups attached to the carbon backbone of a 24-membered lactone ring [36]. Figure 8 lists the structures of macrolactins produced by marine-derived Bacillus species.
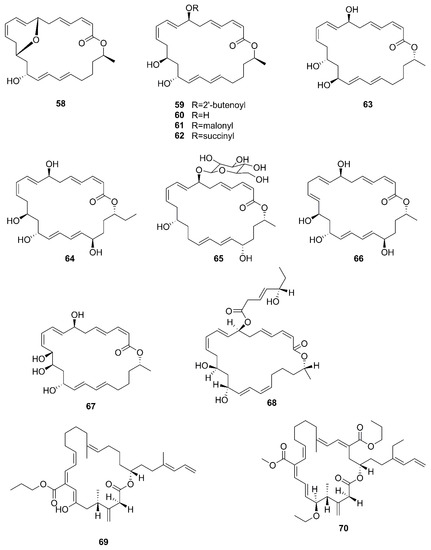
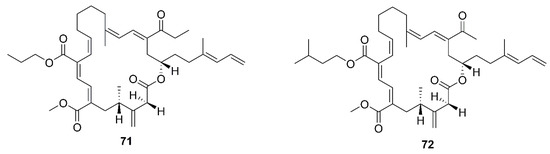
Figure 8.
Macrolactins produced by marine-derived Bacillus species.
A new macrolactin derivative, 7,13-epoxyl-macrolactin A (58), along with four known macrolactins, 7-O-2′E-butenoyl macrolactin A (59), Macrolactin A (60), 7-O-malonyl macrolactin A (61), and 7-O-succinyl macrolactin A (62) were isolated from bacteria B. subtilis B5. It is worth emphasizing that this strain was extracted from deep-sea sediments at depths of 3000 m in the Pacific Ocean [66]. Compounds 60 and 62 were also isolated from seaweed-associated B. amyloliquefaciens MTCC 10456 in the MTCC of Chandigarh, India; this is the first report on the co-production and isolation of anti-Malassezia spp. compounds from marine Bacillus species [44]. In particular, the major difference between compounds 58 and 59–62 is in the epoxy ring. Compound 58 displayed a potent inhibitory effect on the expression of interleukin-1β (IL-1β), interleukin-6 (IL-6) and inducible nitric oxide synthase (iNOS), due to the existence of the epoxy ring [66]. Five novel 24-membered macrolactins named bamemacrolactins A (63), B (64), C (65), D (66) and E (67) were produced by B. siamensis, which was isolated from the gorgonian coral Anthogorgia caerulea gathered from Beihai city (Guangxi, China) [67]. The 7-O-methyl-5′-hydroxy-3′-heptenoate−macrolactin (68), a new macrolactin compound, was obtained from B. subtilis MTCC10403 associated with seaweed Anthophycus longifolius collected from the Gulf of Mannar of Peninsular India [68]. Compounds 69–72 were characterized as four homologous difficidin-type 21-membered macrocyclic lactone, isolated from a heterotrophoic B. amyloliquefaciens MTCC12713 associated with an intertidal macroalga Kappaphycus alverezii collected from the Gulf of Mannar in Peninsular India. In addition, they were established as 18,19-dihydro-6-hydroxy-8-propyl carboxylate difficidin, 5-ethoxy-28-methyl-(9-methyl-19propyl dicarboxylate) difficidin, (6-methyl-9-propyl dicarboxylate)-19-propanone difficidin, and 20-acetyl-(6-methyl-9-isopentyl dicarboxylate) difficidin, respectively [13].
2.7. Other Compounds
Figure 9 lists the other compounds produced by marine-derived Bacillus species. A novel thiopeptide named micrococcin P3 (73) and a known compound named micrococcin P1 (74) were isolated from the fermentation broth of B. stratosphericus [69]. Five new bacillamidins A (75), B (76), C (77), D (78) and E (79), along with two known synthetic analogs, bacillamidins F (80) and G (81), were isolated from the marine-derived B. pumilus strain RJA1515. This strain was extracted from deep-sea sediments at depths of 84 m collected in Bamfield in British Columbia [70]. Ieodoglucomide C (82) and ieodoglycolipid (83), two new glycolipids, were produced by the marine-derived B. licheniformis 09IDYM23 which was isolated from sediments at a depth 20 m collected at Ieodoin the southern reef of the Republic of Korea, both of which were obtained from the fermentation of this strain [71]. According to bioactivity-guided strategy, (-)-sattabacin (84) and (-)-4-hydroxysattabacin (85) were firstly discovered from Bacillus sp. (SCO-147) collected from marine sediments in Suncheon Bay of Korea [72]. Marine-derived B. subtilis AD35, gathered from marine water and sediment at the Alexandria sea shore in Egypt, could yield a previously reported but firstly isolated compound, Di-(2-ethylhexyl) phthalate (DEHP) (86) [73]. Additionally, compound 86 and dibutyl phthalate (DBP) (87) were isolated from the extract broth of marine-derived B. polymyxa L1-9, which was collected in a mud sample from the intertidal mudflat in the Lianyungang Port of China [74].
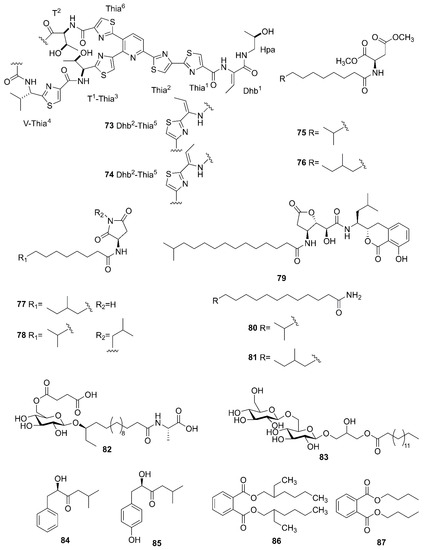
Figure 9.
Other compounds produced by marine-derived Bacillus species.
In this review, a total of 87 secondary metabolites were reported from marine-derived Bacillus species from January 2014 to December 2021. Their chemical structures were classified into cyclic lipopeptides (1–19, among them, 1–2 surfactins, 3–10 belong to iturins, 11 belongs to plipastatin), diketopiperazines (20–27), linear lipopeptides (28–38), nonribosomal peptides (39–51), polyketides (52–57), macrolactins (58–72, among them, 69–72 belong to difficidins), and other compounds (73–87) according to their putative biogenetic sources. As shown in Figure 10A, 21.84% of the compounds reported were CLPs, and these compounds account for an overwhelming majority of all 87 metabolites, followed by macrolactins with 17.24%. Therefore, CLPs are a class of secondary metabolites with structural diversity and pharmacological perspective.
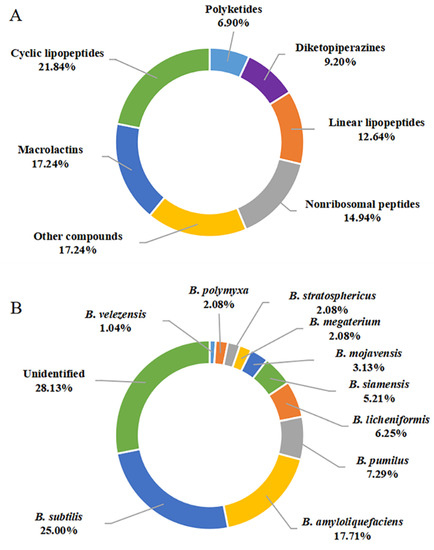
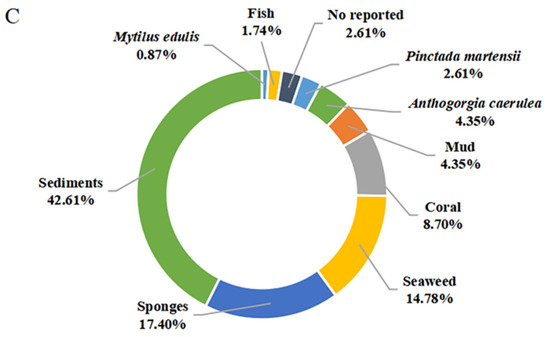
Figure 10.
Quantification of this studies on secondary metabolites from marine-derived Bacillus: (A) Chemical structures categories; (B) Producing strains; (C) Environment sources.
The genus Bacillus comprises more than 350 species, some of which are used as antifungal agents, while others are promising producers of green pesticide [14]. As discussed above, a total of 10 identified species, including B. subtilis, B. amyloliquefaciens, B. megaterium CGMCC7086, B. mojavensis B0621A, B. licheniformis, B. siamensis, B. stratosphericus, B. pumilus RJA1515, B. polymyxa L1-9, and B. velezensis SH-B74 were reported as the producing strains of these described secondary metabolites. Among them, B. subtilis and B. amyloliquefaciens were the most prolific strains, with 24 (20.00%) and 17 (17.71%) metabolites identified, respectively (Figure 10B). CLPs are ubiquitous in several Bacillus strains. However, linear lipopeptides are found predominantly in B. subtilis species, while the macrolactins are more preponderant in B. amyloliquefaciens species, suggesting the species-specific metabolites.
The secondary metabolites of marine-derived Bacillus species could be isolated from marine sediments, marine invertebrates (sponges, molluscs, and corals), and vertebrates (mainly fishes), as well as marine plants (mainly seaweed). Currently, there are 9 reported sources of Bacillus secondary metabolites. As shown in Figure 10C, a total of 49 compounds {1–7,11,15–18,28–37,39–48,58–62,75–85,86 (B. subtilis AD35)} originated from marine sediments, accounting for 42.61% of the sources of Bacillus species. In particular, the producing strains of 58–62 originated from deep-sea sediment at a depth of 3000 m, while the other strains of 75–83 originated from deep-sea sediment at depths less than 100 m. Moreover, the producing strains of 12–14, 86 (B. polymyxa L1-9), and 87 originated from mud. More precisely, the producing strains of 15–16 and 28–37 originated from the Republic of Korea’s southern reef. Twenty compounds (20–27,39–48,50,51), identified from marine sponges, accounted for 17.40% of the reported environmental sources of Bacillus secondary metabolites. It is worth noting that 20 belonging to diketopiperazine were isolated unprecedentedly from a sponge-associated microbe. Moreover, 45–49, 52–56 and 63–67 were also derived from coral, wherein 63–67, whose producing strains were obtained from the gorgonian coral A. caerulea, accounted for 4.35% of the reported environmental sources of Bacillus secondary metabolites. The 8–10 and 19 that originated from pearl oyster P. martensii and M. edulis, respectively, accounted for 3.48% of the reported ones. A total of 17 secondary metabolites {38,45,47,49,52–57,60,62 (B. amyloliquefaciens MTCC 10456) and 68–72} were identified from Bacillus residing in marine plants, accounted for 14.78% of the reported total amount. Thereinto, the producing strains of 52–57 originated from brown seaweed, while the producing strains of 45,47,49,60,62 (B. amyloliquefaciens MTCC 10456), 38,52–57 and 68–72 were collected from seaweed. In addition to these producing strains, only one Bacillus strain (B. megaterium CGMCC7086), which produced 45 and 49, was obtained from the intestines of marine fish and accounted for 1.74% of the reported total amount. Unfortunately, the environmental sources of the producing strain of 47 (B. subtilis B38), 73 and 74 (B. stratosphericus) were not described. From the above analysis, it can be concluded that marine sediments and sponges are more abundant sources of productive strains of marine-derived Bacillus, and which deserved much more attention in subsequent chemical studies.
3. Biological Activities
The producing strains, environmental sources, and biological activities of bioactive compounds from marine-derived Bacillus are listed. Most compounds possess a range of moderate to potent biological activities, including antimicrobial, antifungal, anticancer and other activities. Detailed descriptions of compounds with promising bioactivities are described as follows.
3.1. Antimicrobial Activities
Table 1 lists the potential antimicrobial Bacillus secondary metabolites. Compounds 2–5 exhibited antibacterial activity against Vibrio parahaemolyticus with a minimum inhibitory concentration (MIC) of 50 µg/mL. At the same time, they also displayed significant antibacterial activity against Escherichia coli, Vibrio cholerae, Vibrio harveyi, Pseudomonas aeruginosa, Staphylococcus aureus, and Proteus species, fully illustrating their broad-spectrum bacteriostatic characters [47]. Anti-staphylococcal activity of compounds 15 and 16 was evaluated against clinically methicillin resistant S. aureus (MRSA) strains (ATCC25923, XU212, SA1199B, RN4220) with MIC values from 8 to 32 µg/mL by the broth dilution assay, indicating better potential than that of the positive control norfloxacin (MIC, 2–64 µg/mL) [12]. Compounds 17 and 18 exhibited moderate antibacterial activity against Gram-positive bacteria (S. aureus, B. subtilis), and Gram-negative bacteria (Salmonella typhi and P. aeruginosa) with MIC values ranging from 16 to 32 µg/mL, compared to azithromycin (MIC, 2 µg/mL) [50]. Similarly, compounds 28–37 were active with MICs of 3–64 µg/mL when tested for the same bacteria as above, which proved they could be good biological probes for antimicrobial agents [35,57,58]. Among them, mixed compounds 28 + 29 were more active than individual ones [35]. Surprisingly, compound 19 displayed inhibitory activity against the clinically relevant strains S. aureus and Enterococcus faecalis with MIC values of 12 and 8 µM, respectively, compared to the positive control streptomycin (MIC, 2.09 µM and 5.24 µM), demonstrating the potential of marine microorganism as a hopeful source for the development of new antibiotics [51]. Moreover, compound 20 displayed strong antimicrobial activities against E. coli and S. aureus with MIC values of 16 µg/mL and 22 µg/mL, respectively, compared with ampicillin as positive control. Notably, this was the first report of antibacterial activity of diketopiperazine [53]. Compound 25 exhibited significant antimicrobial activity against fish pathogens, where the MIC was 31.25 mg/mL determined by the broth dilution assay method [55]. Compounds 52 and 53 were reported to display significant antibacterial activity against V. parahaemolyticus ATCC® 17802™, Vibrio vulnificus, and Aeromonas hydrophila [64]. Compounds 54–57 exhibited antibacterial activities against human important clinical pathogens V. vulnificus and V. parahaemolyticus (inhibitory zone diameter greater than 15 mm, 100 mcg on disk) [63]. A new macrolactin 68 showed a moderate effect on A. hydrophila, V. parahemolyticus ATCC 17802 and V. vulnificus with inhibitory zone diameters of 18, 16, and 14 mm at concentration of 100 µg on disk compared with that of the control commercial antibiotics [68]. Difficidin analogues 69–72 displayed significant antibacterial activities against multidrug-resistant (MDR) bacteria containing methicillin-resistant S. aureus, vancomycin-resistant E. faecalis, and other drug-resistant strains, such as P. aeruginosa and Klebsiella pneumonia with the MICs of 0.002–0.009 µM, compared to positive controls (chloramphenicol and ampicillin with MIC of about 0.017–0.049 µM). Thereinto, a drug-likeness score of 70 was greater than those of other difficidin analogues, demonstrating its potential for pharmaceutical uses against the bottleneck of drug-resistant pathogens [13]. Compounds 73 and 74 exhibited higher activities against E. faecalis with MIC values of 0.05–0.8 µg/mL than the positive control (vancomycin and linezolid). In contrast, the antibacterial activities of 73 and 74 against V. parahaemolyticus, Photobacterium damselae, Shewanella algae, Bacillus amyloliquefaciens ssp-plantarum, and Pseudomonas stutzeri (0.5–8 µg/mL) were lower than those of the positive controls. Both compounds did not show cytotoxicity up to a concentration of 10 µM in the MTT assay [69]. Compounds 75–78 displayed antibacterial activity against P. aeruginosa PA-01 and Acinetobacter baumannii ATCC19606 with MIC values 58–64 µg/mL, while the positive control ofloxacin showed MIC values of 1–16 µg/mL [70]. Compounds 82 and 83 displayed good antimicrobial activities against P. aeruginosa, E. coli, Bacillus cereus, B. subtilis, S. typhi, and S. aureus with MICs values of 0.01–0.05 µM, while azithromycin exhibiting an MIC value of 0.003 µM [71]. Compound 86 displayed broad-spectrum antimicrobial activities against Salmonella typhimurium, MRSA, Listeria monocytogenes, A. hydrophila, S. aureus, Staphylococcus epidermidis, E. coli, and P. aeruginosa with MICs as follows: 16 µg/mL, 32 µg/mL, 0.25 µg/mL, 0.5 µg/mL, 8 µg/mL, 4 µg/mL, 4 µg/mL, and 8 µg/mL. Additionally, the minimum bactericidal concentration (MBC) values of 86 observed ranged from 1 to 64 µg/mL, while the lowest MBC value of 86 was 1 µg/mL for L. monocytogenes followed by A. hydrophila (2 µg/mL), E. coli (8 µg/mL), S. aureus (16 µg/mL) and S. epidermidis (16 µg/mL). On the other hand, the highest MBC values were obtained with MRSA (64 µg/mL), S. typhimurium (32 µg/mL) and P. aeruginosa (32 µg/mL) [73]. Compounds 86 and 87 exhibited inhibitory zone diameters of 1.3–9.7 mm against E. coli, B. subtilis, and S. aureus, wherein 87 showed higher inhibitory effect than 86, which proved they could be used as potential candidates for new antibacterial agents [74].

Table 1.
The antimicrobial activities of the secondary metabolites from marine-derived Bacillus.
3.2. Antifungal Activities
The Bacillus secondary metabolites with potential antifungal activities are listed in Table 2. It was the first time reported that a surfactin type CLP 1 displayed an inhibitory effect on appressorium formation of rice blast causal pathogen Magnaporthe oryzae at concentrations of 10 and 50 µM, indicating that 1 may be considered as potential green pesticide against M. oryzae as prospected [46]. Compound 6 had antagonistic activities against several plant pathogens (Valsa mali, Fusarium oxysporum f.sp. cucumerinum and Rhizoctonia solani) when the concentration was over 2 mg/mL by the paper disc-agar diffusion assay [22]. Compounds 8–10 displayed moderate and dose-dependent inhibition of growth with tested F. oxysporum (inhibitory zone diameter 5.31–7.33 mm) via paper disc-agar diffusion assay, when the concentrations ranged from 0.5 to 2.0 mM. The difference in antifungal activity between 8 and the other two compounds was statistically significant (p < 0.05). With respect to activity–structure relationships, a conclusion can be drawn that antifungal activity is closely related to the length of fatty acid chains, as well as C16 and C17 forms of mojavensins [48]. Research revealed that compound 11 has excellent activity in vitro on the suppression of the conidia germination of Botrytis cinerea (MIC, 50 µM) [49]. Compounds 17 (half maximal inhibitory concentration, IC50 = 1 µg/mL) was an approximately 400-times-stronger inhibitor than compound 18 (IC50 = 400 µg/mL) on the motility of zoospores of Phytophthora capsici with dose-dependent and time-dependent manners. It is also noteworthy that the zoospores blocked by 17 whereafter cleaved at a higher concentration (IC50 = 50 µg/mL). It manifested that the methyl group at C-12 of the fatty acid in 17 is vital for activity. Therefore, compound 17 can be used to develop fungicides targeting P. capsici [50]. Moreover, compounds 17 and 18 possessed promising inhibitory activities against R. solani, Colletotrichum acutatum, B. cinerea with MIC values ranging from 4 to 8 µg/mL, which were comparable to that of amphotericin B (MIC, 1 µg/mL) [50]. Similarly, compounds 28–37 were active with MICs of 1–32 µg/mL when testing the same pathogenic fungi as above [35,57,58]. Among them, mixed compounds 28 + 29 were more active than individual ones. Additionally, the four novel non-cytotoxic lipopeptides 34–37 from marine-derived B. subtilis highlighted the research for novel safe antifungal agents [35,58]. Compounds 32, 34–37 could inhibit mycelial growth, conidiogenesis, conidia germination, morphological alterations in the germinated conidia, and wheat blast disease. Additionally, the MICs of the compounds 32, 34–37 were as follows: 1.5 µg/disk (32), 2.5 µg/disk (36), 2.5 µg/disk (37), 10.0 µg/disk (34), and 10.0 µg/disk (35), revealing 32 showed the highest mycelial growth inhibition of M. oryzae Triticum (MoT) among the tested compounds [75]. Compounds 45, 47 and 49 displayed antifungal activity against filamentous fungi and yeasts [45]. Additionally, compound 47 also exhibited antifungal activity against Candida albicans with an MIC value of 12.5 µg/mL [61]. Compounds 38, 60 and 62 showed significant antifungal activity against Malassezia spp. (Malassezia furfur ATCC 44344, Malassezia furfur ATCC 44344, and Malassezia globosa ATCC MYA 4612) with MIC values ranging from 38 to 330 µg/mL, while the positive control (ketoconazole) had MIC values ranging from 0.03 to 0.08 µg/mL. Therefore, these compounds reported against Malassezia spp. could be beneficial for application in the field of cosmetics and dermatology [44]. Macrolactins A-E (63–67) displayed significant antagonistic activities against Sporisorium scitamineum with EC50 values (for 50% of maximal effect) of 0.31–67.99 µg/mL, wherein 65 displayed the highest potency against S. scitamineum compared with azoxystrobin (EC50, 0.26–36.66 µg/mL). The study of structure and activity relationship proved that a hydroxyl group at C-20 and a glycosyl group at C-7 play an important role in 65. Additionally, 65 inhibited the proliferation and mycelial growth of S. scitamineum spores with EC50 values of 0.52, and 3.25 μg/mL, respectively [68]. Compounds 82 and 83 exhibited good inhibitory effect on the plant pathogenic fungi B. cenerea, R. solani, C. acutatum, and Aspergillus niger, with MICs ranging from 0.03 to 0.05 µM compared to positive control amphotericin B (MIC, 0.003 µM). Moreover, 82 and 83 were reported to possess promising antifungal activity against human pathogen C. albicans with MICs ranging from of 0.03 to 0.05 µM, compared with positive control amphotericin B (MIC, 0.003 µM) [71]. Compounds 86 and 87 showed inhibitory zone diameter of 5.3–11.0 mm against Sclerotina sclerotiorum, Bipolaris sorokiniana, R. solani, Alternaria solani, and Bipolaris maydis [74].

Table 2.
The antifungal activities of the secondary metabolites from marine-derived Bacillus.
3.3. Cytotoxic Activities
The potential cytotoxic Bacillus secondary metabolites are listed in Table 3. Compound 12 displayed moderate cytotoxicity against the cancer cell lines including SK-HEP-1 (liver cancer), HCT116 (colorectal cancer), MDA-MB-231 (breast cancer), A549 (lung cancer), SNU638 (stomach cancer) and antimetastatic activity against breast cancer cells (MDA-MB-231) with IC50 ranging from of 28 to 39 µM compared to positive control Etoposide (IC50, 0.42–6.21 µM). By contrast, compounds 13 and 14 did not exhibit antiproliferative and antimetastatic activities against human cancer cells, which indicated 2-N-OH, 16-N-OH, 37-OH (carboxylic acid) in 12 play an important role. More rarely, 12 was inactive towards normal epithelial cells (IC50 > 50µM) [25]. Compounds 28–30 were cytotoxic towards breast cancer (MDA-MB-231), colon cancer (HCT-15), prostate cancer (PC-3), lung cancer (NCI-H23), stomach cancer (NUGC-3), and renal cancer (ACHN), with GI50 values of 4.6 to 23.2 µg/mL compared with the positive control Adriamycin (GI50, 0.33 to 0.91 µg/mL). It is noteworthy that mixed compounds 28 + 29 demonstrated better activity than individuals, especially for lung cancer (NCI-H23) with the GI50 value of 4.6 µg/mL [35]. Compounds 45–48 were observed with moderate cytotoxic activities towards human cancer cell lines MCF7 (breast cancer) and HepG2 (liver cancer) with IC50 values ranging from 8.2 ± 0.2 to 2.9 ± 0.1 µM, whereas compounds 39–44 had no cytotoxic effects, suggesting that a C16-fatty acid of bacillomycin D analogues may be the key moiety with best cytotoxicities [60]. The MTS assay illustrated that compound 58 can promote cell proliferation at the concentration of 10 µM and 58–62 did not display obvious cytotoxic effect in the murine macrophage cell line RAW 264.7 at the employed concentrations (10–40 µM) [66]. Compound 84 possessed the inhibitory effects on human melanoma cell line MNT-1 and melanin synthesis in mouse melanoma cell line B16F10 [72]. Compound 85 displayed enzyme inhibitory activity against melanogenic enzymes, which could modulate melanogenesis by down-regulating melanogenic enzymes expression with minimal cytotoxicity in murine melanoma cells, such as MNT-1, B16F10 and human melanoma cell [72].

Table 3.
The cytotoxic activities of the secondary metabolites from marine-derived Bacillus.
3.4. Other Activities
In the previous study, sponge epiphytic Bacillus was found to have an obvious inhibitory effect on the attachment of many kinds of diatoms. Compounds 21–27 could inhibit the attachment of diatoms to a certain extent in order to help the host sponges realize the chemical defense to Nitzschia closterium, so they can be used as marine natural products antifouling agent with high possibility and selectivity [54]. Compounds 50 and 51 exhibited siderophore activity at concentrations ranging from 1.28 mM to 1.25 µM, which were the first bacterial siderophores containing benzoic acid and nicotinic acid moieties [24]. Owing to the existence of the epoxy ring, compound 58 exhibited a significant inhibitory effect on the expression of various cytokines inducible and nitric oxide, compared with previously known macrolactins (59–62). Additionally, 58 reduced the mRNA expression level of IL-1β with concentration-dependent manner [66]. Of note, in addition to cytotoxicity in melanoma cells, the anti-pigmentary effect of 85 was demonstrated in an artificial human skin model with comparable or superior effects to those of arbutin, bisabolol and kojic acid, indicating it could be potential as hypopigmenting agent [72].
In this view, most of the presented compounds display significant biological activities such as antimicrobial, antifungal, and anticancer. These compounds are also effective to a certain extent in inhibiting the attachment of diatoms, the expression of various cytokines inducible and nitric oxide exhibited siderophore activity, as well as enzyme inhibitory activity. Among them, antimicrobial (41.41%), antifungal (33.33%), and anticancer (10.10%) activities were dominant in evaluating the pharmacological potential of these metabolites (Figure 11). Notably, a high proportion (54.84%) of the 49 marine sediment-derived metabolites exhibited moderate to potent bioactivity. Based on this, these impressive bioactivities indicate their potential to be new antibiotic candidates, biological control agents, and hypopigmenting agents.
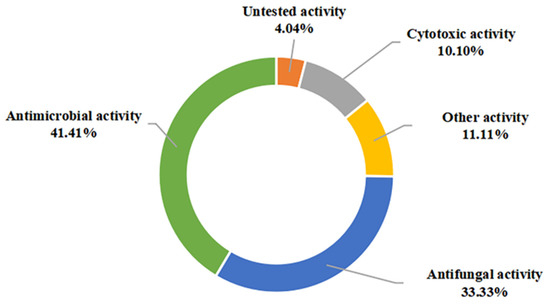
Figure 11.
Bioactivity categories of the secondary metabolites from marine-derived Bacillus.
4. Conclusions
This mini-review summarizes a total of 87 secondary metabolites produced by marine-derived Bacillus species reported in recent years. The described compounds were organized on the basis of their structural diversity, and biological activities. CLPs are ubiquitous in several Bacillus strains, meanwhile linear lipopeptides and macrolactins are found predominantly in B. subtilis and B. amyloliquefaciens species as species-specific metabolites. In addition, marine sediments and sponges are more abundant sources of productive strains of marine-derived Bacillus, and most marine sediment-derived metabolites exhibited moderate to potent bioactivity, which deserves much more attention in subsequent chemical studies. Therefore, marine-derived Bacillus species are a potential promising source for the discovery of new metabolites.
Author Contributions
Conceptualization: S.X. and X.Y.; investigation: N.C., Z.C., M.Z., C.X. and S.Z.; writing-original draft preparation: S.X., N.C., Z.C. and C.X.; writing—review and editing: S.X., X.Y. and M.Z; funding acquisition: X.Y. and S.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31900286 to X.Y.; the Research and innovation Fund of Wuhan Asia General Hospital, grant number 2022KYCX1-A02; and the Youth Innovative Training Fund for College Students of Wuhan University of Science and Technology, grant number 21ZA097.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine Microorganisms as a Promising and Sustainable Source of Bioactive Molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef]
- Barzkar, N. Marine Microbial Alkaline Protease: An Efficient and Essential Tool for Various Industrial Applications. Int. J. Biol. Macromol. 2020, 161, 1216–1229. [Google Scholar] [CrossRef]
- Zheng, L.H.; Zhu, X.J.; Yang, K.L.; Zhu, M.H.; Farooqi, A.A.; Kang, D.L.; Sun, M.; Xu, Y.X.; Lin, X.K.; Feng, Y.G.; et al. PBN11-8, a Cytotoxic Polypeptide Purified from Marine Bacillus, Suppresses Invasion and Migration of Human Hepatocellular Carcinoma Cells by Targeting Focal Adhesion Kinase Pathways. Polymers 2018, 10, 1043. [Google Scholar] [CrossRef]
- De Rop, A.S.; Rombaut, J.; Willems, T.; De Graeve, M.; Vanhaecke, L.; Hulpiau, P.; De Maeseneire, S.L.; De Mol, M.L.; Soetaert, W.K. Novel Alkaloids from Marine Actinobacteria: Discovery and Characterization. Mar. Drugs 2021, 20, 6. [Google Scholar] [CrossRef]
- El-Sersy, N.A.; Abdelwahab, A.E.; Abouelkhiir, S.S.; Abou-Zeid, D.M.; Sabry, S.A. Antibacterial and Anticancer Activity of ε-Poly-L-Lysine (Ε-Pl) Produced by a Marine Bacillus subtilis sp. J. Basic Microbiol. 2012, 52, 513–522. [Google Scholar] [CrossRef]
- Habbu, P.; Warad, V.; Shastri, R.; Madagundi, S.; Kulkarni, V.H. Antimicrobial Metabolites from Marine Microorganisms. Chin. J. Nat. Med. 2016, 14, 101–116. [Google Scholar] [CrossRef]
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine Microorganisms as an Untapped Source of Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar] [CrossRef]
- El-Hossary, E.M.; Cheng, C.; Hamed, M.M.; El-Sayed Hamed, A.N.; Ohlsen, K.; Hentschel, U.; Abdelmohsen, U.R. Antifungal Potential of Marine Natural Products. Eur. J. Med. Chem. 2017, 126, 631–651. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, S.S.; Baindara, P.; Sharma, S.; Khatri, N.; Grover, V.; Patil, P.B.; Korpole, S. Surfactin Like Broad Spectrum Antimicrobial Lipopeptide Co-Produced with Sublancin from Bacillus Subtilis Strain A52: Dual Reservoir of Bioactives. Front. Microbiol. 2020, 11, 1167. [Google Scholar] [CrossRef]
- Mawlankar, R.; Thorat, M.N.; Krishnamurthi, S.; Dastager, S.G. Bacillus cellulasensis sp. nov., Isolated from Marine Sediment. Arch. Microbiol. 2016, 198, 83–89. [Google Scholar] [CrossRef]
- Liu, R.; Huang, Z.B.; Dong, C.M.; Shao, Z.Z. Lottiidibacillus patelloidae Gen. Nov., Sp. Nov., Isolated from the Intestinal Tract of a Marine Limpet and Reclassification of Bacillus taeanensis as Maribacillus taeanensis Gen. Nov., Comb. Nov. Antonie Leeuwenhoek 2019, 112, 797–807. [Google Scholar] [CrossRef]
- Tareq, F.S.; Shin, H.J. Bacilotetrins A and B, Anti-Staphylococcal Cyclic-Lipotetrapeptides from a Marine-Derived Bacillus subtilis. J. Nat. Prod. 2017, 80, 2889–2892. [Google Scholar] [CrossRef]
- Chakraborty, K.; Kizhakkekalam, V.K.; Joy, M.; Dhara, S. Difficidin Class of Polyketide Antibiotics from Marine Macroalga-Associated Bacillus as Promising Antibacterial Agents. Appl. Microbiol. Biotechnol. 2021, 105, 6395–6408. [Google Scholar] [CrossRef]
- Harwood, C.R.; Mouillon, J.M.; Pohl, S.; Arnau, J. Secondary Metabolite Production and the Safety of Industrially Important members of the Bacillus subtilis group. FEMS. Microbiol. Rev. 2018, 42, 721–738. [Google Scholar] [CrossRef]
- Dame, Z.T.; Rahman, M.; Islam, T. Bacilli as Sources of Agrobiotechnology: Recent Advances and Future Directions. Green Chem. Lett. Rev. 2021, 14, 245–270. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Ali, M.S.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K.H. Bacillus velezensis: A Valuable Member of Bioactive Molecules Within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef]
- Bibi, F.; Naseer, M.I.; Azhar, E.I. Assessing the Diversity of Bacterial Communities from Marine Sponges and Their Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 2747–2754. [Google Scholar] [CrossRef]
- Zhang, L.L.; Sun, C.M. Fengycins, Cyclic Lipopeptides from Marine Bacillus subtilis strains, Kill the Plant-Pathogenic Fungus Magnaporthe grisea by Inducing Reactive Oxygen Species Production and Chromatin Condensation. Appl. Environ. Microbiol. 2018, 84, e00418–e00445. [Google Scholar] [CrossRef]
- Kizhakkekalam, V.K.; Chakraborty, K.; Joy, M. Oxygenated Elansolid Type of Polyketide Spanned Macrolides from a Marine Heterotrophic Bacillus as Prospective Antimicrobial Agents Against Multidrug Resistant Pathogens. Int. J. Antimicrob. Agents 2020, 55, 105892. [Google Scholar] [CrossRef]
- Karthik, L.; Sun, W.; Wang, Y.K.; Mulati, N.; Gong, S.Q.; Zhang, F.L.; Li, Z.Y.; Li, Y.X. Biosynthesis In Vitro of Bacillamide Intermediate-Heterocyclic Alacysthiazole by Heterologous Expression of Nonribosomal Peptide Synthetase (NRPS). J. Biotechnol. 2019, 292, 5–11. [Google Scholar] [CrossRef]
- Yi, X.X.; Gan, Y.M.; Jiang, L.; Yu, L.; Liu, Y.H.; Gao, C.H. Rapid Improvement in the Macrolactins Production of Bacillus Sp. Combining Atmospheric Room Temperature Plasma with the Specific Growth Rate Index. J. Biosci. Bioeng. 2020, 130, 48–53. [Google Scholar] [CrossRef]
- Ma, Z.; Hu, J.; Wang, X.; Wang, S. NMR Spectroscopic And MS/MS Spectrometric Characterization of a New Lipopeptide Antibiotic Bacillopeptin B1 Produced by a Marine Sediment-Derived Bacillus amyloliquefaciens SH-B74. J. Antibiot. 2014, 67, 175–178. [Google Scholar] [CrossRef][Green Version]
- Ma, Y.X.; Kong, Q.; Qin, C.; Chen, Y.L.; Chen, Y.J.; Lv, R.H.; Zhou, G.H. Identification of Lipopeptides in Bacillus megaterium by Two-Step Ultrafiltration and LC-ESI-MS/MS. AMB Express 2016, 6, 79. [Google Scholar] [CrossRef]
- Wu, Q.H.; Throckmorton, K.; Maity, M.; Chevrette, M.G.; Braun, D.R.; Rajski, S.R.; Currie, C.R.; Thomas, M.G.; Bugni, T.S. Bacillibactins E and F from a Marine Sponge-Associated Bacillus sp. J. Nat. Prod. 2021, 84, 136–141. [Google Scholar] [CrossRef]
- Shin, D.; Byun, W.S.; Moon, K.; Kwon, Y.; Bae, M.; Um, S.; Lee, S.K.; Oh, D.C. Coculture of Marine Streptomyces sp. With Bacillus sp. Produces a New Piperazic Acid-Bearing Cyclic Peptide. Front. Chem. 2018, 6, 498. [Google Scholar] [CrossRef]
- Stincone, P.; Brandelli, A. Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319. [Google Scholar] [CrossRef]
- Fu, X.T.; Gong, L.F.; Liu, Y.; Lai, Q.L.; Li, G.Y.; Shao, Z.Z. Bacillus pumilus Group Comparative Genomics: Toward Pangenome Features, Diversity, and Marine Environmental Adaptation. Front. Microbiol. 2021, 2, 571212. [Google Scholar] [CrossRef]
- Subramenium, G.A.; Swetha, T.K.; Iyer, P.M.; Balamurugan, K.; Pandian, S.K. 5-Hydroxymethyl-2-Furaldehyde from Marine Bacterium Bacillus Subtilis Inhibits Biofilm and Virulence of Candida albicans. Microbiol. Res. 2018, 207, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.H.; Yu, Q.H.; Yang, S.; Wen, J.; Guo, Z.H.; Wang, X.L.; Wang, N. Ultrafast Recovery of Uranium from Seawater by Bacillus velezensis Strain UUS-1 with Innate Anti-Biofouling Activity. Adv. Sci. (Weinh.) 2019, 6, 1900961. [Google Scholar] [CrossRef]
- Ibrahim, A.Y.; Youness, E.R.; Mahmoud, M.G.; Asker, M.S.; El-Newary, S.A. Acidic Exopolysaccharide Produced from Marine Bacillus Amyloliquefaciens 3MS 2017 For the Protection and Treatment of Breast Cancer. Breast Cancer 2020, 14, 1178223420902075. [Google Scholar] [CrossRef]
- Zhou, H.; Cong, B.L.; Tian, Y.Q.; He, Y.; Yang, H.H. Characterization of Novel Cyclic Lipopeptides Produced by Bacillus Sp. SY27F. Process Biochem. 2019, 83, 206–213. [Google Scholar] [CrossRef]
- Dan, A.K.; Manna, A.; Ghosh, S.; Sikdar, S.; Sahu, R.; Parhi, P.K.; Parida, S. Molecular Mechanisms of the Lipopeptides from Bacillus subtilis in the Apoptosis of Cancer Cells-A Review on Its Current Status in Different Cancer Cell Lines. Adv. Cancer Biol. Met. 2021, 3, 100019. [Google Scholar] [CrossRef]
- Routhu, S.R.; Nagarjuna Chary, R.; Shaik, A.B.; Prabhakar, S.; Kumar, C.G.; Kamal, A. Induction of Apoptosis in Lung Carcinoma Cells by Antiproliferative Cyclic Lipopeptides from Marine Algicolous Isolate Bacillus atrophaeus Strain AKLSR1. Process Biochem. 2019, 79, 142–154. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological Control of Plant Pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, J.-S.; Lee, Y.-J.; Shin, H.J. Gageostatins A–C, Antimicrobial Linear Lipopeptides from a Marine Bacillus subtilis. Mar. Drugs 2014, 12, 871–885. [Google Scholar] [CrossRef]
- Aleti, G.; Sessitsch, A.; Brader, G. Genome mining: Prediction of Lipopeptides and Polyketides from Bacillus and Related Firmicutes. Comput. Struct. Biotechnol. J. 2015, 13, 192–203. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative Analysis of the Complete Genome Sequence of the Plant Growth-Promoting Bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef]
- Chen, X.H.; Scholz, R.; Borriss, M.; Junge, H.; Mögel, G.; Kunz, S.; Borriss, R. Difficidin and Bacilysin Produced by Plant-Associated Bacillus amyloliquefaciens Are Efficient in Controlling Fire Blight Disease. J. Biotechnol. 2009, 140, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhou, Q.N.; Luo, H.Y.; Xia, L.Q.; Li, L.; Sun, M.; Yu, Z.Q. Systemic Nematicidal Activity and Biocontrol Efficacy of Bacillus firmus Against the Root-Knot Nematode Meloidogyne incognita. World J. Microbiol. Biotechnol. 2015, 31, 661–667. [Google Scholar] [CrossRef]
- Xu, K.; Yuan, X.L.; Li, C.; Li, A.X. Recent Discovery of Heterocyclic Alkaloids from Marine-Derived Aspergillus Species. Mar. Drugs 2020, 18, 54. [Google Scholar] [CrossRef]
- Zhang, P.; Wei, Q.; Yuan, X.; Xu, K. Newly Reported Alkaloids Produced by Marine-Derived Penicillium Species (covering 2014-2018). Bioorg. Chem. 2020, 99, 103840. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.R.; He, S.; Yan, X.J. New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade. Mar. Drugs 2017, 15, 132. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2007, 16, 115–125. [Google Scholar] [CrossRef]
- Vairagkar, U.; Mirza, Y. Antagonistic Activity of Antimicrobial Metabolites Produced from Seaweed-Associated Bacillus amyloliquefaciens MTCC 10456 Against Malassezia Spp. Probiotics Antimicrob. Proteins 2021, 13, 1228–1237. [Google Scholar] [CrossRef]
- Nair, D.; Vanuopadath, M.; Nair, B.G.; Pai, J.G.; Nair, S.S. Identification and Characterization of a Library of Surfactins and Fengycins from a Marine Endophytic Bacillus Sp. J. Basic Microbiol. 2016, 56, 1159–1172. [Google Scholar] [CrossRef]
- Ma, Z.W.; Zhang, S.Y.; Zhang, S.H.; Wu, G.Y.; Shao, Y.; Mi, Q.F.; Liang, J.Y.; Hu, J.C. Isolation and Characterization of a New yclic Lipopeptide Surfactin from a Marine-Derived Bacillus velezensis SH-B74. J. Antibiot. 2020, 73, 863–867. [Google Scholar] [CrossRef]
- Chen, Y.L.; Liu, S.L.A.; Mou, H.J.; Ma, Y.X.; Li, M.; Hu, X.K. Characterization of Lipopeptide Biosurfactants Produced by Bacillus licheniformis MB01 from Marine Sediments. Front. Microbiol. 2017, 8, 871. [Google Scholar] [CrossRef]
- Ma, Z.W.; Hu, J.C. Production and Characterization of Iturinic Lipopeptides as Antifungal Agents and Biosurfactants Produced by a Marine Pinctada martensii-Derived Bacillus mojavensis B0621A. Appl. Biochem. Biotechnol. 2014, 173, 705–715. [Google Scholar] [CrossRef]
- Ma, Z.W.; Hu, J.C. Plipastatin A1 Produced by a Marine Sediment-Derived Bacillus amyloliquefaciens SH-B74 Contributes to the Control of Gray Mold Disease in Tomato. 3 Biotech 2018, 8, 125. [Google Scholar] [CrossRef]
- Tareq, F.S.; Hasan, C.M.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Surovy, M.Z.; Islam, M.T.; Shin, H.J. Gageopeptins A and B, New Inhibitors of Zoospore Motility of the Phytopathogen Phytophthora Capsici from a Marine-Derived Bacterium Bacillus Sp. 109GGC020. Bioorg. Med. Chem. Lett. 2015, 25, 3325–3329. [Google Scholar] [CrossRef]
- Wiese, J.; Abdelmohsen, U.R.; Motiei, A.; Humeida, U.H.; Imhoff, J.F. Bacicyclin, a New Antibacterial Cyclic Hexapeptide from Bacillus Sp. Strain BC028 Isolated from Mytilus edulis. Bioorg. Med. Chem. Lett. 2018, 28, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Xia, J. Recent Advances in Diketopiperazines Biosynthesis. Microbiol. China 2012, 41, 111–121. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Lai, T.K.; Saha, A.; Selvin, J.; Mukherjee, J. Structural Elucidation and Antimicrobial Activity of a Diketopiperazine Isolated from A Bacillus Sp. Associated with the Marine Sponge Spongia officinalis. Nat. Prod. Res. 2021, 35, 2315–2323. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yu, S.Y.; Jiang, W.; Zhang, L.K.; Jin, C.L.; Zhou, X.J. Isolation and Identification of Antidiatom Attachment Active Constituents of a Strain of Epiphytic Bacillus Sponge. Mar. Sci. 2016, 40, 23–32. [Google Scholar] [CrossRef]
- Mohan, G.; Thipparamalai Thangappanpillai, A.K.; Ramasamy, B. Antimicrobial Activities of Secondary Metabolites and Phylogenetic Study of Sponge Endosymbiotic Bacteria, Bacillus Sp. at Agatti Island, Lakshadweep Archipelago. Biotechnol. Rep. 2016, 11, 44–52. [Google Scholar] [CrossRef]
- Yan, Y.H.; Li, Y.Z.; Zhang, Z.W.; Wang, X.H.; Niu, Y.Z.; Zhang, S.H.; Xu, W.L.; Ren, C.G. Advances of peptides for antibacterial applications. Colloid. Surf. B 2021, 202, 111682. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Gageotetrins A–C, Noncytotoxic Antimicrobial Linear Lipopeptides from a Marine Bacterium Bacillus subtilis. Org. Lett. 2014, 16, 928–931. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Hasan, C.M.; Islam, M.T.; Shin, H.J. Non-Cytotoxic Antifungal Agents: Isolation and Structures of Gageopeptides A–D from a Bacillus Strain 109GGC020. J. Agric. Food Chem. 2014, 62, 5565–5572. [Google Scholar] [CrossRef]
- Gulick, A.M. Nonribosomal Peptide Synthetase Biosynthetic Clusters of ESKAPE Pathogens. Nat. Prod. Rep. 2017, 34, 981–1009. [Google Scholar] [CrossRef]
- Zhou, M.J.; Liu, F.W.; Yang, X.Y.; Jin, J.; Dong, X.; Zeng, K.-W.; Liu, D.; Zhang, Y.T.; Ma, M.; Yang, D.H. Bacillibactin and Bacillomycin Analogues with Cytotoxicities against Human Cancer Cell Lines from Marine Bacillus Sp. PKU-MA00093 and PKU-MA00092. Mar. Drugs 2018, 16, 22. [Google Scholar] [CrossRef]
- Tabbene, O.; Azaiez, S.; Di Grazia, A.; Karkouch, I.; Ben Slimene, I.; Elkahoui, S.; Alfeddy, M.N.; Casciaro, B.; Luca, V.; Limam, F.; et al. Bacillomycin D And Its Combination with Amphotericin B: Promising Antifungal Compounds with Powerful Antibiofilm Activity and Wound-Healing Potency. J. Appl. Microbiol. 2016, 120, 289–300. [Google Scholar] [CrossRef]
- Miyanaga, A. Structure and Function of Polyketide Biosynthetic Enzymes: Various Strategies for Production of Structurally Diverse Polyketides. Biosci. Biotechnol. Biochem. 2017, 81, 2227–2236. [Google Scholar] [CrossRef]
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Previously Undescribed Antibacterial Polyketides from Heterotrophic Bacillus amyloliquefaciens Associated with Seaweed Padina gymnospora. Appl. Microbiol. Biotechnol. 2018, 184, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Thilakan, B.; Chakraborty, R.D.; Raola, V.K.; Joy, M. O-Heterocyclic Derivatives with Antibacterial Properties from Marine Bacterium Bacillus Subtilis Associated with Seaweed, Sargassum myriocystum. Appl. Microbiol. Biotechnol. 2017, 101, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Mondol, M.A.; Shahidullah Tareq, F.; Kim, J.H.; Lee, M.A.; Lee, H.-S.; Lee, J.S.; Lee, Y.-J.; Shin, H.J. New Antimicrobial Compounds from a Marine-Derived Bacillus sp. J. Antibiot. 2013, 66, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zhou, Y.-X.; Tang, X.-X.; Liu, X.-X.; Yi, Z.-W.; Fang, M.-J.; Wu, Z.; Jiang, F.-Q.; Qiu, Y.-K. Macrolactins from Marine-Derived Bacillus subtilis B5 Bacteria as Inhibitors of Inducible Nitric Oxide and Cytokines Expression. Mar. Drugs 2016, 14, 195. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.H.; Chen, X.Q.; Yu, L.; Jiang, L.; Pan, D.J.; Jiang, S.; Gan, Y.M.; Liu, Y.H.; Yi, X.X. New 24-Membered Macrolactins Isolated from Marine Bacteria Bacillus siamensis as Potent Fungal Inhibitors against Sugarcane Smut. J. Agric. Food Chem. 2021, 69, 4392–4401. [Google Scholar] [CrossRef]
- Chakraborty, K.; Thilakan, B.; Raola, V.K. Polyketide Family of Novel Antibacterial 7-O-Methyl-5’-Hydroxy-3’-Heptenoate-Macrolactin from Seaweed-Associated Bacillus subtilis MTCC 10403. J. Agric. Food Chem. 2014, 62, 12194–12208. [Google Scholar] [CrossRef]
- Wang, W.; Park, K.-H.; Lee, J.; Oh, E.; Park, C.; Kang, E.; Lee, J.; Kang, H. A New Thiopeptide Antibiotic, Micrococcin P3, from a Marine-Derived Strain of the Bacterium Bacillus stratosphericus. Molecules 2020, 25, 4383. [Google Scholar] [CrossRef]
- Zhou, S.-Y.; Hu, Y.-J.; Meng, F.-C.; Qu, S.-Y.; Wang, R.; Andersen, R.J.; Liao, Z.-H.; Chen, M. Bacillamidins A-G from a Marine-Derived Bacillus pumilus. Mar. Drugs 2018, 16, 326. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, H.-S.; Lee, Y.-J.; Lee, J.S.; Shin, H.J. Ieodoglucomide C and Ieodoglycolipid, New Glycolipids from a Marine-Derived Bacterium Bacillus licheniformis 09IDYM23. Lipids 2015, 50, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Leutou, A.S.; Jeong, H.; Kim, D.; Seong, C.N.; Nam, S.-J.; Lim, K.-M. Anti-Pigmentary Effect of (-)-4-Hydroxysattabacin from the Marine-Derived Bacterium Bacillus Sp. Mar. Drugs 2017, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Lotfya, W.A.; Mostafab, S.W.; Adel, A.A.; Ghanem, K.M. Production of Di-(2-Ethylhexyl) Phthalate by Bacillus subtilis AD35: Isolation, Purifification, Characterization and Biological Activities. Microb. Pathog. 2018, 124, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.-Z.; Fu, H.-R.; Wu, S.-J.; Bao, Z.-H.; Wang, S.-F.; Ge, P.-H. Isolation and Structure Elucidation of Antifungal Metabolites from marine Paenibacillus polymyxa Strain L1–9. Acta Pharmacol. Sin. 2014, 44, 486–496. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mahmud, N.U.; Gupta, D.R.; Tareq, F.S.; Shin, H.J.; Islam, T. Inhibitory Effects of Linear Lipopeptides from a Marine Bacillus subtilis on the Wheat Blast Fungus Magnaporthe oryzae Triticum. Front. Microbiol. 2020, 11, 665. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).