A Novel Bi-Functional Fibrinolytic Enzyme with Anticoagulant and Thrombolytic Activities from a Marine-Derived Fungus Aspergillus versicolor ZLH-1
Abstract
1. Introduction
2. Results and Discussion
2.1. Enzyme Purification, SDS-PAGE, and Zymography
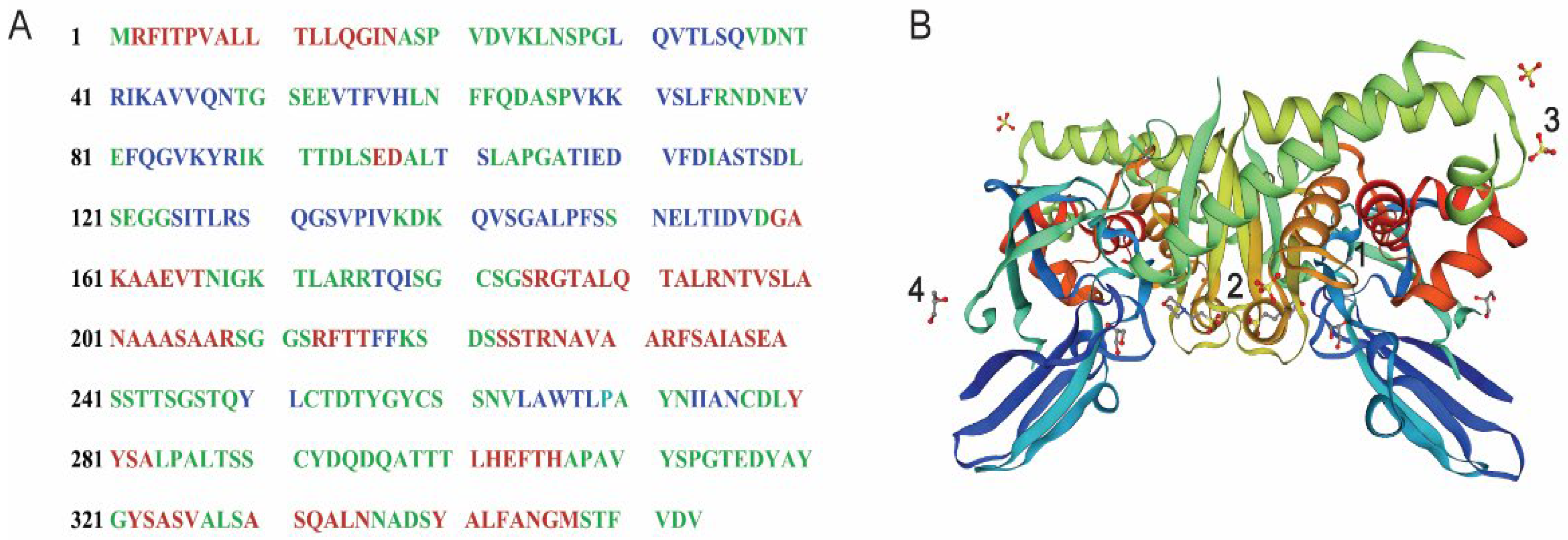
2.2. The Identification and Modeling of Versiase
2.3. Biochemical Properties of Versiase
2.4. Fibrin(ogen)olytic Activity
2.5. Anticoagulant and Thrombolytic Activities
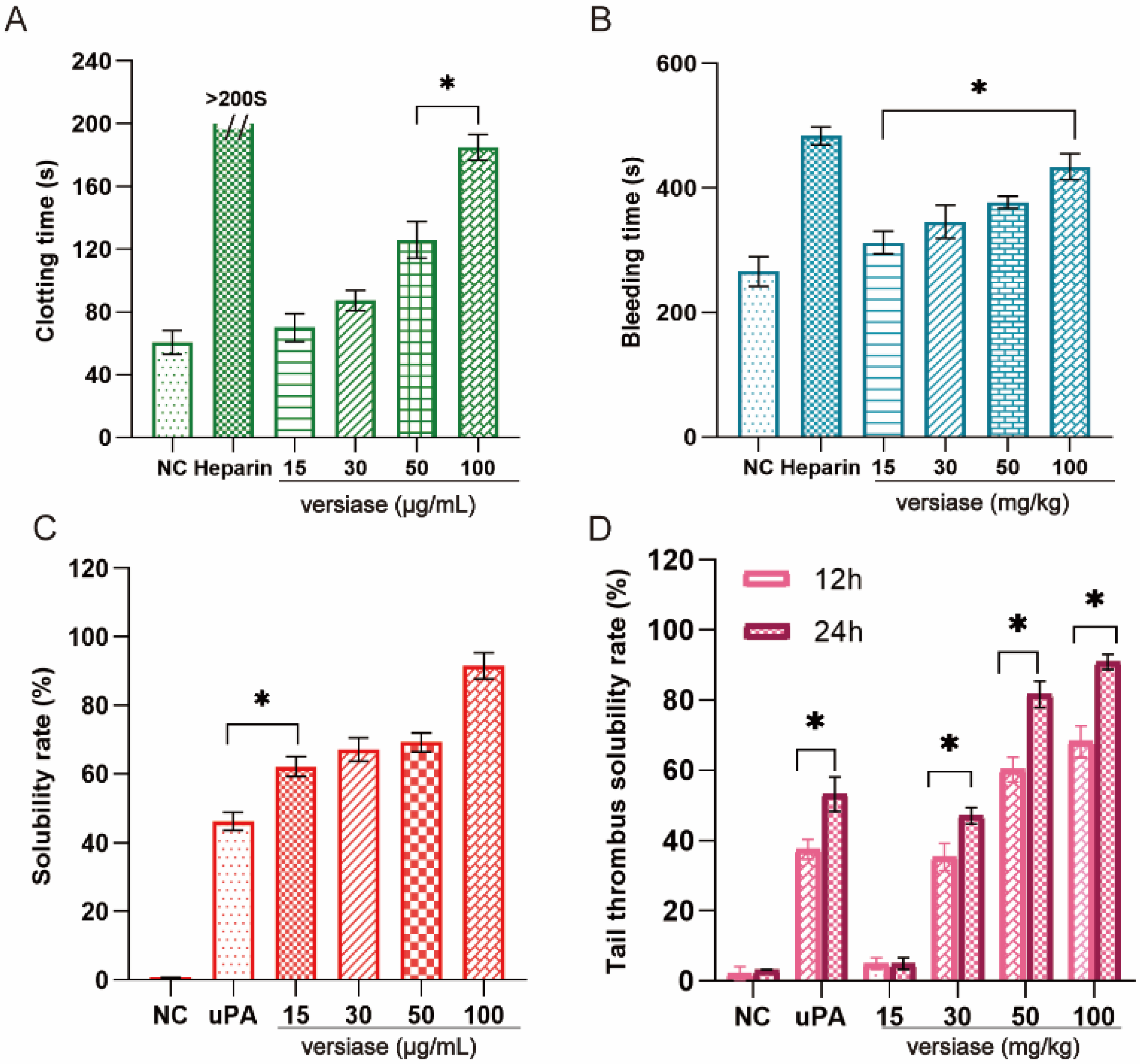
2.5.1. Anticoagulant Activity In Vitro and In Vivo
2.5.2. Thrombolytic Activity In Vitro and In Vivo
2.6. The Safety Evaluation
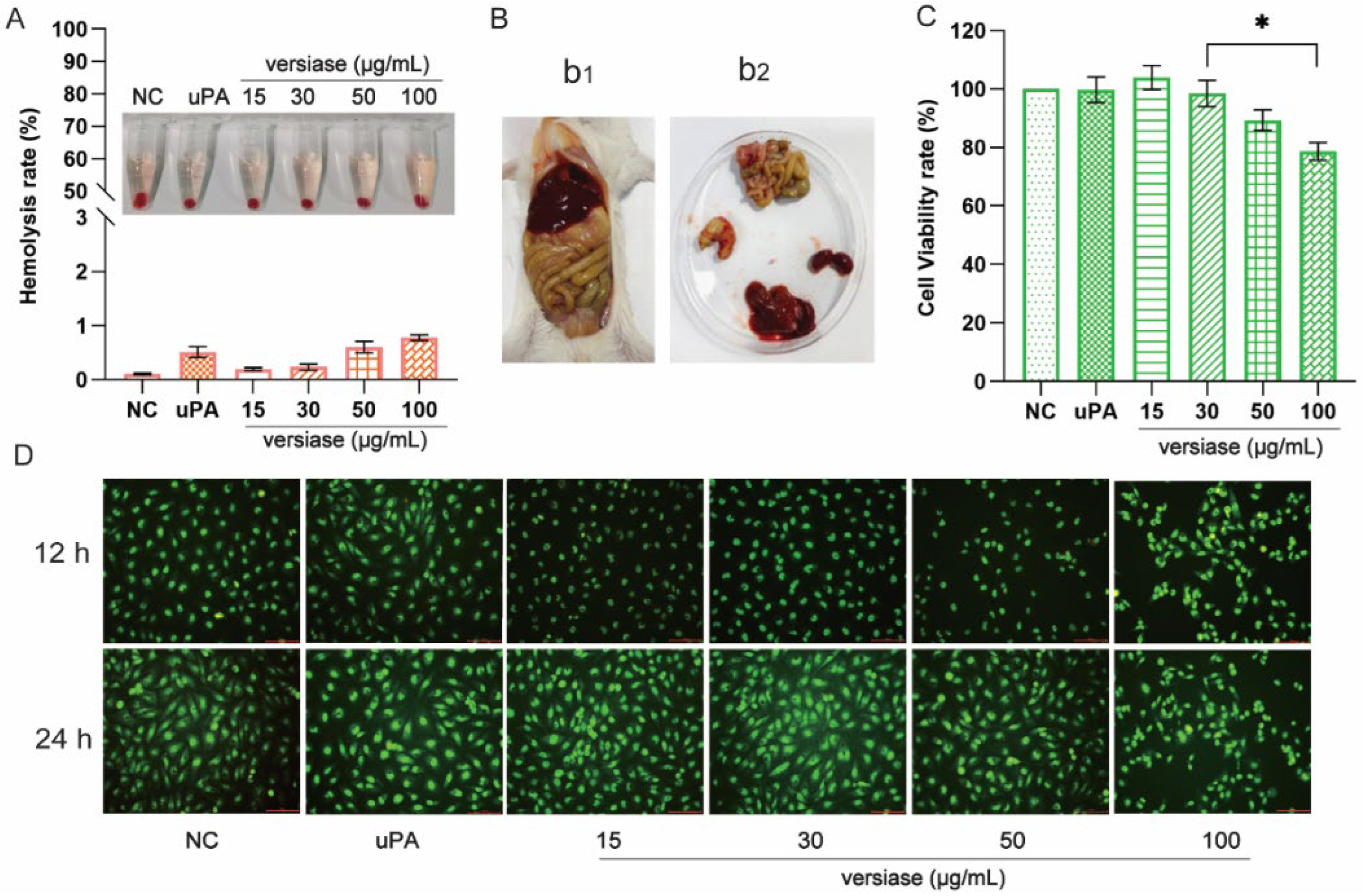
2.6.1. The Hemolysis Test
2.6.2. The Acute Toxicity In Vivo
2.6.3. The Cytotoxicity Assay
3. Materials and Methods
3.1. Microorganism and Cultivation
3.2. Cells and Animals
3.3. Preparation of the Fibrinolytic Enzyme
3.4. Fibrinolytic Enzyme Activity Assay
3.5. SDS-PAGE and Fibrin Zymography
3.6. Protein Identification and Structure Prediction
3.7. Biochemical Characterization
3.8. Detection of Fibrinolytic Activity Using Fibrin Plate
3.9. Fibrin(ogen)olytic Assay
3.10. Anticoagulant Effect of Versiase on Animal Blood In Vitro
3.11. Examination of Anticoagulant Activity In Vivo
3.12. In Vitro Thrombolytic Activities of Versiase
3.13. In Vivo Thrombolytic Test Using the Mouse Tail Thrombosis Model
3.14. The Hemolysis Test
3.15. The Acute Toxicity In Vivo
3.16. Analysis of Cell Viability
3.17. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Cardiovascular Diseases (CVDs), Fact Sheets. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 August 2021).
- Mosesson, M.W. The roles of fibrinogen and fibrin in hemostasis and thrombosis. Semin. Hematol. 1992, 29, 177–188. [Google Scholar] [PubMed]
- Danesh, J.; Lewington, S.; Thompson, S.G.; Lowe, G.D.; Collins, R.; Kostis, J.B.; Wilson, A.C.; Folsom, A.R.; Wu, K.; Benderly, M. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA 2005, 294, 1799–1809. [Google Scholar] [PubMed]
- Schrder, R.; Neuhaus, K.L.; Leizorovicz, A.; Linderer, T.; Tebbe, U. A prospective placebo-controlled double-blind multicenter trial of intravenous streptokinase in acute myocardial infarction (ISAM): Long-term mortality and morbidity. J. Am. Coll. Cardiol. 1987, 9, 197–203. [Google Scholar] [CrossRef][Green Version]
- Lincoff, A.M.; Topol, E.J. Illusion of reperfusion. Does anyone achieve optimal reperfusion during acute myocardial infarction? Circulation 1993, 88, 1361–1374. [Google Scholar] [CrossRef] [PubMed]
- Altaf, F.; Wu, S.; Kasim, V. Role of fibrinolytic enzymes in anti-thrombosis therapy. Front. Mol. Biosci. 2021, 8, 476. [Google Scholar] [CrossRef] [PubMed]
- Wiman, B. Plasminogen activator inhibitor 1 (PAI-1) in plasma: Its role in thrombotic disease. Thromb. Haemost. 1995, 74, 71–76. [Google Scholar] [CrossRef]
- Flemmig, M.; Melzig, M.F. Serine-proteases as plasminogen activators in terms of fibrinolysis. J. Pharm. Pharmacol. 2012, 64, 1025–1039. [Google Scholar] [CrossRef]
- Marder, V.J.; Novokhatny, V. Direct fibrinolytic agents: Biochemical attributes, preclinical foundation and clinical potential. J. Thromb. Haemost. 2010, 8, 433–444. [Google Scholar] [CrossRef]
- Kotb, E. The biotechnological potential of fibrinolytic enzymes in the dissolution of endogenous blood thrombi. Biotechnol. Prog. 2014, 30, 656–672. [Google Scholar] [CrossRef]
- Nascimento, T.P.; Sales, A.E.; Porto, T.S.; Costa, R.M.P.B.; Breydo, L.; Uversky, V.N.; Porto, A.L.F.; Converti, A. Purification, biochemical, and structural characterization of a novel fibrinolytic enzyme from Mucor subtilissimus UCP 1262. Bioproc. Biosyst. Eng. 2017, 40, 1209–1219. [Google Scholar] [CrossRef]
- Choi, J.-H.; Sapkota, K.; Park, S.-E.; Kim, S.; Kim, S.-J. Thrombolytic, anticoagulant and antiplatelet activities of codiase, a bi-functional fibrinolytic enzyme from Codium fragile. Biochimie 2013, 95, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, X.; Cong, S.; Deng, Y.; Zheng, X. A novel serine protease with anticoagulant and fibrinolytic activities from the fruiting bodies of mushroom Agrocybe aegerita. Int. J. Biol. Macromol. 2021, 168, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, X.; Palm, G.J.; Schwenteit, J.; Singh, R.K.; Gudmundsdóttir, B.K.; Hinrichs, W. Structural evidence of intramolecular propeptide inhibition of the aspzincin metalloendopeptidase AsaP1. FEBS Lett. 2016, 590, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Naveena, B.; Gopinath, K.P.; Sakthiselvan, P.; Partha, N. Enhanced production of thrombinase by Streptomyces venezuelae: Kinetic studies on growth and enzyme production of mutant strain. Bioresour. Technol. 2012, 111, 417–424. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.V.; do Nascimento, J.M.; Rodrigues, C.H.; Nascimento, D.C.S.; Costa, R.M.P.B.; Marques, D.d.A.V.; Leite, A.C.L.; Figueiredo, M.d.V.B.; Pastrana, L.; Converti, A. Partial purification of fibrinolytic and fibrinogenolytic protease from Gliricidia sepium seeds by aqueous two-phase system. Biocatal. Agric. Biotechnol. 2020, 27, 101669. [Google Scholar] [CrossRef]
- Al Farraj, D.A.; Kumar, T.S.J.; Vijayaraghavan, P.; Elshikh, M.S.; Alkufeidy, R.M.; Alkubaisi, N.A.; Alshammari, M.K. Enhanced production, purification and biochemical characterization of therapeutic potential fibrinolytic enzyme from a new Bacillus flexus from marine environment. J. King Saud Univ. Sci. 2020, 32, 3174–3180. [Google Scholar] [CrossRef]
- Kumar, S.S.; Haridas, M.; Abdulhameed, S. A novel fibrinolytic enzyme from marine Pseudomonas aeruginosa KU1 and its rapid in vivo thrombolysis with little haemolysis. Int. J. Biol. Macromol. 2020, 162, 470–479. [Google Scholar] [CrossRef]
- Lu, M.; Gao, Z.; Xing, S.; Long, J.; Li, C.; He, L.; Wang, X. Purification, characterization, and chemical modification of Bacillus velezensis SN-14 fibrinolytic enzyme. Int. J. Biol. Macromol. 2021, 177, 601–609. [Google Scholar] [CrossRef]
- Liu, X.; Kopparapu, N.-k.; Shi, X.; Deng, Y.; Zheng, X.; Wu, J. Purification and biochemical characterization of a novel fibrinolytic enzyme from culture supernatant of Cordyceps militaris. J. Agric. Food Chem. 2015, 63, 2215–2224. [Google Scholar] [CrossRef]
- Mihara, H.; Sumi, H.; Yoneta, T.; MizuMOTO, H.; Ikeda, R.; Seiki, M.; Maruyama, M. A novel fibrinolytic enzyme extracted from the earthworm, Lumbricus rubellus. Jpn. J. Physiol. 1991, 41, 461–472. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Xiao, Y.; Wang, Y.; Wu, J.; Liu, C.; Ye, H.; Li, F.; Yu, H.; Lai, R. A bi-functional anti-thrombosis protein containing both direct-acting fibrin (ogen) olytic and plasminogen-activating activities. PLoS ONE 2011, 6, e17519. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Hori, K.; Matsuura, Y.; Miyazawa, K. Purification and characterization of a fibrinolytic enzyme and identification of fibrinogen clotting enzyme in a marine green alga, Codium divaricatum. Comp. Biochem. Physiol. 2000, 125, 137–143. [Google Scholar] [CrossRef]
- Majumdar, S.; Dutta, S.; Das, T.; Chattopadhyay, P.; Mukherjee, A.K. Antiplatelet and antithrombotic activity of a fibrin (ogen) olytic protease from Bacillus cereus strain FF01. Int. J. Biol. Macromol. 2015, 79, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Chesebro, J.; Badimon, L.; Fuster, V. The ischemic risk syndrome following thrombolysis: The problem of arterial re-occlusion. Curr. Opin. Cardiol. 1988, 3, 492–500. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia; China Medical Science and Technology Press: Beijing, China, 2010; p. 92, Appendix XIII H.
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 163, 76–85. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Belur, P.D. A novel fibrinolytic serine metalloprotease from the marine Serratia marcescens subsp. sakuensis: Purification and characterization. Int. J. Biol. Macromol. 2018, 112, 110–118. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural protein during the assembly of the head of bacterio phase T4. Nature 1970, 227, 265–275. [Google Scholar] [CrossRef]
- Kim, S.-H.; Choi, N.-S.; Lee, W.-Y. Fibrin zymography: A direct analysis of fibrinolytic enzymes on gels. Anal. Biochem. 1998, 263, 115–116. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Kim, J.B.; Jung, W.H.; Ryu, J.M.; Lee, Y.J.; Jung, J.K.; Jang, H.W.; Kim, S.W. Identification of a fibrinolytic enzyme by Bacillus vallismortis and its potential as a bacteriolytic enzyme against Streptococcus mutans. Biotechnol. Lett. 2007, 29, 605–610. [Google Scholar] [CrossRef]
- Lassen, M. Heat Denaturation of Plasminogen in the Fibrin Plate Method. Acta Physiol. 1953, 27, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Tucker, E.I.; Verbout, N.G.; Leung, P.Y.; Hurst, S.; McCarty, O.J.; Gailani, D.; Gruber, A. Inhibition of factor XI activation attenuates inflammation and coagulopathy while improving the survival of mouse polymicrobial sepsis. Blood JASN 2012, 119, 4762–4768. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yang, J.; Zhuang, Z.; Yang, Y.; Lin, L.; Wang, S. Thrombolytic effects of Douchi Fibrinolytic enzyme from Bacillus subtilis LD-8547 in vitro and in vivo. BMC Biotechnol. 2012, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Bekemeier, H.; Hirschelmann, R.; Giessler, A. Carrageenin-induced thrombosis in the rat and mouse as a test model of substances influencing thrombosis. Biomed. Biochim. Acta 1984, 43, S347–S350. [Google Scholar]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Amarante-Mendes, G.P.; Finucane, D.; Brunner, T.; Green, D.R. Acridine Orange/Ethidium Bromide (AO/EB) Staining to Detect Apoptosis. CSH Protoc. 2006, 2006, pdb-rot4493. [Google Scholar] [CrossRef]



| Purification Steps | Volume (mL) | Protein (mg) | Activity (U) | Specific Activity (U/mg) | Recovery (%) | Fold |
|---|---|---|---|---|---|---|
| Supernate | 500 | 770 | 24,244.8 | 31.5 | 100.0 | 1.0 |
| AS (90%) | 30 | 104 | 19,164.9 | 184.3 | 79.0 | 5.9 |
| DEAE-(phenyl) | 0.44 | 1.16 | 3905.9 | 3367.2 | 20.4 | 18.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Lin, X.; Fu, J.; Zhang, J.; Tang, W.; He, Z. A Novel Bi-Functional Fibrinolytic Enzyme with Anticoagulant and Thrombolytic Activities from a Marine-Derived Fungus Aspergillus versicolor ZLH-1. Mar. Drugs 2022, 20, 356. https://doi.org/10.3390/md20060356
Zhao L, Lin X, Fu J, Zhang J, Tang W, He Z. A Novel Bi-Functional Fibrinolytic Enzyme with Anticoagulant and Thrombolytic Activities from a Marine-Derived Fungus Aspergillus versicolor ZLH-1. Marine Drugs. 2022; 20(6):356. https://doi.org/10.3390/md20060356
Chicago/Turabian StyleZhao, Lihong, Xiuping Lin, Jingyun Fu, Jun Zhang, Wei Tang, and Zengguo He. 2022. "A Novel Bi-Functional Fibrinolytic Enzyme with Anticoagulant and Thrombolytic Activities from a Marine-Derived Fungus Aspergillus versicolor ZLH-1" Marine Drugs 20, no. 6: 356. https://doi.org/10.3390/md20060356
APA StyleZhao, L., Lin, X., Fu, J., Zhang, J., Tang, W., & He, Z. (2022). A Novel Bi-Functional Fibrinolytic Enzyme with Anticoagulant and Thrombolytic Activities from a Marine-Derived Fungus Aspergillus versicolor ZLH-1. Marine Drugs, 20(6), 356. https://doi.org/10.3390/md20060356







