Abstract
Aquacultured fish are the richest natural source of protein. However, their overproduced biomass is often discarded due to production imbalance, causing considerable losses to the fishery industry. Therefore, it is necessary to utilize surplus fish and add value to overproduced fish. We performed complex enzyme-assisted hydrolysis to determine the correlation between its physical characteristics and anti-hypertensive activity in vitro and in vivo using an SHR model. Protamex-Pepsin assisted hydrolysate from Paralichthys olivaceus (POppH) produced by complex enzyme-assisted hydrolysis contained low-molecular-weight peptides and amino acids with anti-hypertensive activity. POppH regulated blood pressure and serum angiotensin II and angiotensin-I-converting enzyme levels, and histological and ultrasound image analysis revealed substantially reduced thickness and diameter of the carotid aorta in the POppH-administered SHR group. Therefore, we propose to reduce food loss due to overproduction by utilizing the anti-hypertensive activity and physical properties of POppH; the results demonstrate its application as a therapeutic agent.
1. Introduction
Worldwide, approximately one-third of food produced for human consumption is lost or wasted from farm to table, amounting to around 1.3 billion tons per year [1]. In the United States, more than 35 million tons of food went into landfills in 2018 [2]. Wasted food generates severe changes in the marine and terrestrial environments. COVID-19 has exposed the vulnerabilities of food systems and heightened the need to mitigate food loss and waste, both locally and globally [3]. The Food and Agriculture Organization of the United Nations (FAO) stresses the importance of changing perceptions and providing solutions to food loss and wastage [4].
Paralichthys olivaceus (P. olivaceus) or olive flounder, belonging to the genus Paralichthys, is frequently found in sandy bottoms at 10–200 m. Based on the global statistics of olive flounder production, Korea is the major producer of olive flounder. In 2007, 77.6% (44,245 t) of the global olive flounder supply came from Korea. Olive flounder is often referred to as the Korean flatfish and has been the topmost aquacultured finfish in Korea over the past few decades [5]. However, the expansion of aquaculture production has created several problems, including rice reduction, high mortality due to various diseases, and a sudden increase in olive flounder production resulted in a considerable decrease in their price in the domestic aquaculture fish market. In 2019, 10,634 t of over-produced olive flounder was discarded in Jeju, Korea [6]. Earlier studies have shown that overproduction and oversupply lead to a price drop due to an imbalance of demand and supply of fish [6]. Therefore, the utilization and processing of over-produced olive flounders can increase their prices and provide a practical solution to overcome the problems associated with overproduction.
Marine fish-derived protein hydrolysates and peptides have remarkable anti-hypertensive properties [7]. Numerous studies have evaluated the effect of angiotensin-I-converting enzyme (ACE) inhibition in vitro and the anti-hypertensive mechanism underlying the lowering of blood pressure or expansion of blood vessels [8]. Our previous studies reported that a protein hydrolysate of Paralichthys olivaceus inhibited ACE activity and lowered systolic and diastolic blood pressure in spontaneously hypertensive rat [9]. This study focused on the biological properties of hydrolysates obtained from single enzyme-assisted hydrolysis of Paralichthys olivaceus. However, further studies are needed to determine the correlation between their biological activities and physical characteristics, especially the molecular weight of products obtained from single and complex enzyme-assisted hydrolysis of Paralichthys olivaceus.
Garcia et al. reported that products of two-stage protamex-pepsin hydrolysis had higher antioxidant, anti-hypertensive, and anti-inflammatory activities than those obtained by one-step protease hydrolysis [10]. In addition, low-molecular-weight peptides with molecular weights less than 1 kDa have greater mobility and diffusivity than high-molecular-weight molecules [11,12]. The physical and compositional characteristics of these hydrolysates are related to their functionality. In this study, we evaluated the potent anti-hypertensive activities of hydrolysates obtained from single and complex enzyme-assisted hydrolysis of Paralichthys olivaceus and determined the correlation between their physical characteristics and anti-hypertensive activities. In addition, by discovering the functionality of hydrolysates, we identified the utility of over-produced fish as a therapeutic agent, thus preventing food loss.
2. Results
2.1. Preparation of POpH and POppH and Their ACE Inhibitory Activities
Protamex-assisted hydrolysate from Paralichthys olivaceus (POpH) and protamex-pepsin assisted hydrolysate from Paralichthys olivaceus (POppH) were prepared to establish the enzyme-assisted hydrolysis method and optimize the antihypertensive activity of POpH (Figure 1). As shown in Table 1, the angiotensin-I-converting enzyme (ACE) inhibitory activity of POppH was higher than that of POpH. The IC50 values of ACE for POpH and POppH were 127.88 ± 1.32 µg/mL and 103.85 ± 0.97 µg/mL, respectively. These results suggest that ACE inhibition can be increased on the group of complex enzyme-assisted hydrolysis.
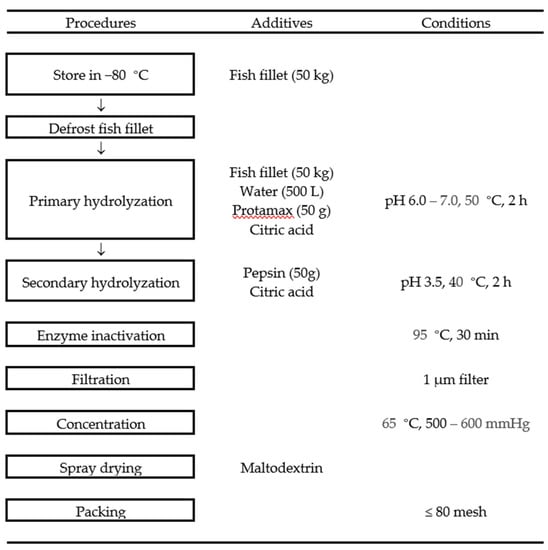
Figure 1.
Preparation of POpH and POppH.

Table 1.
ACE inhibitory activity of POpH and POppH.
2.2. Physical Characterization of POpH and POppH
The physical properties of POpH and POppH were determined using LC-MS, SEM, and viscosity analysis. The molecular distributions of POpH and POppH, summarized in Figure 2A, were found to be widely distributed between 300 and 2399 m/z; smaller molecular distributions were observed in POppH than POpH. The molecular distributions of POpH had a maximum of 300–2399 m/z, and the molecular distributions in POppH ranged from 299–1199 m/z. POpH mainly had distributions in the range 600–899 m/z (36%), whereas POppH mainly had distributions in the range, 300–599 m/z (47%). The molecular mass results of POpH and POppH are presented in Figure S1. The average molecular weights of POpH and POppH determined using MALS revealed that they had molecular masses of g/mol and g/mol, respectively. The surface morphologies of POpH and POppH were observed using field-emission SEM (FE-SEM). The SEM images are shown in Figure 2B. The SEM images of POpH revealed an inconsistent surface and mainly had flattened particles with rough surfaces. A rounded and smooth surface morphology was observed for POppH under 1.00 kx magnification. The temperature- and shear-rate-dependent viscosities were analyzed for POpH and POppH. As shown in Figure 2C, large differences in viscosity η values were found for POpH. However, relatively small differences in viscosity η values were found for POppH. In addition, the shear-rate-dependent viscosity results revealed that the viscosity η of POpH was not detected in the range, − , and its values gradually increased from . The shear-rate-dependent viscosity of POppH revealed constant viscosity η values in POpH. These results suggest that the temperature- and shear-rate-dependent viscosity characteristics were better maintained in POppH than in POpH.
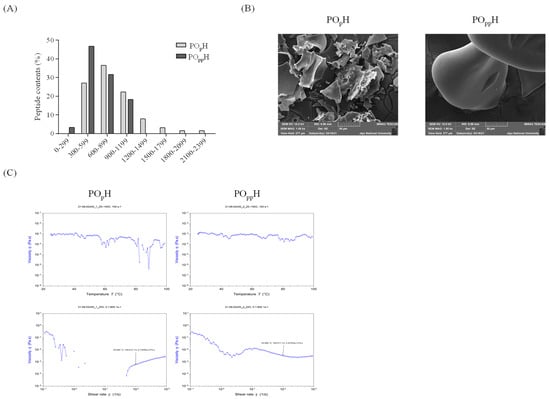
Figure 2.
Physical characteristics of POpH and POppH. (A) Molecular distributions, (B) morphological SEM images, (C) heat and shear activated viscosity.
2.3. Amino Acid Profiles of POpH and POppH
The amino acid compositions of POpH and POppH were analyzed, and the results are summarized in Table 2. According to amino acid profiling, POpH and POppH are composed of 18 amino acids: 12 essential amino acids (histidine; His, arginine; Arg, threonine; Thr, proline; Pro, tyrosine; Try, valine; Val, methionine; Met, isoleucine; Ile, leucine; Leu, phenylalanine; Phe, tryptophan; Trp, lysine; Lys), and 6 non-essential amino acids (cysteine, Cys; aspartic acid, Asp; glutamine, Glu; serine, Ser; glycine, Gly; alanine, and Ala). POpH and POppH had high levels of Ala, Asp, Glu, Arg, Leu, Lys and all these amino acids were slightly increased with complex enzyme assisted hydrolyzation.

Table 2.
Amino acid compositions of POpH and POppH.
2.4. POppH Reduces SBP and DBP in the SHR Model
The changes in SBP and DBP were evaluated as a measure of the antihypertensive activity of POppH during the eight-week experimental period. The initial (week 0) average values of SBP (187.38 ± 12.14 mmHg, n = 8) and DPB (126.92 ± 13.07 mmHg, n = 8) revealed the rats had hypertension at the beginning of the in vivo study. The blood pressure results presented in Figure 3 reveal that SBP and DBP were considerably downregulated from week 7 to 8 in the groups treated with low and high concentrations of POppH compared with those of the SHR groups. L-POppH decreased SBP (160.75 ± 13.82 mmHg) and DBP (93.79 ± 23.10 mmHg), and H-POppH lowered SBP (156.82 ± 28.34 mmHg) and DBP (81.69 ± 12.25 mmHg) at week 8.
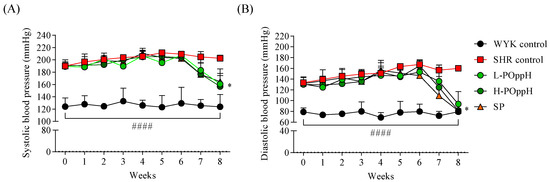
Figure 3.
Changes in systolic and diastolic blood pressure after oral administration. (A) Systolic and (B) diastolic blood pressure. (  ) WYK control (water); (
) WYK control (water); (  ) SHR control (water); (
) SHR control (water); (  ) L-POppH (50 mg/kg of POppH); (
) L-POppH (50 mg/kg of POppH); (  ) H-POppH (100 mg/kg of POppH); (
) H-POppH (100 mg/kg of POppH); (  ) SP (50 mg/kg of SP). Data are expressed as the mean ± standard deviation (SD), (n = 4) in each group. Significant differences were identified at * p < 0.05 as compared to the SHR and #### p < 0.0001 as compared to WYK control.
) SP (50 mg/kg of SP). Data are expressed as the mean ± standard deviation (SD), (n = 4) in each group. Significant differences were identified at * p < 0.05 as compared to the SHR and #### p < 0.0001 as compared to WYK control.
2.5. Effect of POppH on Rat Blood Serum Biochemical Indices
To evaluate the antihypertensive effect of POppH, blood serum angiotensin II (ANG II) and angiotensin-I-converting enzyme (ACE) levels were analyzed to confirm inhibitory effect of POppH on blood ANG II and ACE (Table 3). The ANG II level was remarkably lowered in the L-POppH (1729.47 ± 429.05 pg/mL) and H-POppH (1445.30 ± 253.30 pg/mL) groups. In addition, ACE levels significantly declined in the H-POppH group compared with that in the SHR group. ACE levels considerably decreased to 8.40 ± 0.78 ng/mL in the H-POppH group.

Table 3.
Effect of POppH on serum biochemistry in SHRs.
2.6. Measurement of the Thickness and Ultrasound Imaging of the Carotid Aorta
The effect of POppH on the cross-sectional area of the aorta was observed using H&E staining. As shown in Figure 4A, the H&E results revealed a thicker aorta in the SHR group than in the WKY group. However, the thickness of the aorta was significantly reduced in SHRs in the POppH groups. In particular, the H-POppH group had markedly reduced aorta thickness by 1.38 ± 0.15-fold relative to the SHR group (1.82 ± 0.12-fold, ** p < 0.001). To determine the diameters of the carotid aorta, ultrasound observation was performed by modifying a method by Jin et al. [13]. As shown in Figure 4B, the carotid aorta images revealed a significantly increase in carotid aorta diameter of H-POppH treated group by 1.11 ± 0.03-fold relative to the SHR group (1.06 ± 0.03-fold, **** p < 0.0001).
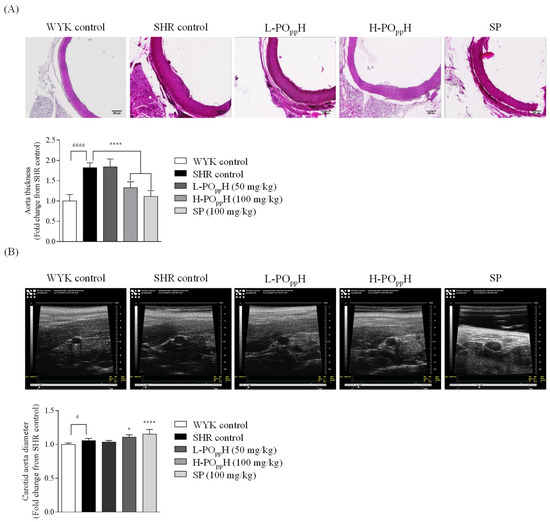
Figure 4.
Histologic and ultrasound graphic analysis of the aorta in SHRs. (A) H&E staining images and (B) ultrasound graphic images. Data are expressed as the mean ± standard deviation (SD), (n = 3) in each group. Significant differences were identified at * p < 0.05 and **** p < 0.0001, as compared to the SHR control, and # p < 0.05 and #### p < 0.0001, as compared to WYK control.
3. Discussion
Hypertension is one of the main mediators of cardiovascular diseases [14]. Elevated central blood flow is highly burdensome and induces damage in tissues and organs, including the heart, kidney, brain, and blood vessels, ultimately resulting in organ dysfunction and failure [15]. Katz et al. reported that most patients with end-organ injuries showed a high rate of chronic and acute hypertension [16]. Therefore, the management and initial control of blood pressure (BP) could help minimize the risk of outbreak of hypertension. Recent research with animals revealed that marine fish hydrolysate and its bioactive peptide have strong angiotensin-I-converting enzyme (ACE) inhibitory activity in vitro and exponentially ameliorate blood pressure [9,17,18,19,20]. However, most studies on marine fish hydrolysate have focused on their biological properties and adopted single-enzyme-assisted hydrolysis to collect fish hydrolysates. Furthermore, the relative biological activities of single and complex enzyme-assisted hydrolysates and the subsequent changes in physical characteristics have not been fully investigated. Here, protamex hydrolysis was adopted as a one-step hydrolysis, and two-step protamex-pepsin hydrolysis was performed on fish fillets from Paralichthys olivaceus to assess their potent antihypertensive activities depending on changes in their physical characteristics.
The molecular distribution results revealed the presence of relatively low molecular weight peptide in POppH compared to that in POpH, implying that the low molecular weight peptide was concentrated in POppH during the two-step hydrolysis. Further, the average molecular weights of POpH and POppH were g/mol and g/mol, respectively. Lin et al. reported the increased potency of ACE inhibitory activity and the potential of antihypertensive properties of low molecular weight protein hydrolysates [21]. Morphologic images of POpH and POppH revealed that the surface morphologies changed during complex enzyme-assisted hydrolysis. The surface of POpH showed comparatively irregular patterns and rough surface particles. However, a smooth and rounded surface was observed for POppH. The temperature- and shear-rate-dependent viscosity results indicated that the physical characteristics, especially temperature- and shear-rate-dependent viscosity, were highly maintained in POppH compared to those in POpH. The amino acid compositions of POpH and POppH showed an increase in the Ala, Asp, Glu, Arg, Leu, Lys in POppH relative to that in POpH. These results correspond with those of previously published reports on the ACE inhibitory activity of marine fish-derived peptides [9,22,23]. Moreover, the POppH contained ACE inhibitory peptides, including Ala and Leu, indicating that POppH might have potential antihypertensive properties [24]. Lee et al. reported that the ACE inhibitory activity is closely associated with the degree of enzyme hydrolysis and peptide sequences and their amino acid composition [25]. The physical analysis results indicated that the physical and chemical characteristics changed with two-step hydrolysis. In particular, the low-molecular-weight peptides and antihypertensive amino acids were found to be concentrated by two-step hydrolysis. In addition, POppH maintained a constant viscosity under temperature- and shear-rate-dependent conditions. These results suggest that compared with single enzyme-assisted hydrolysis, the complex enzyme-assisted hydrolysis markedly increased the antihypertensive potential by increasing the low molecular peptide and antihypertensive amino acid content.
The in vitro ACE inhibitory activities revealed that ACE inhibition was significantly increased in POppH (IC50, 0.43 ± 0.03 mg/mL). Earlier reports by Ko et al. (2016) indicate that the pepsin-assisted hydrolysate from flounder fish showed 50% of ACE inhibition at 1.26 ± 0.14 mg/mL. These results demonstrated the ACE inhibitory activity was increased through the two-step protamex-pepsin enzyme-assisted hydrolysis, thereby influencing the selection of POppH for further in vivo animal antihypertensive studies. In vivo, hypertension was successfully induced in the SHR model (SBP: 187.38 ± 12.14 mmHg, DBP: 126.92 ± 13.07 mmHg). WKY rats maintained an SBP of 123.99 ± 14.13 mmHg and a DBP of 79.14 ± 7.20 mmHg during the initial steps of the experiment. During the eight weeks of SBP and DBP monitoring, high SBP and DBP was maintained in the SHR and POppH groups from weeks 0 to 6. However, SBP and DBP significantly decreased from week 7 in the H-POppH group compared to those in the SHR control group. However, the dose dependent SBP and DBP lowering effect of POppH on the SHR model could not be found. Nonetheless, our findings indicate that the critical concentrations of POppH on SBP and DBP were 100–200 mg/kg. Based on blood serum analysis, serum angiotensin II (ANG II) and ACE levels were significantly decreased in the POppH groups. These results correspond with those of previous reports on ANG II and ACE activation [26,27]. Chappell reported the functions of ANG II and ACE on vasorelaxation in humans [28]. POppH was found to significantly lower SBP and DBP by regulating serum ANG II and ACE levels. Histological analysis indicated that the thickness of the aorta was markedly reduced following H-POppH administration. These results correspond with those of Ashkan et al., who demonstrated the relationship between aortic wall thickness and aortic distensibility [29]. Overall, our findings suggest that H-POppH could ameliorate hypertension-induced aorta or blood vessel hypertrophy in SHRs. Moreover, ultrasound image analysis demonstrated that supplementation with POppH remarkably increased the carotid aorta diameter. Collectively, these results imply that the oral administration of POppH significantly reduced SBP and DBP by regulating ANG II and ACE levels. Furthermore, the oral administration of POppH can reduce the risk of aortic and cardiac hypertrophy.
4. Materials and Methods
4.1. Materials and Chemicals
Commercial protamex was purchased from Novo Co. (Novo Nordisk, Bagsvaerd, Denmark). Pepsin was purchased from Chongqing Jiangxia Biochemistry Pharmaceutical Co., Ltd. (Chongqing, China). The in vitro ACE kit-WST was purchased from Dojindo Inc. (Kumamoto, Japan). Serum angiotensin II (ANG II) and angiotensin-I-converting enzyme (ACE) analysis kits were purchased from LS Bio (Washington, DC, USA). All chemicals and reagents were of analytical grade.
4.2. Preparation of Enzymatic Hydrolysate from Paralichthys olivaceus
Paralichthys olivaceus were obtained from a local fish farm on Jeju Island, Korea. The fish were filleted, washed with tap water, and stored at −80 °C. The frozen fish fillet was defrosted, and 50 kg of fish fillet was hydrolyzed with protamex (50 g) for 2 h under optimal conditions (pH 6.00–7.00, 50 °C). After protamex-assisted hydrolysis, the pH of hydrolysate of Paralichthys olivaceus (POpH) was adjusted to 3.50 with citric acid and additional 50 g of pepsin was added. The pepsin-assisted hydrolysis was continued for 2 h under 40 °C, after which, the protamex-pepsin were inactivated at 95 °C for 30 min. The mixtures were subsequently filtered (pore size: 1 µm), and the filtrate was concentrated in a vacuum concentrator (60 °C, 500–600 mmHg) up to 20 brix. The concentrated solutions were then mixed with maltodextrin and spray-dried under optimal conditions (inlet: 165–180, outlet: 70–90, rpm: 10,000). Thereafter, the spray-dried samples were stored in a freezer at −20 °C before use. Finally, the resulting protamex-pepsin assisted hydrolysate of Paralichthys olivaceus was named as POppH (Lot No. SW1K11SA).
4.3. ACE Inhibitory Activity
The ACE inhibitory effect of POpH and POppH was determined using a commercial ACE assay kit (Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according to the manufacturer’s instructions.
4.4. Molecular Distribution Based on Liquid Chromatography-Mass Spectrometry (LC-MS)
LC-MS analysis was performed to derive the molecular weight distributions of POpH and POppH. The mass spectra were acquired using an UltiMate 3000 system (Dionex, Sunnyvale, CA, USA) coupled with a microQ-TOF III mass spectrometer (Bruker Corporation, 255748, Bremen, Germany). ZORBAX 300SB-C18 (1.0 × 150 mm, 3.5 µm, Agilent) was used as the separation column. The tested samples were directly infused into positive-mode ESI sources at a speed of 100 µL/min. The MS scan range was 200–2000 m/z, and the MS parameters were as follows: capillary voltage, 4500 V; dry temperature: 180 °C; funnel 1RF, 400; funnel 2RF, 400; ISCID energy, 0 eV; Hexapole RF, 250; Ion Energy, 5.0 eV; Low Mass, 300 m/z; Collision Energy, 7 eV; Collision RF, 600; Transfer Time, 80 µs; and Pre Puls Storage, 10 µs). To measure the molecular weight distributions, distilled water with 0.2% formic acid was used as the mobile phase (A), while acetonitrile containing 0.2% formic acid was used as the stationary phase (B). The tested samples were eluted using a gradient of mobile phase. The tested samples were eluted using a gradient of mobile phase (A) and stationary phase (B) at a flow rate of 100 µL/min, and the wavelength of detection was 280 nm. The following gradient elution program was employed: 0–4 min, 95:95–5:5 v/v; 4–5 min, 95:90–5:10 v/v; 5–25 min, 90:70–10:30 v/v; 25–30 min, 70:5–30:95 v/v; 30–40 min, 5:5–95:95 v/v; and 40–46 min, 5:95–95:5 v/v.
4.5. Determining Average Molecular Weight by Multi-Angle Light Scattering (MALS)
To determine the average molecular weight, MALS analysis was performed using DAWN Heleos II multi-angle light scattering coupled with a Shimadzu HPLC system connected to a PL aquagel-OH MIXED-H (7.5 × 300 mm, Agilent Technologies, Santa Clara, CA, USA). The analytical sample was dissolved in 500 mM NaCl and filtered through a membrane filter (pore size: 0.22 µm). The filtered samples were subsequently loaded and eluted with 0.5 mol/L NaCl at a flow rate of 0.5 mL/min. The MALS data were analyzed using the ASTRA 6 software (Wyatt Technologies, Santa Barbara, CA, USA).
4.6. Rheometry
A rotational rheometer (ARES-G2, TA Instruments Ltd., Newcastle, DE, USA) was used to assess the temperature–shear rate dependent viscosity of POpH and POppH. The temperature-dependent viscosities of POpH and POppH were evaluated at 20, 40, 60, 80, and 100 °C, and the shear-rate-dependent viscosity was measured in the range of to . The following parameters were applied: minimum transducer torque in oscillation: 0.05 N·m; minimum transducer torque in steady shear, 0.1 N·m; maximum transducer torque, 200 mN·m; transducer torque resolution, 1 nN·m; strain resolution at drive motor, 0.04; measuring geometry, 25 mm plate; and measuring Gap, 1 mm.
4.7. Scanning Electron Microscopy (SEM)
Surface morphologies were determined using field emission scanning electron microscopy (FE-SEM; MIRA 3 TESCAN, Brno, Czech Republic) coupled with energy dispersive X-ray spectrometry (EDS). The sample was mounted on circular aluminum stubs, coating the carbon tape. After the sample was pretreated, the stub was introduced into the FE-SEM device, and the surface morphology and structure were analyzed using FE-SEM (SEM HV: 15.0 kV, magnification: 1.00 kx).
4.8. Amino Acid Composition
General amino acid profiles were analyzed using an amino acid auto analyzer coupled with an HPLC system (Waters, Milford, MA, USA) equipped with a Pico-Tag reverse-phase column (3.9 × 300 mm, pore size: 4 µm). For amino acid analysis, solvent A (140 mM sodium acetate, 6%(v/v) ACN, pH 5.9) and solvent B (60%(v/v) ACN) were used as mobile phases, and gradient separation was performed at a flow rate of 1 mL/min. The amino acids were detected in a Waters 2487 UV detector at 254 nm. Data were analyzed using Waters Empower 2 software. The following gradient elution of solvent A and B was employed: 0–9 min, 100:0–86:14 v/v; 9–9.2 min, 86:14–80:20 v/v; 9.2–17.5 min, 80:20–54:46 v/v; 17.5–17.7 min, 54:46–0:100 v/v; and 17.7–21 min, 0:100–100:0 v/v.
4.9. Animal Studies
Thirty-two male SHRs and eight male Wistar rats (WKY) (age of rats, 5 weeks old) were purchased from a commercial vendor, Jung Ang Lab Animal Inc. (Seoul, Korea). All animals had free access to tap water and chow diet containing proteins (15.2%), lipids (2.9%), cellulose (4.1%), nitrogen-free extract (60.7%), moisture (12.1%), and mineral ash (5.0%). Rats were housed in a controlled room under optimal temperature (20–22 °C), humidity (40–60%), and a 12:12 light/dark cycle. Animals were allowed to acclimate to the environment for two weeks. Thereafter, the animals were randomly divided into five groups (n = 8 in each group): normal control (WYK), negative control (SHR), positive control (sardine peptide (SP)), low (L-POppH), and high (H-POppH) dosage groups. The L-POppH and H-POppH mice were orally administered 50 and 100 mg/kg of POppH once daily, respectively. The positive control groups were orally administered 100 mg/kg SP once daily, and the normal and negative control groups were administered 0.9% saline. Systolic blood pressure (SBP) and Diastolic blood pressure (DBP) were monitored weekly using a CODATM tail-cuff blood pressure system (Kent Scientific Corp., Torrington, CT, USA). All experimental rats were sacrificed to retrieve their kidney, heart, and aorta tissues for further histological experiments. The animal study was approved by the International Animal Care and Use Committee (IACUC) of Jeju National University (approval number: 2020-0025, 13 July 2020).
4.10. Blood Serum Profiles
Blood was collected from rat heart via a cardiac puncture using an EDTA-rinsed syringe. Subsequently, the blood was transferred into a heparin-coated blood collection tube. Blood serum was allowed to coagulate for 1 h before centrifugation (3000 rpm, 15 min, 4 °C). Thereafter, the supernatant was carefully collected and stored at −80 °C.
4.11. Histology
Histological analysis was performed by dissecting the rat aorta. The isolated aorta tissues were fixed in 10% formalin solution and dehydrated before embedding in paraffin. Subsequently, the blocks of paraffin-embedded aorta tissue were cut into 3-µm sections using a tissue processor machine, placed on an albumin-coated slide, and dried at 37 °C for 24 h. Thereafter, the slides were deparaffinized in xylene, stained with hematoxylin and eosin (H&E) staining, and rinsed three times with deionized water. The slides were mounted with DPX mounting solution (Sigma Chemical Co., St. Louis, MO, USA). Histologic images were obtained using Lionheart FX Automated Microscope (BioTek Instruments, Inc., Winooski, VT, USA). The thickness of rat aorta tissues was measured using ImageJ software (version 1.4).
4.12. Ultrasound Image Analysis
SHRs (8 weeks old) were anesthetized with diethyl ether and O2 gas through a vevo compact anesthesia system. Carotid artery images were observed using the modified methods of Phaeng et al. with a Vevo 770 small animal ultrasound imaging scanner and single-element crystal mechanical imaging transducer (RMV 704; VisualSonics Inc., Toronto, ON, Canada) [30]. The diameter of the carotid aorta was quantified using MATLAB software (Math Works Inc., Natick, MA, USA).
4.13. Statistical Analysis
All measurements were performed in triplicate and are presented as mean ± standard deviation (SD) using the statistical package, GraphPad Prism (Version 6; GraphPad Software Inc., San Diego, CA, USA). One-way ANOVA with Duncan’s test was used to assess differences between the groups. p-values in the following limits were considered significant: * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001 compared with the negative SHR control group; and # p < 0.05, ## p < 0.01, ### p < 0.001, and #### p < 0.0001 compared with the normal WYK control group.
5. Conclusions
In conclusion, our findings revealed that complex enzyme-assisted hydrolysis, similar to two-step protamex-pepsin enzyme-assisted hydrolysis, successfully increased the low molecular weight peptide. Moreover, physical characteristics, such as viscosity, were highly maintained in POppH. The oral administration of POppH potentially caused SBP and DBP lowering by downregulating angiotensin II and down-regulating of angiotensin-I-converting enzyme levels. Taken together, these results indicate that POppH can be utilized as an anti-hypertensive agent. Further, this study provides a rationale for clinical studies on low-molecular-weight peptides from Paralichthys olivaceus used as anti-hypertensive functional food or agents. The anti-hypertensive activity of Paralichthys olivaceus by-products could minimize the loss of aquaculture fisheries and food waste.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/md20060346/s1, Figure S1: Molecular distributions of POpH and POppH.
Author Contributions
H.-G.L.: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing—original draft preparation, Writing—review and editing; J.-Y.O.: Software, Resources; D.-M.C., M.-Y.S. and S.-J.P.: Software, Resources, Data curation; Y.-J.J. and B.-M.R.: Conceptualization, Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a part of the project titled ‘Development of functional food products with natural materials derived from marine resources (No. 20170285)’, funded by the Ministry of Oceans and Fisheries, Korea.
Institutional Review Board Statement
The animal study was approved by the International Animal Care and Use Committee (IACUC) of Jeju National University (approval number: 2020-0025, 13 July 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by a part of the project titled ‘Development of functional food products with natural materials derived from marine resources (No. 20170285)’, funded by the Ministry of Oceans and Fisheries, Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liao, F.; Qing, P.; Hou, M.-H. Analysis on the influencing factors of consumers’ wasting food behaviors: Based on the theory of planned behaviors. Res. Agric. Mod. 2020, 41, 115–124. [Google Scholar]
- Zhongming, Z.; Linong, L.; Wangqiang, Z.; Wei, L. Key Messages on the International Day of Awareness of Food Loss and Waste; United States Department of Agriculture: Washington, DC, USA, 2021.
- Rivera-Ferre, M.G.; López-i-Gelats, F.; Ravera, F.; Oteros-Rozas, E.; di Masso, M.; Binimelis, R.; El Bilali, H. The relation of food systems with the COVID19 pandemic: Causes and consequences. Agric. Syst. 2021, 191, 103134. [Google Scholar] [CrossRef]
- Loboguerrero, A.M.; Campbell, B.M.; Cooper, P.J.; Hansen, J.W.; Rosenstock, T.; Wollenberg, E. Food and earth systems: Priorities for climate change adaptation and mitigation for agriculture and food systems. Sustainability 2019, 11, 1372. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.C.; Lee, S. Culture of olive flounder: Korean perspective. In Practical Flatfish Culture and Stock Enhancement; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 156–168. [Google Scholar]
- Getu, A.; Misganaw, K.; Bazezew, M. Post-harvesting and major related problems of fish production. Fish. Aquac. J. 2015, 6, 1000154. [Google Scholar] [CrossRef]
- UG, Y.; Bhat, I.; Karunasagar, I.; Mamatha, B.S. Antihypertensive activity of fish protein hydrolysates and its peptides. Crit. Rev. Food Sci. Nutr. 2019, 59, 2363–2374. [Google Scholar]
- Ishak, N.H.; Shaik, M.I.; Yellapu, N.K.; Howell, N.K.; Sarbon, N.M. Purification, characterization and molecular docking study of angiotensin-I converting enzyme (ACE) inhibitory peptide from shortfin scad (Decapterus macrosoma) protein hydrolysate. J. Food Sci. Technol. 2021, 58, 1–11. [Google Scholar] [CrossRef]
- Oh, J.-Y.; Kim, E.-A.; Lee, H.; Kim, H.-S.; Lee, J.-S.; Jeon, Y.-J. Antihypertensive effect of surimi prepared from olive flounder (Paralichthys olivaceus) by angiotensin-I converting enzyme (ACE) inhibitory activity and characterization of ACE inhibitory peptides. Process Biochem. 2019, 80, 164–170. [Google Scholar] [CrossRef]
- García, J.; Méndez, D.; Álvarez, M.; Sanmartin, B.; Vázquez, R.; Regueiro, L.; Atanassova, M. Design of novel functional food products enriched with bioactive extracts from holothurians for meeting the nutritional needs of the elderly. LWT 2019, 109, 55–62. [Google Scholar] [CrossRef]
- Ishak, N.; Sarbon, N. A review of protein hydrolysates and bioactive peptides deriving from wastes generated by fish processing. Food Bioprocess Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Karoud, W.; Sila, A.; Krichen, F.; Martinez-Alvarez, O.; Bougatef, A. Characterization, surface properties and biological activities of protein hydrolysates obtained from hake (Merluccius merluccius) heads. Waste Biomass Valorization 2019, 10, 287–297. [Google Scholar] [CrossRef]
- Jin, C.; Nam, K.-H.; Paeng, D.-G. Asymmetric pulsation of rat carotid artery bifurcation in three-dimension observed by ultrasound imaging. Int. J. Cardiovasc. Imaging 2016, 32, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, J. Central hemodynamics and target organ damage in hypertension. Tohoku J. Exp. Med. 2014, 233, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, J.N.; Gore, J.M.; Amin, A.; Anderson, F.A.; Dasta, J.F.; Ferguson, J.J.; Kleinschmidt, K.; Mayer, S.A.; Multz, A.S.; Peacock, W.F. Practice patterns, outcomes, and end-organ dysfunction for patients with acute severe hypertension: The Studying the Treatment of Acute hyperTension (STAT) registry. Am. Heart J. 2009, 158, 599–606.e1. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ryu, B.; Zhang, Y.; Liang, P.; Li, C.; Zhou, C.; Yang, P.; Hong, P.; Qian, Z.J. Comparison of an angiotensin-I-converting enzyme inhibitory peptide from tilapia (Oreochromis niloticus) with captopril: Inhibition kinetics, in vivo effect, simulated gastrointestinal digestion and a molecular docking study. J. Sci. Food Agric. 2020, 100, 315–324. [Google Scholar] [CrossRef]
- Fu, W.; Wang, P.; Wu, H.; Zhang, Z.; Zeng, H.; Zhang, Y.; Zheng, B.; Hu, J. Antihypertensive effects of Trichiurus lepturus myosin hydrolysate in spontaneously hypertensive rats. Food Funct. 2020, 11, 3645–3656. [Google Scholar] [CrossRef]
- Je, J.-G.; Kim, H.-S.; Lee, H.-G.; Oh, J.-Y.; Lu, Y.A.; Wang, L.; Rho, S.; Jeon, Y.-J. Low-molecular weight peptides isolated from seahorse (Hippocampus abdominalis) improve vasodilation via inhibition of angiotensin-converting enzyme in vivo and in vitro. Process Biochem. 2020, 95, 30–35. [Google Scholar] [CrossRef]
- Oh, J.-Y.; Je, J.-G.; Lee, H.-G.; Kim, E.-A.; Kang, S.I.; Lee, J.-S.; Jeon, Y.-J. Anti-Hypertensive Activity of Novel Peptides Identified from Olive Flounder (Paralichthys olivaceus) Surimi. Foods 2020, 9, 647. [Google Scholar] [CrossRef]
- Lin, F.; Chen, L.; Liang, R.; Zhang, Z.; Wang, J.; Cai, M.; Li, Y. Pilot-scale production of low molecular weight peptides from corn wet milling byproducts and the antihypertensive effects in vivo and in vitro. Food Chem. 2011, 124, 801–807. [Google Scholar] [CrossRef]
- Gu, R.-Z.; Li, C.-Y.; Liu, W.-Y.; Yi, W.-X.; Cai, M.-Y. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res. Int. 2011, 44, 1536–1540. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Antihypertensive effect of novel angiotensin I converting enzyme inhibitory peptide from chum salmon (Oncorhynchus keta) skin in spontaneously hypertensive rats. J. Funct. Foods 2014, 7, 381–389. [Google Scholar] [CrossRef]
- Hong, F.; Ming, L.; Yi, S.; Zhanxia, L.; Yongquan, W.; Chi, L. The antihypertensive effect of peptides: A novel alternative to drugs? Peptides 2008, 29, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.J.; Shenoy, V.; Yamazato, Y.; Sriramula, S.; Francis, J.; Yuan, L.; Castellano, R.K.; Ostrov, D.A.; Oh, S.P.; Katovich, M.J. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2009, 179, 1048–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández Prada, J.A.; Ferreira, A.J.; Katovich, M.J.; Shenoy, V.; Qi, Y.; Santos, R.A.; Castellano, R.K.; Lampkins, A.J.; Gubala, V.; Ostrov, D.A. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension 2008, 51, 1312–1317. [Google Scholar] [CrossRef] [Green Version]
- Chappell, M.C. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: More than regulation of blood pressure? Hypertension 2007, 50, 596–599. [Google Scholar] [CrossRef] [Green Version]
- Malayeri, A.A.; Natori, S.; Bahrami, H.; Bertoni, A.G.; Kronmal, R.; Lima, J.A.; Bluemke, D.A. Relation of aortic wall thickness and distensibility to cardiovascular risk factors (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2008, 102, 491–496. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.-Z.; Nam, K.-H.; Paeng, D.-G. The spatio-temporal variation of rat carotid artery bifurcation by ultrasound imaging. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; IEEE: New York, NY, USA, 2014; pp. 1900–1903. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).