Development of a High-Performance Thin-Layer Chromatography Method for the Quantification of Alkyl Glycerolipids and Alkenyl Glycerolipids from Shark and Chimera Oils and Tissues
Abstract
:1. Introduction
2. Results and Discussion
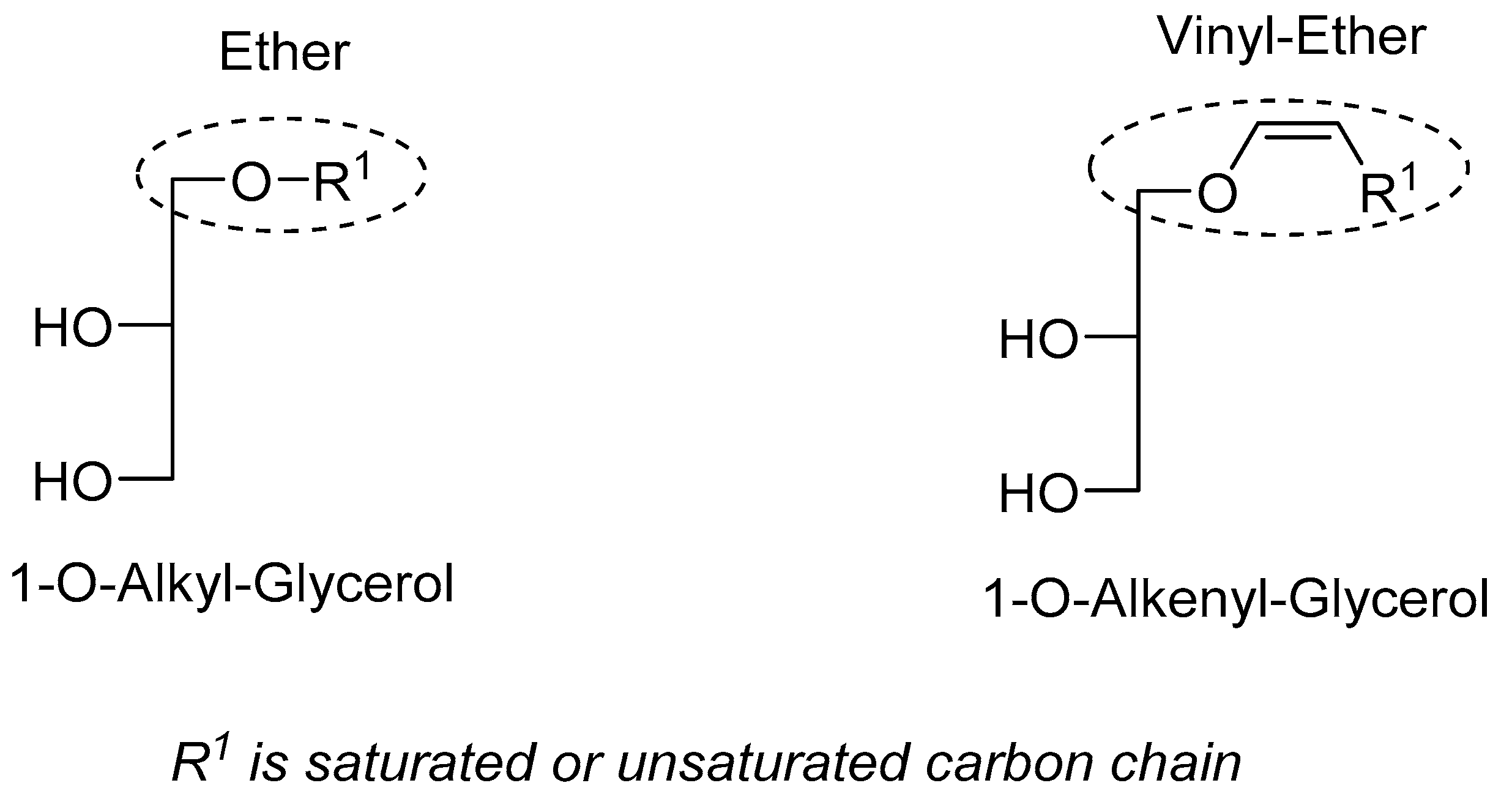
2.1. Principle of the Method
2.2. HPTLC Method Validation Using Standards
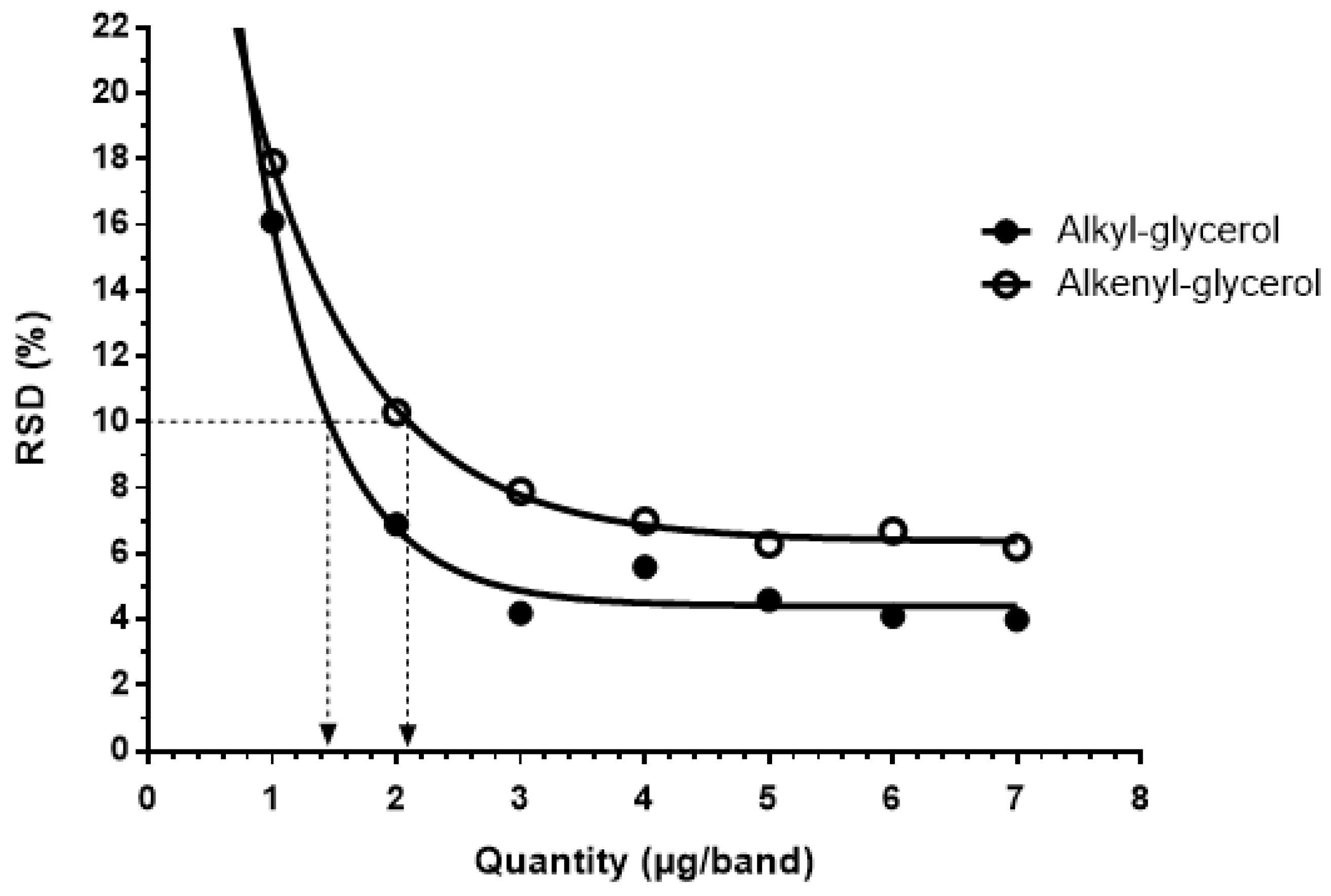
2.3. Statistical Analysis of the Validation
2.4. Application for Biological Samples
2.5. Use of This Method in Combination with Lipidomics
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. HPTLC Apparatus
3.3. Chromatographic Conditions
3.4. Standard Solution Preparation
3.5. Method Validation
3.6. Statistical Tests
3.7. Applications to Biological Samples
3.7.1. Non-Human Primate Heart Samples
3.7.2. Periprostatic Adipose Tissue Samples
3.7.3. Tumor Samples
3.8. Lipid Extraction, Reduction and Purification
3.8.1. Lipid Extraction
3.8.2. Lipid Reduction
3.8.3. Purification
3.9. Acid Hydrolysis
3.10. 1H NMR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nagan, N.; Zoeller, R.A. Plasmalogens: Biosynthesis and Functions. Prog. Lipid Res. 2001, 40, 199–229. [Google Scholar] [CrossRef]
- Magnusson, C.D.; Haraldsson, G.G. Ether Lipids. Chem. Phys. Lipids 2011, 164, 315–340. [Google Scholar] [CrossRef] [PubMed]
- Pradas, I.; Huynh, K.; Cabré, R.; Ayala, V.; Meikle, P.J.; Jové, M.; Pamplona, R. Lipidomics Reveals a Tissue-Specific Fingerprint. Front. Physiol. 2018, 9, 1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, D.H.; Anderson, C.E. Ether-Linked Glycerolipids in Human Brain Tumors. Lipids 1977, 12, 188–192. [Google Scholar] [CrossRef]
- Snyder, F.; Wood, R. Alkyl and Alk-1-Enyl Ethers of Glycerol in Lipids from Normal and Neoplastic Human Tissues. Cancer Res. 1969, 29, 251–257. [Google Scholar]
- Mueller, H.W.; O’Flaherty, J.T.; Greene, D.G.; Samuel, M.P.; Wykle, R.L. 1-O-Alkyl-Linked Glycerophospholipids of Human Neutrophils: Distribution of Arachidonate and Other Acyl Residues in the Ether-Linked and Diacyl Species. J. Lipid Res. 1984, 25, 383–388. [Google Scholar] [CrossRef]
- Chabot, M.C.; Greene, D.G.; Brockschmidt, J.K.; Capizzi, R.L.; Wykle, R.L. Ether-Linked Phosphoglyceride Content of Human Leukemia Cells. Cancer Res. 1990, 50, 7174–7178. [Google Scholar]
- Braverman, N.E.; Moser, A.B. Functions of Plasmalogen Lipids in Health and Disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [Green Version]
- Fontaine, D.; Figiel, S.; Félix, R.; Kouba, S.; Fromont, G.; Mahéo, K.; Potier-Cartereau, M.; Chantôme, A.; Vandier, C. Roles of Endogenous Ether Lipids and Associated PUFAs in the Regulation of Ion Channels and Their Relevance for Disease. J. Lipid Res. 2020, 61, 840–858. [Google Scholar] [CrossRef] [Green Version]
- Dean, J.M.; Lodhi, I.J. Structural and Functional Roles of Ether Lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- Jiménez-Rojo, N.; Riezman, H. On the Road to Unraveling the Molecular Functions of Ether Lipids. FEBS Lett. 2019, 593, 2378–2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorgas, K.; Teigler, A.; Komljenovic, D.; Just, W.W. The Ether Lipid-Deficient Mouse: Tracking down Plasmalogen Functions. Biochim. Biophys. Acta 2006, 1763, 1511–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichosz, G.; Czeczot, H. Milk fat—The only existing for any reason. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2015, 38, 174–179. [Google Scholar]
- Moukarzel, S.; Dyer, R.A.; Keller, B.O.; Elango, R.; Innis, S.M. Human Milk Plasmalogens Are Highly Enriched in Long-Chain PUFAs. J. Nutr. 2016, 146, 2412–2417. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Dilbaz, S.; Coßmann, J.; Hoang, A.C.; Diedrich, V.; Herwig, A.; Harauma, A.; Hoshi, Y.; Moriguchi, T.; Landgraf, K.; et al. Breast Milk Alkylglycerols Sustain Beige Adipocytes through Adipose Tissue Macrophages. J. Clin. Investig. 2019, 129, 2485–2499. [Google Scholar] [CrossRef] [Green Version]
- Pinault, M.; Guimaraes, C.; Couthon, H.; Thibonnet, J.; Fontaine, D.; Chantome, A.; Chevalier, S.; Besson, P.; Jaffres, P.A.; Vandier, C. Synthesis of Alkyl-Glycerolipids Standards for Gas Chromatography Analysis: Application for Chimera and Shark Liver Oils. Mar. Drugs 2018, 16, 101. [Google Scholar] [CrossRef] [Green Version]
- Rangholia, N.; Leisner, T.M.; Holly, S.P. Bioactive Ether Lipids: Primordial Modulators of Cellular Signaling. Metabolites 2021, 11, 41. [Google Scholar] [CrossRef]
- Koch, J.; Lackner, K.; Wohlfarter, Y.; Sailer, S.; Zschocke, J.; Werner, E.R.; Watschinger, K.; Keller, M.A. Unequivocal Mapping of Molecular Ether Lipid Species by LC-MS/MS in Plasmalogen-Deficient Mice. Anal. Chem. 2020, 92, 11268–11276. [Google Scholar] [CrossRef]
- Branch, S.K. Guidelines from the International Conference on Harmonisation (ICH). J. Pharm. Biomed. Anal. 2005, 38, 798–805. [Google Scholar] [CrossRef]
- Renkonen, O. Chromatographic Separation of Plasmalogenic, Alkyl-Acyl, and Diacyl Forms of Ethanolamine Glycerophosphatides. J. Lipid Res. 1968, 9, 34–39. [Google Scholar] [CrossRef]
- Spangenberg, B.; Poole, C.F.; Weins, C. Planning an Analysis and Validation in TLC. In Quantitative Thin-Layer Chromatography; Springer: Berlin/Heidelberg, Germany, 2010; pp. 353–373. ISBN 978-3-642-10727-6. [Google Scholar]
- Shabir, G.A.; John Lough, W.; Arain, S.A.; Bradshaw, T.K. Evaluation and Application of Best Practice in Analytical Method Validation. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 311–333. [Google Scholar] [CrossRef]
- Taguchi, R.; Ishikawa, M. Precise and Global Identification of Phospholipid Molecular Species by an Orbitrap Mass Spectrometer and Automated Search Engine Lipid Search. J. Chromatogr. A 2010, 1217, 4229–4239. [Google Scholar] [CrossRef] [PubMed]
- Mawatari, S.; Hazeyama, S.; Fujino, T. Measurement of Ether Phospholipids in Human Plasma with HPLC-ELSD and LC/ESI-MS After Hydrolysis of Plasma with Phospholipase A1. Lipids 2016, 51, 997–1006. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.J.; Stephens, R.; Jurkowitz-Alexander, M.; Horrocks, L.A. Acidic Hydrolysis of Plasmalogens Followed by High-Performance Liquid Chromatography. Lipids 1993, 28, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.; Byun, H.-S.; Bittman, R. Synthesis of Plasmalogen via 2,3-Bis-O-(4‘-Methoxybenzyl)-Sn-Glycerol. J. Am. Chem. Soc. 1999, 121, 662–668. [Google Scholar] [CrossRef]
- Jayalakshmi, K.; Sonkar, K.; Behari, A.; Kapoor, V.K.; Sinha, N. Lipid Profiling of Cancerous and Benign Gallbladder Tissues by 1H NMR Spectroscopy. NMR Biomed. 2011, 24, 335–342. [Google Scholar] [CrossRef]
- Hardy, R.; Mackie, P.R. Observations on the Chemical Composition and Toxicity of Ratfish (Chimaera monstrosa). J. Sci. Food Agric. 1971, 22, 382–388. [Google Scholar] [CrossRef]
- Cífková, E.; Lísa, M.; Hrstka, R.; Vrána, D.; Gatěk, J.; Melichar, B.; Holčapek, M. Correlation of Lipidomic Composition of Cell Lines and Tissues of Breast Cancer Patients Using Hydrophilic Interaction Liquid Chromatography/Electrospray Ionization Mass Spectrometry and Multivariate Data Analysis. Rapid Commun. Mass Spectrom. RCM 2017, 31, 253–263. [Google Scholar] [CrossRef]
- Pinault, M.; Guimaraes, C.; Ben Hassen, C.; Gutierrez-Pajares, J.L.; Chevalier, S.; Goupille, C.; Bernard-Savary, P.; Frank, P.G. Development of a Novel High-Performance Thin Layer Chromatography-Based Method for the Simultaneous Quantification of Clinically Relevant Lipids from Cells and Tissue Extracts. Lipids 2020, 55, 403–412. [Google Scholar] [CrossRef]
- Khoury, S.; Canlet, C.; Lacroix, M.Z.; Berdeaux, O.; Jouhet, J.; Bertrand-Michel, J. Quantification of Lipids: Model, Reality, and Compromise. Biomolecules 2018, 8, E174. [Google Scholar] [CrossRef] [Green Version]
- Köfeler, H.C.; Fauland, A.; Rechberger, G.N.; Trötzmüller, M. Mass Spectrometry Based Lipidomics: An Overview of Technological Platforms. Metabolites 2012, 2, 19–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, M.; Angelidou, G.; Ni, Z.; Criscuolo, A.; Schiller, J.; Blüher, M.; Fedorova, M. AdipoAtlas: A Reference Lipidome for Human White Adipose Tissue. Cell Rep. Med. 2021, 2, 100407. [Google Scholar] [CrossRef] [PubMed]
- West, A.; Zoni, V.; Teague, W.E.; Leonard, A.N.; Vanni, S.; Gawrisch, K.; Tristram-Nagle, S.; Sachs, J.N.; Klauda, J.B. How Do Ethanolamine Plasmalogens Contribute to Order and Structure of Neurological Membranes? J. Phys. Chem. B 2020, 124, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Chantome, A.; Potier-Cartereau, M.; Clarysse, L.; Fromont, G.; Marionneau-Lambot, S.; Gueguinou, M.; Pages, J.C.; Collin, C.; Oullier, T.; Girault, A.; et al. Pivotal Role of the Lipid Raft SK3-Orai1 Complex in Human Cancer Cell Migration and Bone Metastases. Cancer Res. 2013, 73, 4852–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]



| Ether Glycerol Classes | Petroleum Ether: Diethyl Ether: Acetic Acid (30:70:0.5; v/v/v) | ||
|---|---|---|---|
| Rf Start | Rf Max | Rf End | |
| Alkyl glycerol | 0.294 | 0.337 | 0.379 |
| Alkenyl glycerol | 0.404 | 0.444 | 0.485 |
| Ether Glycerol Classes | Method 1 a | Method 2 b | Method 3 c | |||
|---|---|---|---|---|---|---|
| LOD (ng/band) | LOQ (ng/band) | LOD (ng/band) | LOQ (ng/band) | LOD (ng/band) | LOQ (ng/band) | |
| Alkyl glycerol | 436 | 1459 | 407 | 1235 | / | ~1450 |
| Alkenyl glycerol | 716 | 2388 | 776 | 2352 | / | ~2100 |
| Ether Glycerol Classes | Quantity (ng/band) | Repeatability (%) | Intermediate Precision (%) | LOL (ng/band) | Cochran | ANOVA Validation Fisher’s Test |
|---|---|---|---|---|---|---|
| Ccalc < Cref | ||||||
| (p < 0.01) | ||||||
| Alkyl glycerol | 1000 | 12.4 | 16.1 | 7000 | 0.3192 < 0.397 | Accepted |
| 2000 | 7.1 | 6.9 | ||||
| 3000 | 6.4 | -- | ||||
| 4000 | 6.1 | 5.6 | ||||
| 6000 | 3.8 | 4.1 | ||||
| 7000 | 3.6 | 4.0 | ||||
| Alkenyl glycerol | 1000 | 18.6 | 17.9 | 7000 | 0.3387 < 0.418 | Accepted |
| 2000 | 17.3 | 10.3 | ||||
| 3000 | 15.2 | 7.9 | ||||
| 4000 | 6.5 | 7 | ||||
| 6000 | 5.9 | 6.7 | ||||
| 7000 | 5.7 | 6.2 |
| Ether Glycerol Classes | Amount of Ether Glycerol Spotted (µg/band) | Percentage of Ether Glycerol Added (%) | Theoretical Amount (µg/band) | Amount Determined (Mean ± SD) (µg/band) | % Recovery (Mean ± SD) |
|---|---|---|---|---|---|
| Alkyl glycerol | 2.00 | 50 | 3.00 | 2.92 ± 0.08 | 97.17 ± 2.56 |
| 2.00 | 100 | 4.00 | 3.98 ± 0.06 | 99.62 ± 1.44 | |
| 2.00 | 150 | 5.00 | 5.02 ± 0.07 | 100.66 ± 1.84 | |
| Alkenyl glycerol | 2.00 | 50 | 3.00 | 2.89 ± 0.01 | 96.39 ± 0.26 |
| 2.00 | 100 | 4.00 | 3.95 ± 0.13 | 98.68 ± 3.21 | |
| 2.00 | 150 | 5.00 | 5.04 ± 0.14 | 100.84 ± 2.90 |
| Ether Glycerol Classes | Alkyl Glycerol | Alkenyl Glycerol |
|---|---|---|
| Specificity | Yes | Yes |
| Linearity range (ng/band) a | 1000–7000 | 1000–7000 |
| Linear regression equation | 19,088x + 17,600 | 13,687x + 6778 |
| Slope ± SD | 19,088 ± 3199 | 13,687 ± 3294 |
| Regression coefficient (r2) ± SD | 0.9940 ± 0.0025 | 0.9932 ± 0.0044 |
| Limit of detection (ng/band) b | 407 | 776 |
| Limit of quantification (ng/band) b | 1235 | 2352 |
| Repeatability (%) a | 3.6–12.4 | 5.7–18.6 |
| Intermediate precision (%) a | 4.0–16.1 | 6.2–17.9 |
| Tissue | Tissue Weight (mg) Median [Min–Max] | Total Lipids Weight (mg) Median [Min–Max] |
|---|---|---|
| Chimera liver oil | / | 31.3 [28.9–34.0] |
| Shark liver oil | / | 31.7 [29.5–34.6] |
| PPAT | 966.8 [356.7–1679.0] | 580.2 [182.0–1241.9] |
| Non-Human Primate Heart | 637.6 [565.5–2443.1] | 27.6 [23.6–36.6] |
| Tumors | 335.4 [150.7–440.0] | 15.2 [3.4–23.7] |
| Tissue | Lipids Weight for Reduction/Vitride (mg) Median [Min–Max] | Reduced Lipids Weight (mg) Median [Min–Max] |
|---|---|---|
| Chimera liver oil | 31.3 [28.9–34.0] | 32.5 [29.3–35.4] |
| Shark liver oil | 31.7 [29.5–34.6] | 30.3 [28.2–34.2] |
| PPAT | 36.1 [29.3–40.0] | 31.3 [24.2–35.2] |
| Non-Human Primate Heart | 11.7 [7.7–36.6] | 7.8 [6.8–28.3] |
| Tumors | 16.0 [11.1–23.7] | 13.0 [12.9–19.3] |
| Tissue | Lipids Weight for Purification/SPE (mg) Median [Min–Max] | Solubilization Volume (mL) Median [Min–Max] | Volume Deposited (µL) |
|---|---|---|---|
| Chimera liver oil | / | 10.5 [9.4–11.4] | for alkyl glycerols: 5 for alkenyl glycerols: 20 |
| Shark liver oil | / | 10.8 [10.0–11.8] | 10 |
| PPAT | 31.3 [24.2–35.2] | 0.20 | 20 |
| Non-Human Primate Heart | 7.8 [6.8–28.3] | 0.78 | for alkyl glycerols: 30 for alkenyl glycerols: 15 |
| Tumors | 13.0 [12.9–19.3] | 0.50 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papin, M.; Guimaraes, C.; Pierre-Aue, B.; Fontaine, D.; Pardessus, J.; Couthon, H.; Fromont, G.; Mahéo, K.; Chantôme, A.; Vandier, C.; et al. Development of a High-Performance Thin-Layer Chromatography Method for the Quantification of Alkyl Glycerolipids and Alkenyl Glycerolipids from Shark and Chimera Oils and Tissues. Mar. Drugs 2022, 20, 270. https://doi.org/10.3390/md20040270
Papin M, Guimaraes C, Pierre-Aue B, Fontaine D, Pardessus J, Couthon H, Fromont G, Mahéo K, Chantôme A, Vandier C, et al. Development of a High-Performance Thin-Layer Chromatography Method for the Quantification of Alkyl Glycerolipids and Alkenyl Glycerolipids from Shark and Chimera Oils and Tissues. Marine Drugs. 2022; 20(4):270. https://doi.org/10.3390/md20040270
Chicago/Turabian StylePapin, Marion, Cyrille Guimaraes, Benoit Pierre-Aue, Delphine Fontaine, Jeoffrey Pardessus, Hélène Couthon, Gaëlle Fromont, Karine Mahéo, Aurélie Chantôme, Christophe Vandier, and et al. 2022. "Development of a High-Performance Thin-Layer Chromatography Method for the Quantification of Alkyl Glycerolipids and Alkenyl Glycerolipids from Shark and Chimera Oils and Tissues" Marine Drugs 20, no. 4: 270. https://doi.org/10.3390/md20040270
APA StylePapin, M., Guimaraes, C., Pierre-Aue, B., Fontaine, D., Pardessus, J., Couthon, H., Fromont, G., Mahéo, K., Chantôme, A., Vandier, C., & Pinault, M. (2022). Development of a High-Performance Thin-Layer Chromatography Method for the Quantification of Alkyl Glycerolipids and Alkenyl Glycerolipids from Shark and Chimera Oils and Tissues. Marine Drugs, 20(4), 270. https://doi.org/10.3390/md20040270






